Could BMPs Therapy Be Improved if BMPs Were Used in Composition Acting during Bone Formation in Endochondral Ossification?
Abstract
:1. Introduction
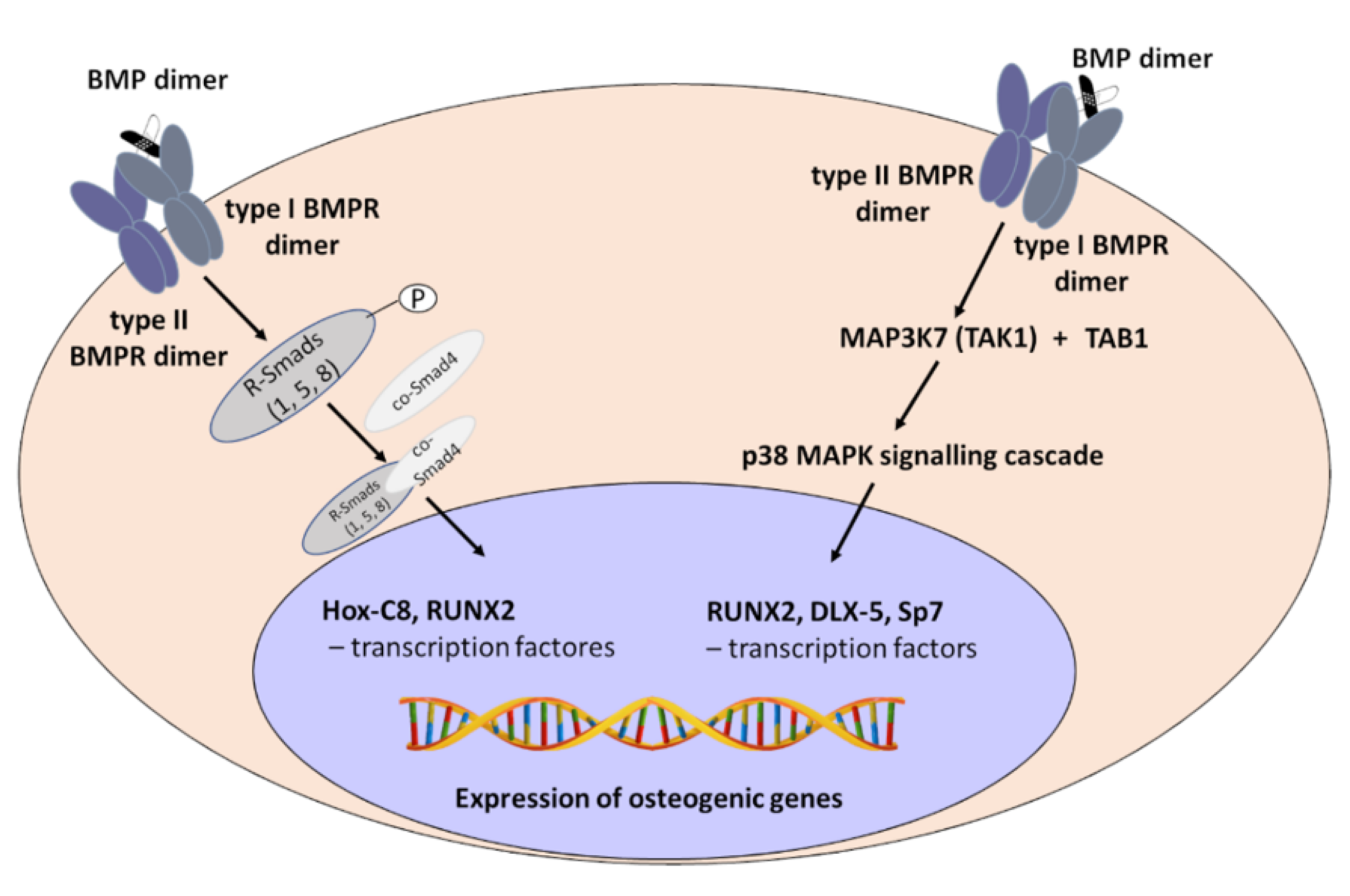
2. Mechanism of Bone Morphogenetic Proteins (BMPs) Action
3. BMPs in Osteogenesis
4. Osteoblast Differentiation
5. BMPs in Clinics—Pros and Cons
6. Synergistic Use of Various BMPs
7. Endochondral Ossification in Fracture Healing
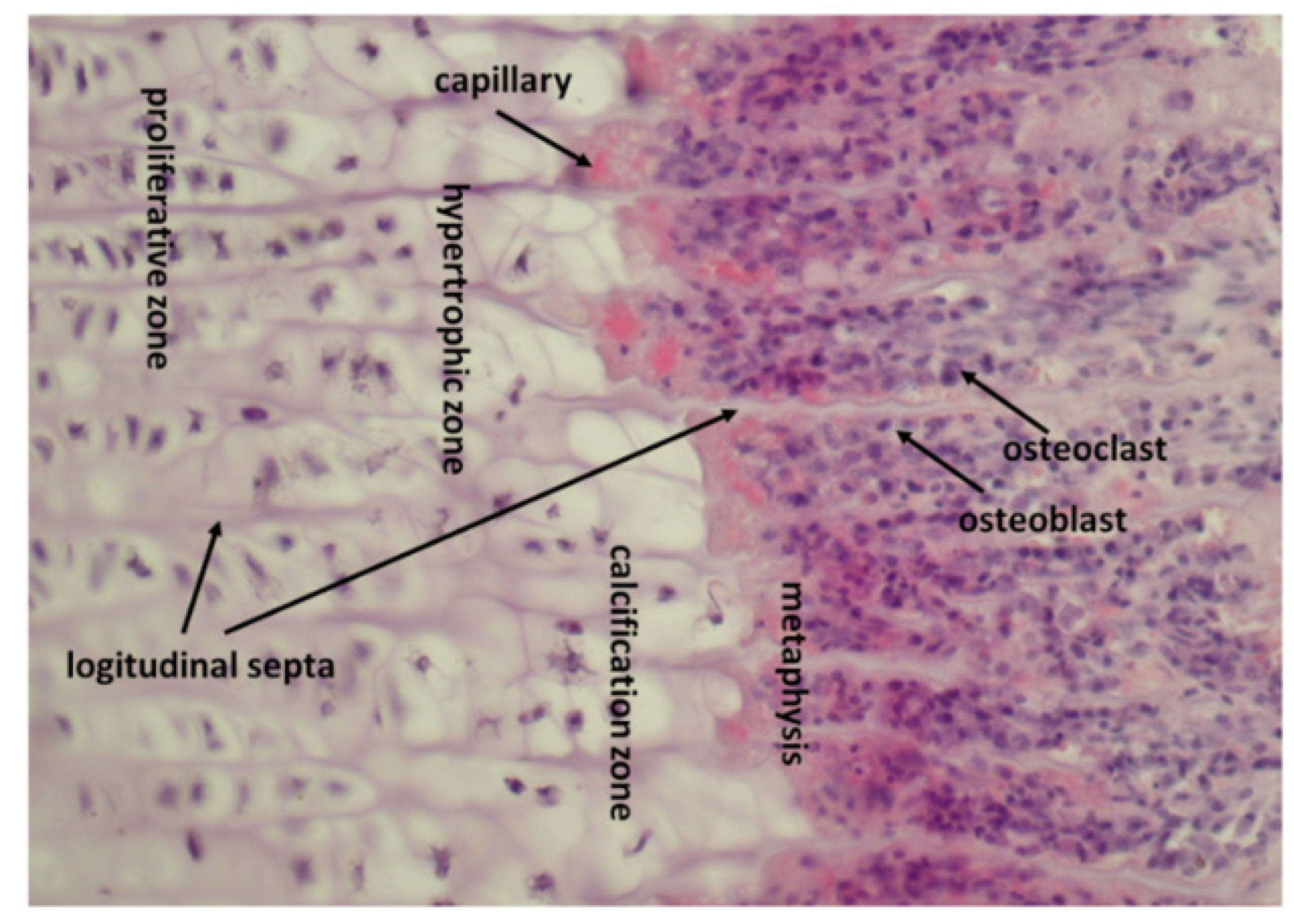
8. How Epiphyseal Growth Plate Produces and Stores Growth Factors
9. Osteoclasts Release Growth Factors from Calcified Cartilage and Transport Them towards Osteoprogenitor Cells
10. Protection of Growth Factors against Denaturation by Proteolytic Enzymes
11. Formation of Osteoclasts Resorbing Calcified Cartilage Is Stimulated by Factors Released from Non-Calcified Cartilage by Septoclasts
12. Isolation of Growth Factors from Calf Costochondral Junctions
13. Growth Factors Responsible for Initial Bone Formation in Human Metaphysis Remain terra incognita—Could Analysis of BMPs mRNA Expression in Chondrocytes from Human Epiphyseal Cartilage Help to Identify Them?
