Electrochemical Biosensor for the Determination of Specific Antibodies against SARS-CoV-2 Spike Protein
Abstract
1. Introduction
2. Results and Discussion
2.1. Cleaning the Gold Electrode
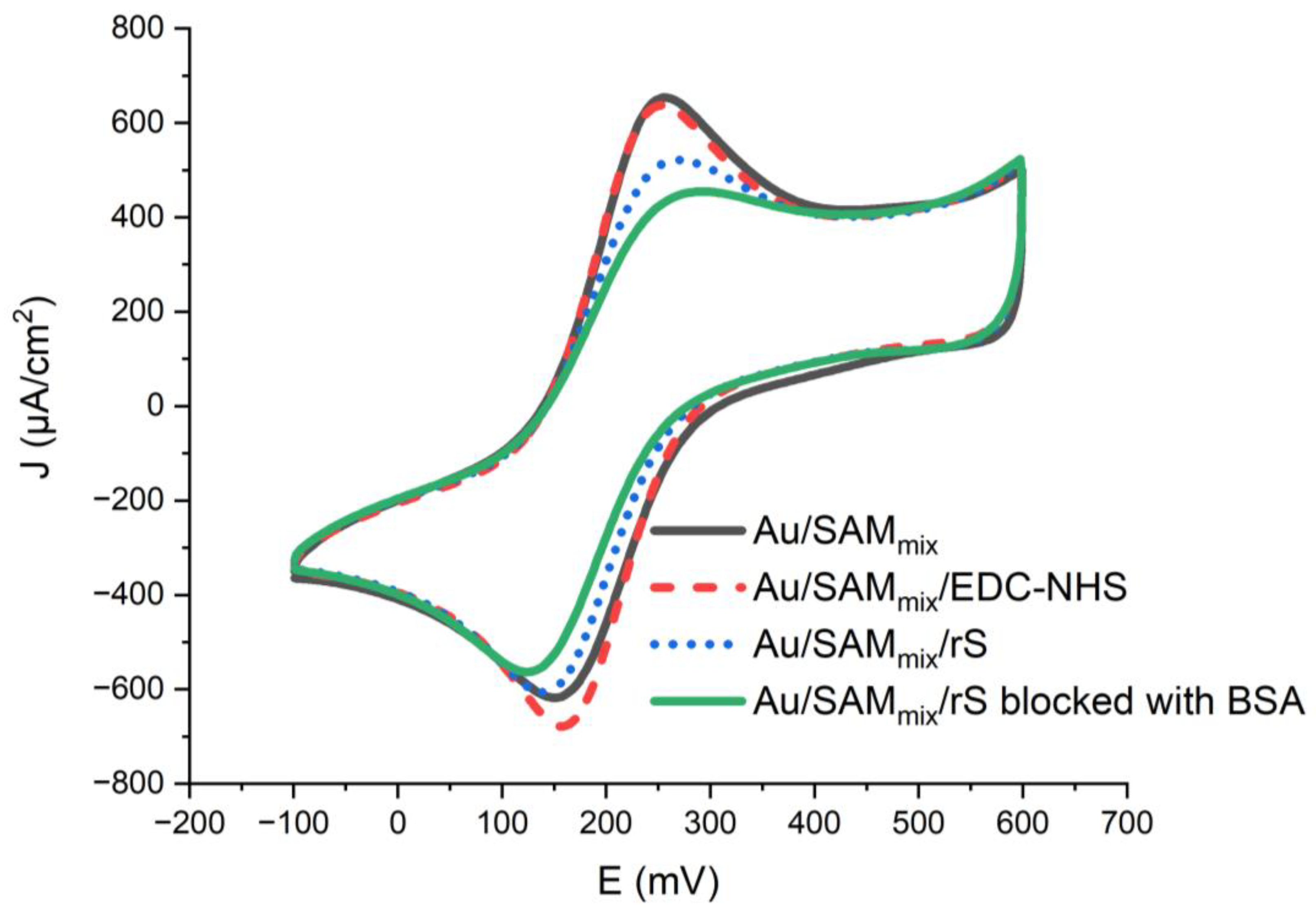
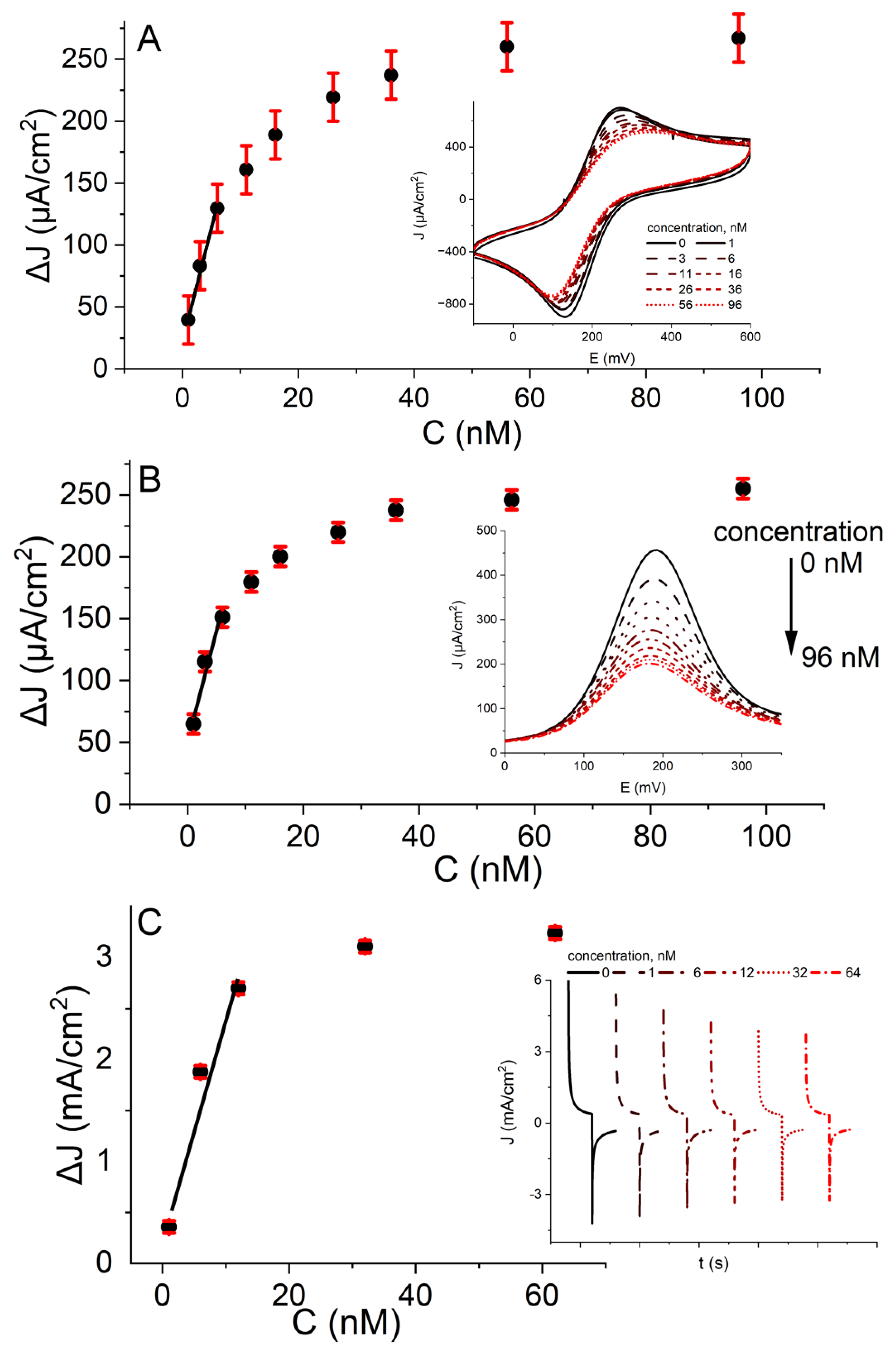
2.2. Electrochemical Assessment of the Developed Biosensor
3. Experimental Setup
3.1. Reagents and Instrumentation
3.2. Serum Sample Collection and Preparation of the Analysis
3.3. Instrumentation
3.4. Cleaning and Assessing of the Gold Disc Electrode
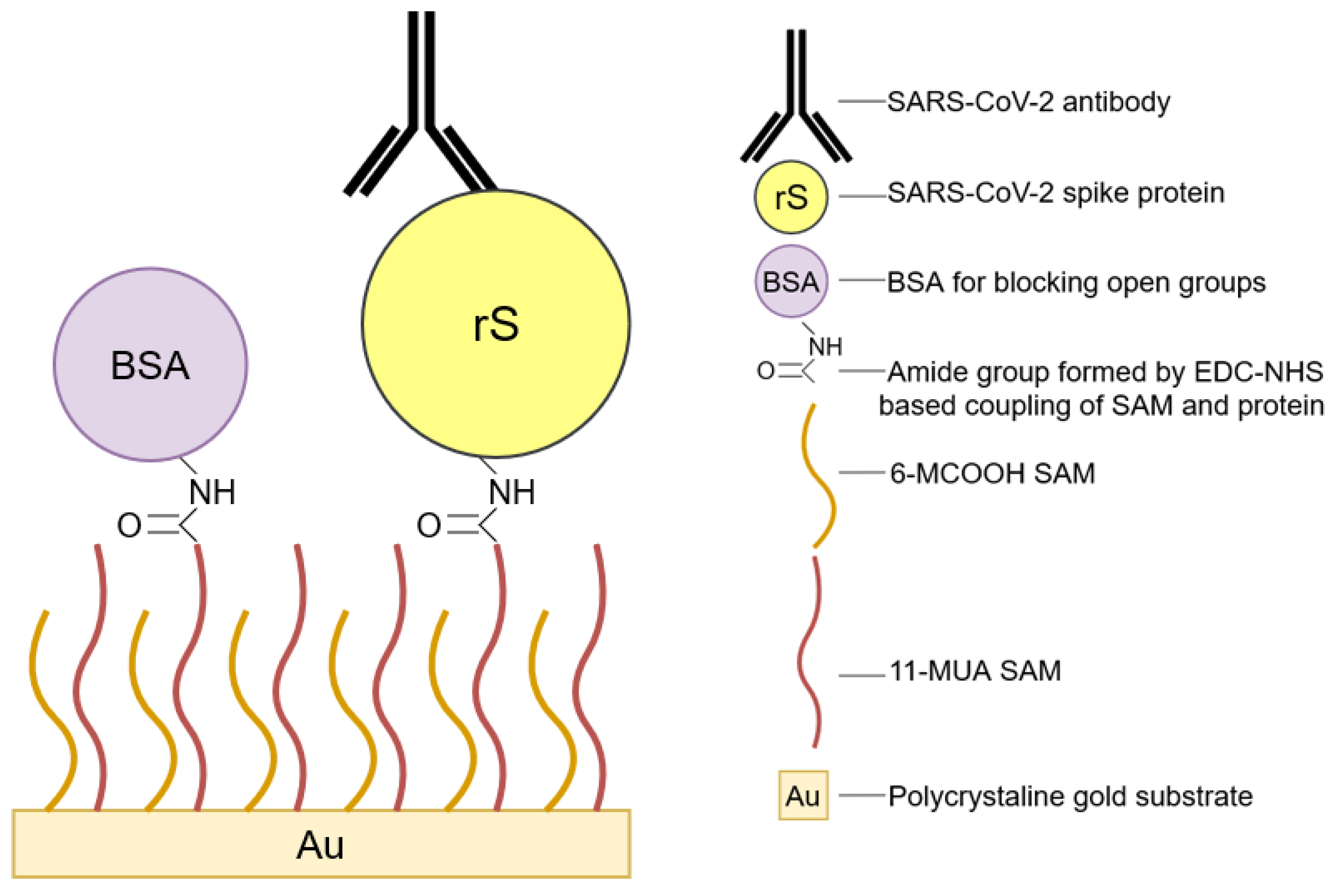
3.5. Protein Immobilization
3.6. Assessment of SAMmix Formation, Protein Immobilization, and Biosensor Response towards Anti-rS Antibodies by Cyclic Voltammetry, Differential Pulse Voltammetry, and Potential Pulsed Amperometry Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liustrovaite, V.; Drobysh, M.; Rucinskiene, A.; Baradoke, A.; Ramanaviciene, A.; Plikusiene, I.; Samukaite-Bubniene, U.; Viter, R.; Chen, C.-F.; Ramanavicius, A. Towards an Electrochemical Immunosensor for the Detection of Antibodies against SARS-CoV-2 Spike Protein. J. Electrochem. Soc. 2022, 169, 037523. [Google Scholar] [CrossRef]
- Drobysh, M.; Liustrovaite, V.; Baradoke, A.; Rucinskiene, A.; Ramanaviciene, A.; Ratautaite, V.; Viter, R.; Chen, C.-F.; Plikusiene, I.; Samukaite-Bubniene, U.; et al. Electrochemical Determination of Interaction between SARS-CoV-2 Spike Protein and Specific Antibodies. Int. J. Mol. Sci. 2022, 23, 6768. [Google Scholar] [CrossRef]
- Białobrzeska, W.; Ficek, M.; Dec, B.; Osella, S.; Trzaskowski, B.; Jaramillo-Botero, A.; Pierpaoli, M.; Rycewicz, M.; Dashkevich, Y.; Łęga, T.; et al. Performance of electrochemical immunoassays for clinical diagnostics of SARS-CoV-2 based on selective nucleocapsid N protein detection: Boron-doped diamond, gold and glassy carbon evaluation. Biosens. Bioelectron. 2022, 209, 114222. [Google Scholar] [CrossRef]
