Dyeing with Hydrotalcite Hybrid Nanoclays and Disperse, Basic and Direct Dyes
Abstract
1. Introduction
2. Results
2.1. Dye Adsorption Performance
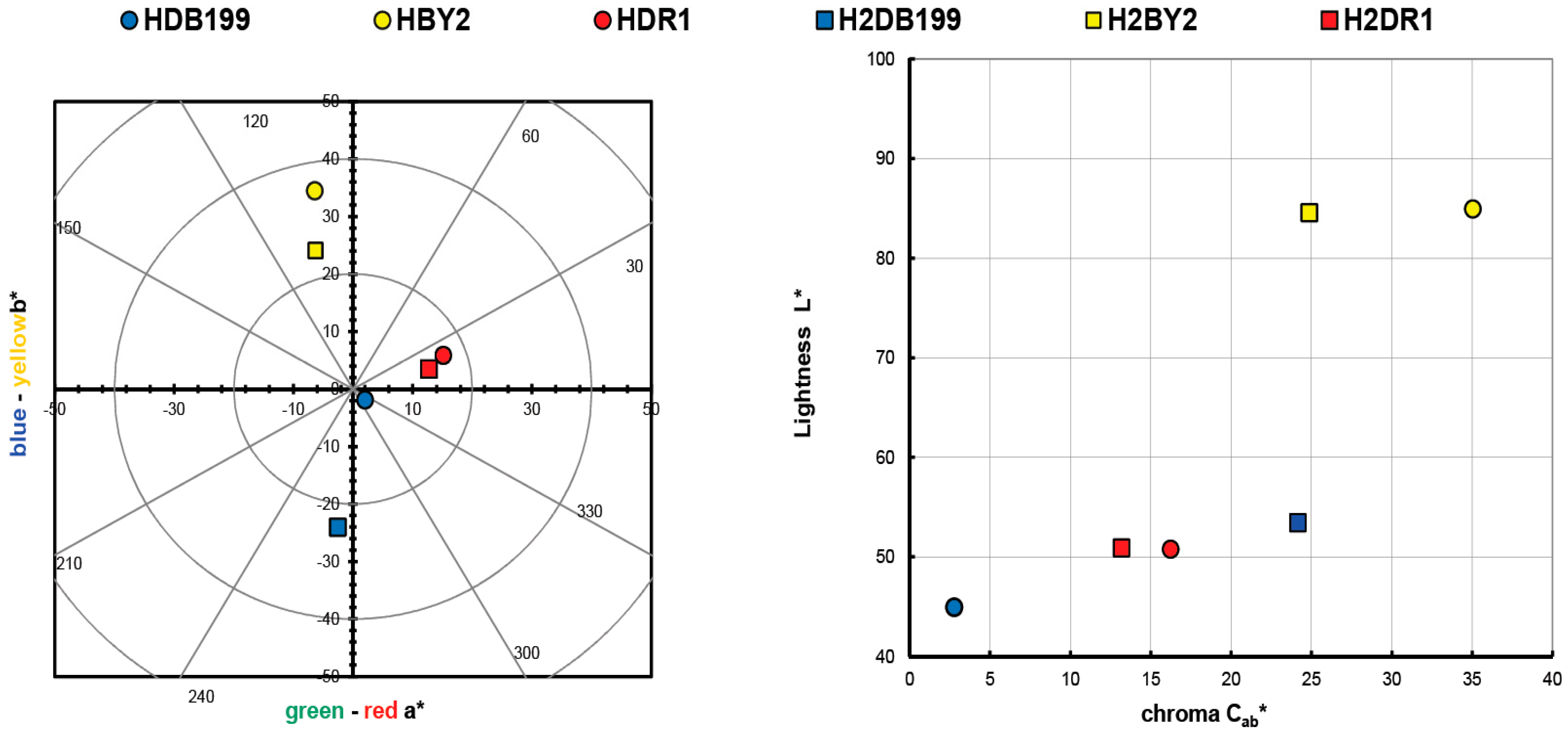
2.2. Hybrid Color Measurements
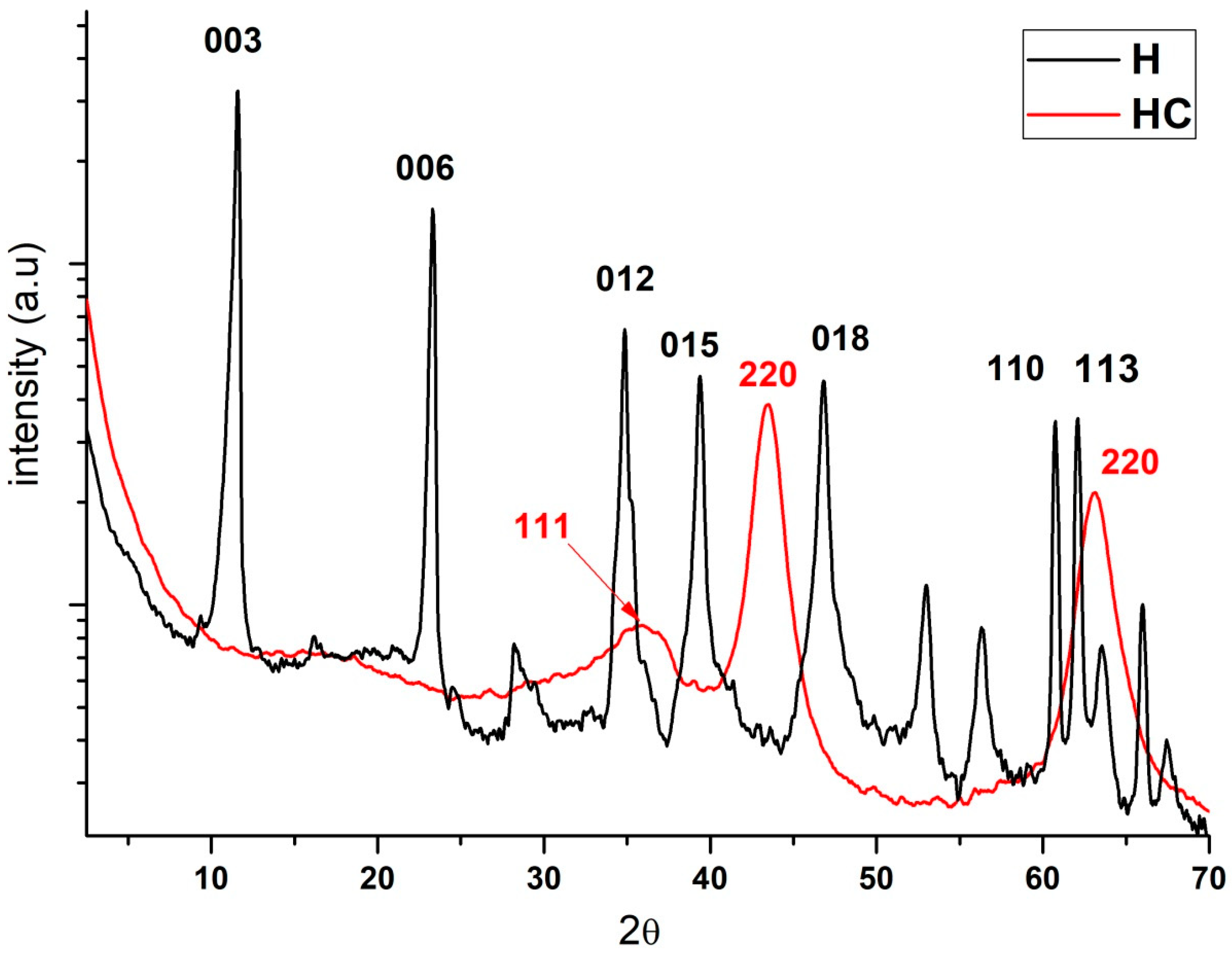
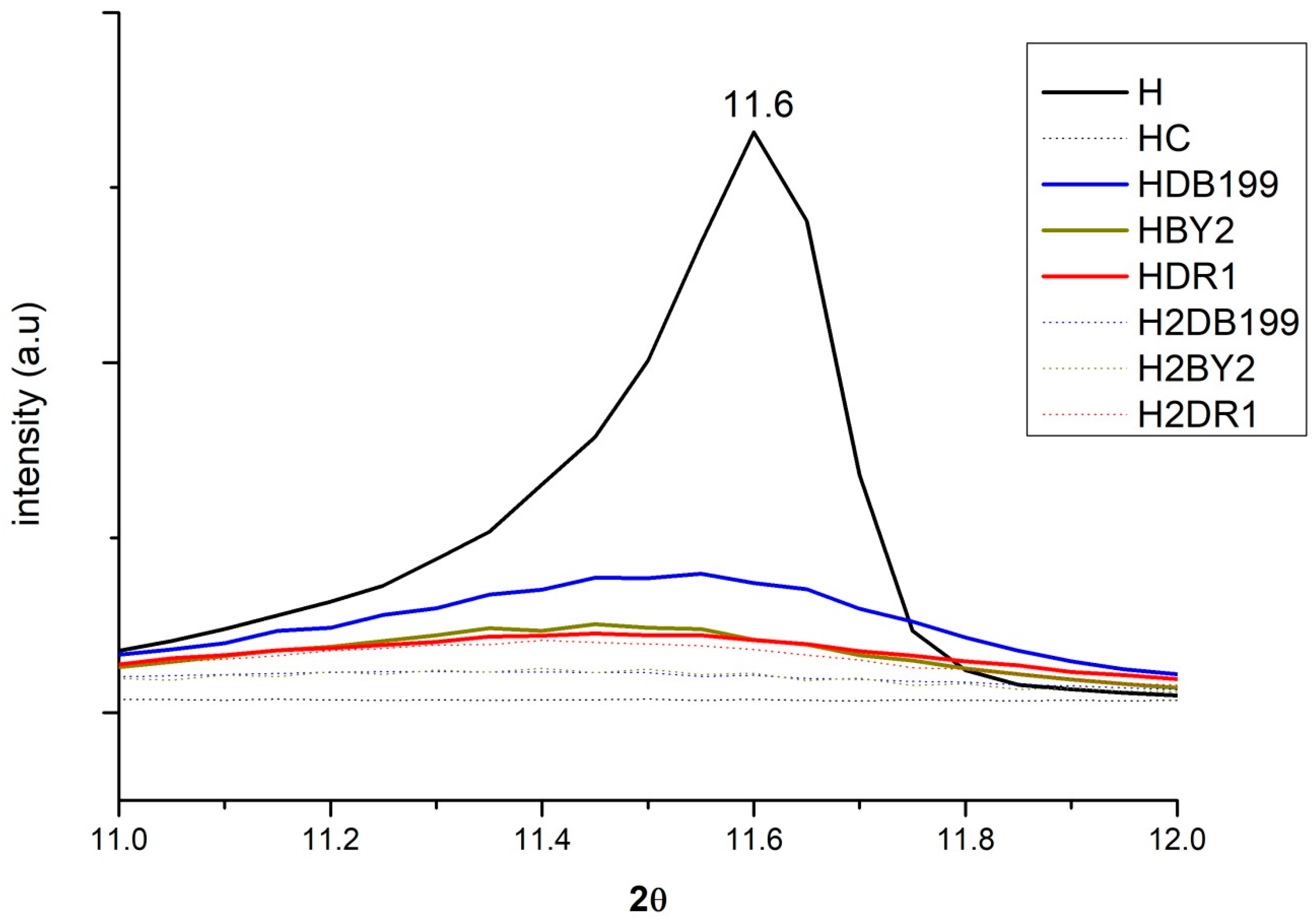
2.3. X-ray Diffraction (XRD)
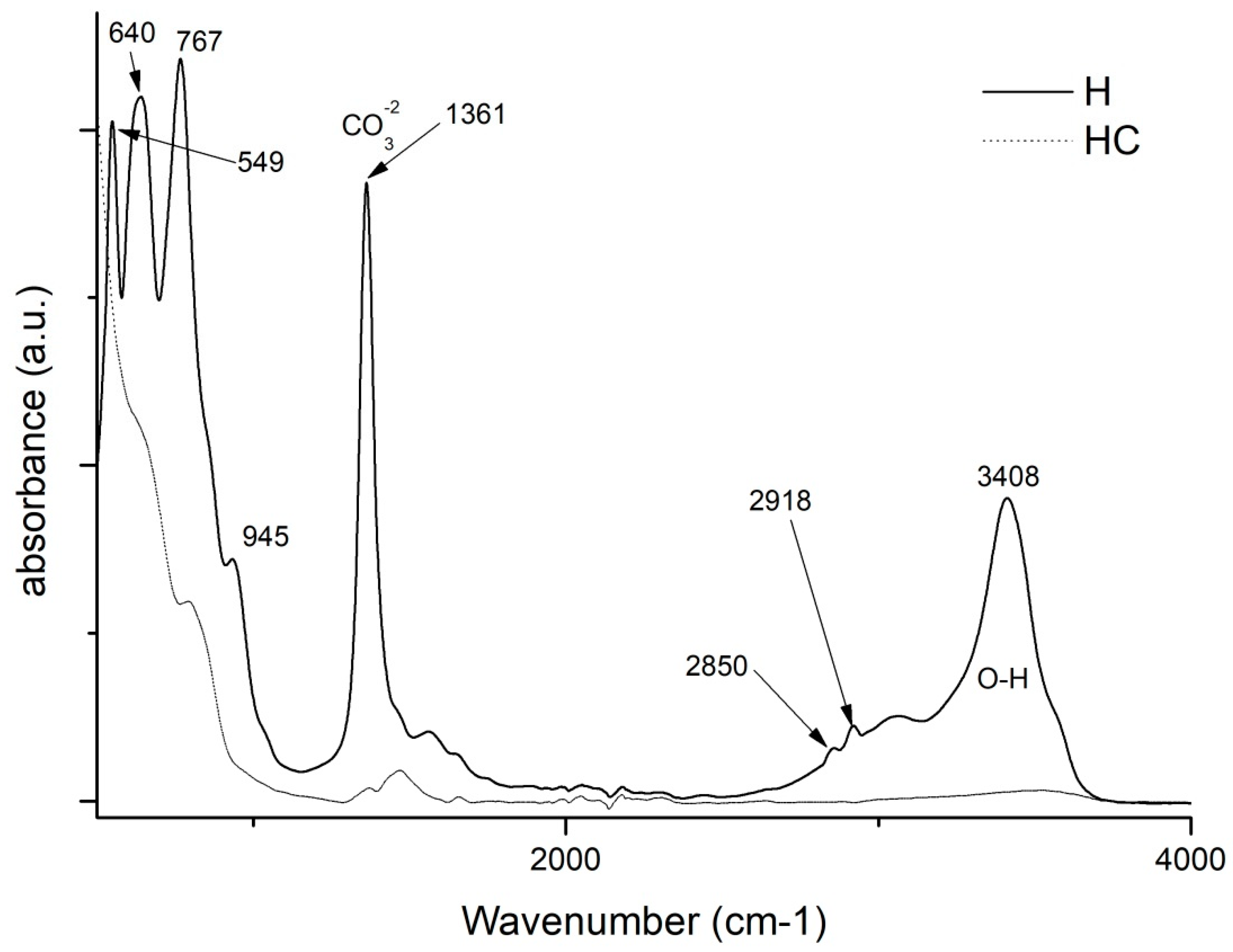
2.4. Fourier Transform Infrared Spectroscopy FTIR-ATR Analysis
2.5. Colour Measurement of the Dyes
2.6. Colour Fastness
2.7. Scanning Electron Microscopy (SEM)
2.8. BET Surface Area and Porosity Measurements
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis Methods
4.3. Characterisation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varadarajan, G.; Venkatachalam, P. Sustainable Textile Dyeing Processes. Environ. Chem. Lett. 2016, 14, 113–122. [Google Scholar] [CrossRef]
- Ramasany, R.; Ahmed, H.A.M.; Karthik, S.S. Development of Microbial Consortium for the Biodegradation and Biodecolorization of Textile Effluents. J. Urban Environ. Eng. 2012, 6, 36–41. [Google Scholar] [CrossRef][Green Version]
- Ponnusami, V.; Srivastava, S.N. Studies on Application of Teak Leaf Powders for the Removal of Color from Synthetic and Industrial Effluents. J. Hazard. Mater. 2009, 169, 1159–1162. [Google Scholar] [CrossRef] [PubMed]
- Ponnusami, V.; Lavanya, N.; Meenal, M.; Raj, R.A.G.; Srivastava, S.N. Application of Nitric Acid Treated Rice Husk for Sorption of Reactive Dye Reactive Black 5: Analysis Using Statistical Experimental Design. Pollut. Res 2008, 27, 45. [Google Scholar]
- Pratibha, R.; Malar, P.; Rajapriya, T.; Balapoornima, S.; Ponnusami, V. Statistical and Equilibrium Studies on Enhancing Biosorption Capacity of Saccharomyces Cerevisiae through Acid Treatment. Desalination 2010, 264, 102–107. [Google Scholar] [CrossRef]
- Hunger, K. Industrial Dyes: Chemistry, Properties, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Luo, Z.; Gao, M.; Ye, Y.; Yang, S. Modification of Reduced-Charge Montmorillonites by a Series of Gemini Surfactants: Characterization and Application in Methyl Orange Removal. Appl. Surf. Sci. 2015, 324, 807–816. [Google Scholar] [CrossRef]
- Gupta, V.K.; Agarwal, S.; Olgun, A.; Demir, H.İ.; Yola, M.L.; Atar, N. Adsorptive Properties of Molasses Modified Boron Enrichment Waste Based Nanoclay for Removal of Basic Dyes. J. Ind. Eng. Chem. 2016, 34, 244–249. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M.; Sánchez, C.; García, F.J.; Medina, J.A.; Bernabé, J.M. Comparison of Saponite and Montmorillonite Behaviour during Static and Stirring Maturation with Seawater for Pelotherapy. Appl. Clay Sci. 2007, 36, 161–173. [Google Scholar] [CrossRef]
- Taleb, K.; Pillin, I.; Grohens, Y.; Saidi-Besbes, S. Gemini Surfactant Modified Clays: Effect of Surfactant Loading and Spacer Length. Appl. Clay Sci. 2018, 161, 48–56. [Google Scholar] [CrossRef]