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wozney, J.M. Overview of bone morphogenetic proteins. Spine 2002, 27, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Kim, S.; Rho, J. Regulation of osteoblast differentiation by cytokine networks. Int. J. Mol. Sci. 2021, 22, 2851. [Google Scholar] [CrossRef] [PubMed]
- Cappariello, A.; Maurizi, A.; Veeriah, V.; Teti, A. The great beauty of the osteoclast. Arch. Biochem. Biophys. 2014, 558, 70–78. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Tan, Z.; To, M.K.T.; Chan, D. Regulation and role of transcription factors in osteogenesis. Int. J. Mol. Sci. 2021, 22, 5445. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Jiang, W.; Phillips, F.M.; Haydon, R.C.; Peng, Y.; Zhou, L.; Luu, H.H.; An, N.; Breyer, B.; Vanichakarn, P.; et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J. Bone Jt. Surg. Am. Vol. 2003, 85, 1544–1552. [Google Scholar] [CrossRef] [PubMed]
- da Silva Madaleno, C.; Jatzlau, J.; Knaus, P. BMP signalling in a mechanical context—Implications for bone biology. Bone 2020, 137, 115416. [Google Scholar] [CrossRef] [PubMed]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone morphogenetic protein-2 in development and bone homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Koosha, E.; Eames, B.F. Two modulators of skeletal development: BMPs and proteoglycans. J. Dev. Biol. 2022, 10, 15. [Google Scholar] [CrossRef]
- Nickel, J.; Mueller, T.D. Specification of BMP signaling. Cells 2019, 8, 1579. [Google Scholar] [CrossRef] [Green Version]
- Ponzetti, M.; Rucci, N. Osteoblast differentiation and signaling: Established concepts and emerging topics. Int. J. Mol. Sci. 2021, 22, 6651. [Google Scholar] [CrossRef] [PubMed]
- Sampath, T.K.; Reddi, A.H. Discovery of bone morphogenetic proteins—A historical perspective. Bone 2020, 140, 115548. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise review: Multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Stenbeck, G.; Horton, M.A. Endocytic trafficking in actively resorbing osteoclasts. J. Cell Sci. 2004, 117, 827–836. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Hyc, A.; Moskalewski, S.; Osiecka-Iwan, A. Growth factors in the initial stage of bone formation, analysis of their expression in chondrocytes from epiphyseal cartilage of rat costochondral junction. Folia Histochem. Cytobiol. 2021, 59, 178–186. [Google Scholar] [CrossRef]
- Iwan, A.; Moskalewski, S.; Hyc, A. Growth factor profile in calcified cartilage from the metaphysis of a calf costochondral junction, the site of initial bone formation. Biomed. Rep. 2021, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Mediouni, M.; Schlatterer, D.R.; Madry, H.; Cucchiarini, M.; Rai, B. A review of translational medicine. The future paradigm: How can we connect the orthopedic dots better? Curr. Med. Res. Opin. 2018, 34, 1217–1229. [Google Scholar] [CrossRef] [PubMed]
- Mediouni, M.; Madiouni, R.; Gardner, M.; Vaughan, N. Translational medicine: Challenges and new orthopaedic vision (Mediouni-Model). Curr. Orthop. Pract. 2019, 31, 196–200. [Google Scholar] [CrossRef]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef]
- Bal, Z.; Kushioka, J.; Kodama, J.; Kaito, T.; Yoshikawa, H.; Korkusuz, P.; Korkusuz, F. BMP and TGFβ use and release in bone regeneration. Turk. J. Med. Sci. 2020, 50, 1707–1722. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Thielen, N.G.M.; van der Kraan, P.M.; van Caam, A.P.M. TGFβ/BMP signaling pathway in cartilage homeostasis. Cells 2019, 8, 969. [Google Scholar] [CrossRef]
