Amelioration of Cyclosporine A-Induced Acute Nephrotoxicity by Cordyceps cicadae Mycelia via Mg+2 Reabsorption and the Inhibition of GRP78-IRE1-CHOP Pathway: In Vivo and In Vitro
Abstract
1. Introduction
2. Results
2.1. Proximate Composition and Polysaccharides of CCM
2.2. Nucleobases and Their Derivatives of CCM
2.3. CCM Decreased Proteinuria in CsA-Induced Rats
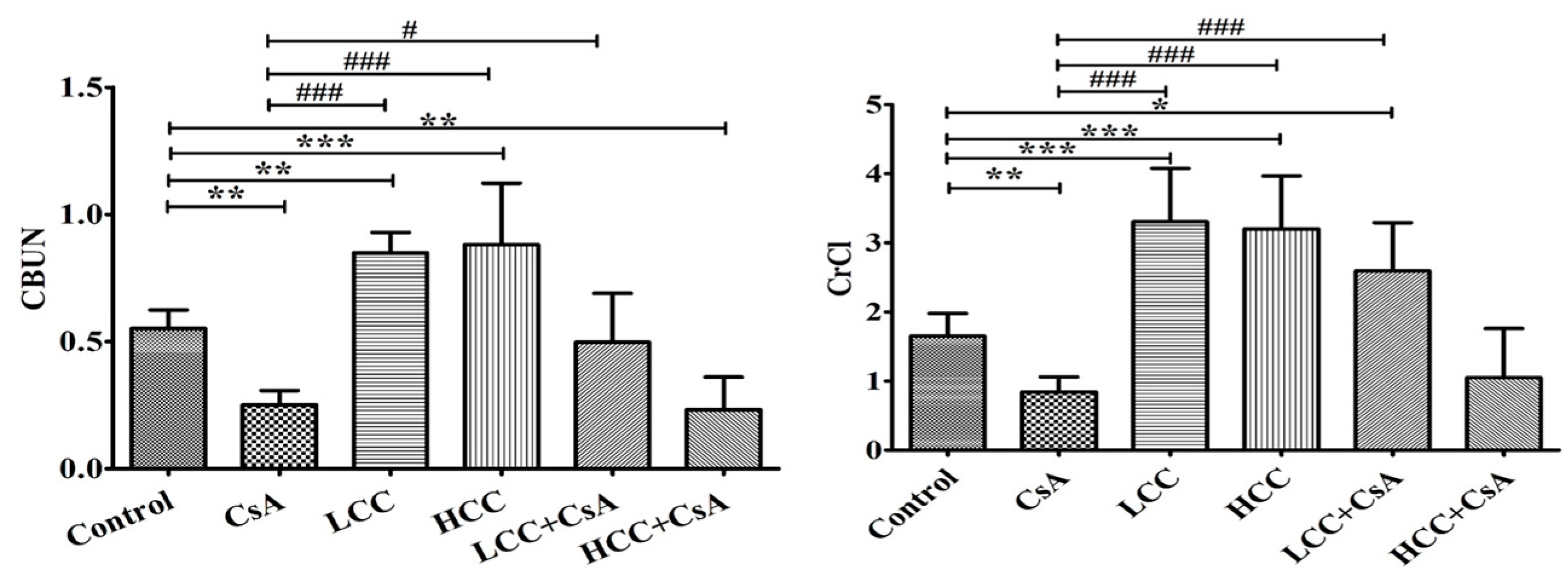
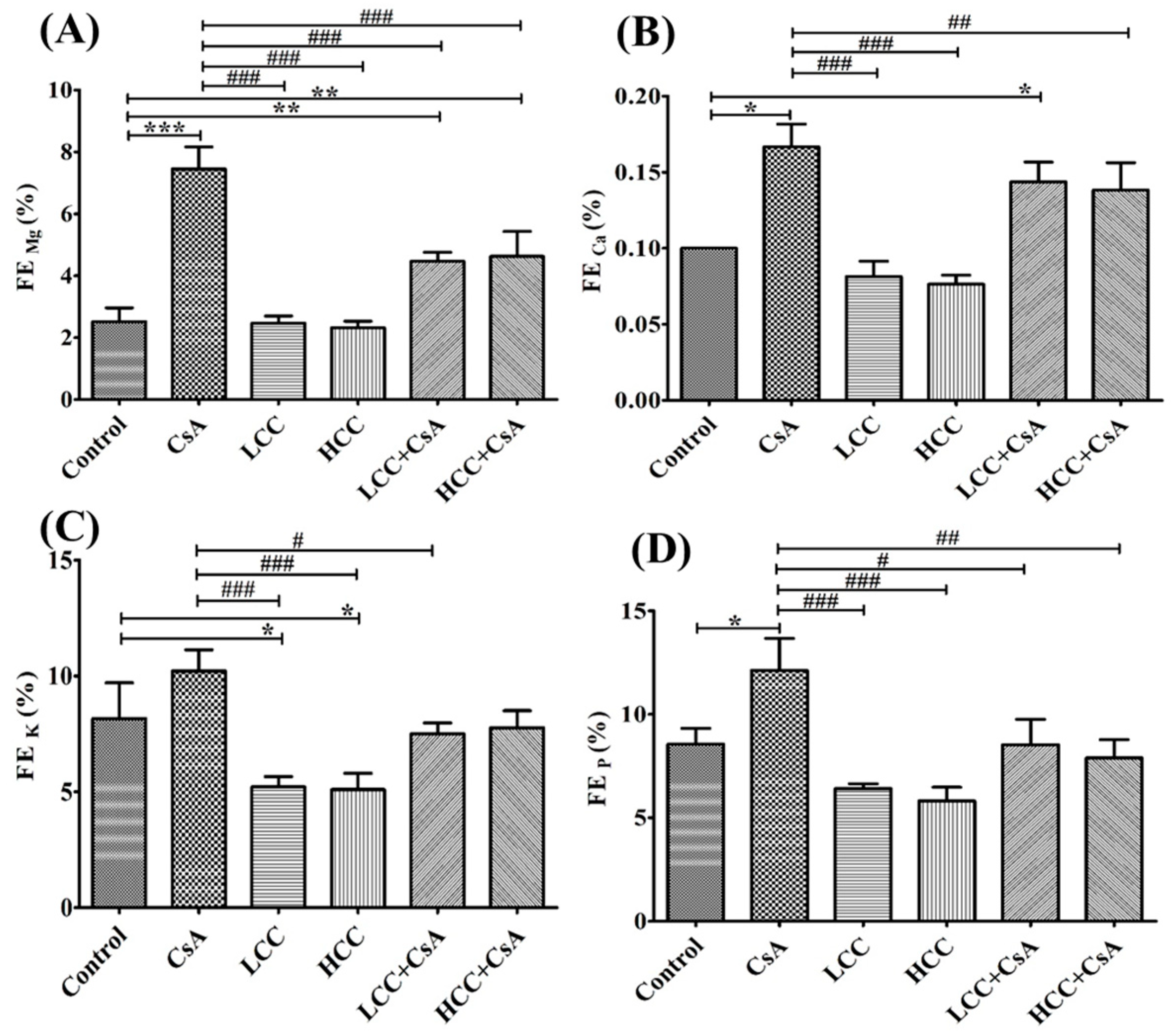
2.4. CCM Improves Tubular Function in CsA-Administered Rats
2.5. CCM Alleviates CsA-Induced Histopathological Changes in Kidney
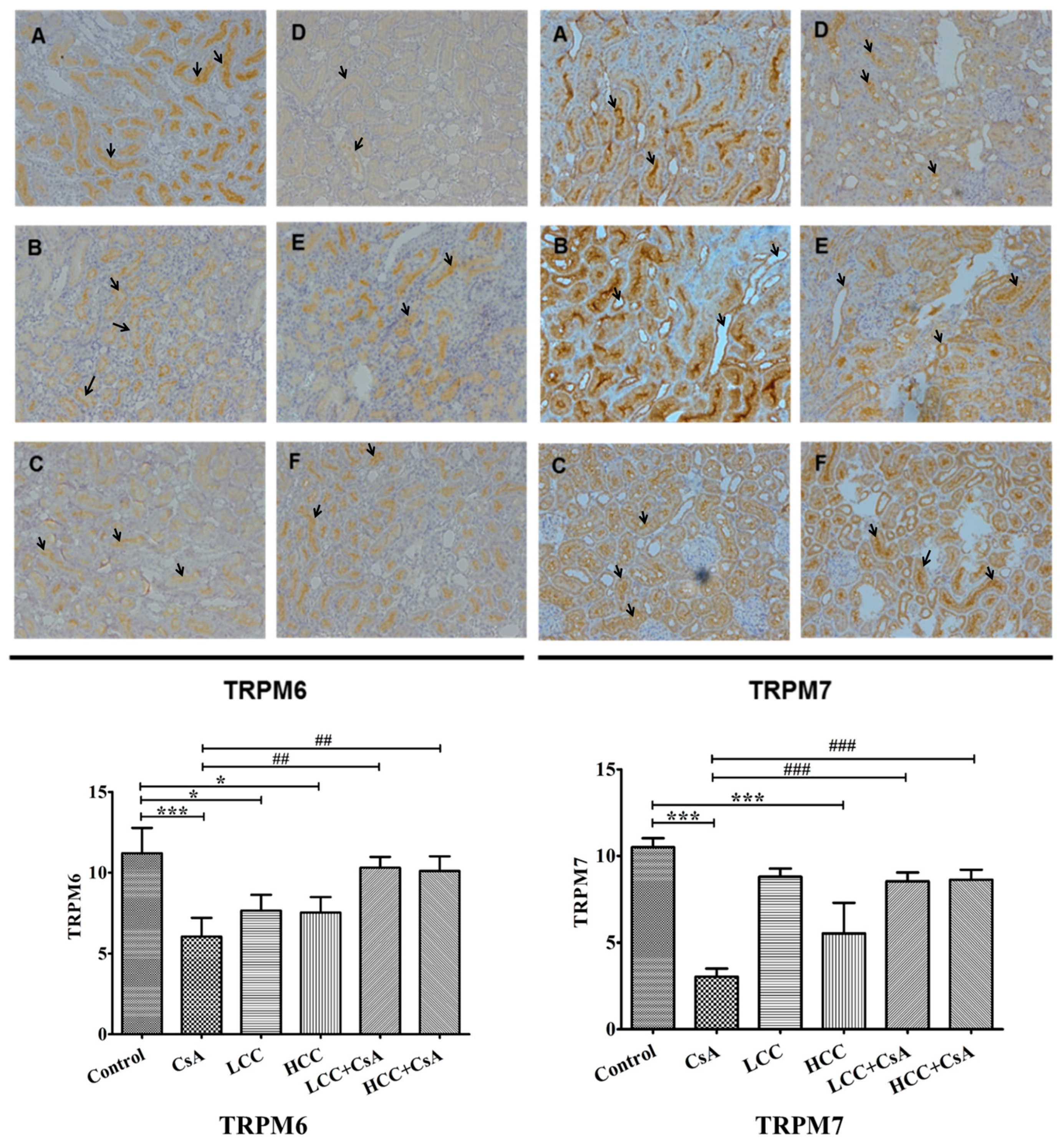
2.6. CCM Up-Regulates TRPM6 and Trpm7protein Expressions Impaired by CsA
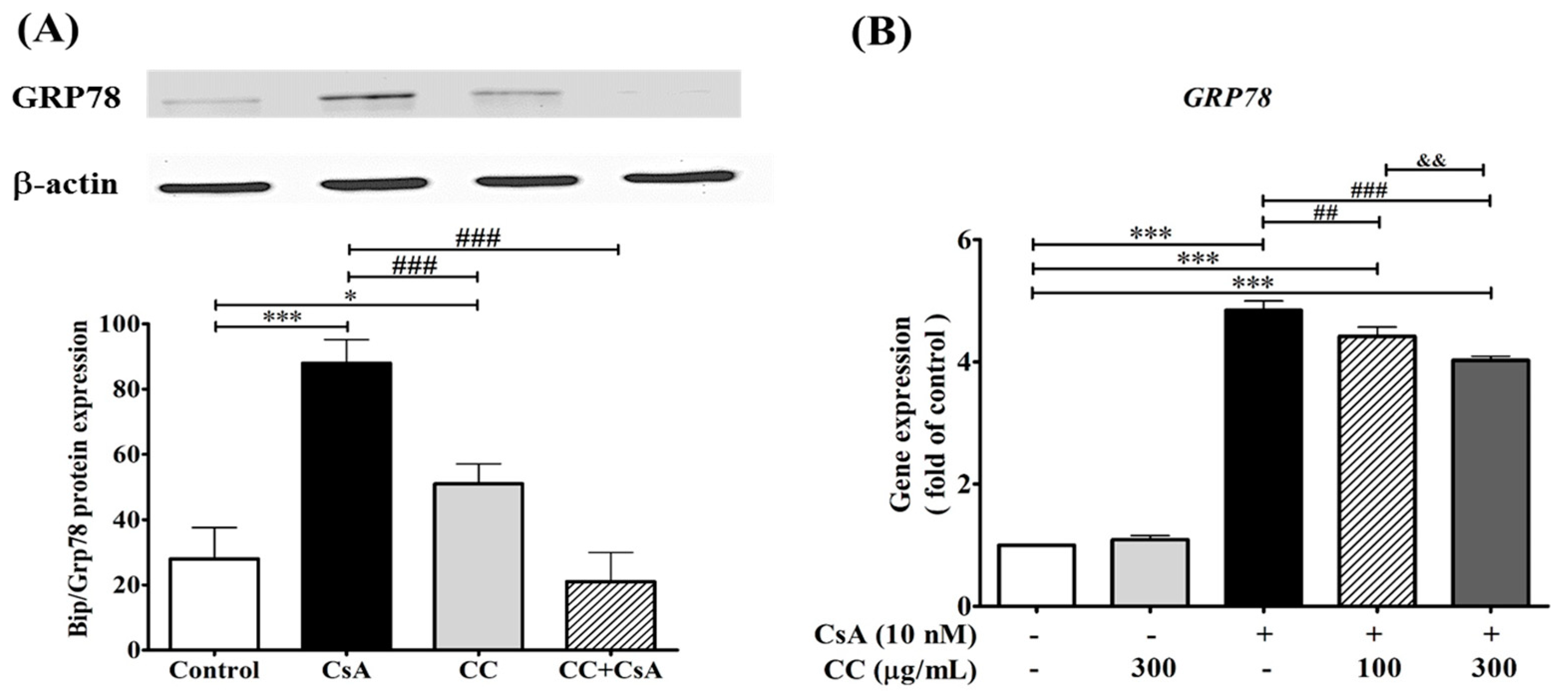
2.7. CCM Reduces Expression of the ER Stress Marker In Vivo
2.8. Effect of CCM and CsA on Cell Viability in HK-2 Cells
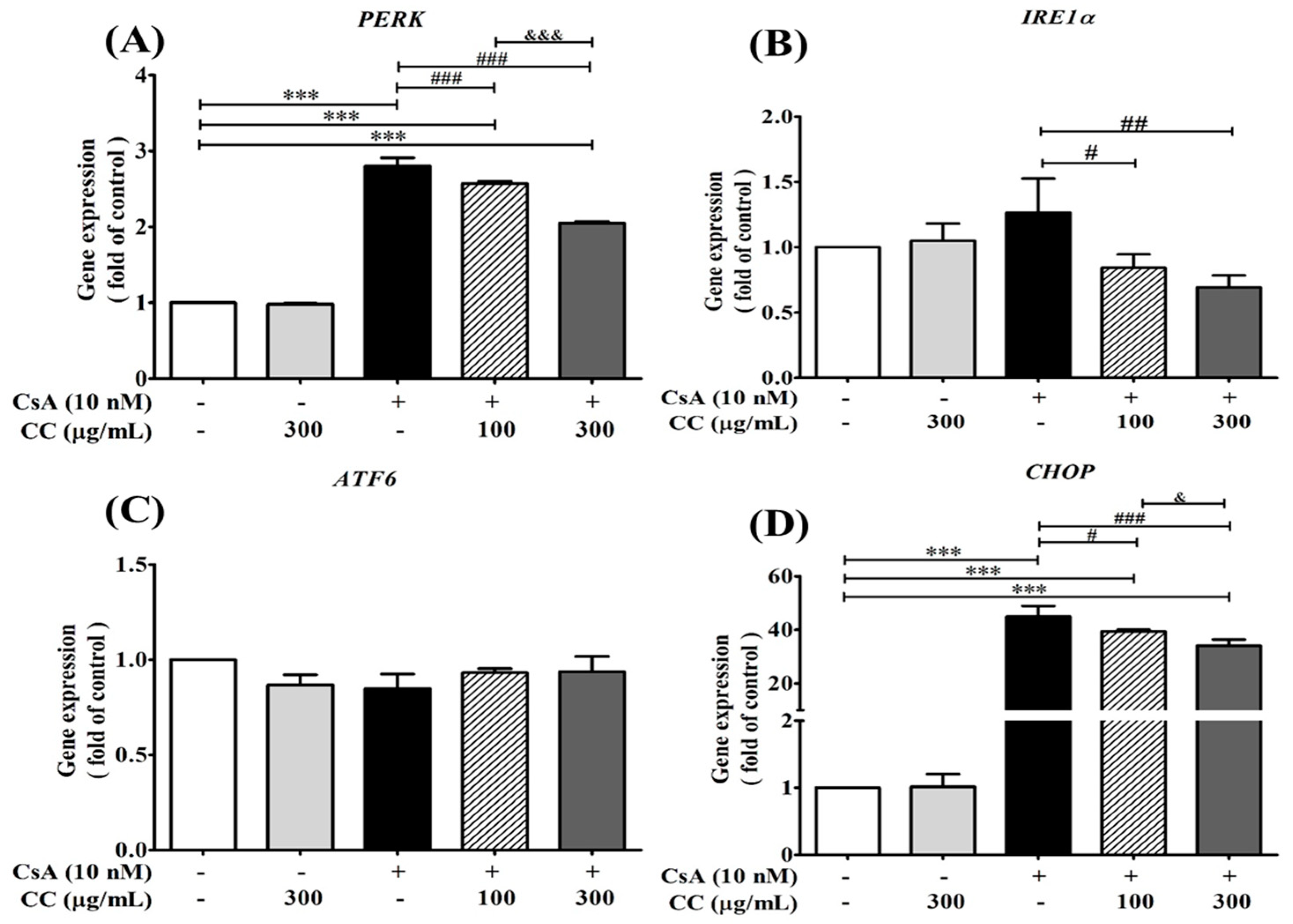
2.9. CCM Reduces Expression of the ER Stress Marker In Vitro
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cultivation and Preparation of C. cicadae Mycelium (CCM)
4.3. Proximate Composition Analysis and Polysaccharide Extraction
4.4. HPLC and Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analyses of Aqueous Extracts of CCM
4.5. Animal Studies
4.6. Biochemical Analysis
4.7. Histological Analysis
4.8. Immunohistochemistry Staining of TRPM6 and TRPM7
4.9. Western Blot Analysis of GRP 78 in Rat Kidney
4.10. In Vitro Study of m-RNA Expression
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopp, J.B.; Klotman, P.E. Cellular and molecular mechanisms of cyclosporin nephrotoxicity. J. Am. Soc. Nephrol. 1990, 1, 162–179. [Google Scholar] [CrossRef] [PubMed]
