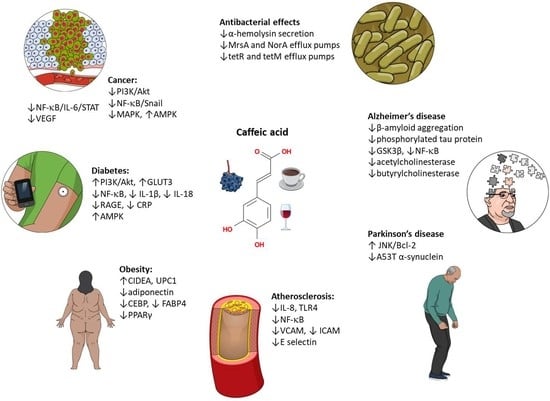
Caffeic Acid and Diseases—Mechanisms of Action
Abstract
1. Introduction
2. Caffeic Acid as an Antioxidant
3. Caffeic Acid and Cancer
3.1. Cancer Prevention
3.2. Liver Cancer
3.3. Breast Cancer
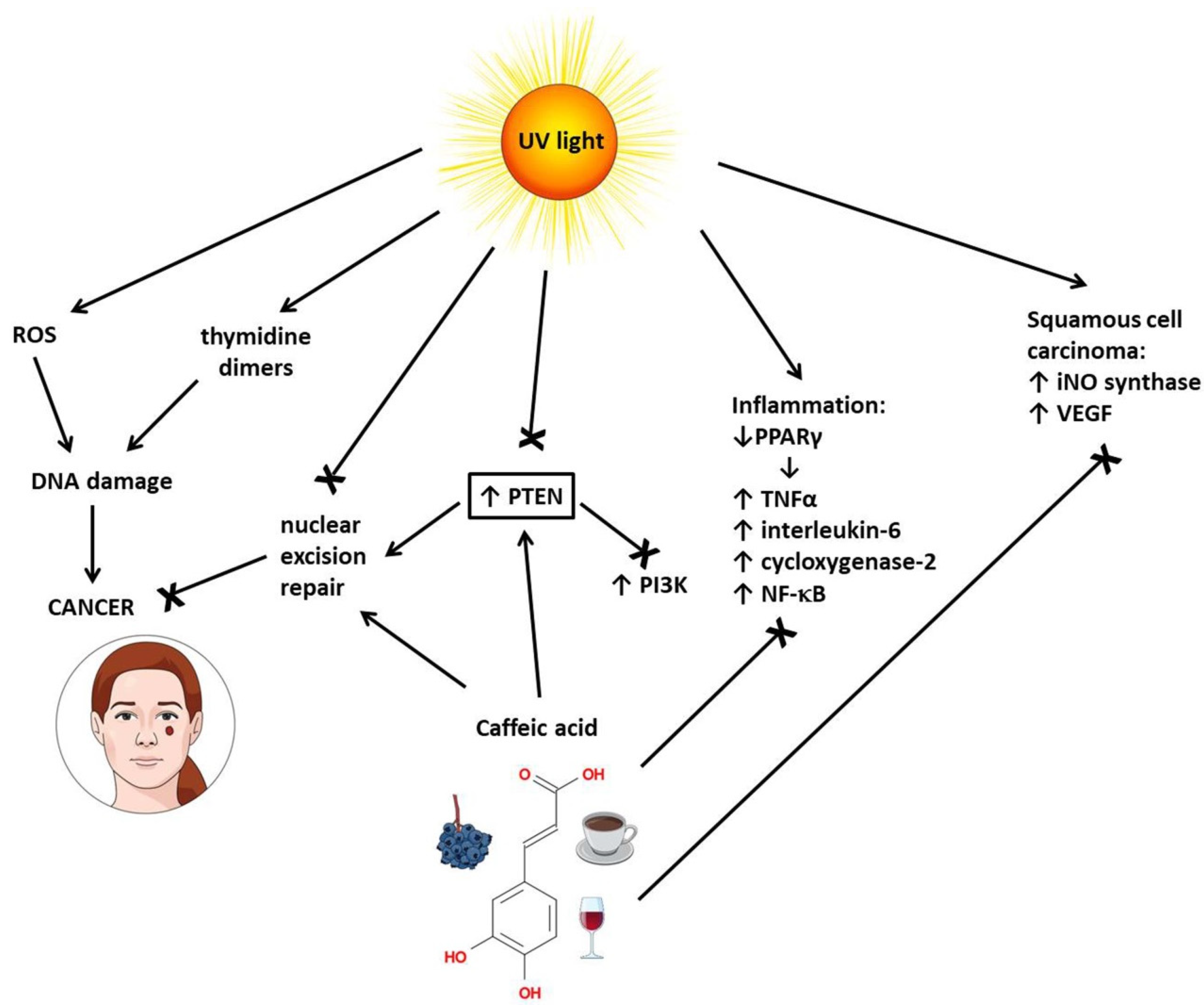
3.4. Skin Cancer
3.5. Lung Cancer
3.6. Oral Cancer
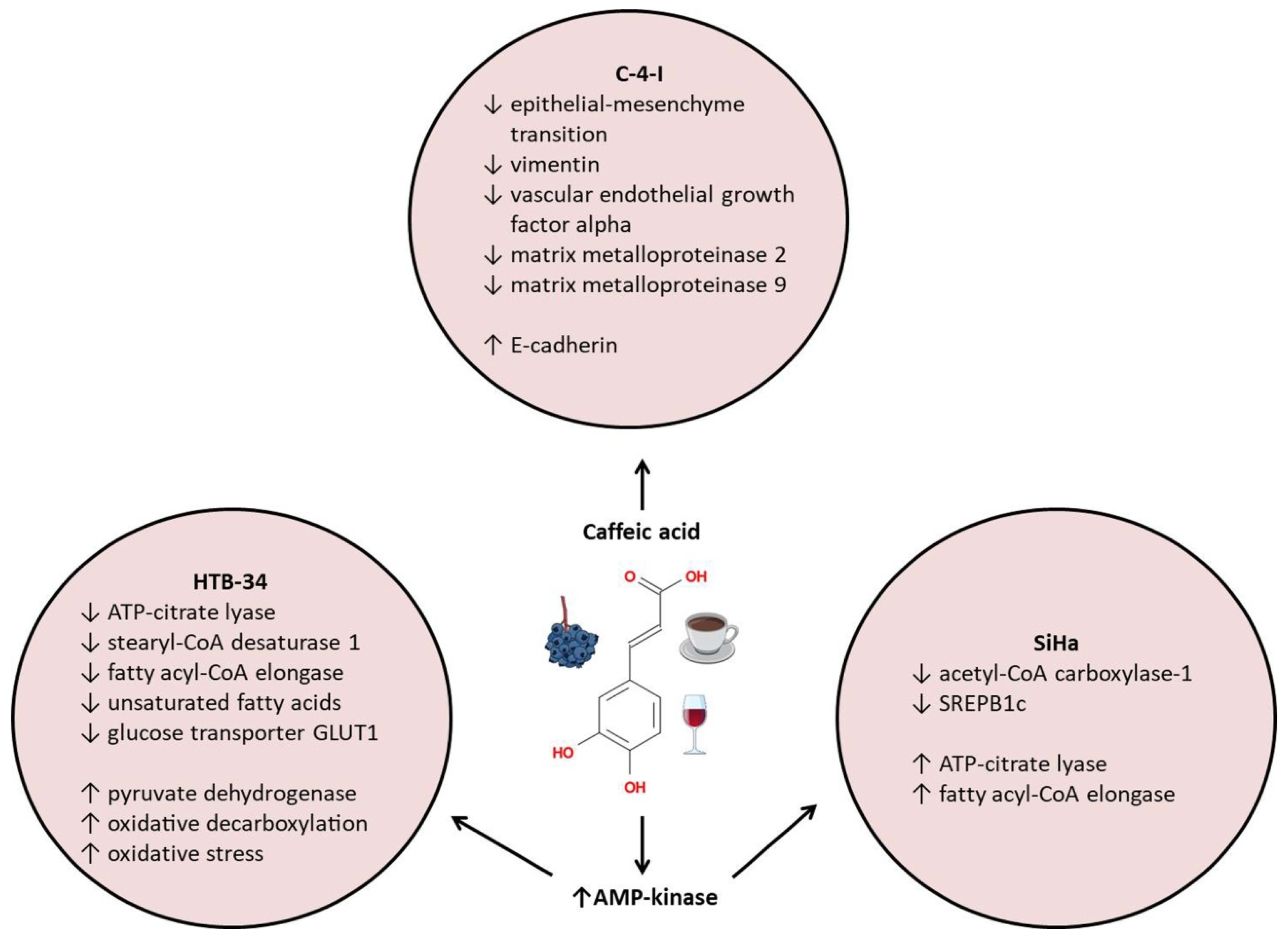
3.7. Cervical Cancer
4. Caffeic Acid and Diabetes, Obesity, and Metabolic Syndrome
4.1. Diabetes
4.2. Obesity
4.3. Atherosclerosis
5. Effects of Caffeic Acid on Brain-Related Diseases
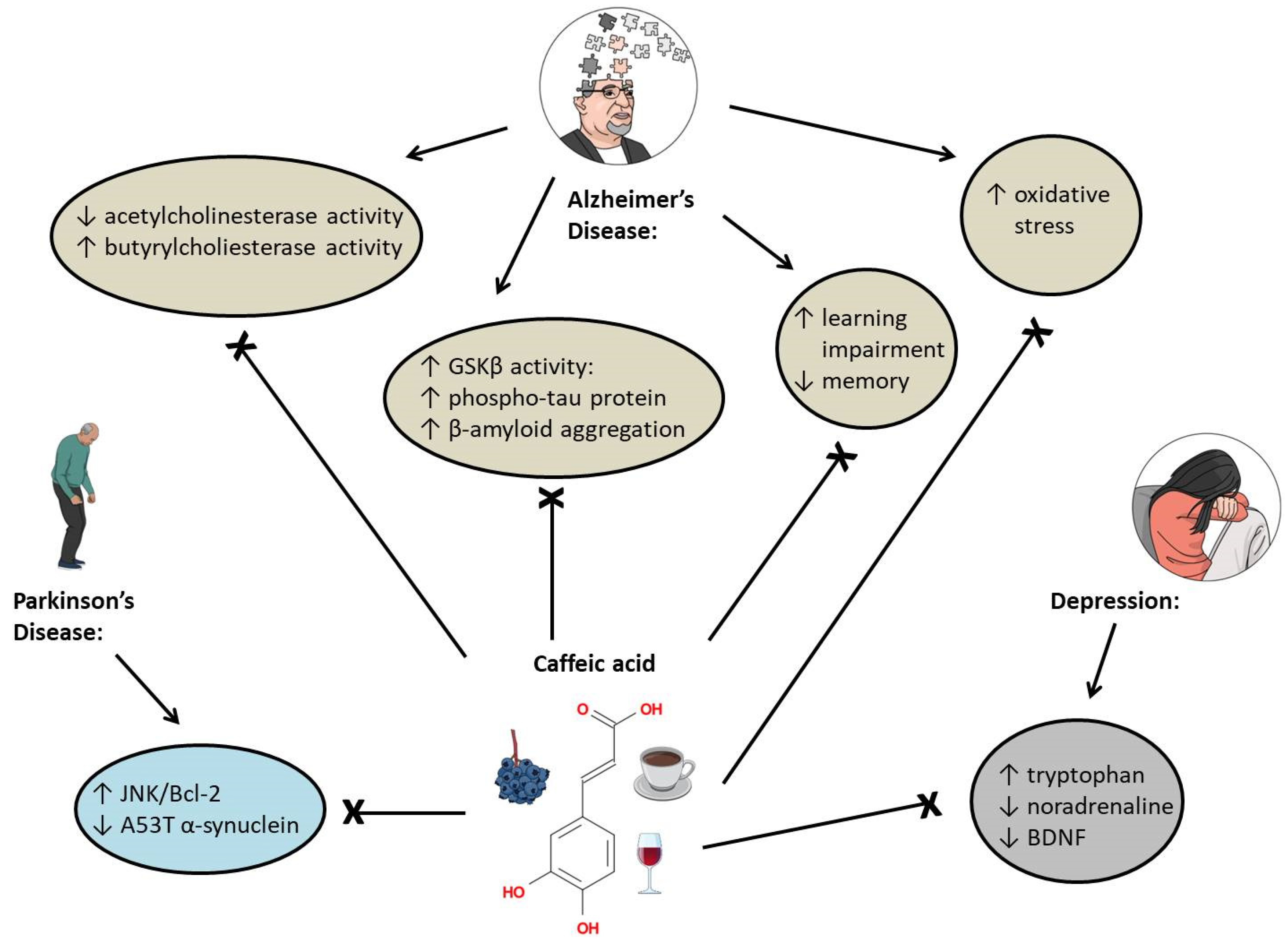
5.1. Alzheimer’s Disease
5.2. Depression
5.3. Parkinson’s Disease
6. Antibacterial and Antiviral Activity of Caffeic Acid
6.1. Antibacterial Activity
6.2. Antiviral Activity
7. Summary
Funding
Conflicts of Interest
References
- El-Seedi, H.R.; El-Said, A.M.; Khalifa, S.A.; Goransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef] [PubMed]
- Trandafir, I.; Nour, V.; Ionica, M.E. Antioxidant capacity, phenolic acids and caffeine contents of some commercial coffees available on the Romanian market. Arch. Latinoam. De Nutr. 2013, 63, 87–94. [Google Scholar]
- Lafay, S.; Morand, C.; Manach, C.; Besson, C.; Scalbert, A. Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats. Br. J. Nutr. 2006, 96, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Osakabe, N.; Natsume, M.; Terao, J. Orally administered rosmarinic acid is present as the conjugated and/or methylated forms in plasma, and is degraded and metabolized to conjugated forms of caffeic acid, ferulic acid and m-coumaric acid. Life Sci. 2004, 75, 165–178. [Google Scholar] [CrossRef]
- Celli, N.; Dragani, L.K.; Murzilli, S.; Pagliani, T.; Poggi, A. In vitro and in vivo stability of caffeic acid phenethyl ester, a bioactive compound of propolis. J. Agric. Food Chem. 2007, 55, 3398–3407. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Farahani, M.V.; Hamzehlou, S.; Far, F.B.; Sharifzadeh, S.O.; Samarghandian, S.; Khan, H.; et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacol. Res. 2021, 171, 105759. [Google Scholar] [CrossRef]
- Simonetti, P.; Gardana, C.; Pietta, P. Plasma levels of caffeic acid and antioxidant status after red wine intake. J. Agric. Food Chem. 2001, 49, 5964–5968. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A.; Murtaza, G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food. Drug. Anal. 2016, 24, 695–702. [Google Scholar] [CrossRef]