- Yang, P.; Troncone, L.; Augur, Z.M.; Kim, S.S.J.; McNeil, M.E.; Yu, P.B. The role of bone morphogenetic protein signaling in vascular calcification. Bone 2020, 141, 115542. [Google Scholar] [CrossRef]
- Nakashima, K.; de Crombrugghe, B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003, 19, 458–466. [Google Scholar] [CrossRef]
- Shen, B.; Wei, A.; Whittaker, S.; Williams, L.A.; Tao, H.; Ma, D.D.; Diwan, A.D. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J. Cell. Biochem. 2010, 109, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Bruderer, M.; Richards, R.G.; Alini, M.; Stoddart, M.J. Role and regulation of RUNX2 in osteogenesis. Eur. Cells Mater. 2014, 28, 269–286. [Google Scholar] [CrossRef]
- Sinha, K.M.; Zhou, X. Genetic and molecular control of osterix in skeletal formation. J. Cell. Biochem. 2013, 114, 975–984. [Google Scholar] [CrossRef]
- Fu, H.L.; Pan, H.X.; Zhao, B.; Dong, B.C.; Shao, L.; Fu, G.S.; Wang, Q.; Li, M. MicroRNA-100 inhibits bone morphogenetic protein-induced osteoblast differentiation by targeting Smad1. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3911–3919. [Google Scholar]
- Garcia, J.; Delany, A.M. MicroRNAs regulating TGFβ and BMP signaling in the osteoblast lineage. Bone 2021, 143, 115791. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem. Cell Biol. 2018, 149, 313–323. [Google Scholar] [CrossRef]
- Hojo, H.; Ohba, S. Sp7 Action in the skeleton: Its mode of action, functions, and relevance to skeletal diseases. Int. J. Mol. Sci. 2022, 23, 5647. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, M.; Wang, S.; Xiao, Z.; Xiong, Y.; Wang, G. Recent advances of osterix transcription factor in osteoblast differentiation and bone formation. Front. Cell Dev. Biol. 2020, 8, 601224. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yuan, Y.; Wu, L.; Ho, T.V.; Jing, J.; Sugii, H.; Li, J.; Han, X.; Feng, J.; Guo, C.; et al. BMP-IHH-mediated interplay between mesenchymal stem cells and osteoclasts supports calvarial bone homeostasis and repair. Bone Res. 2018, 6, 30. [Google Scholar] [CrossRef]
- Moskalewski, S.; Malejczyk, J.; Osiecka-Iwan, A.; Hyc, A. Osteoblasts from calvarial and endochondral bone transplanted intramuscularly produce bone similar to that of their origin. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2022, 37, 1209–1210. [Google Scholar] [CrossRef]
- Aslani, S.; Abhari, A.; Sakhinia, E.; Sanajou, D.; Rajabi, H.; Rahimzadeh, S. Interplay between microRNAs and Wnt, transforming growth factor-β, and bone morphogenic protein signaling pathways promote osteoblastic differentiation of mesenchymal stem cells. J. Cell. Physiol. 2019, 234, 8082–8093. [Google Scholar] [CrossRef]
- Mazziotta, C.; Lanzillotti, C.; Iaquinta, M.R.; Taraballi, F.; Torreggiani, E.; Rotondo, J.C.; Otòn-Gonzalez, L.; Mazzoni, E.; Frontini, F.; Bononi, I.; et al. MicroRNAs modulate signaling pathways in osteogenic differentiation of mesenchymal stem cells. Int. J. Mol. Sci. 2021, 22, 2362. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Srinaath, N.; Rohini, M.; Selvamurugan, N. Regulation of Runx2 by MicroRNAs in osteoblast differentiation. Life Sci. 2019, 232, 116676. [Google Scholar] [CrossRef] [PubMed]
- Hrdlicka, H.C.; Lee, S.K.; Delany, A.M. MicroRNAs are critical regulators of osteoclast differentiation. Curr. Mol. Biol. Rep. 2019, 5, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.; Duroux-Richard, I.; Firat, H.; Schordan, E.; Apparailly, F. MicroRNAs: Key regulators to understand osteoclast differentiation? Front. Immunol. 2019, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Weivoda, M.M.; Lee, S.K.; Monroe, D.G. miRNAs in osteoclast biology. Bone 2021, 143, 115757. [Google Scholar] [CrossRef] [PubMed]
