Mild Hypophosphatemia-Associated Conditions in Children: The Need for a Comprehensive Approach
Abstract
1. Introduction
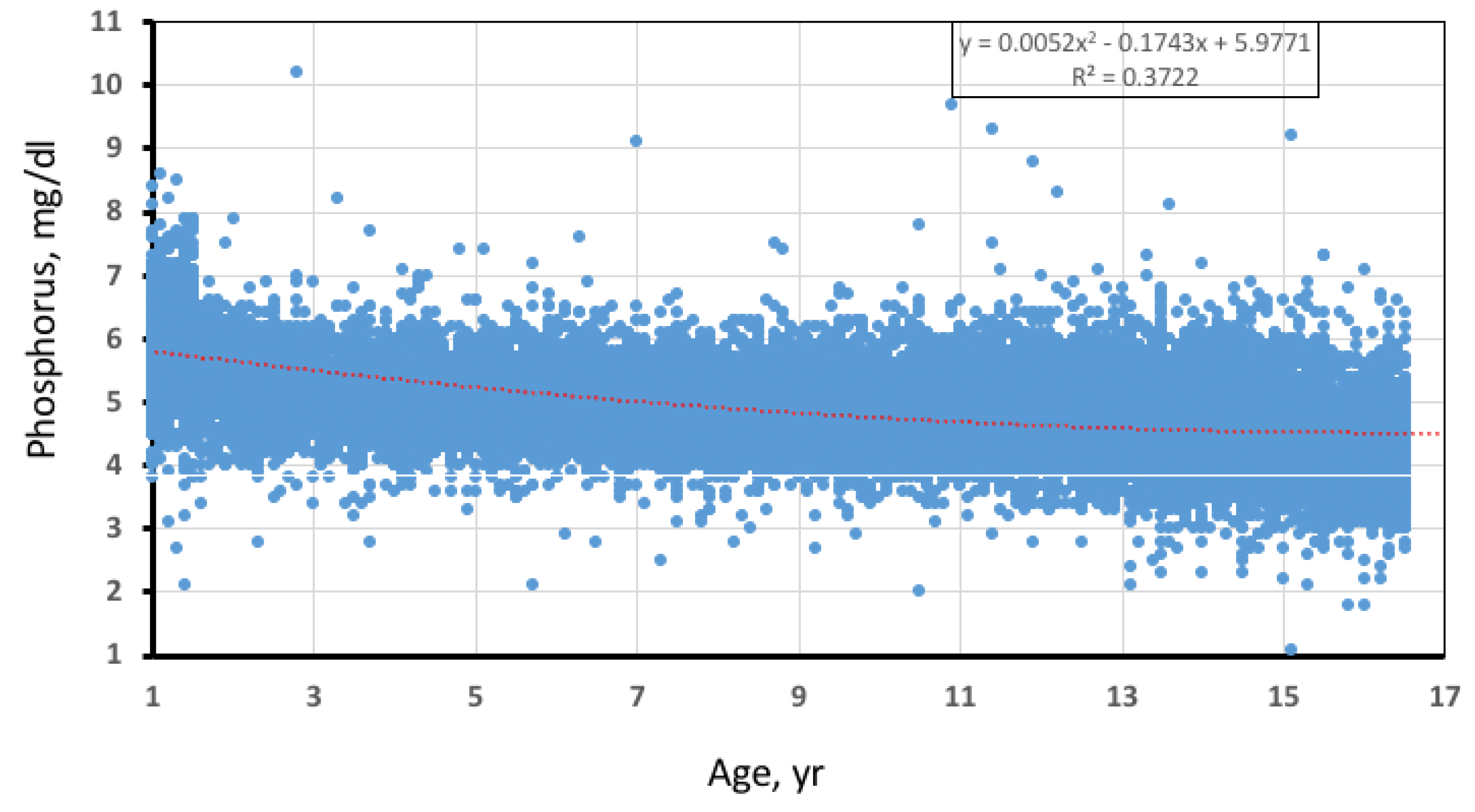
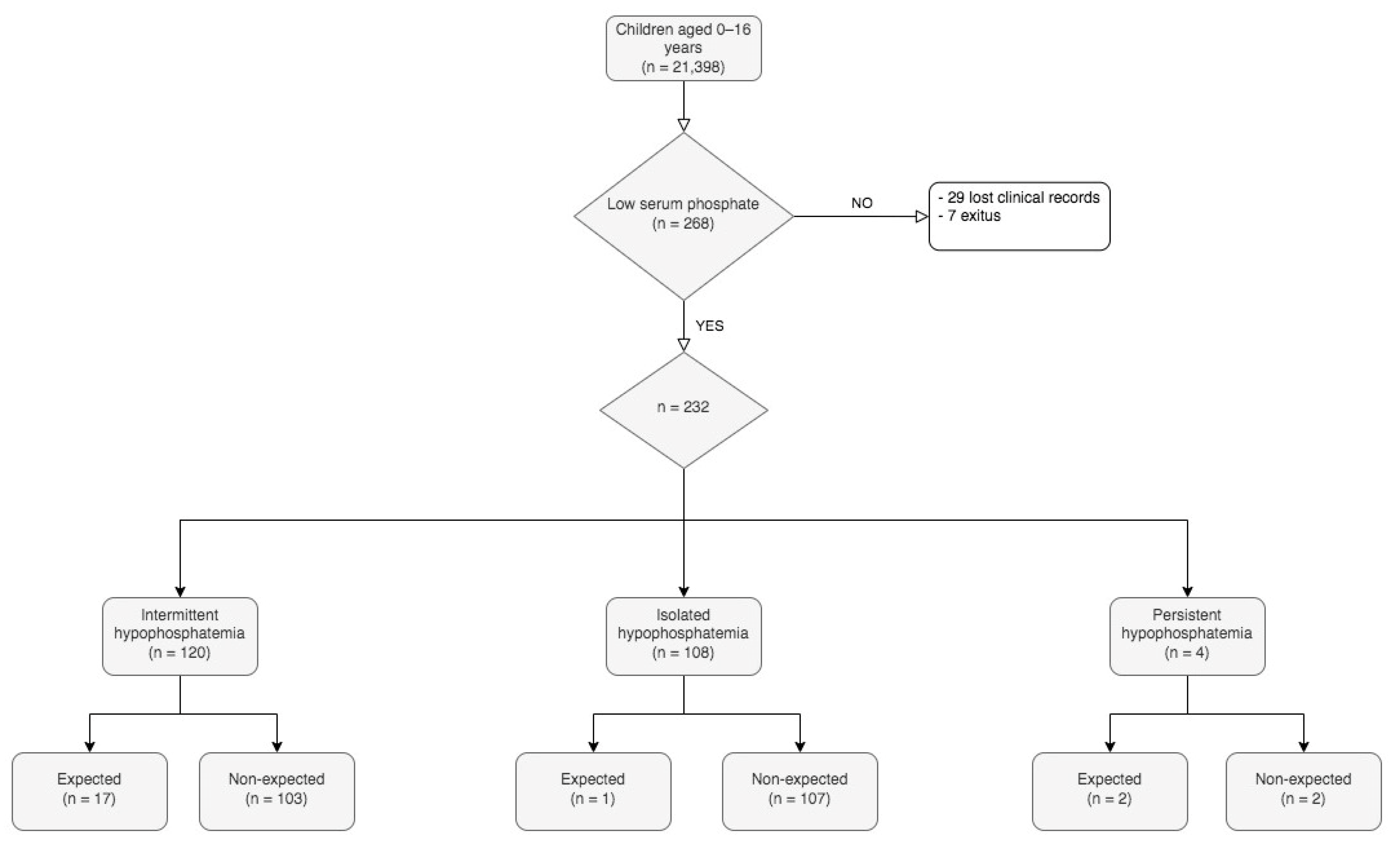
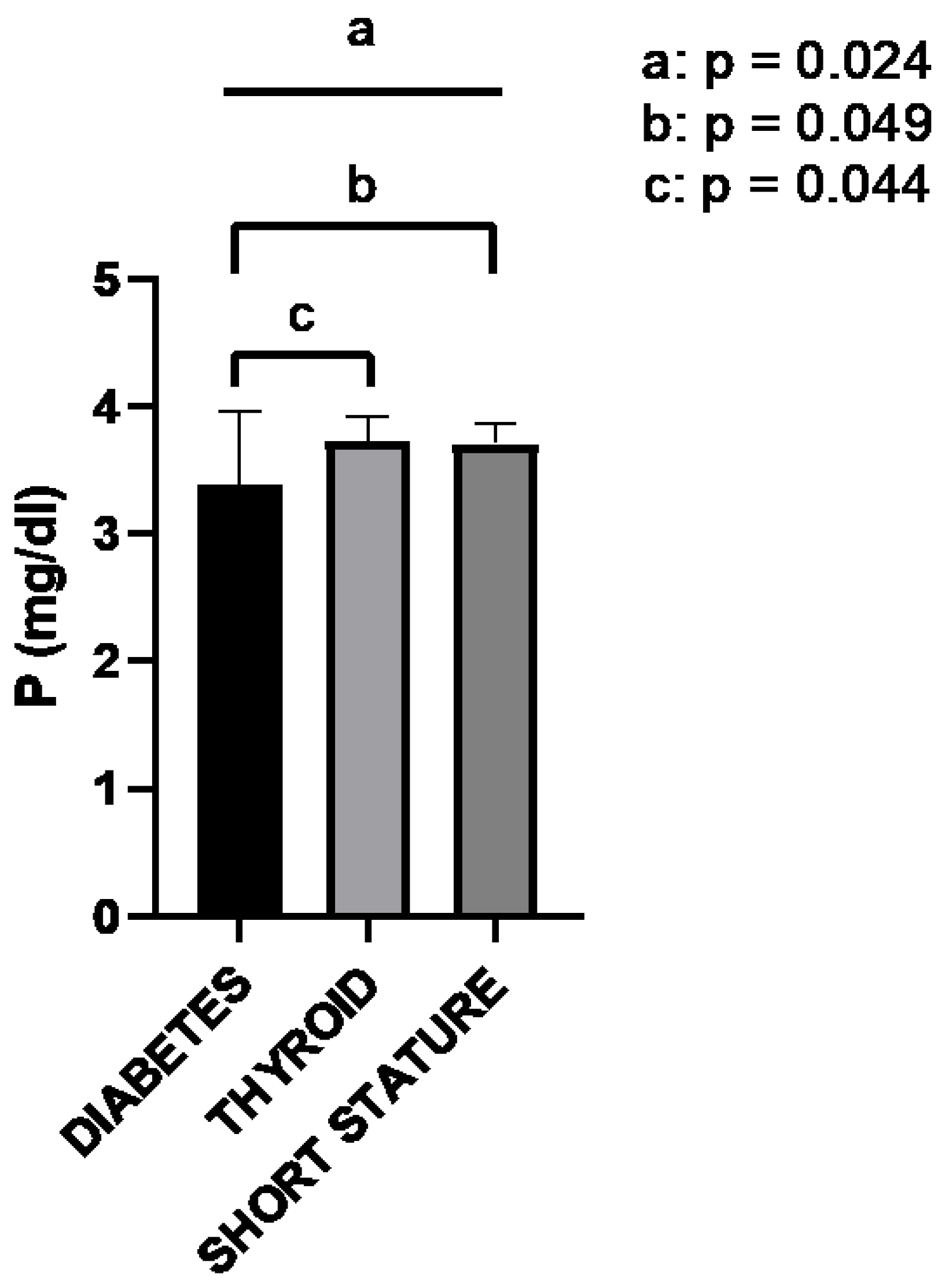
2. Results
3. Discussion
4. Patients and Methods
4.1. Study Design
4.2. Laboratory Procedures
4.3. Data Analysis
4.4. Ethical Aspects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Florenzano, P.; Cipriani, C.; Roszko, K.L.; Fukumoto, S.; Collins, M.T.; Minisola, S.; Pepe, J. Approach to patients with hypophosphataemia. Lancet Diabetes Endocrinol. 2020, 8, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Megapanou, E.; Florentin, M.; Milionis, H.; Elisaf, M.; Liamis, G. Drug-Induced Hypophosphatemia: Current Insights. Drug Saf. 2020, 43, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Imel, E.A. Congenital Conditions of Hypophosphatemia in Children. Calcif Tissue Int. 2021, 108, 74–90. [Google Scholar] [CrossRef]
- Quarles, L.D. FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Kumar Shah, S.; Irshad, M.; Gupta, N.; Kumar Kabra, S.; Lodha, R. Hypophosphatemia in Critically Ill Children: Risk Factors, Outcome and Mechanism. Indian J. Pediatr. 2016, 83, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros LF, G.; Ma, N.S.; Gordon, R.J.; Ward, L.; Backeljauw, P.; Wasserman, H.; Weber, D.R.; DiMeglio, L.A.; Gagne, J.; Stein, R.; et al. Unexpected widespread hypophosphatemia and bone disease associated with elemental formula use in infants and children. Bone 2017, 97, 287–292. [Google Scholar] [CrossRef][Green Version]
- DeLuca, H.F. The metabolism and functions of vitamin D. Adv. Exp. Med. Biol. 1986, 196, 361–375. [Google Scholar]
- Sabbagh, Y.; Giral, H.; Caldas, Y.; Levi, M.; Schiavi, S.C. Intestinal Phosphate Transport. Adv. Chronic Kidney Dis. 2011, 18, 85–90. [Google Scholar] [CrossRef]
