The Role of Mononuclear Phagocytes in the Testes and Epididymis
Abstract
1. Introduction
2. Methods
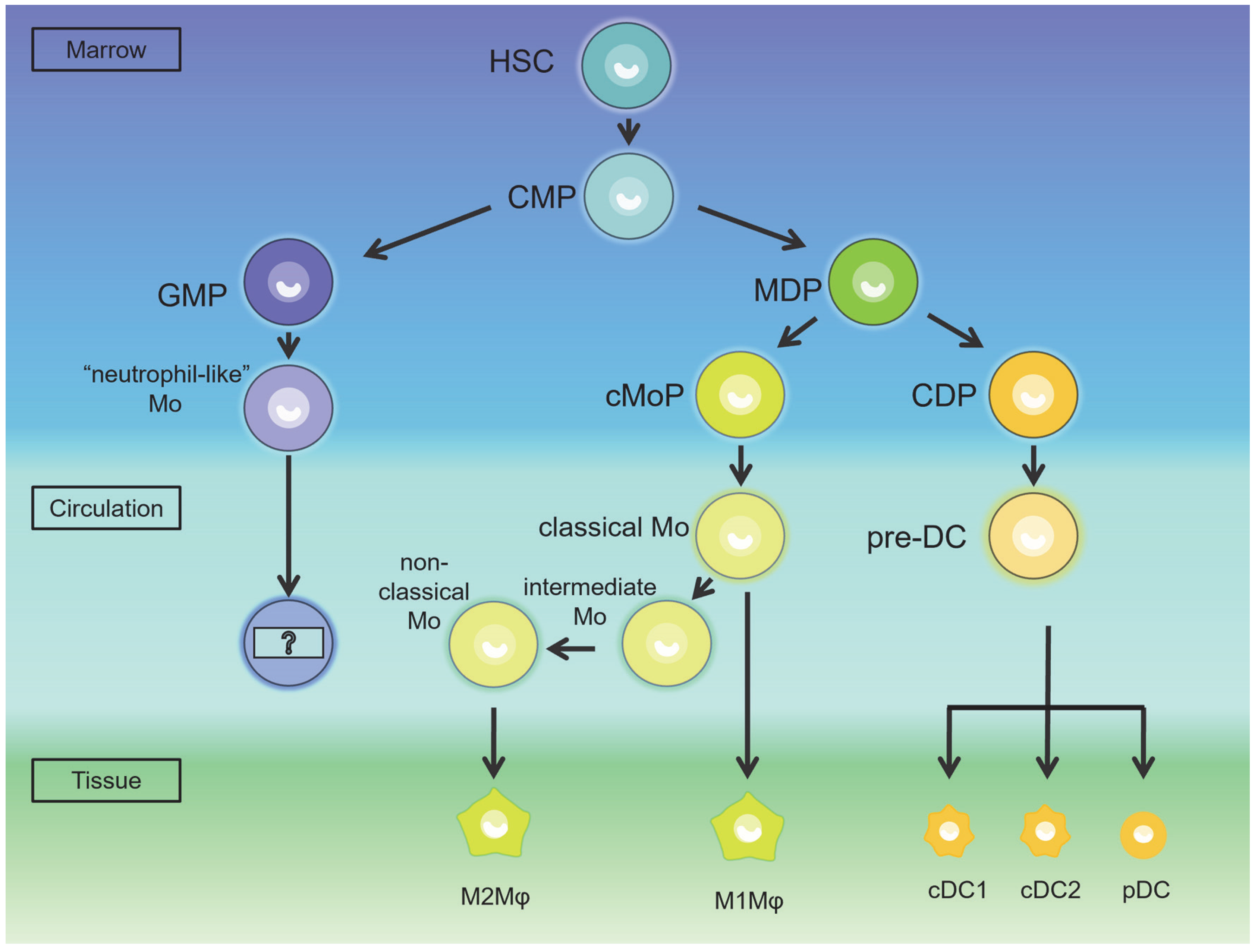
3. The Occurrence of the MPS in Testes and Epididymis
4. The Characteristic of the MPS in the Testes and Epididymis
4.1. DCs in the Testes and Epididymis
4.1.1. DCs in the Testes
The Classification and Distribution of DCs in the Testes
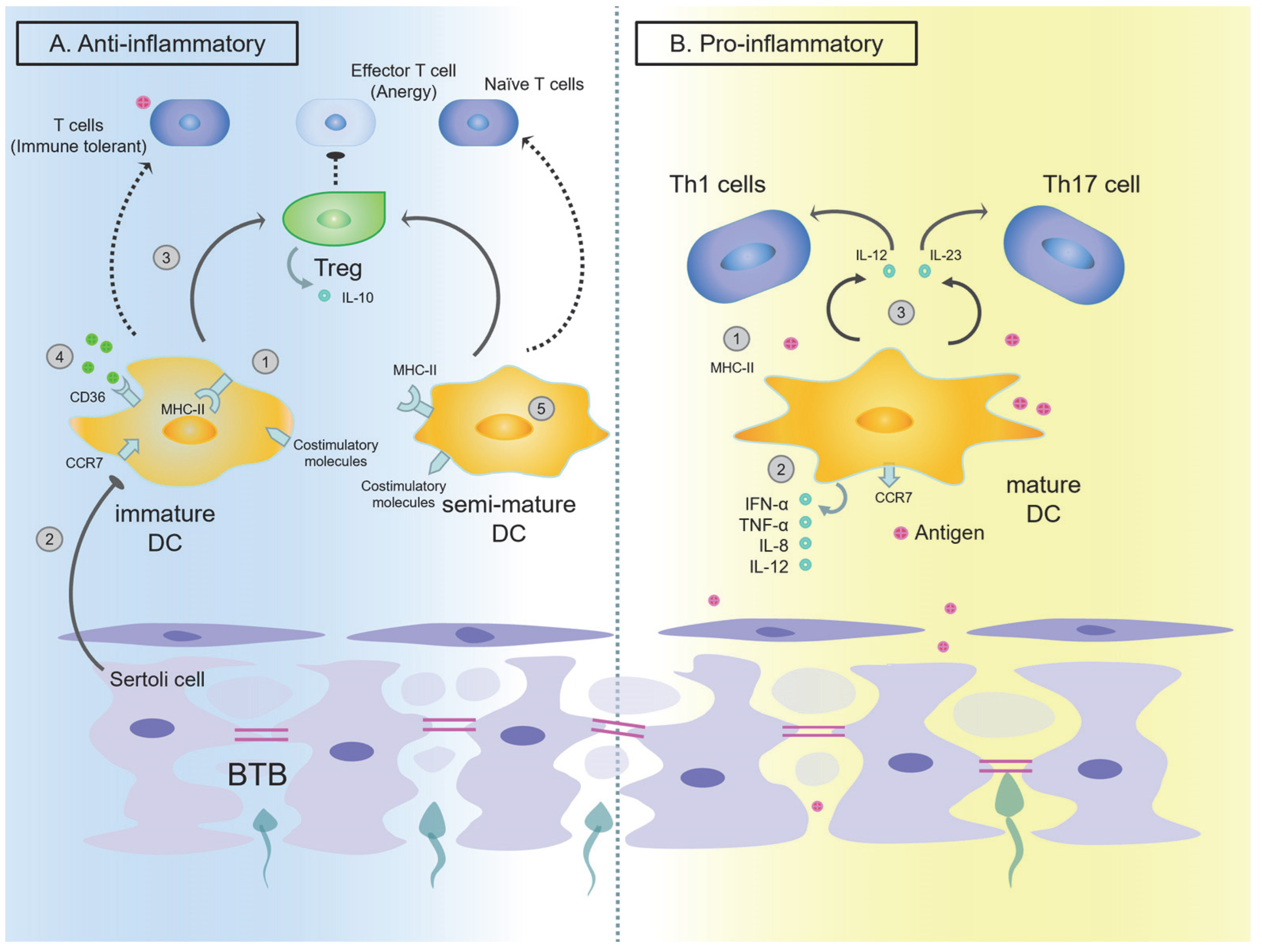
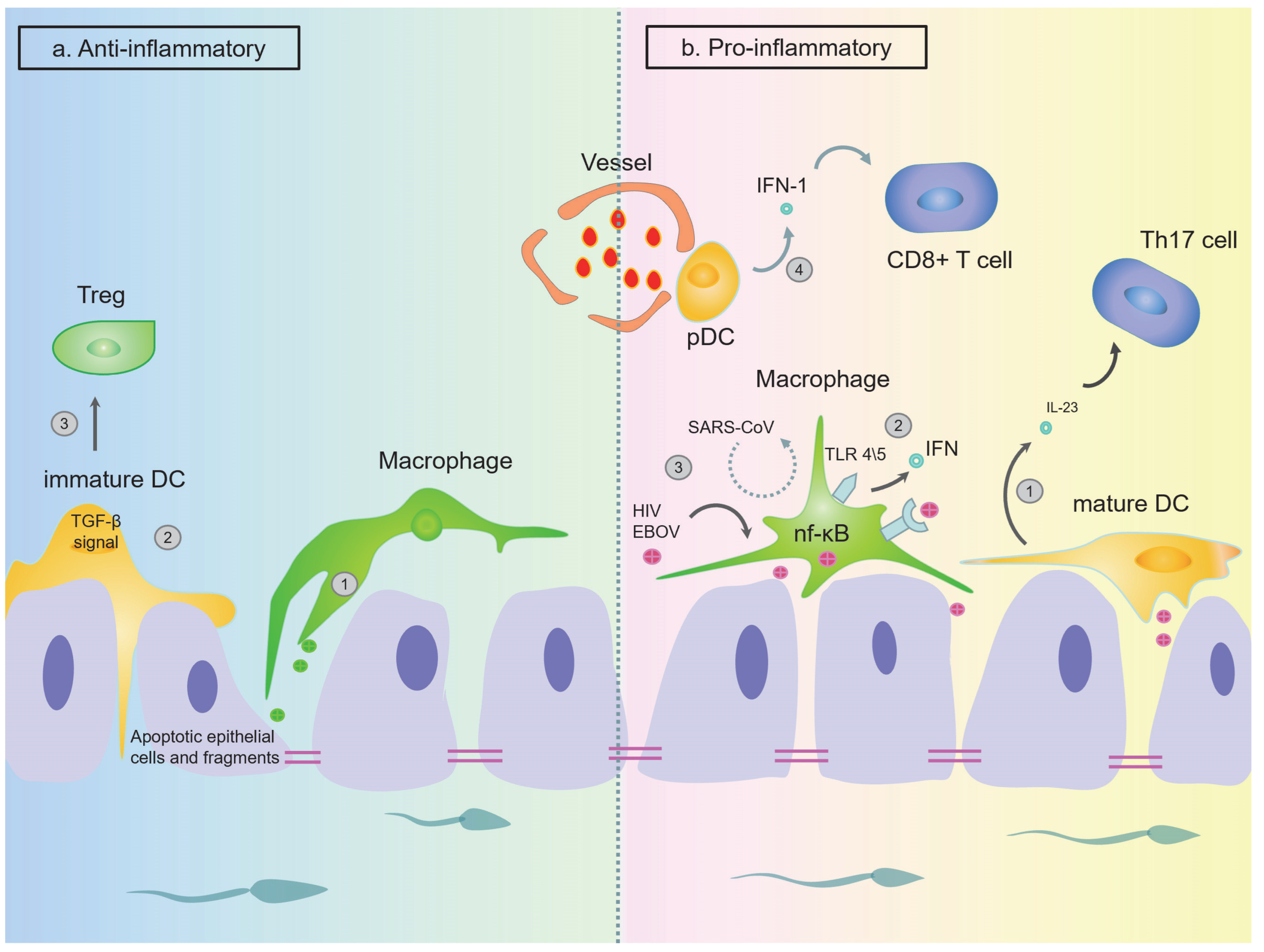
The Function of DCs in the Testes
4.1.2. DCs in the Epididymis
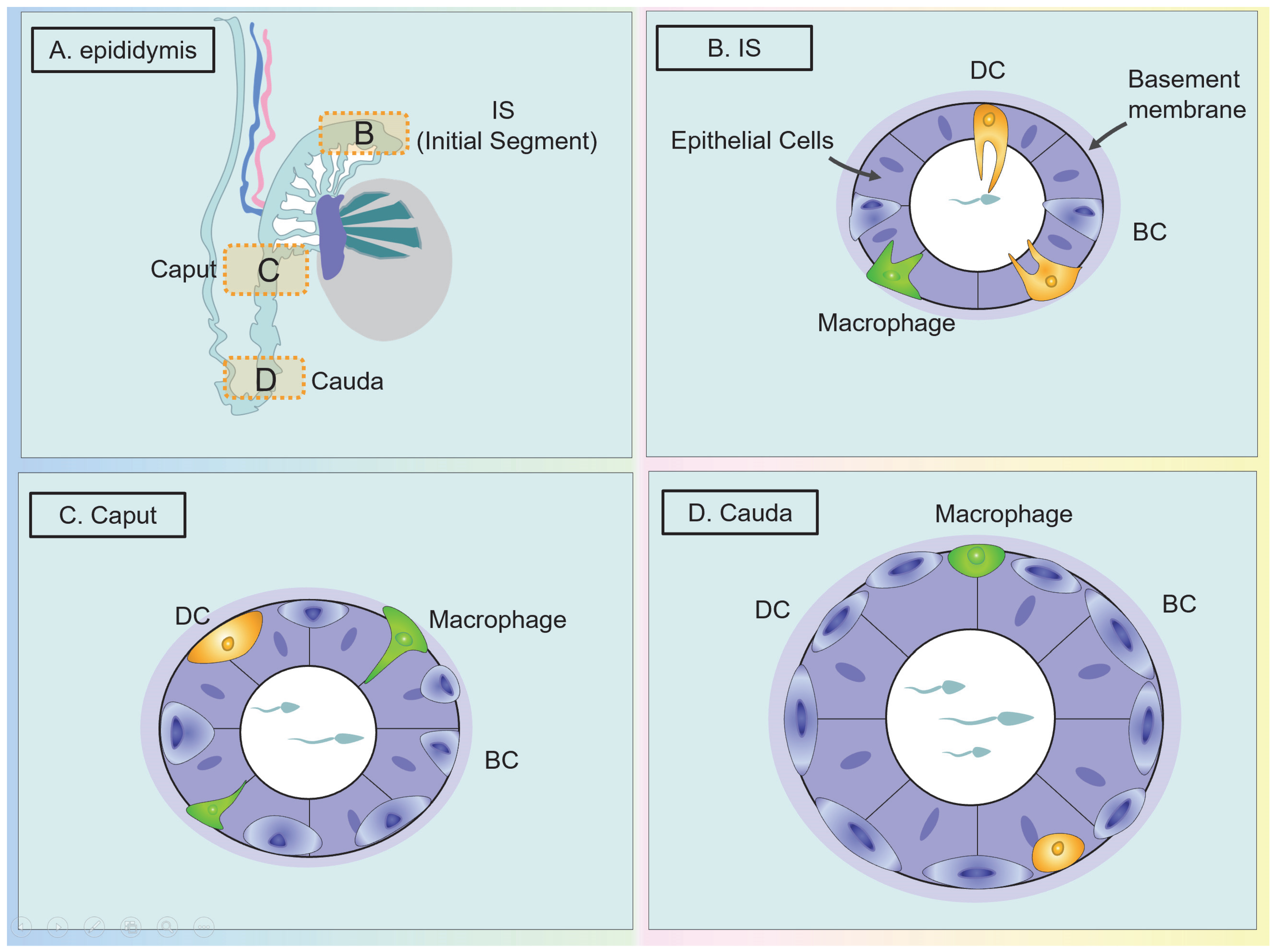
The Classification and Distribution of DCs in the Epididymis
The Function of DCs in the Epididymis
4.2. Macrophage in Testes and Epididymis
4.2.1. Macrophages in the Testes
The Classification and Distribution of Macrophages in the Testes
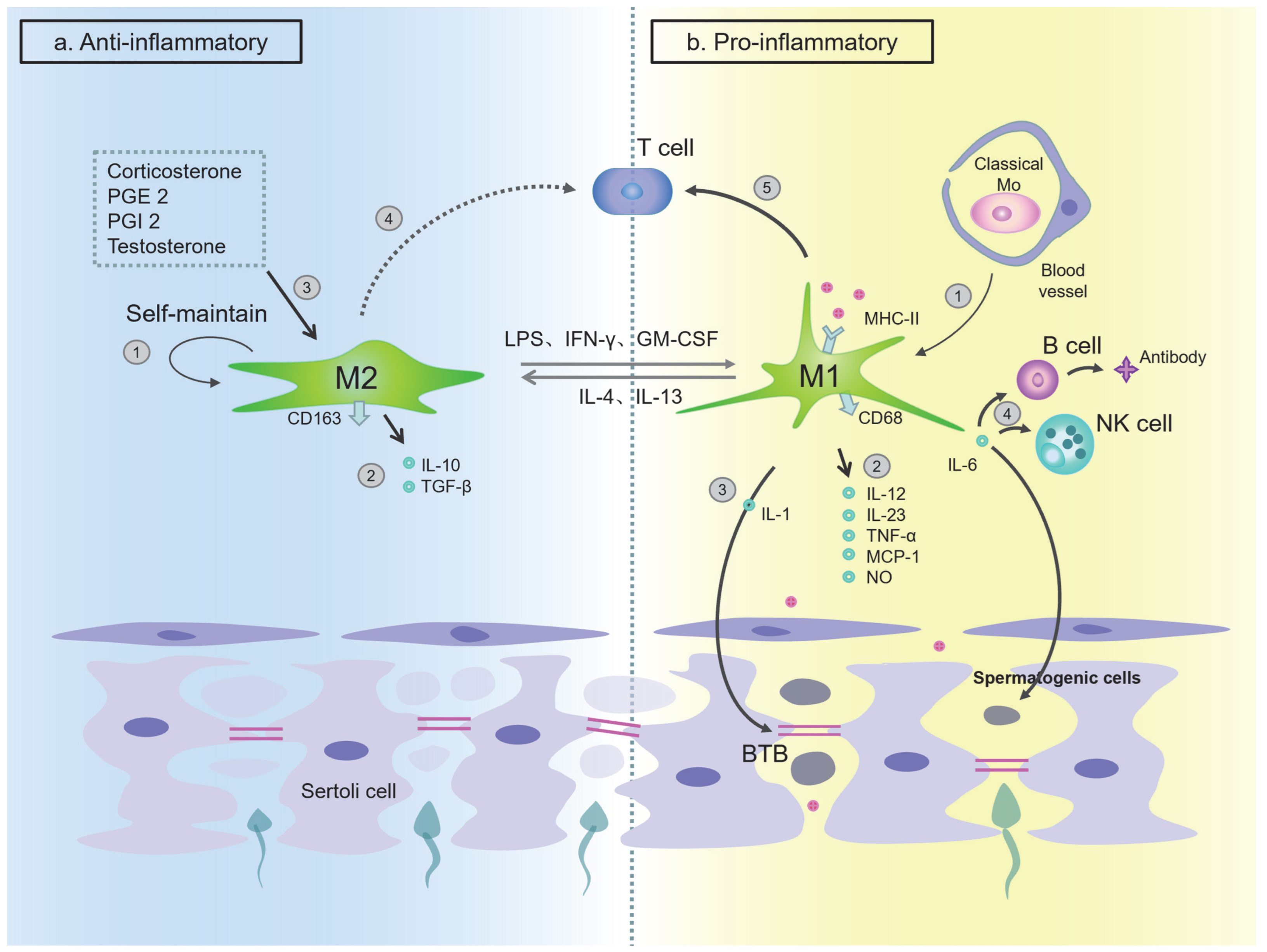
The Function of Macrophages in Testes
4.2.2. Macrophages in Epididymis
The Classification and Distribution of Macrophages in the Epididymis
The Function of Macrophages in the Epididymis
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Witkin, S.S.; Jeremias, J.; Bongiovanni, A.M.; Munoz, M.G. Immune regulation in the male genital tract. Infect. Dis. Obstet. Gynecol. 1996, 4, 131–135. [Google Scholar] [CrossRef]
- Naz, R.K.; Menge, A.C. Antisperm antibodies: Origin, regulation, and sperm reactivity in human infertility. Fertil. Steril. 1994, 61, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Schagdarsurengin, U.; Western, P.; Steger, K.; Meinhardt, A. Developmental origins of male subfertility: Role of infection, inflammation, and environmental factors. Semin. Immunopathol. 2016, 38, 765–781. [Google Scholar] [CrossRef]
- van Furth, R.; Cohn, Z.A.; Hirsch, J.G.; Humphrey, J.H.; Spector, W.G.; Langevoort, H.L. The mononuclear phagocyte system: A new classification of macrophages, monocytes, and their precursor cells. Bull. World Health Organ. 1972, 46, 845–852. [Google Scholar]
- Cui, X.; Ye, Z.; Wang, D.; Yang, Y.; Jiao, C.; Ma, J.; Tang, N.; Zhang, H. Aryl hydrocarbon receptor activation ameliorates experimental colitis by modulating the tolerogenic dendritic and regulatory T cell formation. Cell Biosci. 2022, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Kabat, A.M.; Pearce, E.J. Inflammation by way of macrophage metabolism. Science 2017, 356, 488–489. [Google Scholar] [CrossRef] [PubMed]
- Pierucci-Alves, F.; Midura-Kiela, M.T.; Fleming, S.D.; Schultz, B.D.; Kiela, P.R. Transforming Growth Factor Beta Signaling in Dendritic Cells Is Required for Immunotolerance to Sperm in the Epididymis. Front. Immunol. 2018, 9, 1882. [Google Scholar] [CrossRef]
- Da Silva, N.; Smith, T.B. Exploring the role of mononuclear phagocytes in the epididymis. Asian J. Androl. 2015, 17, 591–596. [Google Scholar] [CrossRef]
- Hedger, M.P. Immunophysiology and pathology of inflammation in the testis and epididymis. J. Androl. 2011, 32, 625–640. [Google Scholar] [CrossRef]
- Leir, S.H.; Yin, S.; Kerschner, J.L.; Cosme, W.; Harris, A. An atlas of human proximal epididymis reveals cell-specific functions and distinct roles for CFTR. Life Sci. Alliance 2020, 3, e202000744. [Google Scholar] [CrossRef]
- Hermo, L.; Schellenberg, M.; Liu, L.Y.; Dayanandan, B.; Zhang, T.; Mandato, C.A.; Smith, C.E. Membrane domain specificity in the spatial distribution of aquaporins 5, 7, 9, and 11 in efferent ducts and epididymis of rats. J. Histochem. Cytochem. 2008, 56, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Auffray, C.; Sieweke, M.H.; Geissmann, F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009, 27, 669–692. [Google Scholar] [CrossRef] [PubMed]
- Dress, R.J.; Wong, A.Y.; Ginhoux, F. Homeostatic control of dendritic cell numbers and differentiation. Immunol. Cell Biol. 2018, 96, 463–476. [Google Scholar] [CrossRef]
- Yanez, A.; Coetzee, S.G.; Olsson, A.; Muench, D.E.; Berman, B.P.; Hazelett, D.J.; Salomonis, N.; Grimes, H.L.; Goodridge, H.S. Granulocyte-Monocyte Progenitors and Monocyte-Dendritic Cell Progenitors Independently Produce Functionally Distinct Monocytes. Immunity 2017, 47, 890–902.e4. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.; Liu, Y.-J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Ginhoux, F.; Jakubzick, C.; Naik, S.H.; Onai, N.; Schraml, B.U.; Segura, E.; Tussiwand, R.; Yona, S. Dendritic cells, monocytes and macrophages: A unified nomenclature based on ontogeny. Nat. Rev. Immunol. 2014, 14, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Jung, S.; Littman, D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003, 19, 71–82. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Fu, M.; Xin, H.B. Polarizing Macrophages In Vitro. Methods Mol. Biol. 2018, 1784, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef]