- Olvera, R.C.; Silva, S.L.; Robles-Belmont, E.; Lau, E.Z. Review of Nanotechnology Value Chain for Water Treatment Applications in Mexico. Resour. Technol. 2017, 3, 1–11. [Google Scholar] [CrossRef]
- López-Rodríguez, D.; Micó-Vicent, B.; Jordán-Núñez, J.; Bonet-Aracil, M.; Bou-Belda, E. Uses of Nanoclays and Adsorbents for Dye Recovery: A Textile Industry Review. Appl. Sci. 2021, 11, 11422. [Google Scholar] [CrossRef]
- Gasser, M.S.; Mekhamer, H.S.; Abdel Rahman, R.O. Optimization of the Utilization of Mg/Fe Hydrotalcite like Compounds in the Removal of Sr(II) from Aqueous Solution. J. Environ. Chem. Eng. 2016, 4, 4619–4630. [Google Scholar] [CrossRef]
- Xi, H.; Li, Q.; Yang, Y.; Zhang, J.; Guo, F.; Wang, X.; Xu, S.; Ruan, S. Highly Effective Removal of Phosphate from Complex Water Environment with Porous Zr-Bentonite Alginate Hydrogel Beads: Facile Synthesis and Adsorption Behavior Study. Appl. Clay Sci. 2021, 201, 105919. [Google Scholar] [CrossRef]
- de Queiroga, L.N.F.; França, D.B.; Rodrigues, F.; Santos, I.M.G.; Fonseca, M.G.; Jaber, M. Functionalized Bentonites for Dye Adsorption: Depollution and Production of New Pigments. J. Environ. Chem. Eng. 2019, 7, 103333. [Google Scholar] [CrossRef]
- Kanani-Jazi, M.H.; Akbari, S. Amino-Dendritic and Carboxyl Functionalized Halloysite Nanotubes for Highly Efficient Removal of Cationic and Anionic Dyes: Kinetic, Isotherm, and Thermodynamic Studies. J. Environ. Chem. Eng. 2021, 9, 105214. [Google Scholar] [CrossRef]
- Parades-Aguilar, J.; Reyes-Martínez, V.; Bustamante, G.; Almendáriz-Tapia, F.J.; Martínez-Meza, G.; Vílchez-Vargas, R.; Link, A.; Certucha-Barragán, M.T.; Calderón, K. Removal of Nickel (II) from Wastewater Using a Zeolite-Packed Anaerobic Bioreactor: Bacterial Diversity and Community Structure Shifts. J. Environ. Manag. 2021, 279, 111558. [Google Scholar] [CrossRef]
- Schoonheydt, R.A.; Heughebaert, L. Clay Adsorbed Dyes: Methylene Blue on Laponite. Clay Miner. 1992, 27, 91–100. [Google Scholar] [CrossRef]
- Yi, J.-Z.; Zhang, L.-M. Removal of Methylene Blue Dye from Aqueous Solution by Adsorption onto Sodium Humate/Polyacrylamide/Clay Hybrid Hydrogels. Bioresour. Technol. 2008, 99, 2182–2186. [Google Scholar] [CrossRef]
- Mittal, H.; Babu, R.; Dabbawala, A.A.; Stephen, S.; Alhassan, S.M. Zeolite-Y Incorporated Karaya Gum Hydrogel Composites for Highly Effective Removal of Cationic Dyes. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124161. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Zhuang, G.; Jiang, R. A New Method to Prepare ‘Maya Red’ Pigment from Sepiolite and Basic Red 46. Appl. Clay Sci. 2019, 174, 38–46. [Google Scholar] [CrossRef]