- Damasceno, S.S.; Dantas, B.B.; Ribeiro-Filho, J.; Antonio, M.A.D.; Galberto, M.d.C.J. Chemical Properties of Caffeic and Ferulic Acids in Biological System: Implications in Cancer Therapy. A Review. Curr. Pharm. Des. 2017, 23, 3015–3023. [Google Scholar] [CrossRef]
- Zheng, L.F.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z.L. Prooxidant activity of hydroxycinnamic acids on DNA damage in the presence of Cu(II) ions: Mechanism and structure-activity relationship. Food Chem. Toxicol. 2008, 46, 149–156. [Google Scholar] [CrossRef]
- Bhat, S.H.; Azmi, A.S.; Hadi, S.M. Prooxidant DNA breakage induced by caffeic acid in human peripheral lymphocytes: Involvement of endogenous copper and a putative mechanism for anticancer properties. Toxicol. Appl. Pharmacol. 2007, 218, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Huang, X.J.; Xu, S.T.; Shen, H.; Zhang, P.F.; Huang, Y.; Jiang, J.Y.; Sun, Y.J.; Jiang, B.; Wu, X.M.; et al. Discovery of novel hybrids of diaryl-1,2,4-triazoles and caffeic acid as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase for cancer therapy. Eur. J. Med. Chem. 2016, 108, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Adeyeye, S.A.O. Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons in Cooked Meat Products: A Review. Polycycl. Aromat. Compd. 2018, 40, 1557–1567. [Google Scholar] [CrossRef]
- Felton, J.S.; Knize, M.G.; Wu, R.W.; Colvin, M.E.; Hatch, F.T.; Malfatti, M.A. Mutagenic potency of food-derived heterocyclic amines. Mutat. Res. 2007, 616, 90–94. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, Y.F.; Zhao, Y.L.; Fan, D.M.; Li, L.J.; Yan, B.W.; Tao, G.; Zhao, J.X.; Zhang, H.; Wang, M.F. Caffeic acid assists microwave heating to inhibit the formation of mutagenic and carcinogenic PhIP. Food Chem. 2020, 317, 8. [Google Scholar] [CrossRef]
- Cheng, K.W.; Wong, C.C.; Chao, J.; Lo, C.; Chen, F.; Chu, I.K.; Che, C.-M.; Ho, C.-T.; Wang, M. Inhibition of mutagenic PhIP formation byepigallocatechin gallateviascavenging ofphenylacetaldehyde. Mol. Nutr. Food Res. 2009, 53, 716–725. [Google Scholar] [CrossRef]
- Hong, Y.J.; Yang, S.Y.; Nam, M.H.; Koo, Y.C.; Lee, K.W. Caffeic Acid Inhibits the Uptake of 2-Amino-1-methyl-6-phenylimidazo 4,5-b pyridine (PhIP) by Inducing the Efflux Transporters Expression in Caco-2 Cells. Biol. Pharm. Bull. 2015, 38, 201–207. [Google Scholar] [CrossRef][Green Version]
- web3. Available online: https://www.wcrf.org/cancer-trends/liver-cancer-statistics/ (accessed on 8 August 2022).
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef]