- Winnall, W.R.; Hedger, M.P. Phenotypic and functional heterogeneity of the testicular macrophage population: A new regulatory model. J. Reprod. Immunol. 2013, 97, 147–158. [Google Scholar] [CrossRef]
- Serbina, N.V.; Salazar-Mather, T.P.; Biron, C.A.; Kuziel, W.A.; Pamer, E.G. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003, 19, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Mass, E.; Ballesteros, I.; Farlik, M.; Halbritter, F.; Gunther, P.; Crozet, L.; Jacome-Galarza, C.E.; Händler, K.; Klughammer, J.; Kobayashi, Y.; et al. Specification of tissue-resident macrophages during organogenesis. Science 2016, 353, aaf4238. [Google Scholar] [CrossRef] [PubMed]
- Gomez Perdiguero, E.; Klapproth, K.; Schulz, C.; Busch, K.; Azzoni, E.; Crozet, L.; Garner, H.; Trouillet, C.; de Bruijn, M.F.; Geissmann, F.; et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015, 518, 547–551. [Google Scholar] [CrossRef]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, M.B.; Leboeuf, M.; Becker, C.D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Mossadegh-Keller, N.; Gentek, R.; Gimenez, G.; Bigot, S.; Mailfert, S.; Sieweke, M.H. Developmental origin and maintenance of distinct testicular macrophage populations. J. Exp. Med. 2017, 214, 2829–2841. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Cansever, D.; Wang, Y.; Kantores, C.; Messiaen, S.; Moison, D.; Livera, G.; Chakarov, S.; Weinberger, T.; et al. Two populations of self-maintaining monocyte-independent macrophages exist in adult epididymis and testis. Proc. Natl. Acad. Sci. USA 2021, 118, e2013686117. [Google Scholar] [CrossRef]
- Lokka, E.; Lintukorpi, L.; Cisneros-Montalvo, S.; Makela, J.A.; Tyystjarvi, S.; Ojasalo, V.; Gerke, H.; Toppari, J.; Rantakari, P.; Salmi, M. Generation, localization and functions of macrophages during the development of testis. Nat. Commun. 2020, 11, 4375. [Google Scholar] [CrossRef]
- Serre, V.; Robaire, B. Distribution of immune cells in the epididymis of the aging Brown Norway rat is segment-specific and related to the luminal content. Biol. Reprod. 1999, 61, 705–714. [Google Scholar] [CrossRef]
- Villani, A.C.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356, eaah4573. [Google Scholar] [CrossRef]
- Dzionek, A.; Fuchs, A.; Schmidt, P.; Cremer, S.; Zysk, M.; Miltenyi, S.; Buck, D.W.; Schmitz, J. BDCA-2, BDCA-3, and BDCA-4: Three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 2000, 165, 6037–6046. [Google Scholar] [CrossRef]
- Haniffa, M.; Shin, A.; Bigley, V.; McGovern, N.; Teo, P.; See, P.; Wasan, P.S.; Wang, X.-N.; Malinarich, F.; Malleret, B.; et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 2012, 37, 60–73. [Google Scholar] [CrossRef]
- den Haan, J.M.; Lehar, S.M.; Bevan, M.J. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 2000, 192, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Meixlsperger, S.; Leung, C.S.; Ramer, P.C.; Pack, M.; Vanoaica, L.D.; Breton, G.; Pascolo, S.; Salazar, A.M.; Dzionek, A.; Schmitz, J.; et al. CD141+ dendritic cells produce prominent amounts of IFN-α after dsRNA recognition and can be targeted via DEC-205 in humanized mice. Blood 2013, 121, 5034–5044. [Google Scholar] [CrossRef] [PubMed]
- Schlitzer, A.; McGovern, N.; Ginhoux, F. Dendritic cells and monocyte-derived cells: Two complementary and integrated functional systems. Semin. Cell. Dev. Biol. 2015, 41, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Guazzone, V.A. Exploring the role of antigen presenting cells in male genital tract. Andrologia 2018, 50, e13120. [Google Scholar] [CrossRef]
- Fucikova, J.; Palova-Jelinkova, L.; Bartunkova, J.; Spisek, R. Induction of Tolerance and Immunity by Dendritic Cells: Mechanisms and Clinical Applications. Front. Immunol. 2019, 10, 2393. [Google Scholar] [CrossRef]