- Calne, R.Y.; White, D.J.; Thiru, S.; Evans, D.B.; McMaster, P.; Dunn, D.C.; Craddock, G.N.; Pentlow, B.D.; Rolles, K. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet 1978, 2, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Burdmann, E.A.; Andoh, T.F.; Yu, L.; Bennett, W.M. Cyclosporine nephrotoxicity. Semin. Nephrol. 2003, 23, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.E.; Yang, C.W. Established and newly proposed mechanisms of chronic cyclosporine nephropathy. Korean J. Intern. Med. 2009, 24, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Inagi, R.; Nangaku, M.; Onogi, H.; Ueyama, H.; Kitao, Y.; Nakazato, K.; Ogawa, S.; Kurokawa, K.; Couser, W.G.; Miyata, T. Involvement of endoplasmic reticulum (ER) stress in podocyte injury induced by excessive protein accumulation. Kidney Int. 2005, 68, 2639–2650. [Google Scholar] [CrossRef]
- Ni, M.; Lee, A.S. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007, 581, 3641–3651. [Google Scholar] [CrossRef]
- Kitamura, M. Induction of the unfolded protein response by calcineurin inhibitors: A double-edged sword in renal transplantation. Nephrol. Dial. Transplant. 2010, 25, 6–9. [Google Scholar] [CrossRef]
- Giménez-Mascarell, P.; Schirrmacher, C.E.; Martínez-Cruz, L.A.; Müller, D. Novel aspects of renal magnesium homeostasis. Front. Pediatr. 2018, 6, 77. [Google Scholar] [CrossRef]
- Chyau, C.C.; Chen, C.C.; Chen, J.C.; Yang, T.C.; Shu, K.H.; Cheng, C.H. Mycelia glycoproteins from Cordyceps sobolifera ameliorate cyclosporine-induced renal tubule dysfunction in rats. J. Ethnopharmacol. 2014, 153, 650–658. [Google Scholar] [CrossRef]
- Brandao, K.; Deason-Towne, F.; Zhao, X.; Perraud, A.L.; Schmitz, C. TRPM6 kinase activity regulates TRPM7 trafficking and inhibits cellular growth under hypomagnesic conditions. Cell Mol. Life Sci. 2014, 71, 4853–4867. [Google Scholar] [CrossRef]