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Ramanaviciene, A.; Ciplys, E.; Juozapaitis, M.; Slibinskas, R.; Bechelany, M.; Ramanavicius, A. Molecularly Imprinted Polypyrrole based Sensor for the Detection of SARS-CoV-2 Spike Glycoprotein. Electrochim. Acta 2022, 403, 139581. [Google Scholar] [CrossRef]
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Plausinaitis, D.; Ramanavicius, S.; Samukaite-Bubniene, U.; Bechelany, M.; Ramanavicius, A. Evaluation of the interaction between SARS-CoV-2 spike glycoproteins and the molecularly imprinted polypyrrole. Talanta 2023, 253, 123981. [Google Scholar] [CrossRef]
- Avelino, K.Y.P.S.; dos Santos, G.S.; Frías, I.A.M.; Silva-Junior, A.G.; Pereira, M.C.; Pitta, M.G.R.; de Araújo, B.C.; Errachid, A.; Oliveira, M.D.L.; Andrade, C.A.S. Nanostructured sensor platform based on organic polymer conjugated to metallic nanoparticle for the impedimetric detection of SARS-CoV-2 at various stages of viral infection. J. Pharm. Biomed. Anal. 2021, 206, 114392. [Google Scholar] [CrossRef] [PubMed]
- Ratautaite, V.; Nesladek, M.; Ramanaviciene, A.; Baleviciute, I.; Ramanavicius, A. Evaluation of Histamine Imprinted Polypyrrole Deposited on Boron Doped Nanocrystalline Diamond. Electroanalysis 2014, 26, 2458–2464. [Google Scholar] [CrossRef]
- Hartl, A.; Schmich, E.; Garrido, J.A.; Hernando, J.; Catharino, S.C.R.; Walter, S.; Feulner, P.; Kromka, A.; Steinmuller, D.; Stutzmann, M. Protein-modified nanocrystalline diamond thin films for biosensor applications. Nat. Mater. 2004, 3, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Witt, S.; Rogien, A.; Werner, D.; Siegenthaler, J.; Lesiyon, R.; Kurien, N.; Rechenberg, R.; Baule, N.; Hardy, A.; Becker, M. Boron doped diamond thin films for the electrochemical detection of SARS-CoV-2 S1 protein. Diam. Relat. Mater. 2021, 118, 108542. [Google Scholar] [CrossRef]