- Espindola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Rosario, A.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Gu, W.T.; Yang, Y.; Zhang, C.; Zhang, Y.J.; Chen, L.J.; Shen, J.; Li, G.Y.; Li, Z.; Li, L.; Li, Y.; et al. Caffeic acid attenuates the angiogenic function of hepatocellular carcinoma cells via reduction in JNK-1-mediated HIF-1 alpha stabilization in hypoxia. RSC Adv. 2016, 6, 82774–82782. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, X.X.; Liu, Q.Q.; Shen, J.; Li, Z.; Li, Y.; Zhang, J.P. Inhibition of TGF-beta/SMAD3/NF-kappa B signaling by microRNA-491 is involved in arsenic trioxide-induced anti-angiogenesis in hepatocellular carcinoma cells. Toxicol. Lett. 2014, 231, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Lu, M.; Yi, M.; Chen, L.J.; Shen, J.; Li, Z.; Li, L.; Yang, Y.; Zhang, J.P.; Li, Y. Caffeic acid attenuates the autocrine IL-6 in hepatocellular carcinoma via the epigenetic silencing of the NF-kappa B-IL-6-STAT-3 feedback loop. RSC Adv. 2015, 5, 52952–52957. [Google Scholar] [CrossRef]
- Chung, T.W.; Moon, S.K.; Chang, Y.C.; Ko, J.H.; Lee, Y.C.; Cho, G.; Kim, S.H.; Kim, J.G.; Kim, C.H. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: Complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004, 18, 1670–1681. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, M.; Dai, Y.; Shan, W.Q.; Chen, S.; Cai, R.; Yang, H.J.; Tang, L.M.; Li, L. Involvement and Targeted Intervention of Mortalin-Regulated Proteome Phosphorylated-Modification in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Brautigan, D.L.; Gielata, M.; Heo, J.; Kubicka, E.; Wilkins, L.R. Selective toxicity of caffeic acid in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2018, 505, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, L.R.; Brautigan, D.L.; Wu, H.P.; Yarmohammadi, H.; Kubicka, E.; Serbulea, V.; Leitinger, N.; Liu, W.; Haaga, J.R. Cinnamic Acid Derivatives Enhance the Efficacy of Transarterial Embolization in a Rat Model of Hepatocellular Carcinoma. Cardiovasc. Interv. Radiol. 2017, 40, 430–437. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.; Qiao, S.L.; Wu, X.Y.; Cao, S.Y.; Wang, L.; Su, X.J.; Li, L. Metabolic and microbial signatures in rat hepatocellular carcinoma treated with caffeic acid and chlorogenic acid. Sci. Rep. 2017, 7, 10. [Google Scholar] [CrossRef]
- Bunz, F. Principles of Cancer Genetics, 2nd ed.; Springer Science+Business Media: Dordrecht, The Netherland, 2016. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- web2. Available online: https://www.wcrf.org/cancer-trends/breast-cancer-statistics/ (accessed on 15 August 2022).