- Elmoslemany, A.M.; El-Magd, M.A.; Ghamry, H.I.; Alshahrani, M.Y.; Zidan, N.S.; Zedan, A.M.G. Avocado seeds relieve oxidative stress-dependent nephrotoxicity but enhance immunosuppression induced by cyclosporine in rats. Antioxidants 2021, 10, 1194. [Google Scholar] [CrossRef] [PubMed]
- Ezejiofor, A.N.; Udowelle, N.A.; Orisakwe, O.E. Nephroprotective and antioxidant effect of aqueous leaf extract of Costus Afer Ker gawl on cyclosporin-a (Csa) induced nephrotoxicity. Clin. Phytosci. 2017, 2, 11. [Google Scholar] [CrossRef]
- Shashidhar, M.G.; Giridhar, P.; Udaya Sankar, K.; Manohar, B. Bioactive principles from Cordyceps sinensis: A potent food supplement—A review. J. Funct. Foods 2013, 5, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.-B.; Yu, H.; Ge, F.; Yang, J.-Y.; Chen, Z.-H.; Wang, Y.-B.; Dai, Y.-D.; Adams, A. Distribution of nucleosides in populations of Cordyceps cicadae. Molecules 2014, 19, 6123–6141. [Google Scholar] [CrossRef]
- Paterson, R.R. Cordyceps: A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry 2008, 69, 1469–1495. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Chen, Y.P.; Deng, Y.Y.; Zheng, R.; Zhong, Y.F.; Wang, L.; Du, L.P. Cordyceps cicadae extracts ameliorate renal malfunction in a remnant kidney model. J. Zhejiang Univ. Sci. B 2011, 12, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, J.; Wang, D.; Yu, X.; Olatunji, O.J.; Ouyang, Z.; Wei, Y. Neuroprotective effects of butanol fraction of Cordyceps cicadae on glutamate-induced damage in PC12 Cells involving oxidative toxicity. Chem. Biodivers. 2018, 15, e1700385. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Wei, Y.; Ouyang, Z.; Su, Z. Polysaccharides purified from Cordyceps cicadae protects PC12 cells against glutamate-induced oxidative damage. Carbohydr. Polym. 2016, 153, 187–195. [Google Scholar] [CrossRef]