- Hata, A.; Kang, H. Functions of the bone morphogenetic protein signaling pathway through microRNAs. Int. J. Mol. Med. 2015, 35, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New technologies for analysis of extracellular vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef] [PubMed]
- Davies, O.G.; Cox, S.C.; Azoidis, I.; McGuinness, A.J.A.; Cooke, M.; Heaney, L.M.; Davis, E.T.; Jones, S.W.; Grover, L.M. Osteoblast-derived vesicle protein content is temporally regulated during osteogenesis: Implications for regenerative therapies. Front. Bioeng. Biotechnol. 2019, 7, 92. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef]
- Hu, H.; Wang, D.; Li, L.; Yin, H.; He, G.; Zhang, Y. Role of microRNA-335 carried by bone marrow mesenchymal stem cells-derived extracellular vesicles in bone fracture recovery. Cell Death Dis. 2021, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Balakrishnan, L.; Sharma, K.; Khan, A.A.; Advani, J.; Gowda, H.; Tripathy, S.P.; Suar, M.; Pandey, A.; Gandotra, S.; et al. A network map of Interleukin-10 signaling pathway. J. Cell Commun. Signal. 2016, 10, 61–67. [Google Scholar] [CrossRef]
- Urist, M.R.; Lietze, A.; Mizutani, H.; Takagi, K.; Triffitt, J.T.; Amstutz, J.; DeLange, R.; Termine, J.; Finerman, G.A. A bovine low molecular weight bone morphogenetic protein (BMP) fraction. Clin. Orthop. Relat. Res. 1982, 162, 219–232. [Google Scholar] [CrossRef]
- Sampath, T.K.; Muthukumaran, N.; Reddi, A.H. Isolation of osteogenin, an extracellular matrix-associated, bone-inductive protein, by heparin affinity chromatography. Proc. Natl. Acad. Sci. USA 1987, 84, 7109–7113. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Bigham-Sadegh, A. Bone morphogenetic proteins: A powerful osteoinductive compound with non-negligible side effects and limitations. Biofactors 2014, 40, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Li, X. Growth differentiation factor 5 regulation in bone regeneration. Curr. Pharm. Des. 2013, 19, 3364–3373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zara, J.; Siu, R.K.; Ting, K.; Soo, C. The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. J. Dent. Res. 2010, 89, 865–878. [Google Scholar] [CrossRef]
- Termaat, M.F.; Den Boer, F.C.; Bakker, F.C.; Patka, P.; Haarman, H.J. Bone morphogenetic proteins. Development and clinical efficacy in the treatment of fractures and bone defects. J. Bone Jt. Surg. Am. Vol. 2005, 87, 1367–1378. [Google Scholar] [CrossRef]
- McKay, W.F.; Peckham, S.M.; Badura, J.M. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int. Orthop. 2007, 31, 729–734. [Google Scholar] [CrossRef]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- El Bialy, I.; Jiskoot, W.; Reza Nejadnik, M. Formulation, delivery and stability of bone morphogenetic proteins for effective bone regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef]
- Fukuda, T.; Fukuda, R.; Tanabe, R.; Koinuma, D.; Koyama, H.; Hashizume, Y.; Moustakas, A.; Miyazono, K.; Heldin, C.H. BMP signaling is a therapeutic target in ovarian cancer. Cell Death Discov. 2020, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ye, Y.; Long, X.; Xiao, P.; Ren, X.; Yu, J. BMP signaling and its paradoxical effects in tumorigenesis and dissemination. Oncotarget 2016, 7, 78206–78218. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E. Basic science and spine literature document bone morphogenetic protein increases cancer risk. Surg. Neurol. Int. 2014, 5, S552–S560. [Google Scholar] [CrossRef]