- Vici, P.; Pizzuti, L.; Natoli, C.; Gamucci, T.; Di Lauro, L.; Barba, M.; Sergi, D.; Botti, C.; Michelotti, A.; Moscetti, L.; et al. Triple positive breast cancer: A distinct subtype? Cancer Treat. Rev. 2015, 41, 69–76. [Google Scholar] [CrossRef]
- Rosendahl, A.H.; Perks, C.M.; Zeng, L.; Markkula, A.; Simonsson, M.; Rose, C.; Ingvar, C.; Holly, J.M.P.; Jernstrom, H. Caffeine and Caffeic Acid Inhibit Growth and Modify Estrogen Receptor and Insulin-like Growth Factor I Receptor Levels in Human Breast Cancer. Clin. Cancer Res. 2015, 21, 1877–1887. [Google Scholar] [CrossRef]
- Rezaei-Seresht, H.; Cheshomi, H.; Falanji, F.; Movahedi-Motlagh, F.; Hashemian, M.; Mireskandari, E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: An in silico and in vitro study. Avicenna J. Phytomedicine 2019, 9, 574–586. [Google Scholar]
- Kabala-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Jastrzebska-Stojko, Z.; Stojko, R.; Wojtyczka, R.D.; Stojko, J. Comparison of Two Components of Propolis: Caffeic Acid (CA) and Caffeic Acid Phenethyl Ester (CAPE) Induce Apoptosis and Cell Cycle Arrest of Breast Cancer Cells MDA-MB-231. Molecules 2017, 22, 1554. [Google Scholar] [CrossRef] [PubMed]
- Kabala-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Caffeic Acid Versus Caffeic Acid Phenethyl Ester in the Treatment of Breast Cancer MCF-7 Cells: Migration Rate Inhibition. Integr. Cancer Ther. 2018, 17, 1247–1259. [Google Scholar] [CrossRef]
- Balupillai, A.; Nagarajan, R.P.; Ramasamy, K.; Govindasamy, K.; Muthusamy, G. Caffeic acid prevents UVB radiation induced photocarcinogenesis through regulation of PTEN signaling in human dermal fibroblasts and mouse skin. Toxicol. Appl. Pharmacol. 2018, 352, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.; He, Y.Y. PTEN in DNA damage repair. Cancer Lett. 2012, 319, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Balupillai, A.; Prasad, R.N.; Ramasamy, K.; Muthusamy, G.; Shanmugham, M.; Govindasamy, K.; Gunaseelan, S. Caffeic Acid Inhibits UVB-induced Inflammation and Photocarcinogenesis Through Activation of Peroxisome Proliferator-activated Receptor- in Mouse Skin. Photochem. Photobiol. 2015, 91, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Fu, Y.; Malakhova, M.; Kurinov, I.; Zhu, F.; Yao, K.; Li, H.T.; Chen, H.Y.; Li, W.; Lim, D.Y.; et al. Caffeic Acid Directly Targets ERK1/2 to Attenuate Solar UV-Induced Skin Carcinogenesis. Cancer Prev. Res. 2014, 7, 1056–1066. [Google Scholar] [CrossRef]
- Pelinson, L.P.; Assmann, C.E.; Palma, T.V.; da Cruz, I.B.M.; Pillat, M.M.; Manica, A.; Stefanello, N.; Weis, G.C.C.; Alves, A.D.; de Andrade, C.M.; et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019, 46, 2085–2092. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Wang, K.B.; Wang, Y.; Yin, W.Q.; Li, L. P38/NF-kappa B/Snail Pathway Is Involved in Caffeic Acid-Induced Inhibition of Cancer Stem Cells-Like Properties and Migratory Capacity in Malignant Human Keratinocyte. PLoS ONE 2013, 8, e58915. [Google Scholar]
- Wu, Y.; Zhou, B.P. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br. J. Cancer 2010, 102, 639–644. [Google Scholar] [CrossRef]
- web1. Available online: https://www.wcrf.org/cancer-trends/lung-cancer-statistics/ (accessed on 8 August 2022).