- Ahammad, A.J.S.; Li, X.B.; Rahman, M.M.; Noh, K.M.; Lee, J.J. Characteristics of a Poly (thionine) Modified Glassy Carbon Electrode and the Detection of Dopamine and Uric acid. Int. J. Electrochem. Sci. 2013, 8, 7806–7815. [Google Scholar]
- Ratautaite, V.; Brazys, E.; Ramanaviciene, A.; Ramanavicius, A. Electrochemical Sensors based on L-Tryptophan Molecularly Imprinted Polypyrrole and Polyaniline. J. Electroanal. Chem. 2022, 917, 116389. [Google Scholar] [CrossRef]
- de Lima, L.F.; Ferreira, A.L.; Torres, M.D.T.; de Araujo, W.R.; de la Fuente-Nunez, C. Minute-scale detection of SARS-CoV-2 using a low-cost biosensor composed of pencil graphite electrodes. Proc. Natl. Acad. Sci. USA 2021, 118, e2106724118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tao, J.; Zhu, Z.; Zhang, Y.; Wang, H.; Pang, P.; Wang, H.; Yang, W. A Sensitive Electrochemical Assay for T4 Polynucleotide Kinase Activity Based on Fe3O4@TiO2 and Gold Nanoparticles Hybrid Probe Modified Magnetic Electrode. J. Electrochem. Soc. 2022, 169, 027504. [Google Scholar] [CrossRef]
- Wang, H.-B.; Zhang, H.-D.; Zhang, Y.-H.; Chen, H.; Xu, L.-L.; Huang, K.-J.; Liu, Y.-M. Tungsten Disulfide Nano-Flowers/Silver Nanoparticles Composites Based Electrochemical Sensor for Theophylline Determination. J. Electrochem. Soc. 2015, 162, B173. [Google Scholar] [CrossRef]
- Wang, H.-B.; Zhang, H.-D.; Xu, L.-L.; Gan, T.; Huang, K.-J.; Liu, Y.-M. Electrochemical biosensor for simultaneous determination of guanine and adenine based on dopamine-melanin colloidal nanospheres–graphene composites. J. Solid State Electrochem. 2014, 18, 2435–2442. [Google Scholar] [CrossRef]