- Huang, Y.S.; Wang, X.; Feng, Z.; Cui, H.; Zhu, Z.; Xia, C.; Han, X.; Liu, W.J.; Liu, Y.N. Cordyceps cicadae prevents renal tubular epithelial cell apoptosis by regulating the SIRT1/p53 pathway in hypertensive renal injury. Evid. Based Complement. Alternat. Med. 2020, 2020, 7202519. [Google Scholar] [CrossRef]
- Horng, C.T.; Yang, Y.L.; Chen, C.C.; Huang, Y.S.; Chen, C.; Chen, F.A. Intraocular pressure-lowering effect of Cordyceps cicadae mycelia extract in a glaucoma rat model. Int. J. Med. Sci. 2021, 18, 1007. [Google Scholar] [CrossRef]
- Chen, Y.L.; Yeh, S.H.; Lin, T.W.; Chen, C.C.; Chen, C.S.; Kuo, C.F. A 90-day subchronic toxicity study of submerged mycelial culture of Cordyceps cicadae (Ascomycetes) in rats. Int. J. Med. Mushrooms 2015, 17, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.S.; Hsu, J.H.; Lin, D.P.; Chang, H.H.; Chang, W.J.; Chen, Y.L.; Chen, C.C. Safety Assessment of HEA-Enriched Cordyceps cicadae Mycelium: A Randomized Clinical Trial. J. Am. Coll. Nutr. 2021, 40, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Moravcová, D.; Haapala, M.; Planeta, J.; Hyötyläinen, T.; Kostiainen, R.; Wiedmer, S.K. Separation of nucleobases, nucleosides, and nucleotides using two zwitterionic silica-based monolithic capillary columns coupled with tandem mass spectrometry. J. Chromatogr. A 2014, 1373, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.S.; Barseghyan, G.S.; Asatiani, M.D.; Wasser, S.P. Chemical composition and medicinal value of fruiting bodies and submerged cultured mycelia of caterpillar medicinal fungus Cordyceps militaris CBS-132098 (Ascomycetes). Int. J. Med. Mushrooms 2015, 17, 649–659. [Google Scholar] [CrossRef]
- Hsu, T.H.; Shiao, L.H.; Hsieh, C.Y.; Chang, D.M. A comparison of the chemical composition and bioactive ingredients of the Chinese medicinal mushroom DongChongXiaCao, its counterfeit and mimic, and fermented mycelium of Cordyceps sinensis. Food Chem. 2002, 78, 463–469. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Yang, B.; Leng, Q.; Li, J.; Wang, X.; Lu, J.; Olatunji, O.J.; Tang, J. Alleviation of liver dysfunction, oxidative stress, and inflammation underlines the protective effects of polysaccharides from Cordyceps cicadae on high sugar/high fat diet-induced metabolic syndrome in rats. Chem. Biodivers. 2021, 18, e2100065. [Google Scholar] [CrossRef]
- Chyau, C.-C.; Wu, H.-L.; Peng, C.-C.; Huang, S.-H.; Chen, C.-C.; Chen, C.-H.; Peng, R.Y. Potential protection effect of ER homeostasis of N6-(2-hydroxyethyl)adenosine isolated from Cordyceps cicadae in nonsteroidal anti-inflammatory drug-stimulated human proximal tubular cells. Int. J. Mol. Sci. 2021, 22, 1577. [Google Scholar] [CrossRef]
- Lu, M.Y.; Chen, C.C.; Lee, L.Y.; Lin, T.W.; Kuo, C.F. N(6)-(2-Hydroxyethyl)adenosine in the Medicinal Mushroom Cordyceps cicadae Attenuates Lipopolysaccharide-Stimulated Pro-inflammatory Responses by Suppressing TLR4-Mediated NF-κB Signaling Pathways. J. Nat. Prod. 2015, 78, 2452–2460. [Google Scholar] [CrossRef]
- Zheng, R.; Zhu, R.; Li, X.; Li, X.; Shen, L.; Chen, Y.; Zhong, Y.; Deng, Y. N6-(2-Hydroxyethyl) adenosine from Cordyceps cicadae ameliorates renal interstitial fibrosis and prevents inflammation via TGF-β1/Smad and NF-κB signaling pathway. Front. Physiol. 2018, 9, 1229. [Google Scholar] [CrossRef]
- Haberland, A.; Henke, W.; Grune, T.; Siems, W.; Jung, K.; Schimke, I. Differential response of oxygen radical metabolism in rat heart, liver and kidney to cyclosporine A treatment. Inflamm. Res. 1997, 46, 452–454. [Google Scholar] [CrossRef]