- Celeste, A.J.; Iannazzi, J.A.; Taylor, R.C.; Hewick, R.M.; Rosen, V.; Wang, E.A.; Wozney, J.M. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc. Natl. Acad. Sci. USA 1990, 87, 9843–9847. [Google Scholar] [CrossRef]
- Wutzl, A.; Rauner, M.; Seemann, R.; Millesi, W.; Krepler, P.; Pietschmann, P.; Ewers, R. Bone morphogenetic proteins 2, 5, and 6 in combination stimulate osteoblasts but not osteoclasts in vitro. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2010, 28, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Luu, H.H.; Song, W.X.; Luo, X.; Manning, D.; Luo, J.; Deng, Z.L.; Sharff, K.A.; Montag, A.G.; Haydon, R.C.; He, T.C. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2007, 25, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.S.; Long, M.W.; Hankenson, K.D. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J. Cell. Biochem. 2006, 98, 538–554. [Google Scholar] [CrossRef]
- Stokovic, N.; Ivanjko, N.; Maticic, D.; Luyten, F.P.; Vukicevic, S. Bone morphogenetic proteins, carriers, and animal models in the development of novel bone regenerative therapies. Materials 2021, 14, 3513. [Google Scholar] [CrossRef] [PubMed]
- Vukicevic, S.; Grgurevic, L. BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev. 2009, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef]
- Qasim, M.; Chae, D.S.; Lee, N.Y. Bioengineering strategies for bone and cartilage tissue regeneration using growth factors and stem cells. J. Biomed. Mater. Res. Part A 2020, 108, 394–411. [Google Scholar] [CrossRef] [PubMed]
- Brighton, C.T. Structure and function of the growth plate. Clin. Orthop. Relat. Res. 1978, 136, 22–32. [Google Scholar] [CrossRef]
- Nahar, N.N.; Missana, L.R.; Garimella, R.; Tague, S.E.; Anderson, H.C. Matrix vesicles are carriers of bone morphogenetic proteins (BMPs), vascular endothelial gana, L.R.; Garimella, R.; Tague, S.E.; Anderson, H.C. Matrix vesicles are carriers of bone morphorowth factor (VEGF), and noncollagenous matrix proteins. J. Bone Miner. Metab. 2008, 26, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Jaroszewicz, J.; Bazarnik, P.; Osiecka-Iwan, A.; Hyc, A.; Choinska, E.; Chlanda, A.; Swieszkowski, W.; Moskalewski, S. From matrix vesicles to miniature rocks: Evolution of calcium deposits in calf costochondral junctions. Cartilage 2021, 13, 326s–335s. [Google Scholar] [CrossRef] [PubMed]
- Schenk, R.K.; Spiro, D.; Wiener, J. Cartilage resorption in the tibial epiphyseal plate of growing rats. J. Cell Biol. 1967, 34, 275–291. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.E.; Parker, J. Invasion and resorption in enchondral ossification. An electron microscopic study. J. Bone Jt. Surg. Am. Vol. 1966, 48, 899–914. [Google Scholar] [CrossRef]
- Lewinson, D.; Silbermann, M. Chondroclasts and endothelial cells collaborate in the process of cartilage resorption. Anat. Rec. 1992, 233, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Suzuki, S.; Yamashita, Y. An ultrastructural study of cartilage resorption at the site of initial endochondral bone formation in the fetal mouse mandibular condyle. J. Anat. 1997, 191 Pt 1, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Lakkakorpi, P.T.; Väänänen, H.K. Cytoskeletal changes in osteoclasts during the resorption cycle. Microsc. Res. Tech. 1996, 33, 171–181. [Google Scholar] [CrossRef]
- Takito, J.; Inoue, S.; Nakamura, M. The sealing zone in osteoclasts: A self-organized structure on the bone. Int. J. Mol. Sci. 2018, 19, 984. [Google Scholar] [CrossRef]