- Drobysh, M.; Liustrovaite, V.; Baradoke, A.; Viter, R.; Chen, C.-F.; Ramanavicius, A.; Ramanaviciene, A. Determination of rSpike Protein by Specific Antibodies with Screen-Printed Carbon Electrode Modified by Electrodeposited Gold Nanostructures. Biosensors 2022, 12, 593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, L.; Zhang, Y. Highly sensitive electrochemical determination of the SARS-COV-2 antigen based on a gold/graphene imprinted poly-arginine sensor. Anal. Methods 2021, 13, 5772–5776. [Google Scholar] [CrossRef]
- Roy, V.; Fischinger, S.; Atyeo, C.; Slein, M.; Loos, C.; Balazs, A.; Luedemann, C.; Astudillo, M.G.; Yang, D.; Wesemann, D.R.; et al. SARS-CoV-2-specific ELISA development. J. Immunol. Methods 2020, 484–485, 112832. [Google Scholar] [CrossRef]
- Tan, L.L.; Loganathan, N.; Agarwalla, S.; Yang, C.; Yuan, W.; Zeng, J.; Wu, R.; Wang, W.; Duraiswamy, S. Current commercial dPCR platforms: Technology and market review. Crit. Rev. Biotechnol. 2022, 1–32. [Google Scholar] [CrossRef]
- Ahrens, P.; Zander, M.; Hasse, U.; Wulff, H.; Jeyabharathi, C.; Kruth, A.; Scholz, F. Electrochemical Formation of Gold Nanoparticles on Polycrystalline Gold Electrodes during Prolonged Potential Cycling. ChemElectroChem 2018, 5, 943–957. [Google Scholar] [CrossRef]
- Carvalhal, R.F.; Sanches Freire, R.; Kubota, L.T. Polycrystalline Gold Electrodes: A Comparative Study of Pretreatment Procedures Used for Cleaning and Thiol Self-Assembly Monolayer Formation. Electroanalysis 2005, 17, 1251–1259. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chiu, P.-H.; Wang, Y.-H.; Chen, K.-L.; Linn, J.-J.; Yang, C.-F. Electrochemically Controlling the Size of Gold Nanoparticles. J. Electrochem. Soc. 2006, 153, D193. [Google Scholar] [CrossRef]