- Min, J.; Shen, H.; Xi, W.; Wang, Q.; Yin, L.; Zhang, Y.F.; Yu, Y.; Yang, Q.; Wang, Z.N. Synergistic Anticancer Activity of Combined Use of Caffeic Acid with Paclitaxel Enhances Apoptosis of Non-Small-Cell Lung Cancer H1299 Cells in Vivo and in Vitro. Cell. Physiol. Biochem. 2018, 48, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Choy, P.M.; Bubici, C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene 2019, 38, 2223–2240. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Chen, R.F.; Chen, J.Y.F.; Chu, Y.C.; Wang, H.M.; Chou, H.L.; Chang, W.C.; Fong, Y.; Chang, W.T.; Wu, C.Y.; et al. Protective Effect of Caffeic Acid on Paclitaxel Induced Anti-Proliferation and Apoptosis of Lung Cancer Cells Involves NF-kappa B Pathway. Int. J. Mol. Sci. 2012, 13, 6236–6245. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Li, S.T.; Liu, X.Y.; An, H.L.; Kang, X.J.; Guo, S. Caffeic Acid, an Active Ingredient in Coffee, Combines with DOX for Multitarget Combination Therapy of Lung Cancer. J. Agric. Food Chem. 2022, 70, 8326–8337. [Google Scholar] [CrossRef]
- Crottes, D.; Jan, L.Y. The multifaceted role of TMEM16A in cancer. Cell Calcium 2019, 82, 102050. [Google Scholar] [CrossRef]
- Gupta, B.; Johnson, N.W.; Kumar, N. Global Epidemiology of Head and Neck Cancers: A Continuing Challenge. Oncology 2016, 91, 13–23. [Google Scholar] [CrossRef]
- Dziedzic, A.; Kubina, R.; Kabala-Dzik, A.; Wojtyczka, R.D.; Morawiec, T.; Buldak, R.J. Caffeic Acid Reduces the Viability and Migration Rate of Oral Carcinoma Cells (SCC-25) Exposed to Low Concentrations of Ethanol. Int. J. Mol. Sci. 2014, 15, 18725–18741. [Google Scholar] [CrossRef]
- Dziedzic, A.; Kubina, R.; Kabala-Dzik, A.; Tanasiewicz, M. Induction of Cell Cycle Arrest and Apoptotic Response of Head and Neck Squamous Carcinoma Cells (Detroit 562) by Caffeic Acid and Caffeic Acid Phenethyl Ester Derivative. Evid. Based Complement. Altern. Med. 2017, 2017, 6793456. [Google Scholar] [CrossRef]
- Celinska-Janowicz, K.; Zareba, I.; Lazarek, U.; Teul, J.; Tomczyk, M.; Palka, J.; Miltyk, W. Constituents of Propolis: Chrysin, Caffeic Acid, p-Coumaric Acid, and Ferulic Acid Induce PRODH/POX-Dependent Apoptosis in Human Tongue Squamous Cell Carcinoma Cell (CAL-27). Front. Pharmacol. 2018, 9, 336. [Google Scholar] [CrossRef]
- Phang, J.M.; Liu, W.; Hancock, C.; Christian, K.J. The proline regulatory axis and cancer. Front. Oncol. 2012, 2, 60. [Google Scholar] [CrossRef]
- Vu, M.; Yu, J.; Awolude, O.A.; Chuang, L. Cervical cancer worldwide. Curr. Probl. Cancer 2018, 42, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Koraneekit, A.; Limpaiboon, T.; Sangka, A.; Boonsiri, P.; Daduang, S.; Daduang, J. Synergistic effects of cisplatin-caffeic acid induces apoptosis in human cervical cancer cells via the mitochondrial pathways. Oncol. Lett. 2018, 15, 7397–7402. [Google Scholar] [CrossRef] [PubMed]
- Tyszka-Czochara, M.; Konieczny, P.; Majka, M. Caffeic Acid Expands Anti-Tumor Effect of Metformin in Human Metastatic Cervical Carcinoma HTB-34 Cells: Implications of AMPK Activation and Impairment of Fatty Acids De Novo Biosynthesis. Int. J. Mol. Sci. 2017, 18, 462. [Google Scholar] [CrossRef] [PubMed]
- Zannella, V.E.; Cojocari, D.; Hilgendorf, S.; Vellanki, R.N.; Chung, S.; Wouters, B.G.; Koritzinsky, M. AMPK regulates metabolism and survival in response to ionizing radiation. Radiother. Oncol. 2011, 99, 293–299. [Google Scholar] [CrossRef]
- Chomanicova, N.; Gazova, A.; Adamickova, A.; Valaskova, S.; Kyselovic, J. The role of AMPK/mTOR signaling pathway in anticancer activity of metformin. Physiol. Res. 2021, 70, 501–508. [Google Scholar] [CrossRef]
- Tyszka-Czochara, M.; Bukowska-Strakova, K.; Majka, M. Metformin and caffeic acid regulate metabolic reprogramming in human cervical carcinoma SiHa/HTB-35 cells and augment anticancer activity of Cisplatin via cell cycle regulation. Food Chem. Toxicol. 2017, 106, 260–272. [Google Scholar] [CrossRef]
- Tyszka-Czochara, M.; Lasota, M.; Majka, M. Caffeic Acid and Metformin Inhibit Invasive Phenotype Induced by TGF-beta1 in C-4I and HTB-35/SiHa Human Cervical Squamous Carcinoma Cells by Acting on Different Molecular Targets. Int. J. Mol. Sci. 2018, 19, 266. [Google Scholar] [CrossRef]
- Zheng, H.; Takahashi, H.; Murai, Y.; Cui, Z.; Nomoto, K.; Niwa, H.; Tsuneyama, K.; Takano, Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006, 26, 3579–3583. [Google Scholar]
- Castro, M.F.V.; Stefanello, N.; Assmann, C.E.; Baldissarelli, J.; Bagatini, M.D.; da Silva, A.D.; da Costa, P.; Borba, L.; da Cruz, I.B.M.; Morsch, V.M.; et al. Modulatory effects of caffeic acid on purinergic and cholinergic systems and oxi-inflammatory parameters of streptozotocin-induced diabetic rats. Life Sci. 2021, 277, 12. [Google Scholar] [CrossRef]
- Xu, W.G.; Luo, Q.; Wen, X.Y.; Xiao, M.; Mei, Q.J. Antioxidant and anti-diabetic effects of caffeic acid in a rat model of diabetes. Trop. J. Pharm. Res. 2020, 19, 1227–1232. [Google Scholar] [CrossRef]
- Orsolic, N.; Sirovina, D.; Odeh, D.; Gajski, G.; Balta, V.; Sver, L.; Jembrek, M.J. Efficacy of Caffeic Acid on Diabetes and Its Complications in the Mouse. Molecules 2021, 26, 3262. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.K.; Wang, H.; Su, W.H. Protective Effect of Caffeic Acid on Streptozotocin Induced Gestational Diabetes Mellitus in Rats: Possible Mechanism. Pak. J. Zool. 2021, 53, 1045–1052. [Google Scholar] [CrossRef]