- Rival, C.; Guazzone, V.A.; von Wulffen, W.; Hackstein, H.; Schneider, E.; Lustig, L.; Meinhardt, A.; Fijak, M. Expression of co-stimulatory molecules, chemokine receptors and proinflammatory cytokines in dendritic cells from normal and chronically inflamed rat testis. Mol. Hum. Reprod. 2007, 13, 853–861. [Google Scholar] [CrossRef]
- Cella, M.; Sallusto, F.; Lanzavecchia, A. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 1997, 9, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Rival, C.; Lustig, L.; Iosub, R.; Guazzone, V.A.; Schneider, E.; Meinhardt, A.; Fijak, M. Identification of a dendritic cell population in normal testis and in chronically inflamed testis of rats with autoimmune orchitis. Cell Tissue Res. 2006, 324, 311–318. [Google Scholar] [CrossRef] [PubMed]
- De Rose, R.; Fernandez, C.S.; Hedger, M.P.; Kent, S.J.; Winnall, W.R. Characterisation of macaque testicular leucocyte populations and T-lymphocyte immunity. J. Reprod. Immunol. 2013, 100, 146–156. [Google Scholar] [CrossRef]
- Winnall, W.R.; Lloyd, S.B.; De Rose, R.; Alcantara, S.; Amarasena, T.H.; Hedger, M.P.; Girling, J.E.; Kent, S.J. Simian immunodeficiency virus infection and immune responses in the pig-tailed macaque testis. J. Leukoc. Biol. 2015, 97, 599–609. [Google Scholar] [CrossRef]
- Flores-Romo, L. In vivo maturation and migration of dendritic cells. Immunology 2001, 102, 255–262. [Google Scholar] [CrossRef]
- Pollanen, P.; Cooper, T.G. Immunology of the testicular excurrent ducts. J. Reprod. Immunol. 1994, 26, 167–216. [Google Scholar] [CrossRef]
- Shreedhar, V.; Moodycliffe, A.M.; Ullrich, S.E.; Bucana, C.; Kripke, M.L.; Flores-Romo, L. Dendritic cells require T cells for functional maturation in vivo. Immunity 1999, 11, 625–636. [Google Scholar] [CrossRef]
- Sanchez, P.J.; McWilliams, J.A.; Haluszczak, C.; Yagita, H.; Kedl, R.M. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J. Immunol. 2007, 178, 1564–1572. [Google Scholar] [CrossRef]
- Maldonado, R.A.; von Andrian, U.H. How tolerogenic dendritic cells induce regulatory T cells. Adv. Immunol. 2010, 108, 111–165. [Google Scholar] [CrossRef]
- Mahnke, K.; Johnson, T.S.; Ring, S.; Enk, A.H. Tolerogenic dendritic cells and regulatory T cells: A two-way relationship. J. Dermatol. Sci. 2007, 46, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Le Texier, L.; Durand, J.; Lavault, A.; Hulin, P.; Collin, O.; Le Bras, Y.; Cuturi, M.C.; Chiffoleau, E. LIMLE, a new molecule over-expressed following activation, is involved in the stimulatory properties of dendritic cells. PLoS ONE 2014, 9, e93894. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.G.; Wang, P.; Zheng, W.; Zhang, Q.; Huang, W.; Jin, F.; Cai, Z. Characterisation of dendritic cell subsets in chronically inflamed human epididymis. Andrologia 2016, 48, 431–440. [Google Scholar] [CrossRef]
- Loveland, K.L.; Klein, B.; Pueschl, D.; Indumathy, S.; Bergmann, M.; Loveland, B.E.; Hedger, M.P.; Schuppe, H.-C. Cytokines in Male Fertility and Reproductive Pathologies: Immunoregulation and Beyond. Front. Endocrinol. 2017, 8, 307. [Google Scholar] [CrossRef]
- Gao, J.; Wang, X.; Wang, Y.; Han, F.; Cai, W.; Zhao, B.; Li, Y.; Han, S.; Wu, X.; Hu, D. Murine Sertoli cells promote the development of tolerogenic dendritic cells: A pivotal role of galectin-1. Immunology 2016, 148, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Menges, M.; Rossner, S.; Voigtlander, C.; Schindler, H.; Kukutsch, N.A.; Bogdan, C.; Erb, K.J.; Schuler, G.; Lutz, M.B. Repetitive injections of dendritic cells matured with tumor necrosis factor α induce antigen-specific protection of mice from autoimmunity. J. Exp. Med. 2002, 195, 15–21. [Google Scholar] [CrossRef]
- Yang, J.S.; Xu, L.Y.; Huang, Y.M.; Van Der Meide, P.H.; Link, H.; Xiao, B.G. Adherent dendritic cells expressing high levels of interleukin-10 and low levels of interleukin-12 induce antigen-specific tolerance to experimental autoimmune encephalomyelitis. Immunology 2000, 101, 397–403. [Google Scholar] [CrossRef]
- Guazzone, V.A.; Hollwegs, S.; Mardirosian, M.; Jacobo, P.; Hackstein, H.; Wygrecka, M.; Schneider, E.; Meinhardt, A.; Lustig, L.; Fijak, M. Characterization of dendritic cells in testicular draining lymph nodes in a rat model of experimental autoimmune orchitis. Int. J. Androl. 2011, 34, 276–289. [Google Scholar] [CrossRef]