- Fernandez-Blanco, C.; Heras, A.; Ruiz, V.; Colina, A. Spectroelectrochemical synthesis of gold nanoparticles using cyclic voltammetry in the presence of a protective agent. RSC Adv. 2014, 4, 45168–45173. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Peng, H.-H. Fabricating Gold Nanocomplexes of Controllable Size Using Electrochemical Oxidation−Reduction Cycling of Gold Substrates in Aqueous Solution. J. Phys. Chem. B 2004, 108, 16654–16658. [Google Scholar] [CrossRef]
- Chen, L.H.; Chan, C.C.; Yuan, W.; Goh, S.K.; Sun, J. High performance chitosan diaphragm-based fiber-optic acoustic sensor. Sens. Actuat A-Phys. 2010, 163, 42–47. [Google Scholar] [CrossRef]
- Yuan, W.; Dong, H.; Li, C.M.; Cui, X.; Yu, L.; Lu, Z.; Zhou, Q. pH-Controlled Construction of Chitosan/Alginate Multilayer Film: Characterization and Application for Antibody Immobilization. Langmuir 2007, 23, 13046–13052. [Google Scholar] [CrossRef]
- Rezek, B.; Ukraintsev, E.; Michalíková, L.; Kromka, A.; Zemek, J.; Kalbacova, M. Adsorption of fetal bovine serum on H/O-terminated diamond studied by atomic force microscopy. Diam. Relat. Mater. 2009, 18, 918–922. [Google Scholar] [CrossRef]
- Tsugimura, K.; Ohnuki, H.; Endo, H.; Tsuya, D.; Izumi, M. Protein-G-based human immunoglobulin G biosensing by electrochemical impedance spectroscopy. Jpn. J. Appl. Phys. 2016, 55, 02BE06. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiols and dithiols on gold: New challenges for a well-known system. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef]
- Bhadra, P.; Siu, S.W.I. Effect of Concentration, Chain Length, Hydrophobicity, and an External Electric Field on the Growth of Mixed Alkanethiol Self-Assembled Monolayers: A Molecular Dynamics Study. Langmuir 2021, 37, 1913–1924. [Google Scholar] [CrossRef]