- Akil, J.; Ciotonea, C.; Siffert, S.; Royer, S.; Pirault-Roy, L.; Cousin, R.; Poupin, C. NO Reduction by CO under Oxidative Conditions over CoCuAl Mixed Oxides Derived from Hydrotalcite-like Compounds: Effect of Water. Catal. Today 2021, 384–286, 97–105. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Zhou, X.; Liu, Z.; Liu, Q.; Li, X. Different Dye Removal Mechanisms between Monodispersed and Uniform Hexagonal Thin Plate-like MgAl–CO32−-LDH and Its Calcined Product in Efficient Removal of Congo Red from Water. J. Alloys Compd. 2016, 673, 265–271. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, S.; Chen, H.; He, H.; Sun, C. Adsorption Behavior and Mechanism of Reactive Brilliant Red X-3B in Aqueous Solution over Three Kinds of Hydrotalcite-like LDHs. Appl. Surf. Sci. 2014, 301, 329–337. [Google Scholar] [CrossRef]
- Shan, R.; Yan, L.; Yang, Y.; Yang, K.; Yu, S.; Yu, H.; Zhu, B.; Du, B. Highly Efficient Removal of Three Red Dyes by Adsorption onto Mg–Al-Layered Double Hydroxide. J. Ind. Eng. Chem. 2015, 21, 561–568. [Google Scholar] [CrossRef]
- Heraldy, E.; Santosa, S.J.; Triyono, T.; Wijaya, K. Anionic and Cationic Dyes Removal from Aqueous Solutions by Adsorption onto Synthetic Mg/Al Hydrotalcite-like Compound. Indones. J. Chem. 2015, 15, 234–241. [Google Scholar] [CrossRef]
- dos Santos, R.M.M.; Gonçalves, R.G.L.; Constantino, V.R.L.; da Costa, L.M.; da Silva, L.H.M.; Tronto, J.; Pinto, F.G. Removal of Acid Green 68:1 from Aqueous Solutions by Calcined and Uncalcined Layered Double Hydroxides. Appl. Clay Sci. 2013, 80–81, 189–195. [Google Scholar] [CrossRef]
- Orthman, J.; Zhu, H.Y.; Lu, G.Q. Use of Anion Clay Hydrotalcite to Remove Coloured Organics from Aqueous Solutions. Sep. Purif. Technol. 2003, 31, 53–59. [Google Scholar] [CrossRef]
- Grum, F.; Witzel, R.F.; Stensby, P. Evaluation of Whiteness. JOSA 1974, 64, 210–215. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Rodríguez, L.; Muñoz, G.; Martin, A.J.; Folgado, M.A.; Daza, L. Biogas Reforming over La-NiMgAl Catalysts Derived from Hydrotalcite-like Structure: Influence of Calcination Temperature. Catal. Commun. 2011, 12, 961–967. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Jordán, J.; Perales, E.; Martínez-Verdú, F.; Cases, F. Finding the Additives Incorporation Moment in Hybrid Natural Pigments Synthesis to Improve Bioresin Properties. Coatings 2019, 9, 34. [Google Scholar] [CrossRef]
- Millange, F.; Walton, R.I.; O’Hare, D. Time-Resolved in Situ X-Ray Diffraction Study of the Liquid-Phase Reconstruction of Mg–Al–Carbonate Hydrotalcite-like Compounds. J. Mater. Chem. 2000, 10, 1713–1720. [Google Scholar] [CrossRef]
- Stanimirova, T.S.; Vergilov, I.; Kirov, G.; Petrova, N. Thermal Decomposition Products of Hydrotalcite-like Compounds: Low-Temperature Metaphases. J. Mater. Sci. 1999, 34, 4153–4161. [Google Scholar] [CrossRef]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-Type Anionic Clays: Preparation, Properties and Applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Dahdah, E.; Estephane, J.; Taleb, Y.; El Khoury, B.; El Nakat, J.; Aouad, S. The Role of Rehydration in Enhancing the Basic Properties of Mg–Al Hydrotalcites for Biodiesel Production. Sustain. Chem. Pharm. 2021, 22, 100487. [Google Scholar] [CrossRef]