- Greenwald, R.J.; Boussiotis, V.A.; Lorsbach, R.B.; Abbas, A.K.; Sharpe, A.H. CTLA-4 regulates induction of anergy in vivo. Immunity 2001, 14, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Salomon, B.; Lenschow, D.J.; Rhee, L.; Ashourian, N.; Singh, B.; Sharpe, A.; Bluestone, J.A. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 2000, 12, 431–440. [Google Scholar] [CrossRef]
- Pilatz, A.; Fijak, M.; Wagenlehner, F.; Schuppe, H.C. Hodenentzündung. Urologe A 2019, 58, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Jacobo, P.; Guazzone, V.A.; Theas, M.S.; Lustig, L. Testicular autoimmunity. Autoimmun. Rev. 2011, 10, 201–204. [Google Scholar] [CrossRef]
- Taneichi, A.; Shibahara, H.; Hirano, Y.; Suzuki, T.; Obara, H.; Fujiwara, H.; Takamizawa, S.; Sato, I. Sperm immobilizing antibodies in the sera of infertile women cause low fertilization rates and poor embryo quality in vitro. Am. J. Reprod. Immunol. 2002, 47, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Pilatz, A.; Hossain, H.; Kaiser, R.; Mankertz, A.; Schuttler, C.G.; Domann, E.; Schuppe, H.-C.; Chakraborty, T.; Weidner, W.; Wagenlehner, F. Acute epididymitis revisited: Impact of molecular diagnostics on etiology and contemporary guideline recommendations. Eur. Urol. 2015, 68, 428–435. [Google Scholar] [CrossRef]
- Burne, J. Case of Epidemic Mumps: Complicated with Parotitis, Orchitis, Nephritis, Albuminuria, Convulsions: Recovery. Prov. Med. Surg. J. 1851, 15, 623–625. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fijak, M.; Pilatz, A.; Hedger, M.P.; Nicolas, N.; Bhushan, S.; Michel, V.; Tung, K.S.K.; Schuppe, H.-C.; Meinhardt, A. Infectious, inflammatory and ‘autoimmune’ male factor infertility: How do rodent models inform clinical practice? Hum. Reprod. Update 2018, 24, 416–441. [Google Scholar] [CrossRef]
- Tung, K.S.; Yule, T.D.; Mahi-Brown, C.A.; Listrom, M.B. Distribution of histopathology and Ia positive cells in actively induced and passively transferred experimental autoimmune orchitis. J. Immunol. 1987, 138, 752–759. [Google Scholar] [CrossRef]
- Duan, Y.G.; Yu, C.F.; Novak, N.; Bieber, T.; Zhu, C.H.; Schuppe, H.C.; Haidl, G.; Allam, J.-P. Immunodeviation towards a Th17 immune response associated with testicular damage in azoospermic men. Int. J. Androl. 2011, 34 Pt 2, e536–e545. [Google Scholar] [CrossRef]
- Adams, S.; O’Neill, D.W.; Bhardwaj, N. Erratum: Recent advances in dendritic cell biology. J. Clin. Immunol. 2005, 25, 177–188. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sozzani, S.; Allavena, P.; Vecchi, A.; Mantovani, A. Chemokines and dendritic cell traffic. J. Clin. Immunol. 2000, 20, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Fijak, M.; Meinhardt, A. The testis in immune privilege. Immunol. Rev. 2006, 213, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Fijak, M.; Bhushan, S.; Meinhardt, A. Immunoprivileged sites: The testis. Methods Mol. Biol. 2011, 677, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.B.; Schuler, G. Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol. 2002, 23, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Theas, M.S.; Guazzone, V.A.; Jacobo, P.; Wang, M.; Fijak, M.; Meinhardt, A.; Lustig, L. Immune Cell Subtypes and Their Function in the Testis. Front. Immunol. 2020, 11, 583304. [Google Scholar] [CrossRef] [PubMed]
- Randolph, G.J.; Angeli, V.; Swartz, M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 2005, 5, 617–628. [Google Scholar] [CrossRef]
- Gately, M.K.; Renzetti, L.M.; Magram, J.; Stern, A.S.; Adorini, L.; Gubler, U.; Presky, D.H. The interleukin-12/interleukin-12-receptor system: Role in normal and pathologic immune responses. Annu. Rev. Immunol. 1998, 16, 495–521. [Google Scholar] [CrossRef]
- Gutcher, I.; Becher, B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J. Clin. Investig. 2007, 117, 1119–1127. [Google Scholar] [CrossRef]
- Collins, M.M.; Stafford, R.S.; O’Leary, M.P.; Barry, M.J. How common is prostatitis? A national survey of physician visits. J. Urol. 1998, 159, 1224–1228. [Google Scholar] [CrossRef]
- McConaghy, J.R.; Panchal, B. Epididymitis: An Overview. Am. Fam. Phys. 2016, 94, 723–726. [Google Scholar]
- Da Silva, N.; Cortez-Retamozo, V.; Reinecker, H.C.; Wildgruber, M.; Hill, E.; Brown, D.; Swirski, F.K.; Pittet, M.J.; Breton, S. A dense network of dendritic cells populates the murine epididymis. Reproduction 2011, 141, 653–663. [Google Scholar] [CrossRef]