- Guo, Q.; Li, F. Self-assembled alkanethiol monolayers on gold surfaces: Resolving the complex structure at the interface by STM. Phys. Chem. Chem. Phys. 2014, 16, 19074–19090. [Google Scholar] [CrossRef] [PubMed]
- Drobysh, M.; Ramanaviciene, A.; Viter, R.; Chen, C.-F.; Samukaite-Bubniene, U.; Ratautaite, V.; Ramanavicius, A. Biosensors for the Determination of SARS-CoV-2 Virus and Diagnosis of COVID-19 Infection. Int. J. Mol. Sci. 2022, 23, 666. [Google Scholar] [CrossRef] [PubMed]
- Pandey, L.M. Design of engineered surfaces for prospective detection of SARS-CoV-2 using quartz crystal microbalance-based techniques. Expert Rev. Proteom. 2020, 17, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Sukeri, A.; Bertotti, M. Nanoporous gold surface: An efficient platform for hydrogen evolution reaction at very low overpotential. J. Braz. Chem. Soc. 2018, 29, 226–231. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. A comparative study of short chain and long chain mercapto acids used in biosensor fabrication: A VEGF-R1-based immunosensor as a model system. Artif. Cells Nanomed. Biotechnol. 2016, 44, 462–470. [Google Scholar] [CrossRef]
- Vadlamani, B.S.; Uppal, T.; Verma, S.C.; Misra, M. Functionalized TiO2 Nanotube-Based Electrochemical Biosensor for Rapid Detection of SARS-CoV-2. Sensors 2020, 20, 5871. [Google Scholar] [CrossRef]
- Fabiani, L.; Saroglia, M.; Galatà, G.; De Santis, R.; Fillo, S.; Luca, V.; Faggioni, G.; D’Amore, N.; Regalbuto, E.; Salvatori, P.; et al. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Gumus, Z.P.; Soylak, M.; Erk, N. Electrochemical immunosensor for rapid and highly sensitive detection of SARS-CoV-2 antigen in the nasal sample. Talanta 2022, 240, 123211. [Google Scholar] [CrossRef]