- Zhu, M.-X.; Li, Y.-P.; Xie, M.; Xin, H.-Z. Sorption of an Anionic Dye by Uncalcined and Calcined Layered Double Hydroxides: A Case Study. J. Hazard. Mater. 2005, 120, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Lakraimi, M.; Legrouri, A.; Barroug, A.; Besse, J.-P. Removal of Pesticides from Water by Anionic Clays. J. Chim. Phys. Phys.-Chim. Biol. 1999, 96, 470–478. [Google Scholar] [CrossRef]
- Sato, T.; Kato, K.; Endo, T.; Shimada, M. Preparation and Chemical Properties of Magnesium Aluminium Oxide Solid Solutions. React. Solids 1986, 2, 253–260. [Google Scholar] [CrossRef]
- Bernard, E.; Zucha, W.J.; Lothenbach, B.; Mäder, U. Stability of Hydrotalcite (Mg-Al Layered Double Hydroxide) in Presence of Different Anions. Cem. Concr. Res. 2022, 152, 106674. [Google Scholar] [CrossRef]
- Geetha Bhavani, A.; Wani, T.A.; Ma’Aruf, A.; Prasad, T. Effect of Ageing Process on Crystal Morphology of Co-Mg-Al Hydrotalcite. Mater. Today Proc. 2021, 44, 2277–2282. [Google Scholar] [CrossRef]
- Wiyantoko, B.; Kurniawati, P.; Purbaningtias, T.E.; Fatimah, I. Synthesis and Characterization of Hydrotalcite at Different Mg/Al Molar Ratios. Procedia Chem. 2015, 17, 21–26. [Google Scholar] [CrossRef]
- Roelofs, J.C.A.A.; van Bokhoven, J.A.; Van Dillen, A.J.; Geus, J.; de Jong, K.P. The Thermal Decomposition of Mg-A1 Hydrotalcites: Effects of Interlayer Anions and Charateristics of the Final Structure. Chem. Eur. J. 2002, 8, 5571–5579. [Google Scholar] [CrossRef] [PubMed]
- Ziyat, H.; Elmzioui, S.; Naciri Bennani, M.; Houssaini, J.; Allaoui, S.; Arhzaf, S. Kinetic, Isotherm, and Mechanism Investigations of the Removal of Nitrate and Nitrite from Water by the Synthesized Hydrotalcite Mg–Al. Res. Chem. Intermed. 2021, 47, 2605–2627. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, J.; He, H.; Yuan, P.; Shen, W.; Liu, D. Infrared Investigation of Organo-Montmorillonites Prepared from Different Surfactants. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 76, 122–129. [Google Scholar] [CrossRef]
- Lopez, T.; Bosch, P.; Asomoza, M.; Gómez, R.; Ramos, E. DTA-TGA and FTIR Spectroscopies of Sol-Gel Hydrotalcites: Aluminum Source Effect on Physicochemical Properties. Mater. Lett. 1997, 31, 311–316. [Google Scholar] [CrossRef]
- de Souza, T.N.V.; de Carvalho, S.M.L.; Vieira, M.G.A.; da Silva, M.G.C.; Brasil, D.D.S.B. Adsorption of Basic Dyes onto Activated Carbon: Experimental and Theoretical Investigation of Chemical Reactivity of Basic Dyes Using DFT-Based Descriptors. Appl. Surf. Sci. 2018, 448, 662–670. [Google Scholar] [CrossRef]
- López-Rodríguez, D.; Micó-Vicent, B.; Bonet-Aracil, M.; Cases, F.; Bou-Belda, E. The Optimal Concentration of Nanoclay Hydrotalcite for Recovery of Reactive and Direct Textile Colorants. Int. J. Mol. Sci. 2022, 23, 9671. [Google Scholar] [CrossRef]