- Arrighi, S. Are the basal cells of the mammalian epididymis still an enigma? Reprod. Fertil. Dev. 2014, 26, 1061–1071. [Google Scholar] [CrossRef]
- Battistone, M.A.; Mendelsohn, A.C.; Spallanzani, R.G.; Brown, D.; Nair, A.V.; Breton, S. Region-specific transcriptomic and functional signatures of mononuclear phagocytes in the epididymis. Mol. Hum. Reprod. 2020, 26, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Voisin, A.; Whitfield, M.; Damon-Soubeyrand, C.; Goubely, C.; Henry-Berger, J.; Saez, F.; Kocer, A.; Drevet, J.R.; Guiton, R. Comprehensive overview of murine epididymal mononuclear phagocytes and lymphocytes: Unexpected populations arise. J. Reprod. Immunol. 2018, 126, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Shum, W.W.; Smith, T.B.; Cortez-Retamozo, V.; Grigoryeva, L.S.; Roy, J.W.; Hill, E.; Pittet, M.J.; Breton, S.; Da Silva, N. Epithelial basal cells are distinct from dendritic cells and macrophages in the mouse epididymis. Biol. Reprod. 2014, 90, 90. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.B.; Cortez-Retamozo, V.; Grigoryeva, L.S.; Hill, E.; Pittet, M.J.; Da Silva, N. Mononuclear phagocytes rapidly clear apoptotic epithelial cells in the proximal epididymis. Andrology 2014, 2, 755–762. [Google Scholar] [CrossRef]
- Ritchie, A.W.; Hargreave, T.B.; James, K.; Chisholm, G.D. Intra-epithelial lymphocytes in the normal epididymis. A mechanism for tolerance to sperm auto-antigens? Br. J. Urol. 1984, 56, 79–83. [Google Scholar] [CrossRef]
- de Witte, L.; Nabatov, A.; Geijtenbeek, T.B. Distinct roles for DC-SIGN+-dendritic cells and Langerhans cells in HIV-1 transmission. Trends Mol. Med. 2008, 14, 12–19. [Google Scholar] [CrossRef]
- de Witte, L.; Nabatov, A.; Pion, M.; Fluitsma, D.; de Jong, M.A.W.P.; de Gruijl, T.; Piguet, V.; Van Kooyk, Y.; Geijtenbeek, T.B.H. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 2007, 13, 367–371. [Google Scholar] [CrossRef]
- Coombes, J.L.; Siddiqui, K.R.; Arancibia-Carcamo, C.V.; Hall, J.; Sun, C.M.; Belkaid, Y.; Powrie, F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 2007, 204, 1757–1764. [Google Scholar] [CrossRef]
- Matteoli, G.; Mazzini, E.; Iliev, I.D.; Mileti, E.; Fallarino, F.; Puccetti, P.; Chieppa, M.; Rescigno, M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut 2010, 59, 595–604. [Google Scholar] [CrossRef]
- Li, K.; Wei, X.; Zhang, G.; Li, M.; Zhang, X.; Zhou, C.; Hou, J.; Yuan, H. Different expression of B7-H3 in the caput, corpus, and cauda of the epididymis in mouse. BMC Urol. 2017, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Suh, W.K.; Wang, S.X.; Jheon, A.H.; Moreno, L.; Yoshinaga, S.K.; Ganss, B.; Sodek, J.; Grynpas, M.D.; Mak, T.W. The immune regulatory protein B7-H3 promotes osteoblast differentiation and bone mineralization. Proc. Natl. Acad. Sci. USA 2004, 101, 12969–12973. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wei, X.; Zhang, G.; Li, C.; Zhang, X.; Hou, J. B7-H3 over expression in prostate cancer promotes tumor cell progression. J. Urol. 2011, 186, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Chapoval, A.I.; Ni, J.; Lau, J.S.; Wilcox, R.A.; Flies, D.B.; Liu, D.; Dong, H.; Sica, G.L.; Zhu, G.; Tamada, K.; et al. B7-H3: A costimulatory molecule for T cell activation and IFN-γ production. Nat. Immunol. 2001, 2, 269–274. [Google Scholar] [CrossRef]
- Lupu, C.M.; Eisenbach, C.; Kuefner, M.A.; Schmidt, J.; Lupu, A.D.; Stremmel, W.; Encke, J. An orthotopic colon cancer model for studying the B7-H3 antitumor effect in vivo. J. Gastrointest. Surg. 2006, 10, 635–645. [Google Scholar] [CrossRef]
- Ling, V.; Wu, P.W.; Spaulding, V.; Kieleczawa, J.; Luxenberg, D.; Carreno, B.M.; Collins, M. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: Divergent history of functional redundancy and exon loss. Genomics 2003, 82, 365–377. [Google Scholar] [CrossRef]
- Suh, W.K.; Gajewska, B.U.; Okada, H.; Gronski, M.A.; Bertram, E.M.; Dawicki, W.; Duncan, G.S.; Bukczynski, J.; Plyte, S.; Elia, A.J.; et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat. Immunol. 2003, 4, 899–906. [Google Scholar] [CrossRef]
- Wang, P.; Duan, Y.G. The role of dendritic cells in male reproductive tract. Am. J. Reprod. Immunol. 2016, 76, 186–192. [Google Scholar] [CrossRef]