- Efthymiadou, A.; Kritikou, D.; Mantagos, S.; Chrysis, D. The effect of GH treatment on serum FGF23 and Klotho in GH-deficient children. Eur. J. Endocrinol. 2016, 174, 473–479. [Google Scholar] [CrossRef]
- Peacock, M. Phosphate Metabolism in Health and Disease. Calcif. Tissue Int. 2021, 108, 3–15. [Google Scholar] [CrossRef]
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Acedemies Press: Washington, DC, USA, 1997; Available online: https://nap.nationalacademies.org/catalog/5776/dietary-reference-intakes-for-calcium-phosphorus-magnesium-vitamin-d-and-fluoride (accessed on 3 December 2022).
- Allgrove, J. Physiology of Calcium, Phosphate, Magnesium and Vitamin D. Endocr. Dev. 2015, 28, 7–32. [Google Scholar] [PubMed]
- Koljonen, L.; Enlund-Cerullo, M.; Hauta-Alus, H.; Holmlund-Suila, E.; Valkama, S.; Rosendahl, J.; Andersson, S.; Pekkinen, M.; Mäkitie, O. Phosphate Concentrations and Modifying Factors in Healthy Children From 12 to 24 Months of Age. J. Clin. Endocrinol. Metab. 2021, 106, 2865–2875. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R. Phosphate excretion Cp/Ccr vs. PEI. N. Engl. J. Med. 1976, 294, 559. [Google Scholar]
- Penido, M.G.; Alon, U.S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2012, 27, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- García Ospina, C.A.; Holguín, M.C.; Cáceres Escobar, D.; Restrepo Valencia, C.A.V. Importance of hyperphosphatemia in chronic kidney disease, how to avoid it and treat it by nutritional measures. Rev. Colomb. Nefrol. 2017, 4, 38–56. [Google Scholar]
- Bhadada, S.K.; Rao, S.D. Role of Phosphate in Biomineralization. Calcif. Tissue Int. 2021, 108, 32–40. [Google Scholar] [CrossRef]
- Michigami, T.; Kawai, M.; Yamazaki, M.; Ozono, K. Phosphate as a signaling molecule and its sensing mechanism. Physiol. Rev. 2018, 98, 2317–2348. [Google Scholar] [CrossRef]