- Chang, W.C.; Kuo, P.L.; Chen, C.W.; Wu, J.S.B.; Shen, S.C. Caffeic acid improves memory impairment and brain glucose metabolism via ameliorating cerebral insulin and leptin signaling pathways in high-fat diet-induced hyperinsulinemic rats. Food Res. Int. 2015, 77, 24–33. [Google Scholar] [CrossRef]
- Cao, X.Y.; Xia, Y.; Zeng, M.; Wang, W.Y.; He, Y.; Liu, J.L. Caffeic Acid Inhibits the Formation of Advanced Glycation End Products (AGEs) and Mitigates the AGEs-Induced Oxidative Stress and Inflammation Reaction in Human Umbilical Vein Endothelial Cells (HUVECs). Chem. Biodivers. 2019, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Fratantonio, D.; Speciale, A.; Canali, R.; Natarelli, L.; Ferrari, D.; Saija, A.; Virgili, F.; Cimino, F. Low nanomolar caffeic acid attenuates high glucose-induced endothelial dysfunction in primary human umbilical-vein endothelial cells by affecting NF-B and Nrf2 pathways. Biofactors 2017, 43, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Natarelli, L.; Ranaldi, G.; Leoni, G.; Roselli, M.; Guantario, B.; Comitato, R.; Ambra, R.; Cimino, F.; Speciale, A.; Virgili, F.; et al. Nanomolar Caffeic Acid Decreases Glucose Uptake and the Effects of High Glucose in Endothelial Cells. PLoS ONE 2015, 10, 19. [Google Scholar] [CrossRef]
- Toma, L.; Sanda, G.M.; Niculescu, L.S.; Deleanu, M.; Stancu, C.S.; Sima, A.V. Caffeic acid attenuates the inflammatory stress induced by glycated LDL in human endothelial cells by mechanisms involving inhibition of AGE-receptor, oxidative, and endoplasmic reticulum stress. Biofactors 2017, 43, 685–697. [Google Scholar] [CrossRef]
- Choudhary, S.; Mourya, A.; Ahuja, S.; Sah, S.P.; Kumar, A. Plausible anti-inflammatory mechanism of resveratrol and caffeic acid against chronic stress-induced insulin resistance in mice. Inflammopharmacology 2016, 24, 347–361. [Google Scholar] [CrossRef]
- Salau, V.F.; Erukainure, O.L.; Ibeji, C.U.; Koorbanally, N.A.; Islam, M.S. Ferric-Induced Pancreatic Injury Involves Exacerbation of Cholinergic and Proteolytic Activities, and Dysregulation of Metabolic Pathways: Protective Effect of Caffeic Acid. Biol. Trace Elem. Res. 2020, 196, 517–527. [Google Scholar] [CrossRef]
- Tsuda, S.; Egawa, T.; Ma, X.; Oshima, R.; Kurogi, E.; Hayashi, T. Coffee polyphenol caffeic acid but not chlorogenic acid increases 5’ AMP-activated protein kinase and insulin-independent glucose transport in rat skeletal muscle. J. Nutr. Biochem. 2012, 23, 1403–1409. [Google Scholar] [CrossRef]
- Virtanen, K.A.; Nuutila, P. Brown adipose tissue in humans. Curr. Opin. Lipidol. 2011, 22, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, L.V.; Savova, M.S.; Amirova, K.M.; Balcheva-Sivenova, Z.; Ferrante, C.; Orlando, G.; Wabitsch, M.; Georgiev, M.I. Caffeic and Chlorogenic Acids Synergistically Activate Browning Program in Human Adipocytes: Implications of AMPK- and PPAR-Mediated Pathways. Int. J. Mol. Sci. 2020, 21, 9740. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kim, Y.; Lee, E.S.; Huh, J.H.; Chung, C.H. Caffeic acid ameliorates hepatic steatosis and reduces ER stress in high fat diet-induced obese mice by regulating autophagy. Nutrition 2018, 55–56, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Mariana, B.D.; Tiago, L.S.; Ramon, R.P.P.B.d.M.; Jamile, M.F.; Tiago, S.M.; Richard, R.C.M.; Hector, G.R.; Dânya, B.L.; Alice, M.C.M.; Maria, G.R.d.Q. Caffeic acid reduces lipid accumulation and reactive oxygen species production in adipocytes. Afr. J. Pharm. Pharmacol. 2018, 12, 263–268. [Google Scholar] [CrossRef]
- Lutfi, E.; Babin, P.J.; Gutierrez, J.; Capilla, E.; Navarro, I. Caffeic acid and hydroxytyrosol have anti-obesogenic properties in zebrafish and rainbow trout models. PLoS ONE 2017, 12, 21. [Google Scholar] [CrossRef]
- Lee, J.E.; Ge, K. Transcriptional and epigenetic regulation of PPARgamma expression during adipogenesis. Cell Biosci. 2014, 4, 29. [Google Scholar] [CrossRef]
- Pamukcu, B.; Lip, G.Y.; Devitt, A.; Griffiths, H.; Shantsila, E. The role of monocytes in atherosclerotic coronary artery disease. Ann. Med. 2010, 42, 394–403. [Google Scholar] [CrossRef]
- Lee, E.S.; Park, S.H.; Kim, M.S.; Han, S.Y.; Kim, H.S.; Kang, Y.H. Caffeic Acid Disturbs Monocyte Adhesion onto Cultured Endothelial Cells Stimulated by Adipokine Resistin. J. Agric. Food Chem. 2012, 60, 2730–2739. [Google Scholar] [CrossRef]
- Moon, M.K.; Lee, Y.J.; Kim, J.S.; Kang, D.G.; Lee, H.S. Effect of Caffeic Acid on Tumor Necrosis Factor-Alpha-Induced Vascular Inflammation in Human Umbilical Vein Endothelial Cells. Biol. Pharm. Bull. 2009, 32, 1371–1377. [Google Scholar] [CrossRef]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 222–231. [Google Scholar] [CrossRef]
- Wang, Y.; Kaur, G.; Kumar, M.; Kushwah, A.S.; Kabra, A.; Kainth, R. Caffeic Acid Prevents Vascular Oxidative Stress and Atherosclerosis against Atherosclerogenic Diet in Rats. Evid. Based Complement. Altern. Med. 2022, 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Vacaresse, N.; Vieira, O.; Robbesyn, F.; Jurgens, G.; Salvayre, R.; Negre-Salvayre, A. Phenolic antioxidants trolox and caffeic acid modulate the oxidized LDL-induced EGF-receptor activation. Br. J. Pharmacol. 2001, 132, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, F.; Lucas, J.J.; Avila, J. GSK3 and tau: Two convergence points in Alzheimer’s disease. J. Alzheimer Dis. 2013, 33 (Suppl. S1), S141–S144. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.C.; Huang, D.W.; Lo, Y.M.; Tee, Q.Q.; Kuo, P.; Wu, J.S.; Huang, W.C.; Shen, Z.C. Protective Effect of Caffeic Acid against Alzheimer’s Disease Pathogenesis via Modulating Cerebral Insulin Signaling, beta-Amyloid Accumulation, and Synaptic Plasticity in Hyperinsulinemic Rats. J. Agric. Food Chem. 2019, 67, 7684–7693. [Google Scholar] [CrossRef]