- Rival, C.; Theas, M.S.; Guazzone, V.A.; Lustig, L. Interleukin-6 and IL-6 receptor cell expression in testis of rats with autoimmune orchitis. J. Reprod. Immunol. 2006, 70, 43–58. [Google Scholar] [CrossRef]
- Allam, J.P.; Duan, Y.; Heinemann, F.; Winter, J.; Gotz, W.; Deschner, J.; Wenghoefer, M.; Bieber, T.; Jepsen, S.; Novak, N. IL-23-producing CD68+ macrophage-like cells predominate within an IL-17-polarized infiltrate in chronic periodontitis lesions. J. Clin. Periodontol. 2011, 38, 879–886. [Google Scholar] [CrossRef]
- Voisin, A.; Saez, F.; Drevet, J.R.; Guiton, R. The epididymal immune balance: A key to preserving male fertility. Asian J. Androl. 2019, 21, 531–539. [Google Scholar] [CrossRef]
- Dijkstra, C.D.; Dopp, E.A.; Joling, P.; Kraal, G. The heterogeneity of mononuclear phagocytes in lymphoid organs: Distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology 1985, 54, 589–599. [Google Scholar]
- Funes, S.C.; Rios, M.; Escobar-Vera, J.; Kalergis, A.M. Implications of macrophage polarization in autoimmunity. Immunology 2018, 154, 186–195. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Fijak, M.; Damm, L.J.; Wenzel, J.P.; Aslani, F.; Walecki, M.; Wahle, E.; Eisel, F.; Bhushan, S.; Hackstein, H.; Baal, N.; et al. Influence of Testosterone on Inflammatory Response in Testicular Cells and Expression of Transcription Factor Foxp3 in T Cells. Am. J. Reprod. Immunol. 2015, 74, 12–25. [Google Scholar] [CrossRef]
- Wang, M.; Fijak, M.; Hossain, H.; Markmann, M.; Nusing, R.M.; Lochnit, G.; Hartmann, M.F.; Wudy, S.A.; Zhang, L.; Gu, H.; et al. Characterization of the Micro-Environment of the Testis that Shapes the Phenotype and Function of Testicular Macrophages. J. Immunol. 2017, 198, 4327–4340. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS−) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Vats, D.; Mukundan, L.; Odegaard, J.I.; Zhang, L.; Smith, K.L.; Morel, C.R.; Wagner, R.A.; Greaves, D.R.; Murray, P.J.; Chawla, A. Oxidative metabolism and PGC-1β attenuate macrophage-mediated inflammation. Cell Metab. 2006, 4, 13–24. [Google Scholar] [CrossRef]
- Nashan, D.; Jantos, C.; Ahlers, D.; Bergmann, M.; Schiefer, H.G.; Sorg, C.; Nieschlag, E. Immuno-competent cells in the murine epididymis following infection with Escherichia coli. Int. J. Androl. 1993, 16, 47–52. [Google Scholar] [CrossRef]
- DeFalco, T.; Potter, S.J.; Williams, A.V.; Waller, B.; Kan, M.J.; Capel, B. Macrophages Contribute to the Spermatogonial Niche in the Adult Testis. Cell Rep. 2015, 12, 1107–1119. [Google Scholar] [CrossRef]
- Mossadegh-Keller, N.; Sieweke, M.H. Characterization of Mouse Adult Testicular Macrophage Populations by Immunofluorescence Imaging and Flow Cytometry. Bio-Protocol 2019, 9, e3178. [Google Scholar] [CrossRef]
- Hedger, M.P. Macrophages and the immune responsiveness of the testis. J. Reprod. Immunol. 2002, 57, 19–34. [Google Scholar] [CrossRef]
- Mossadegh-Keller, N.; Sieweke, M.H. Testicular macrophages: Guardians of fertility. Cell. Immunol. 2018, 330, 120–125. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Zhang, Y.; Zhang, Y.; Yan, Y.; Bhushan, S.; Meinhardt, A.; Qin, Z.; Wang, M. Corticosterone Enhances the AMPK-Mediated Immunosuppressive Phenotype of Testicular Macrophages During Uropathogenic Escherichia coli Induced Orchitis. Front. Immunol. 2020, 11, 583276. [Google Scholar] [CrossRef]
- Chelen, C.J.; Fang, Y.; Freeman, G.J.; Secrist, H.; Marshall, J.D.; Hwang, P.T.; Frankel, L.R.; DeKruyff, R.H.; Umetsu, D.T. Human alveolar macrophages present antigen ineffectively due to defective expression of B7 costimulatory cell surface molecules. J. Clin. Investig. 1995, 95, 1415–1421. [Google Scholar] [CrossRef]
- Rogler, G.; Hausmann, M.; Vogl, D.; Aschenbrenner, E.; Andus, T.; Falk, W.; Andreesen, R.; Schölmerich, J.; Gross, V. Isolation and phenotypic characterization of colonic macrophages. Clin. Exp. Immunol. 1998, 112, 205–215. [Google Scholar] [CrossRef]
- Indumathy, S.; Pueschl, D.; Klein, B.; Fietz, D.; Bergmann, M.; Schuppe, H.C.; Da Silva, N.; Loveland, B.; Hickey, M.; Hedger, M.; et al. Testicular immune cell populations and macrophage polarisation in adult male mice and the influence of altered activin A levels. J. Reprod. Immunol. 2020, 142, 103204. [Google Scholar] [CrossRef]