- Westheimer, F.H. Nature Chose Phosphates The Role of Phosphates The Importance of Being Ionized. Science 1987, 235, 1173–1178. [Google Scholar] [CrossRef]
- Pesta, D.H.; Tsirigotis, D.N.; Befroy, D.E.; Caballero, D.; Jurczak, M.J.; Rahimi, Y.; Cline, G.W.; Dufour, S.; Birkenfeld, A.L.; Rothman, D.L.; et al. Hypophosphatemia promotes lower rates of muscle ATP synthesis. FASEB J. 2016, 30, 3378–3387. [Google Scholar] [CrossRef]
- Subramanian, R.; Khardori, R. Severe Hypophosphatemia. Pathophysiologic implications, clinical presentations and treatment. Medicine 2000, 79, 1–8. [Google Scholar] [CrossRef]
- Brunelli, S.M.; Goldfarb, S. Hypophosphatemia: Clinical consequences and management. J. Am. Soc. Nephrol. 2007, 18, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Amanzadeh, J.; Reilly, R.F. Hypophosphatemia: An evidence-based approach to its clinical consequences and management. Nat. Clin. Pract. Nephrol. 2006, 2, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Kliegman, R. Nelson Textbook of Pediatrics, 20th ed.; Elsevier: Philadelphia, PA, USA, 2016; pp. 383–387. [Google Scholar]
- Tiosano, D.; Hochberg, Z. Hypophosphatemia: The common denominator of all rickets. J. Bone Miner. Metab. 2009, 27, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Michigami, T.; Ozono, K. Roles of phosphate in skeleton. Front. Endocrinol. 2019, 10, 180. [Google Scholar] [CrossRef] [PubMed]
- Rendenbach, C.; Yorgan, T.A.; Heckt, T.; Otto, B.; Baldauf, C.; Jeschke, A.; Streichert, T.; David, J.P.; Amling, M.; Schinke, T. Effects of extracellular phosphate on gene expression in murine osteoblasts. Calcif. Tissue Int. 2014, 94, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Gillis, D.; Hefter, A. Optimal 25-OH-Vitamin D level in children derived from metabolic parameters. Horm. Res. Paediatr. 2022, 95 (Suppl. S2), 453. [Google Scholar]