- Karaman, C.; Yola, B.B.; Karaman, O.; Atar, N.; Polat, İ.; Yola, M.L. Sensitive sandwich-type electrochemical SARS-CoV-2 nucleocapsid protein immunosensor. Microchim. Acta 2021, 188, 425. [Google Scholar] [CrossRef]
- Zhao, J.; Fu, Z.; Li, H.; Xiong, Y.; Cai, S.; Wang, C.; Chen, Y.; Han, N.; Yang, R. Magnet-assisted electrochemical immunosensor based on surface-clean Pd-Au nanosheets for sensitive detection of SARS-CoV-2 spike protein. Electrochim. Acta 2022, 404, 139766. [Google Scholar] [CrossRef]
- Rahmati, Z.; Roushani, M.; Hosseini, H.; Choobin, H. An electrochemical immunosensor using SARS-CoV-2 spike protein-nickel hydroxide nanoparticles bio-conjugate modified SPCE for ultrasensitive detection of SARS-CoV-2 antibodies. Microchem. J. 2021, 170, 106718. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Pan, Y.; Li, Z.; Qin, Z.; Rini, J.M.; Liu, X. SPEEDS: A portable serological testing platform for rapid electrochemical detection of SARS-CoV-2 antibodies. Biosens. Bioelectron. 2022, 197, 113762. [Google Scholar] [CrossRef] [PubMed]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N.; Chailapakul, O.; Chaiyo, S. Paper-based electrochemical biosensor for diagnosing COVID-19: Detection of SARS-CoV-2 antibodies and antigen. Biosens. Bioelectron. 2021, 176, 112912. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Biological Standards and Control, 20/136WHO International Standard (2020). Available online: https://www.nibsc.org/documents/ifu/20-136.pdf (accessed on 25 September 2022).
- Immundiagnostik AG 2021 Manual. Available online: https://www.immundiagnostik.com/media/pages/testkits/k-5004/e859ef5a82-1647482477/k5004_2021-08-10_idk_sars-cov-2-igg.pdf (accessed on 25 September 2022).