- Huang, J.; Yao, X.; Weng, G.; Qi, H.; Ye, X. Protective effect of curcumin against cyclosporine A-induced rat nephrotoxicity. Mol. Med. Rep. 2018, 17, 6038–6044. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.J.; Feng, Y.; Olatunji, O.O.; Tang, J.; Ouyang, Z.; Su, Z. Cordycepin protects PC12 cells against 6-hydroxydopamine induced neurotoxicity via its antioxidant properties. Biomed. Pharmacother. 2016, 81, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Diskin, C.J.; Stokes, T.J.; Dansby, L.M.; Radcliff, L.; Carter, T.B. The comparative benefits of the fractional excretion of urea and sodium in various azotemic oliguric states. Nephron. Clin. Pract. 2010, 114, c145–c150. [Google Scholar] [CrossRef]
- Komada, T.; Usui, F.; Shirasuna, K.; Kawashima, A.; Kimura, H.; Karasawa, T.; Nishimura, S.; Sagara, J.; Noda, T.; Taniguchi, S.; et al. ASC in renal collecting duct epithelial cells contributes to inflammation and injury after unilateral ureteral obstruction. Am. J. Pathol. 2014, 184, 1287. [Google Scholar] [CrossRef]
- Nozue, T.; Kobayashi, A.; Kodama, T.; Uemasu, F.; Endoh, H.; Sako, A.; Takagi, Y. Pathogenesis of cyclosporine-induced hypomagnesemia. J. Pediatr. 1992, 120 Pt 1, 638–640. [Google Scholar] [CrossRef]
- Hsiao, P.J.; Liao, C.Y.; Kao, Y.H.; Chan, J.S.; Lin, Y.F.; Chuu, C.P.; Chen, J.S. Comparison of fractional excretion of electrolytes in patients at different stages of chronic kidney disease: A cross-sectional study. Medicine (Baltimore) 2020, 99, e18709. [Google Scholar] [CrossRef]
- Jin, Z.H.; Chen, Y.P. Clinical observation on Cordyceps cicadae Shing tang in preventing the progression of chronic renal failure. Chin. Arch. Tradit. Chin. Med. 2006, 24, 1457–1459. [Google Scholar]
- Li, L.; Zhang, T.; Li, C.; Xie, L.; Li, N.; Hou, T.; Wang, Y.; Wang, B. Potential therapeutic effects of Cordyceps cicadae and Paecilomyces cicadae on adenine-induced chronic renal failure in rats and their phytochemical analysis. Drug Des. Devel. Ther. 2018, 13, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Hosokawa, H.; Matsumura, K.; Kobayashi, S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur. J. Neurosci. 2008, 27, 1131–1142. [Google Scholar] [CrossRef]