- van der Vaart, A.; Waanders, F.; van Beek, A.P.; Vriesendorp, T.M.; Wolffenbutel, B.H.R.; van Dijk, P.R. Incidence and determinants of hypophosphatemia in diabetic ketoacidosis: An observational study. BMJ Open Diabetes Res. Care 2021, 9, e002018. [Google Scholar] [CrossRef]
- Levine, B.; Ho, K.; Kraut, J.; Coburn, J.; Kurokawa, K. Effect of metabolic acidosis on phosphate transport by the renal brush-border membrane. Biochim. Biophys. Acta 1983, 727, 7–12. [Google Scholar] [CrossRef]
- Liamis, G. Diabetes mellitus and electrolyte disorders. World J. Clin. Cases 2014, 2, 488–496. [Google Scholar] [CrossRef]
- Kebler, R.; McDonald, F.D.; Cadnapaphornchai, P. Dynamic changes in serum phosphorus levels in diabetic ketoacidosis. Am. J. Med. 1985, 79, 571–576. [Google Scholar] [CrossRef]
- Shen, T.; Braude, S. Changes in Serum phosphate during treatment of diabetic ketoacidosis: Predictive significance of severity of acidosis on presentation. Intern. Med. J. 2012, 42, 1347–1350. [Google Scholar] [CrossRef] [PubMed]
- Şimşek, D.; Okan Bakır, B. Determination of malnutrition status of children with diabetes during diagnosis. Hum. Nutr. Metab. 2021, 24, 200123. [Google Scholar] [CrossRef]
- Nansel, T.R.; Haynie, D.L.; Lipsky, L.M.; Laffel, L.M.B.; Mehta, S.N. Multiple Indicators of Poor Diet Quality in Children and Adolescents with Type 1 Diabetes Are Associated with Higher Body Mass Index Percentile but not Glycemic Control. J. Acad. Nutr. Diet. 2012, 112, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Svoren, B.M.; Volkening, L.K.; Wood, J.R.; Laffel, L.M.B. Significant Vitamin D Deficiency in Youth with Type 1 Diabetes Mellitus. J. Pediatr. 2009, 154, 132–134. [Google Scholar] [CrossRef][Green Version]
- Janner, M.; Flueck, C.E.; Mullis, P.E. High prevalence of vitamin D deficiency in children and adolescents with type 1 diabetes. Bone 2009, 46, 183–189. [Google Scholar] [CrossRef][Green Version]