- Hafshejani, M.K.; Ogugbue, C.J.; Morad, N. Application of Response Surface Methodology for Optimization of Decolorization and Mineralization of Triazo Dye Direct Blue 71 by Pseudomonas Aeruginosa. 3 Biotech 2014, 4, 605–619. [Google Scholar] [CrossRef]
- Extremera, R.; Pavlovic, I.; Pérez, M.R.; Barriga, C. Removal of Acid Orange 10 by Calcined Mg/Al Layered Double Hydroxides from Water and Recovery of the Adsorbed Dye. Chem. Eng. J. 2012, 213, 392–400. [Google Scholar] [CrossRef]
- Hu, C.; Jimmy, C.Y.; Hao, Z.; Wong, P.K. Photocatalytic Degradation of Triazine-Containing Azo Dyes in Aqueous TiO2 Suspensions. Appl. Catal. B Environ. 2003, 42, 47–55. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C. Spectrometric Identification of Organic Compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Öztürk, A.; Malkoc, E. Adsorptive Potential of Cationic Basic Yellow 2 (BY2) Dye onto Natural Untreated Clay (NUC) from Aqueous Phase: Mass Transfer Analysis, Kinetic and Equilibrium Profile. Appl. Surf. Sci. 2014, 299, 105–115. [Google Scholar] [CrossRef]
- Çalışkan, Y.; Harbeck, S.; Bektaş, N. Adsorptive Removal of Basic Yellow Dye Using Bigadiç Zeolites: FTIR Analysis, Kinetics, and Isotherms Modeling. Environ. Prog. Sustain. Energy 2019, 38, S185–S195. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, M.; Chen, L.; Qian, G. Synthesis and Spectroscopic Characterization of an Alkoxysilane Dye Containing C. I. Disperse Red 1. Dye. Pigment. 2004, 62, 43–47. [Google Scholar] [CrossRef]
- Taunaumang, H.; Herman, H.; Tjia, M.O. Molecular Orientation in Disperse Red 1 Thin Film Produced by PVD Method. Opt. Mater. 2001, 18, 343–350. [Google Scholar] [CrossRef]
- Duffner, H.; Bach, E.; Cleve, E.; Schollmeyer, E. New Mathematical Model for Determining Time-Dependent Adsorption and Diffusion of Dyes into Fibers through Dye Sorption Curves in Combination Shades: Part II: Kinetic Data from Dyeing Cotton with a Trichrome Direct Dye System. Text. Res. J. 2000, 70, 223–229. [Google Scholar] [CrossRef]
- Hauser, P.J.; Tabba, A.H. Improving the Environmental and Economic Aspects of Cotton Dyeing Using a Cationised Cotton. Color. Technol. 2001, 117, 282–288. [Google Scholar] [CrossRef]
- Daruwalla, E.H.; Kulkarni, G.G. Studies in the Equilibrium Dyeing of Cotton with Direct Dyes: The Determination of the Activity and Affinity of Direct Dyes for Cellulose. Bull. Chem. Soc. Jpn. 1964, 37, 1250–1261. [Google Scholar] [CrossRef]
- Aspland, J.R. The Application of Basic Dye Cations to Anionic Fibers: Dyeing Acrylic and Other Fibers with Basic Dyes. Text. Chem. Color. 1993, 25, 21–26. [Google Scholar]
- Van Der Kraan, M.; Vanesa Fernandez Cid, M.; Woerlee, G.F.; Veugelers, W.J.T.; Witkamp, G.-J. Equilibrium Study on the Disperse Dyeing of Polyester Textile in Supercritical Carbon Dioxide. Text. Res. J. 2007, 77, 550–558. [Google Scholar] [CrossRef]