- Sul, D.; Kim, H.S.; Lee, D.; Joo, S.S.; Hwang, K.W.; Park, S.Y. Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci. 2009, 84, 257–262. [Google Scholar] [CrossRef]
- Andrade, S.; Loureiro, J.A.; Pereira, M.C. Caffeic acid for the prevention and treatment of Alzheimer’s disease: The effect of lipid membranes on the inhibition of aggregation and disruption of A beta fibrils. Int. J. Biol. Macromol. 2021, 190, 853–861. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, Y.T.; Li, J.F.; Hua, L.L.; Han, B.; Zhang, Y.Z.; Yang, X.P.; Zeng, Z.L.; Bai, H.Y.; Yin, H.L.; et al. Effects of caffeic acid on learning deficits in a model of Alzheimer’s disease. Int. J. Mol. Med. 2016, 38, 869–875. [Google Scholar] [CrossRef]
- Deshmukh, R.; Kaundal, M.; Bansal, V.; Samardeep. Caffeic acid attenuates oxidative stress, learning and memory deficit in intra-cerebroventricular streptozotocin induced experimental dementia in rats. Biomed. Pharmacother. 2016, 81, 56–62. [Google Scholar] [CrossRef]
- Khan, K.A.; Kumar, N.; Nayak, P.G.; Nampoothiri, M.; Shenoy, R.R.; Krishnadas, N.; Rao, C.M.; Mudgal, J. Impact of caffeic acid on aluminium chloride-induced dementia in rats. J. Pharm. Pharmacol. 2013, 65, 1745–1752. [Google Scholar] [CrossRef]
- Kim, J.H.; Wang, Q.; Choi, J.M.; Lee, S.; Cho, E.J. Protective role of caffeic acid in an A beta(25-35)-induced Alzheimer’s disease model. Nutr. Res. Pract. 2015, 9, 480–488. [Google Scholar] [CrossRef]
- Brimijoin, S. Molecular forms of acetylcholinesterase in brain, nerve and muscle: Nature, localization and dynamics. Prog. Neurobiol. 1983, 21, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Inhibitors of acetylcholinesterase and butyrylcholinesterase meet immunity. Int. J. Mol. Sci. 2014, 15, 9809–9825. [Google Scholar] [CrossRef] [PubMed]
- Knez, D.; Coquelle, N.; Pislar, A.; Zakelj, S.; Jukic, M.; Sova, M.; Mravljak, J.; Nachon, F.; Brazzolotto, X.; Kos, J.; et al. Multi-target-directed ligands for treating Alzheimer’s disease: Butyrylcholinesterase inhibitors displaying antioxidant and neuroprotective activities. Eur. J. Med. Chem. 2018, 156, 598–617. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative Study on the Inhibitory Effect of Caffeic and Chlorogenic Acids on Key Enzymes Linked to Alzheimer’s Disease and Some Pro-oxidant Induced Oxidative Stress in Rats’ Brain-In Vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Bradley, M.A.; Markesbery, W.R.; Lovell, M.A. Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radic. Biol. Med. 2010, 48, 1570–1576. [Google Scholar] [CrossRef]
- Huang, Y.J.; Jin, M.H.; Pi, R.B.; Zhang, J.J.; Chen, M.H.; Ouyang, Y.; Liu, A.M.; Chao, X.J.; Liu, P.Q.; Liu, J.; et al. Protective effects of caffeic acid and caffeic acid phenethyl ester against acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Neurosci. Lett. 2013, 535, 146–151. [Google Scholar] [CrossRef]
- Liang, G.J.; Shi, B.; Luo, W.N.; Yang, J.Q. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015, 11, 10. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Zhang, P.; Yang, J.Q.; Su, Q.A. 5-lipoxygenase expression in a brain damage model induced by chronic oral administration of aluminum. Neural Regen. Res. 2010, 5, 1634–1638. [Google Scholar]
- Song, Y.; Wei, E.Q.; Zhang, W.P.; Zhang, L.; Liu, J.R.; Chen, Z. Minocycline protects PC12 cells from ischemic-like injury and inhibits 5-lipoxygenase activation. Neuroreport 2004, 15, 2181–2184. [Google Scholar] [CrossRef]
- Yang, J.Q.; Zhou, Q.X.; Liu, B.Z.; He, B.C. Protection of mouse brain from aluminum-induced damage by caffeic acid. CnsNeurosci. Ther. 2008, 14, 10–16. [Google Scholar] [CrossRef]
- Huang, D.; Zhang, L.; Yang, J.Q.; Luo, Y.; Cui, T.; Du, T.T.; Jiang, X.H. Evaluation on monoamine neurotransmitters changes in depression rats given with sertraline, meloxicam or/and caffeic acid. Genes Dis. 2019, 6, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Dzitoyeva, S.; Imbesi, M.; Uz, T.; Dimitrijevic, N.; Manev, H.; Manev, R. Caffeic acid attenuates the decrease of cortical BDNF transcript IV mRNA induced by swim stress in wild-type but not in 5-lipoxygenase-deficient mice. J. Neural Transm. 2008, 115, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Huang, T.L. Brain-derived neurotrophic factor and mental disorders. Biomed. J. 2020, 43, 134–142. [Google Scholar] [CrossRef]
- Maroteaux, L.; Campanelli, J.T.; Scheller, R.H. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988, 8, 2804–2815. [Google Scholar] [CrossRef] [PubMed]