- Read, S.; Grundy, E. Allostatic Load—A Challenge to Measure Multisystem Physiological Dysregulation; National Centre for Research Methods: London, UK, 2012. [Google Scholar]
- Martínez Redondo, I. Deficiencia De Vitamina D En Niños Aragoneses Sanos. Nutr. Hosp. 2018, 35, 782–788. [Google Scholar] [CrossRef]
- González-Padilla, E.; López, A.S.; González-Rodríguez, E.; García-Santana, S.; Mirallave-Pescador, A.; Marco, M.D.V.G.; Saavedra, P.; Gómez, J.M.Q.; Henríquez, M.S. Elevada prevalencia de hipovitaminosis D en los estudiantes de medicina de Gran Canaria, Islas Canarias (España). Endocrinol. Nutr. 2011, 58, 267–273. [Google Scholar] [CrossRef]
- Togo, A.; Espadas Maciá, D.; Blanes Segura, S.; Sivó Díaz, N.; Villalba Martínez, C. Existe déficit de vitamina D en los niños de una ciudad soleada del Mediterráneo? An. Pediatr. (Engl. Ed.) 2016, 84, 163–169. [Google Scholar] [CrossRef]
- Jacquillet, G.; Unwin, R.J. Physiological regulation of phosphate by vitamin D, parathyroid hormone (PTH) and phosphate (Pi). Pflügers Arch. 2019, 471, 83–98. [Google Scholar] [CrossRef]
- Lederer, E. Regulation of serum phosphate. J. Physiol. 2014, 592, 3985–3995. [Google Scholar] [CrossRef]
- Garabedian, M. Regulation of phosphate homeostasis in infants, children, and adolescents, and the role of phosphatonins in this process. Curr. Opin. Pediatr. 2007, 19, 488–491. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Reference Interval | Number of Patients | Patients with Abnormal Results (%) | Most Extreme Value a | Mean | Median (IQR) |
|---|---|---|---|---|---|---|
| Phosphate (mg/dL) | <12 years: 4–6 ≥12 years: 3–5.4 | 232 | 100 | 1.4 | 3.6 | 3.8 (3.5–3.9) |
| Albumin (g/dL) | 3.8–5.1 | 173 | 17 | 1.8 | 4.3 | 4.4 (4.02–4.7) |
| Corrected Ca (mg/dL) | 8.7–10.4 | 173 | 12 | 7.1 | 9.2 | 9.2 (8.83–9.4) |