- Dietzen, D.J. 13–Amino Acids, Peptides, and Proteins. In Principles and Applications of Molecular Diagnostics; Rifai, N., Horvath, A.R., Wittwer, C.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]



| LOD, nM | LOQ, nM | Linear Range | |
|---|---|---|---|
| Cyclic voltammetry | 0.34 | 1.04 | 1.0–6.0 nM |
| Differential pulse voltammetry | 0.61 | 1.86 | 1.0–6.0 nM |
| Potential pulsed amperometry | 0.70 | 2.11 | 1.0–12.0 nM |
| No | Electrode and Modification | Experimental Conditions | Electrochemical Methods | Sample | Analyte Specification | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1. | Ti with Co-TNT* | 20 mM sodium phosphate, 500 mM NaCl, 8 M urea, 200 mM imidazole, pH 4.0 | CA | Nasal secretions and saliva samples | SARS-CoV-2 S-RBD | 0.7 nM | 14–1400 nM | [36] |
| 2. | Carbon black-based SPEs with commercial CB N220 dispersion | PBS, pH 7.4 | DPV | Saliva | SARS-CoV-2 S (subunit S1) SARS-CoV-2 N | 19 ng/mL 8 ng/mL | 0.04–10 μg/mL | [37] |
| 3. | SPCE with SiO2@UiO-66 | - | EIS | Nasal sample | SARS-CoV-2 S | 100.0 fg/mL | 100.0 fg/mL–10.0 ng/mL | [38] |
| 4. | GCE with bismuth tungstate/bismuth sulfide composite decorated with gold nanoparticles | PBS, pH 7.0 | DPV | Product | SARS-CoV-2 N | 3.00 fg/mL | 0.01–1.00 pg/ mL | [39] |
| 5. | GCE with Pd-Au nanosheets | PBS with 10 mM H2O2 | DPV | Product | SARS-CoV-2 S | 0.072 ng/mL | 0.01–1000 ng mL−1 | [40] |
| 6. | SPCE with Ni(OH)2 nanoparticles | PBS, pH 7.4, with redox probe | DPV | Human blood | IgM/IgG | 0.3 fg/mL | 1 fg/mL–1 μg/mL | [41] |
| 7. | SPCE with streptavidine | 1,4-benzoquinone | CA | Human monoclonal anti | IgG IgM | 10.1 ng/mL 1.64 ng/mL | 10.1 pg/mL–60 μg/mL 1.64 ng/mL–50 μg/mL | [42] |
| 8. | ePAD with graphene oxide-activated EDC/NHS | PBS, pH 7.4, with redox probe | SWV | IgG IgM | 0.96 ng/mL 0.14 ng/mL | 1–1000 ng/mL | [43] | |
| 9. | Vaporized Au with SAM | PBS, pH 7.4, with redox probe** | CV EIS | Human serum polyclonal anti rS | Mixture Ig | 2.35 nM 1.99 nM | 30–150 nM | [1] |
| 10. | Polycrystalline Au with SAM | PBS, pH 7.4, with redox probe | CV DPV PPA | Human serum polyclonal anti rS | Mixture Ig | 0.344 nM (CV), 0.613 nM (DPV), 0.695 nM (PPA) | 1–90 nM | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zukauskas, S.; Rucinskiene, A.; Ratautaite, V.; Ramanaviciene, A.; Pilvenyte, G.; Bechelany, M.; Ramanavicius, A. Electrochemical Biosensor for the Determination of Specific Antibodies against SARS-CoV-2 Spike Protein. Int. J. Mol. Sci. 2023, 24, 718. https://doi.org/10.3390/ijms24010718
Zukauskas S, Rucinskiene A, Ratautaite V, Ramanaviciene A, Pilvenyte G, Bechelany M, Ramanavicius A. Electrochemical Biosensor for the Determination of Specific Antibodies against SARS-CoV-2 Spike Protein. International Journal of Molecular Sciences. 2023; 24(1):718. https://doi.org/10.3390/ijms24010718
Chicago/Turabian StyleZukauskas, Sarunas, Alma Rucinskiene, Vilma Ratautaite, Almira Ramanaviciene, Greta Pilvenyte, Mikhael Bechelany, and Arunas Ramanavicius. 2023. "Electrochemical Biosensor for the Determination of Specific Antibodies against SARS-CoV-2 Spike Protein" International Journal of Molecular Sciences 24, no. 1: 718. https://doi.org/10.3390/ijms24010718
APA StyleZukauskas, S., Rucinskiene, A., Ratautaite, V., Ramanaviciene, A., Pilvenyte, G., Bechelany, M., & Ramanavicius, A. (2023). Electrochemical Biosensor for the Determination of Specific Antibodies against SARS-CoV-2 Spike Protein. International Journal of Molecular Sciences, 24(1), 718. https://doi.org/10.3390/ijms24010718