- Kim, T.-K.; Son, Y.-A.; Lim, Y.-J. Thermodynamic Parameters of Disperse Dyeing on Several Polyester Fibers Having Different Molecular Structures. Dye. Pigment. 2005, 67, 229–234. [Google Scholar] [CrossRef]
- Ketema, A.; Worku, A. Review on Intermolecular Forces between Dyes Used for Polyester Dyeing and Polyester Fiber. J. Chem. 2020, 2020, 6628404. [Google Scholar] [CrossRef]
- Tichit, D.; Lhouty, M.H.; Guida, A.; Chiche, B.H.; Figueras, F.; Auroux, A.; Bartalini, D.; Garrone, E. Textural Properties and Catalytic Activity of Hydrotalcites. J. Catal. 1995, 151, 50–59. [Google Scholar] [CrossRef]
- Liu, Y.; Lotero, E.; Goodwin, J.G.; Mo, X. Transesterification of Poultry Fat with Methanol Using Mg-Al Hydrotalcite Derived Catalysts. Appl. Catal. A Gen. 2007, 331, 138–148. [Google Scholar] [CrossRef]
- Shen, J.; Tu, M.; Hu, C. Structural and Surface Acid/Base Properties of Hydrotalcite-Derived MgAlO Oxides Calcined at Varying Temperatures. J. Solid State Chem. 1998, 137, 295–301. [Google Scholar] [CrossRef]
- Ulibarri, M.A.; Pavlovic, I.; Barriga, C.; Hermosın, M.C.; Cornejo, J. Adsorption of Anionic Species on Hydrotalcite-like Compounds: Effect of Interlayer Anion and Crystallinity. Appl. Clay Sci. 2001, 18, 17–27. [Google Scholar] [CrossRef]
- Bish, D.L. Anion-Exchange in Takovite: Applications to Other Hydroxide Minerals. Bull. Mineral. 1980, 103, 170–175. [Google Scholar] [CrossRef]
- Miyata, S. Physico-Chemical Properties of Synthetic Hydrotalcites in Relation to Composition. Clays Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Lian, Z.Y.; Zhang, Y.; Wang, X.Z. Manufacturing of High Quality Hydrotalcite by Computational Fluid Dynamics Simulation of an Impinging Jet Crystallizer. Ceram. Int. 2022, 48, 15470–15482. [Google Scholar] [CrossRef]
- Bou-Belda, E.; López-Rodríguez, D.; Micó-Vicent, B.; Bonet-Aracil, M. Direct and Reactive Dyes Recovery in Textile Wastewater Using Calcinated Hydrotalcite. In Materials Science Forum; Trans Tech Publications: Bäch, Switzerland, 2022; Volume 1063, pp. 233–242. [Google Scholar]
- Bigman, J.L. Monitoring of Chemicals and Water. In Handbook of Silicon Wafer Cleaning Technology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 619–657. [Google Scholar] [CrossRef]
- Silva, M.M.F.; Oliveira, M.M.; Avelino, M.C.; Fonseca, M.G.; Almeida, R.K.S.; Silva Filho, E.C. Adsorption of an Industrial Anionic Dye by Modified-KSF-Montmorillonite: Evaluation of the Kinetic, Thermodynamic and Equilibrium Data. Chem. Eng. J. 2012, 203, 259–268. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Martínez Verdú, F.M. Method for Optimising the Synthesis of Hybrid Nanopigments. ES2568833A1 Patent, 4 May 2017. [Google Scholar]
- H. Fischer, L.F.B. Coloring Pigment, 2001. Available online: http://www.artiscreation.com/yellow.html#.Y6pd6hVByUl (accessed on 23 October 2022).