| Alkaline phosphatase (U/L) | 1–3 years: 104–345 4–6 years: 93–309 7–9 years: 86–315 10–12 years: 42–362 >12 years: 44–147 | 197 | 3 a | 11,325 a | 328 | 218 (165–288) |
| Estimated Glomerular Filtration Rate (mL/min/1.73 m2) | 100–120 b | 199 | 4 | 15 | 104 | 105 (95–120) |
| 25-hydroxy-vitamin D (ng/mL) | 20–50 | 36 | 53 | 8 | 20 | 19 (15–25.5) |
| Parathyroid hormone (pg/mL) | 18–88 | 15 | 27 a | 108 a | 49 | 33 (17–35) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Docio, P.; Llorente-Pelayo, S.; García-Unzueta, M.T.; Lavin-Gómez, B.A.; Puente, N.; Mateos, F.; Riancho-Zarrabeitia, L.; Gonzalez-Lamuño, D.; Riancho, J.A. Mild Hypophosphatemia-Associated Conditions in Children: The Need for a Comprehensive Approach. Int. J. Mol. Sci. 2023, 24, 687. https://doi.org/10.3390/ijms24010687
Docio P, Llorente-Pelayo S, García-Unzueta MT, Lavin-Gómez BA, Puente N, Mateos F, Riancho-Zarrabeitia L, Gonzalez-Lamuño D, Riancho JA. Mild Hypophosphatemia-Associated Conditions in Children: The Need for a Comprehensive Approach. International Journal of Molecular Sciences. 2023; 24(1):687. https://doi.org/10.3390/ijms24010687
Chicago/Turabian StyleDocio, Pablo, Sandra Llorente-Pelayo, María Teresa García-Unzueta, Bernardo A. Lavin-Gómez, Nuria Puente, Fátima Mateos, Leyre Riancho-Zarrabeitia, Domingo Gonzalez-Lamuño, and José A. Riancho. 2023. "Mild Hypophosphatemia-Associated Conditions in Children: The Need for a Comprehensive Approach" International Journal of Molecular Sciences 24, no. 1: 687. https://doi.org/10.3390/ijms24010687
APA StyleDocio, P., Llorente-Pelayo, S., García-Unzueta, M. T., Lavin-Gómez, B. A., Puente, N., Mateos, F., Riancho-Zarrabeitia, L., Gonzalez-Lamuño, D., & Riancho, J. A. (2023). Mild Hypophosphatemia-Associated Conditions in Children: The Need for a Comprehensive Approach. International Journal of Molecular Sciences, 24(1), 687. https://doi.org/10.3390/ijms24010687