- O’Bryan, M.K.; Gerdprasert, O.; Nikolic-Paterson, D.J.; Meinhardt, A.; Muir, J.A.; Foulds, L.M.; Phillips, D.J.; De Kretser, D.M.; Hedger, M.P. Cytokine profiles in the testes of rats treated with lipopolysaccharide reveal localized suppression of inflammatory responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1744–R1755. [Google Scholar] [CrossRef]
- Winnall, W.R.; Muir, J.A.; Hedger, M.P. Rat resident testicular macrophages have an alternatively activated phenotype and constitutively produce interleukin-10 in vitro. J. Leukoc. Biol. 2011, 90, 133–143. [Google Scholar] [CrossRef]
- Bhushan, S.; Tchatalbachev, S.; Lu, Y.; Frohlich, S.; Fijak, M.; Vijayan, V.; Chakraborty, T.; Meinhardt, A. Differential activation of inflammatory pathways in testicular macrophages provides a rationale for their subdued inflammatory capacity. J. Immunol. 2015, 194, 5455–5464. [Google Scholar] [CrossRef]
- Kern, S.; Robertson, S.A.; Mau, V.J.; Maddocks, S. Cytokine secretion by macrophages in the rat testis. Biol. Reprod. 1995, 53, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Bergh, A.; Damber, J.E.; van Rooijen, N. The human chorionic gonadotrophin-induced inflammation-like response is enhanced in macrophage-depleted rat testes. J. Endocrinol. 1993, 136, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Meinhardt, A. The macrophages in testis function. J. Reprod. Immunol. 2017, 119, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Wang, J.; Yang, L.; An, Y.; Zhu, H. AMPK activation enhances the anti-atherogenic effects of high density lipoproteins in apoE−/− mice. J. Lipid Res. 2017, 58, 1536–1547. [Google Scholar] [CrossRef]
- Klein, B.; Bhushan, S.; Gunther, S.; Middendorff, R.; Loveland, K.L.; Hedger, M.P.; Meinhardt, A. Differential tissue-specific damage caused by bacterial epididymo-orchitis in the mouse. Mol. Hum. Reprod. 2020, 26, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Hossain, H.; Lu, Y.; Geisler, A.; Tchatalbachev, S.; Mikulski, Z.; Schuler, G.; Klug, J.; Pilatz, A.; Wagenlehner, F.; et al. Uropathogenic E. coli induce different immune response in testicular and peritoneal macrophages: Implications for testicular immune privilege. PLoS ONE 2011, 6, e28452. [Google Scholar] [CrossRef]
- Dejucq, N.; Jegou, B. Viruses in the mammalian male genital tract and their effects on the reproductive system. Microbiol. Mol. Biol. Rev. 2001, 65, 208–231. [Google Scholar] [CrossRef]
- Xu, J.; Qi, L.; Chi, X.; Yang, J.; Wei, X.; Gong, E.; Peh, S.; Gu, J. Orchitis: A complication of severe acute respiratory syndrome (SARS). Biol. Reprod. 2006, 74, 410–416. [Google Scholar] [CrossRef]
- Seymen, C.M. The other side of COVID-19 pandemic: Effects on male fertility. J. Med. Virol. 2021, 93, 1396–1402. [Google Scholar] [CrossRef]
- Stopsack, K.H.; Mucci, L.A.; Antonarakis, E.S.; Nelson, P.S.; Kantoff, P.W. TMPRSS2 and COVID-19: Serendipity or Opportunity for Intervention? Cancer Discov. 2020, 10, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Massarotti, C.; Garolla, A.; Maccarini, E.; Scaruffi, P.; Stigliani, S.; Anserini, P.; Foresta, C. SARS-CoV-2 in the semen: Where does it come from? Andrology 2021, 9, 39–41. [Google Scholar] [CrossRef]
- Dosch, S.F.; Mahajan, S.D.; Collins, A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-κB pathway in human monocyte macrophages in vitro. Virus Res. 2009, 142, 19–27. [Google Scholar] [CrossRef]
- Douglas, G.C.; O’Bryan, M.K.; Hedger, M.P.; Lee, D.K.; Yarski, M.A.; Smith, A.I.; Lew, R.A. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology 2004, 145, 4703–4711. [Google Scholar] [CrossRef] [PubMed]
- Jung, A.; Schuppe, H.C. Influence of genital heat stress on semen quality in humans. Andrologia 2007, 39, 203–215. [Google Scholar] [CrossRef]
- Lustig, L.; Guazzone, V.A.; Theas, M.S.; Pleuger, C.; Jacobo, P.; Perez, C.V.; Meinhardt, A.; Fijak, M. Pathomechanisms of Autoimmune Based Testicular Inflammation. Front. Immunol. 2020, 11, 583135. [Google Scholar] [CrossRef]
- Rival, C.; Theas, M.S.; Suescun, M.O.; Jacobo, P.; Guazzone, V.; van Rooijen, N.; Lustig, L. Functional and phenotypic characteristics of testicular macrophages in experimental autoimmune orchitis. J. Pathol. 2008, 215, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Hedger, M.P.; Meinhardt, A. Cytokines and the immune-testicular axis. J. Reprod. Immunol. 2003, 58, 1–26. [Google Scholar] [CrossRef]