- Park, J.W.; Bae, E.H.; Kim, I.J.; Ma, S.K.; Choi, C.; Lee, J.; Kim, S.W. Paricalcitol attenuates cyclosporine-induced kidney injury in rats. Kidney Int. 2010, 77, 1076–1085. [Google Scholar] [CrossRef]
- Cheng, C.H.; Shu, K.H.; Chang, H.R.; Chou, M.C. Cyclosporine-Induced Tubular Vacuolization: The Role of Bip/Grp78. Nephron. Exp. Nephrol. 2012, 122, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pallet, N.; Bouvier, N.; Bendjallabah, A.; Rabant, M.; Flinois, J.P.; Hertig, A.; Legendre, C.; Beaune, P.; Thervet, E.; Anglicheau, D. Cyclosporine-induced endoplasmic reticulum stress triggers tubular phenotypic changes and death. Am. J. Transplant. 2008, 8, 2283–2296. [Google Scholar] [CrossRef]
- Lee, A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 2005, 35, 373–381. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Hartree, E.F. Determination of protein: A modification of the Lowry method that gives a linear photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Mastrototaro, L.; Trapani, V.; Boninsegna, A.; Martin, H.; Devaux, S.; Berthelot, A.; Cittadini, A.; Wolf, F.I. Dietary Mg2+ regulates the epithelial Mg2+ channel TRPM6 in rat mammary tissue. Magnes. Res. 2011, 24, S122–S129. [Google Scholar] [CrossRef] [PubMed]



| PeakNo. a | RT (min) | Assigned Identity | Precursor Ion, m/z | Product Ion, m/z | Fragmentor (V) | Collision Energy (V) | Content (μg/g) |
|---|---|---|---|---|---|---|---|
| 1 | 3.03 | Cytosine c | 112 | 95 | 160 | 20 | 54.65 |
| 2 | 3.50 | Cytidine c | 244 | 112 | 110 | 10 | 33.43 |
| 3 | 3.99 | Guanine b | 152 | 135 | 160 | 20 | 110.03 |
| 4 | 4.78 | Adenine b | 136 | 119 | 160 | 20 | 20.13 |
| 5 | 7.31 | Inosine c | 269 | 137 | 115 | 10 | 60.14 |