- Ozansoy, M.; Basak, A.N. The central theme of Parkinson’s disease: Alpha-synuclein. Mol. Neurobiol. 2013, 47, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Q.M.; Zhang, L.; Wang, Q.; Yang, Z.X.; Liu, J.; Feng, L.Y. Caffeic acid reduces A53T alpha-synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol. Res. 2019, 150, 14. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food. Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.F.S.; Tintino, S.R.; de Freitas, T.S.; Campina, F.F.; Irwin, R.D.A.; Siqueira-Junior, J.P.; Coutinho, H.D.M.; Cunha, F.A.B. In vitro e in silico evaluation of the inhibition of Staphylococcus aureus efflux pumps by caffeic and gallic acid. Comp. Immunol. Microbiol. Infect. Dis. 2018, 57, 22–28. [Google Scholar] [CrossRef]
- Kwon, Y.I.; Apostolidis, E.; Labbe, R.G.; Shetty, K. Inhibition of Staphylococcus aureusby Phenolic Phytochemicals of Selected Clonal Herbs Species ofLamiaceaeFamily and Likely Mode of Action through Proline Oxidation. Food Biotechnol. 2007, 21, 71–89. [Google Scholar] [CrossRef]
- Servet, C.; Ghelis, T.; Richard, L.; Zilberstein, A.; Savoure, A. Proline dehydrogenase: A key enzyme in controlling cellular homeostasis. Front. Biosci. (Landmark Ed.) 2012, 17, 607–620. [Google Scholar] [CrossRef]
- Luis, A.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, S.; Girija, A.S.S.; Priyadharsini, J.V. Evaluation of the inhibitory effect of caffeic acid and gallic acid on tetR and tetM efflux pumps mediating tetracycline resistance in Streptococcus sp. using computational approach. J. King Saud Univ. Sci. 2020, 32, 904–909. [Google Scholar] [CrossRef]
- Saavedra, M.J.; Borges, A.; Dias, C.; Aires, A.; Bennett, R.N.; Rosa, E.S.; Simoes, M. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. Med. Chem. 2010, 6, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Ferreira, I.C.; Barros, L.; Carvalho, A.M.; Soares, G.; Henriques, M. Antibacterial potential of northeastern Portugal wild plant extracts and respective phenolic compounds. Biomed. Res. Int. 2014, 2014, 814590. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Ichinosei, M.; Ikeda, K.; Uozaki, M.; Morishita, J.; Kuwahara, T.; Koyama, A.H.; Yamasaki, H. Inhibition by caffeic acid of the influenza A virus multiplication in vitro. Int. J. Mol. Med. 2014, 34, 1020–1024. [Google Scholar] [CrossRef]
- Shen, J.; Wang, G.F.; Zuo, J.P. Caffeic acid inhibits HCV replication via induction of IFN alpha antiviral response through p62-mediated Keap1/Nrf2 signaling pathway. Antivir. Res. 2018, 154, 166–173. [Google Scholar] [CrossRef]
- Wang, G.F.; Shi, L.P.; Ren, Y.D.; Liu, Q.F.; Liu, H.F.; Zhang, R.J.; Li, Z.; Zhu, F.H.; He, P.L.; Tang, W.; et al. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef]
- Ikeda, K.; Tsujimoto, K.; Uozaki, M.; Nishide, M.; Suzuki, Y.; Koyama, A.H.; Yamasaki, H. Inhibition of multiplication of herpes simplex virus by caffeic acid. Int. J. Mol. Med. 2011, 28, 595–598. [Google Scholar]
- Langland, J.; Jacobs, B.; Wagner, C.E.; Ruiz, G.; Cahill, T.M. Antiviral activity of metal chelates of caffeic acid and similar compounds towards herpes simplex, VSV-Ebola pseudotyped and vaccinia viruses. Antivir. Res. 2018, 160, 143–150. [Google Scholar] [CrossRef]
- Ogawa, M.; Shirasago, Y.; Ando, S.; Shimojima, M.; Saijo, M.; Fukasawa, M. Caffeic acid, a coffee-related organic acid, inhibits infection by severe fever with thrombocytopenia syndrome virus in vitro. J. Infect. Chemother. 2018, 24, 597–601. [Google Scholar] [CrossRef]
- Ogawa, M.; Shirasago, Y.; Tanida, I.; Kakuta, S.; Uchiyama, Y.; Shimojima, M.; Hanada, K.; Saijo, M.; Fukasawa, M. Structural basis of antiviral activity of caffeic acid against severe fever with thrombocytopenia syndrome virus. J. Infect. Chemother. 2021, 27, 397–400. [Google Scholar] [CrossRef] [PubMed]

| Caffeic Acid | ||
|---|---|---|
| hepatocellular carcinoma | ||
| HepG2, HCC97H | 20 μM | ↓HIF-1α |
| HepG2 | 100 μM | ↓NF-κB/IL-6/STAT3 |
| ↓VEGF, MM-9 | ||
| HepG2, Hep3B, sorafenib-resistant HuH7 | 20 μM | ↓mortalin |
| breast cancer | ||
| MCF7 | 50 μM | ↓ER, PKB/Akt |
| ↓IGF-1R | ||
| MCF7 | 171 μg/mL | ↑p21 mRNA |
| ↑MCL1 mRNA | ||
| skin cancer | ||
| human dermal fibroblasts and mouse skin | 40 μM | ↑XPC, XPA, PTEN |
| ↑TFIIH-p44, ERCC1 | ||
| squamous cell carcinoma | 15 mg/kg | ↓iNOS, VEGF |
| ↑p53 | ||
| A431, SK-MEL-5, SK-MEL-28 | 40 μM | ↓ERK1/2 |
| HaCaT | 100 μM | ↓NF-kB/Snail |
| lung cancer | ||
| H1299 cells and H1299-xenografts | 100 μM | ↑Bid, Bax |
| (with paclitaxel) | ↑ cas-3/7, cas-9 | |
| ↑p-JNK, p-ERK1/2 | ||
| A549 | 100 μM | ↑survivin, Bcl-2 |
| LA-795 | 60 μM | ↓p-MEK1/2, p-ERK1/2 |
| ↓cyclin D, vimentin | ||
| ↓beta-catenin | ||
| ↓TMEM16A | ||
| oral cancer | ||
| CAL-27 | 65 μg/mL | ↑p53 |
| ↑PRODH | ||
| cervical cancer | ||
| HTB-34 (ATCC-CRL1550) | 100 μM | ↑AMPK, GLUT1 |
| ↓ACLY, SCD1, ELOVL6 | ||
| SiHa | 100 μM | ↑AMPK |
| ↓ACC1, SREPB1c | ||
| ↑ACLY, ELOVL6 | ||
| C-4I | 100 μM | ↑E-cadherin |
| ↓vimentin | ||
| ↑TIMP-1 and -2 mRNA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlíková, N. Caffeic Acid and Diseases—Mechanisms of Action. Int. J. Mol. Sci. 2023, 24, 588. https://doi.org/10.3390/ijms24010588
Pavlíková N. Caffeic Acid and Diseases—Mechanisms of Action. International Journal of Molecular Sciences. 2023; 24(1):588. https://doi.org/10.3390/ijms24010588
Chicago/Turabian StylePavlíková, Nela. 2023. "Caffeic Acid and Diseases—Mechanisms of Action" International Journal of Molecular Sciences 24, no. 1: 588. https://doi.org/10.3390/ijms24010588
APA StylePavlíková, N. (2023). Caffeic Acid and Diseases—Mechanisms of Action. International Journal of Molecular Sciences, 24(1), 588. https://doi.org/10.3390/ijms24010588