- Stenbeck, G. Formation and function of the ruffled border in osteoclasts. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2002; Volume 13, pp. 285–292. [Google Scholar] [CrossRef]
- Baron, R.; Neff, L.; Louvard, D.; Courtoy, P.J. Cell-mediated extracellular acidification and bone resorption: Evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J. Cell Biol. 1985, 101, 2210–2222. [Google Scholar] [CrossRef] [PubMed]
- Blair, H.C.; Teitelbaum, S.L.; Ghiselli, R.; Gluck, S. Osteoclastic bone resorption by a polarized vacuolar proton pump. Science 1989, 245, 855–857. [Google Scholar] [CrossRef]
- Silver, I.A.; Murrills, R.J.; Etherington, D.J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res. 1988, 175, 266–276. [Google Scholar] [CrossRef]
- Everts, V.; Delaissé, J.M.; Korper, W.; Niehof, A.; Vaes, G.; Beertsen, W. Degradation of collagen in the bone-resorbing compartment underlying the osteoclast involves both cysteine-proteinases and matrix metalloproteinases. J. Cell. Physiol. 1992, 150, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Mukai, S.; Yorita, K.; Kawagoe, Y.; Katayama, Y.; Nakahara, K.; Kamibeppu, T.; Sugie, S.; Tukino, H.; Kamoto, T.; Kataoka, H. Matriptase and MET are prominently expressed at the site of bone metastasis in renal cell carcinoma: Immunohistochemical analysis. Hum. Cell 2015, 28, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Tseng, I.C.; Xu, H.; Chou, F.P.; Li, G.; Vazzano, A.P.; Kao, J.P.; Johnson, M.D.; Lin, C.Y. Matriptase activation, an early cellular response to acidosis. J. Biol. Chem. 2010, 285, 3261–3270. [Google Scholar] [CrossRef] [PubMed]
- Friess, W.; Uludag, H.; Foskett, S.; Biron, R. Bone regeneration with recombinant human bone morphogenetic protein-2 (rhBMP-2) using absorbable collagen sponges (ACS): Influence of processing on ACS characteristics and formulation. Pharm. Dev. Technol. 1999, 4, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Salo, J.; Lehenkari, P.; Mulari, M.; Metsikkö, K.; Väänänen, H.K. Removal of osteoclast bone resorption products by transcytosis. Science 1997, 276, 270–273. [Google Scholar] [CrossRef]
- Griffith, D.L.; Keck, P.C.; Sampath, T.K.; Rueger, D.C.; Carlson, W.D. Three-dimensional structure of recombinant human osteogenic protein 1: Structural paradigm for the transforming growth factor beta superfamily. Proc. Natl. Acad. Sci. USA 1996, 93, 878–883. [Google Scholar] [CrossRef] [PubMed]
- Colgrave, M.L.; Craik, D.J. Thermal, chemical, and enzymatic stability of the cyclotide kalata B1: The importance of the cyclic cystine knot. Biochemistry 2004, 43, 5965–5975. [Google Scholar] [CrossRef]
- Kikuchi, K.; Sugiura, M.; Kimura, T. High proteolytic resistance of spider-derived inhibitor cystine knots. Int. J. Pept. 2015, 2015, 537508. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.S.; Mancera, R.L. Prediction of heparin binding sites in bone morphogenetic proteins (BMPs). Biochim. Biophys. Acta 2012, 1824, 1374–1381. [Google Scholar] [CrossRef] [PubMed]