- Limousin, G.; Gaudet, J.-P.; Charlet, L.; Szenknect, S.; Barthes, V.; Krimissa, M. Sorption Isotherms: A Review on Physical Bases, Modeling and Measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Voudrias, E.; Fytianos, K.; Bozani, E. Sorption–Desorption Isotherms of Dyes from Aqueous Solutions and Wastewaters with Different Sorbent Materials. Glob. Nest Int. J. 2002, 4, 75–83. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Porous Structure and Adsorptive Properties of Pineapple Peel Based Activated Carbons Prepared via Microwave Assisted KOH and K2CO3 Activation. Microporous Mesoporous Mater. 2012, 148, 191–195. [Google Scholar] [CrossRef]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Kousha, M.; Naushad, M.; Bhatnagar, A. Desorption of Methylene Blue Dye from Brown Macroalga: Effects of Operating Parameters, Isotherm Study and Kinetic Modeling. J. Clean. Prod. 2017, 152, 443–453. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A Useful Adsorption Isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Momina; Mohammad, S.; Suzylawati, I. Study of the Adsorption/Desorption of MB Dye Solution Using Bentonite Adsorbent Coating. J. Water Process Eng. 2020, 34, 101155. [Google Scholar] [CrossRef]
- Di Natale, C.; Monti, D.; Paolesse, R. Chemical Sensitivity of Porphyrin Assemblies. Mater. Today 2010, 13, 46–52. [Google Scholar] [CrossRef]
- Pálková, H.; Madejová, J.; Zimowska, M.; Bielańska, E.; Olejniczak, Z.; Lityńska-Dobrzyńska, L.; Serwicka, E.M. Laponite-Derived Porous Clay Heterostructures: I. Synthesis and Physicochemical Characterization. Microporous Mesoporous Mater. 2010, 127, 228–236. [Google Scholar] [CrossRef]
- Zhuo, W.; Xie, Y.; Benson, M.T.; Ge, J.; Mariani, R.D.; Zhang, J. XRD and SEM/EDS Characterization of Two Quaternary Fuel Alloys (U-2.5Mo-2.5Ti-5.0Zr and U-1.5Mo-1.5Ti-7.0Zr in Wt. %) for Fast Reactors. Mater. Charact. 2020, 170, 110696. [Google Scholar] [CrossRef]





| Sample | Polarity | Initial Conc. g·L−1 | Final Conc. g·L−1 | Ads (%) |
|---|---|---|---|---|
| HDB199 | Anionic | 1 | 6.57 × 10−4 ± 3.2 × 10−8 | 99.316 |
| HBY2 | Cationic | 1 | 6.22 × 10−4 ± 1.1 × 10−8 | 99.949 |
| HDR1 | Non-ionic | 1 | 6.32 × 10−3 ± 2.9 × 10−9 | 98.122 |
| Sample | L* | a* | b* | C*ab | h |
|---|---|---|---|---|---|
| HDB199 | 44.94 | 2.03 | 1.88 | 2.77 | 317.16 |