| 6 | 7.50 | Guanosine b | 284 | 152 | 140 | 10 | 15.12 |
| 7 | 9.05 | Adenosine b | 268 | 136 | 135 | 20 | 28.04 |
| 8 | 9.47 | Cordycepin c | 252 | 136 | 135 | 20 | 6.17 |
| IS | 9.79 | IS e | 409 | 215 | 180 | 20 | 5.00 |
| 9 | 10.10 | HEA b | 312 | 180 | 135 | 20 | 103.15 |
| 10 | 15.11 | M.W. 430 d | 431 | 255 | 125 | 15 | 90.16 |
| 11 | 16.82 | M.W. 446 d | 447 | 271 | 125 | 15 | 78.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-H.; Chiu, C.-H.; Chen, C.-C.; Chyau, C.-C.; Cheng, C.-H. Amelioration of Cyclosporine A-Induced Acute Nephrotoxicity by Cordyceps cicadae Mycelia via Mg+2 Reabsorption and the Inhibition of GRP78-IRE1-CHOP Pathway: In Vivo and In Vitro. Int. J. Mol. Sci. 2023, 24, 772. https://doi.org/10.3390/ijms24010772
Wu Z-H, Chiu C-H, Chen C-C, Chyau C-C, Cheng C-H. Amelioration of Cyclosporine A-Induced Acute Nephrotoxicity by Cordyceps cicadae Mycelia via Mg+2 Reabsorption and the Inhibition of GRP78-IRE1-CHOP Pathway: In Vivo and In Vitro. International Journal of Molecular Sciences. 2023; 24(1):772. https://doi.org/10.3390/ijms24010772
Chicago/Turabian StyleWu, Zong-Han, Chun-Hung Chiu, Chin-Chu Chen, Charng-Cherng Chyau, and Chi-Hung Cheng. 2023. "Amelioration of Cyclosporine A-Induced Acute Nephrotoxicity by Cordyceps cicadae Mycelia via Mg+2 Reabsorption and the Inhibition of GRP78-IRE1-CHOP Pathway: In Vivo and In Vitro" International Journal of Molecular Sciences 24, no. 1: 772. https://doi.org/10.3390/ijms24010772
APA StyleWu, Z.-H., Chiu, C.-H., Chen, C.-C., Chyau, C.-C., & Cheng, C.-H. (2023). Amelioration of Cyclosporine A-Induced Acute Nephrotoxicity by Cordyceps cicadae Mycelia via Mg+2 Reabsorption and the Inhibition of GRP78-IRE1-CHOP Pathway: In Vivo and In Vitro. International Journal of Molecular Sciences, 24(1), 772. https://doi.org/10.3390/ijms24010772