- De Pasquale, V.; Pavone, L.M. Heparan sulfate proteoglycans: The sweet side of development turns sour in mucopolysaccharidoses. Biochim. Biophys. Acta. Mol. Basis Dis. 2019, 1865, 165539. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Kim, C.L.; Kim, Y.S.; Jang, J.W.; Lee, G.M. Selective endocytosis of recombinant human BMPs through cell surface heparan sulfate proteoglycans in CHO cells: BMP-2 and BMP-7. Sci. Rep. 2021, 11, 3378. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.R.; Lamplugh, L.; Shepard, N.L.; Mort, J.S. The septoclast, a cathepsin B-rich cell involved in the resorption of growth plate cartilage. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1995, 43, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Odgren, P.R.; Witwicka, H.; Reyes-Gutierrez, P. The cast of clasts: Catabolism and vascular invasion during bone growth, repair, and disease by osteoclasts, chondroclasts, and septoclasts. Connect. Tissue Res. 2016, 57, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Bando, Y.; Sakashita, H.; Taira, F.; Miyake, G.; Ogasawara, Y.; Sakiyama, K.; Owada, Y.; Amano, O. Origin and development of septoclasts in endochondral ossification of mice. Histochem. Cell Biol. 2018, 149, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, E.; Hasegawa, T.; Hongo, H.; Yamamoto, T.; Abe, M.; Yoshida, T.; Zhao, S.; Tsuboi, K.; Udagawa, N.; Henrique Luiz de Freitas, P.; et al. Histochemical assessment on the cellular interplay of vascular endothelial cells and septoclasts during endochondral ossification in mice. Microscopy 2021, 70, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Sivaraj, K.K.; Majev, P.G.; Jeong, H.W.; Dharmalingam, B.; Zeuschner, D.; Schröder, S.; Bixel, M.G.; Timmen, M.; Stange, R.; Adams, R.H. Mesenchymal stromal cell-derived septoclasts resorb cartilage during developmental ossification and fracture healing. Nat. Commun. 2022, 13, 571. [Google Scholar] [CrossRef]
- Gerber, H.P.; Vu, T.H.; Ryan, A.M.; Kowalski, J.; Werb, Z.; Ferrara, N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999, 5, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Olsen, B.R. Distinct VEGF functions during bone development and homeostasis. Arch. Immunol. Ther. Exp. 2014, 62, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Byard, R.W.; Foster, B.K.; Byers, S. Immunohistochemical characterisation of the costochondral junction in SIDS. J. Clin. Pathol. 1993, 46, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, O.; Parker, E.A.; Hegde, A.; Chau, M.; Barnes, K.M.; Baron, J. Gradients in bone morphogenetic protein-related gene expression across the growth plate. J. Endocrinol. 2007, 193, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Academy of Medical Royal Colleges. Ethical Issues in Paediatric Organ Donation—A Position Paper by the UK Donation Ethics Committee (UKDEC); Academy of Medical Royal Colleges: London, UK, 2015; Volume 4. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyc, A.; Osiecka-Iwan, A.; Moskalewski, S. Could BMPs Therapy Be Improved if BMPs Were Used in Composition Acting during Bone Formation in Endochondral Ossification? Int. J. Mol. Sci. 2022, 23, 10327. https://doi.org/10.3390/ijms231810327
Hyc A, Osiecka-Iwan A, Moskalewski S. Could BMPs Therapy Be Improved if BMPs Were Used in Composition Acting during Bone Formation in Endochondral Ossification? International Journal of Molecular Sciences. 2022; 23(18):10327. https://doi.org/10.3390/ijms231810327
Chicago/Turabian StyleHyc, Anna, Anna Osiecka-Iwan, and Stanislaw Moskalewski. 2022. "Could BMPs Therapy Be Improved if BMPs Were Used in Composition Acting during Bone Formation in Endochondral Ossification?" International Journal of Molecular Sciences 23, no. 18: 10327. https://doi.org/10.3390/ijms231810327
APA StyleHyc, A., Osiecka-Iwan, A., & Moskalewski, S. (2022). Could BMPs Therapy Be Improved if BMPs Were Used in Composition Acting during Bone Formation in Endochondral Ossification? International Journal of Molecular Sciences, 23(18), 10327. https://doi.org/10.3390/ijms231810327