| HBY2 | 84.94 | 6.41 | 34.46 | 35.05 | 100.53 |
| HDR1 | 50.77 | 15.14 | 5.86 | 16.24 | 21.17 |
| H2DB199 | 53.43 | −2.53 | 24.03 | 24.17 | 263.98 |
| H2BY2 | 84.59 | 6.26 | 24.07 | 24.87 | 104.57 |
| H2DR1 | 50.89 | 12.73 | 3.45 | 13.19 | 15.16 |
| Sample | Dye Shade | L* | a* | b* | C*ab | h |
|---|---|---|---|---|---|---|
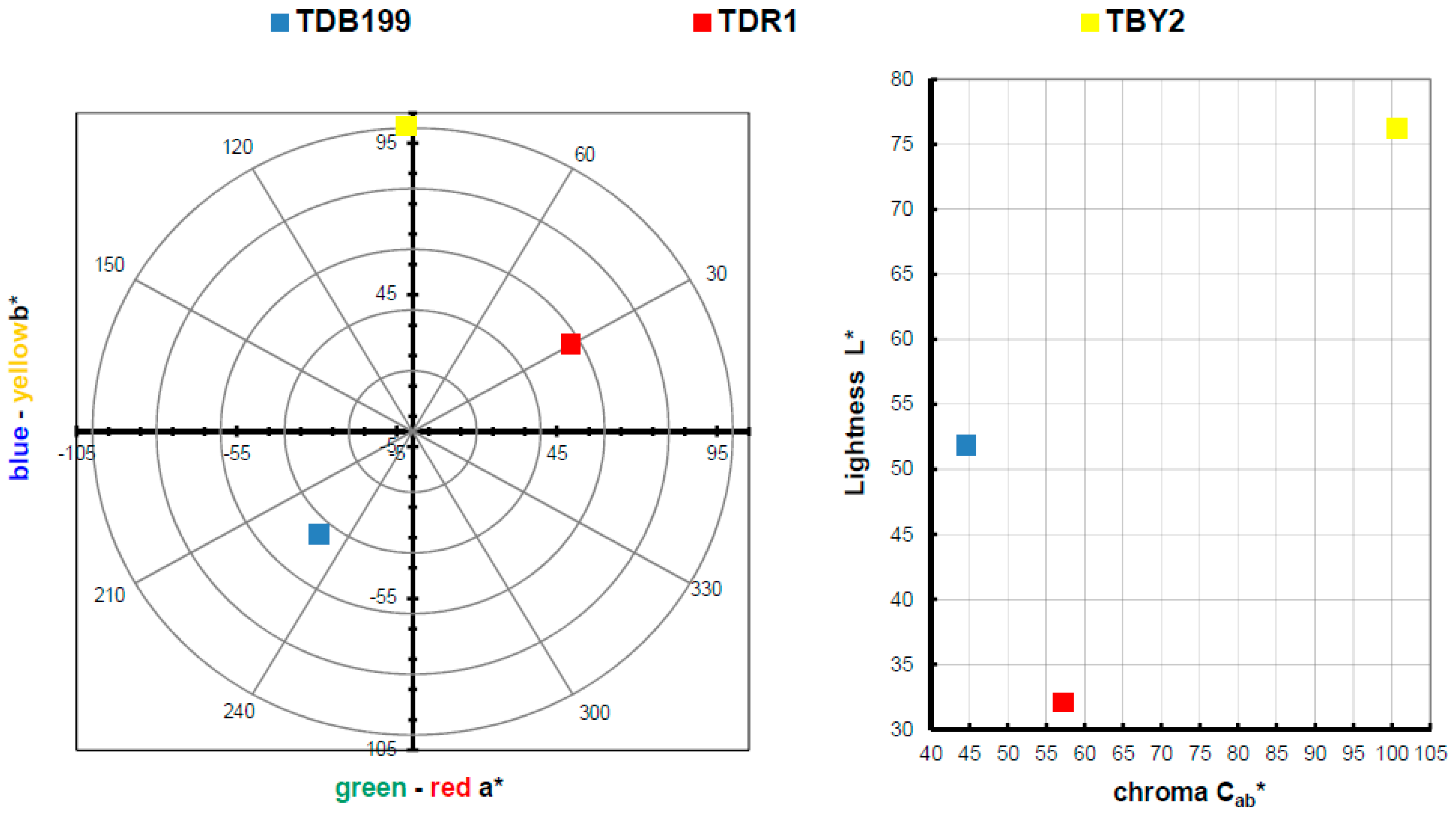
| TDB199 |  | 51.86 | −29.17 | −33.79 | 44.63 | 229.20 |
| TBY2 |  | 76.23 | −1.97 | 100.61 | 100.63 | 91.12 |
| TDR1 |  | 32.05 | 49.45 | 28.75 | 57.20 | 30.18 |
| Colour Fastness | ||||||
|---|---|---|---|---|---|---|
| Wash | Rub | Iron | ||||
| Sample | Dry | Wet | Dry | Humid | Wet | |
| TDB199 | 3–4 | 4–5 | 4 | 4–5 | 4 | 3–4 |
| TBY2 | 4 | 4 | 4–5 | 2 | 3 | 3 |
| TDR1 | 4–5 | 4–5 | 4–5 | 4 | 4 | 3–4 |
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| H | 114.3 | 0.21 | 10.07 |
| HC | 239.6 | 0.37 | 18.7 |
| HDR1 | 102.2 | 0.15 | 11.8 |
| HDB199 | 93.1 | 0.22 | 10.15 |
| HBY2 | 98.5 | 0.18 | 11.25 |
| Dye | Equation | R2 |
|---|---|---|
| Direct Blue 199 (DB199) | y = 21.784 x − 0.015 | 0.9982 |
| Basic Yellow 2 (BY2) | y = 18.023 x − 0.0112 | 0.9972 |
| Disperse Red 1 (DR1) | y = 25.411 x − 0.0244 | 0.9989 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Rodríguez, D.; Jordán-Núñez, J.; Gisbert-Paya, J.; Díaz-García, P.; Bou-Belda, E. Dyeing with Hydrotalcite Hybrid Nanoclays and Disperse, Basic and Direct Dyes. Int. J. Mol. Sci. 2023, 24, 808. https://doi.org/10.3390/ijms24010808
López-Rodríguez D, Jordán-Núñez J, Gisbert-Paya J, Díaz-García P, Bou-Belda E. Dyeing with Hydrotalcite Hybrid Nanoclays and Disperse, Basic and Direct Dyes. International Journal of Molecular Sciences. 2023; 24(1):808. https://doi.org/10.3390/ijms24010808
Chicago/Turabian StyleLópez-Rodríguez, Daniel, Jorge Jordán-Núñez, Jaime Gisbert-Paya, Pablo Díaz-García, and Eva Bou-Belda. 2023. "Dyeing with Hydrotalcite Hybrid Nanoclays and Disperse, Basic and Direct Dyes" International Journal of Molecular Sciences 24, no. 1: 808. https://doi.org/10.3390/ijms24010808
APA StyleLópez-Rodríguez, D., Jordán-Núñez, J., Gisbert-Paya, J., Díaz-García, P., & Bou-Belda, E. (2023). Dyeing with Hydrotalcite Hybrid Nanoclays and Disperse, Basic and Direct Dyes. International Journal of Molecular Sciences, 24(1), 808. https://doi.org/10.3390/ijms24010808