- Da Silva, N.; Barton, C.R. Macrophages and dendritic cells in the post-testicular environment. Cell Tissue Res. 2016, 363, 97–104. [Google Scholar] [CrossRef][Green Version]
- Sarkar, O.; Mathur, P.P.; Cheng, C.Y.; Mruk, D.D. Interleukin 1 alpha (IL1A) is a novel regulator of the blood-testis barrier in the rat. Biol. Reprod. 2008, 78, 445–454. [Google Scholar] [CrossRef]
- Frungieri, M.B.; Weidinger, S.; Meineke, V.; Kohn, F.M.; Mayerhofer, A. Proliferative action of mast-cell tryptase is mediated by PAR2, COX2, prostaglandins, and PPARγ: Possible relevance to human fibrotic disorders. Proc. Natl. Acad. Sci. USA 2002, 99, 15072–15077. [Google Scholar] [CrossRef] [PubMed]
- Theas, M.S.; Rival, C.; Jarazo-Dietrich, S.; Jacobo, P.; Guazzone, V.A.; Lustig, L. Tumour necrosis factor-α released by testicular macrophages induces apoptosis of germ cells in autoimmune orchitis. Hum. Reprod. 2008, 23, 1865–1872. [Google Scholar] [CrossRef]
- McGovern, N.; Schlitzer, A.; Janela, B.; Ginhoux, F. Protocols for the Identification and Isolation of Antigen-Presenting Cells in Human and Mouse Tissues. Methods Mol. Biol. 2016, 1423, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.Y.; Ma, T.; Lock, E.J.; Hao, Q.; Kristiansen, K.; Frøyland, L.; Madsen, L. Depot-dependent effects of adipose tissue explants on co-cultured hepatocytes. PLoS ONE 2011, 6, e20917. [Google Scholar] [CrossRef]
- Mendelsohn, A.C.; Sanmarco, L.M.; Spallanzani, R.G.; Brown, D.; Quintana, F.J.; Breton, S.; Battistone, M.A. From initial segment to cauda: A regional characterization of mouse epididymal CD11c+ mononuclear phagocytes based on immune phenotype and function. Am. J. Physiol. Cell Physiol. 2020, 319, C997–C1010. [Google Scholar] [CrossRef]
- Dutertre, C.A.; Becht, E.; Irac, S.E.; Khalilnezhad, A.; Narang, V.; Khalilnezhad, S.; Ng, P.Y.; van den Hoogen, L.L.; Leong, J.Y.; Lee, B.; et al. Single-Cell Analysis of Human Mononuclear Phagocytes Reveals Subset-Defining Markers and Identifies Circulating Inflammatory Dendritic Cells. Immunity 2019, 51, 573–589.e8. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Ibeas, P.; Pericuesta, E.; Fernandez-Gonzalez, R.; Ramirez, M.A.; Gutierrez-Adan, A. Most regions of mouse epididymis are able to phagocytose immature germ cells. Reproduction 2013, 146, 481–489. [Google Scholar] [CrossRef]
- Cheng, L.; Chen, Q.; Zhu, W.; Wu, H.; Wang, Q.; Shi, L.; Zhao, X.; Han, D. Toll-like Receptors 4 and 5 Cooperatively Initiate the Innate Immune Responses to Uropathogenic Escherichia coli Infection in Mouse Epididymal Epithelial Cells. Biol. Reprod. 2016, 94, 58. [Google Scholar] [CrossRef]
- Rodrigues, A.; Queiroz, D.B.; Honda, L.; Silva, E.J.; Hall, S.H.; Avellar, M.C. Activation of toll-like receptor 4 (TLR4) by in vivo and in vitro exposure of rat epididymis to lipopolysaccharide from Escherichia coli. Biol. Reprod. 2008, 79, 1135–1147. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, G.; Hayden, M.S.; Greenblatt, M.B.; Bussey, C.; Flavell, R.A.; Ghosh, S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science 2004, 303, 1522–1526. [Google Scholar] [CrossRef]
- Silva, E.J.R.; Ribeiro, C.M.; Mirim, A.F.M.; Silva, A.A.S.; Romano, R.M.; Hallak, J.; Avellar, M.C.W. Lipopolysaccharide and lipotheicoic acid differentially modulate epididymal cytokine and chemokine profiles and sperm parameters in experimental acute epididymitis. Sci. Rep. 2018, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.J.; Vogel, P.; Alexander, N.J.; Dandekar, S.; Hendrickx, A.G.; Marx, P.A. Pathology and localization of simian immunodeficiency virus in the reproductive tract of chronically infected male rhesus macaques. Lab. Investig. 1994, 70, 255–262. [Google Scholar]
- Mullen, T.E., Jr.; Kiessling, R.L.; Kiessling, A.A. Tissue-specific populations of leukocytes in semen-producing organs of the normal, hemicastrated, and vasectomized mouse. AIDS Res. Hum. Retrovir. 2003, 19, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Albanesi, C.; Fairchild, H.R.; Madonna, S.; Scarponi, C.; De Pita, O.; Leung, D.Y.M.; Howell, M.D. IL-4 and IL-13 negatively regulate TNF-α- and IFN-γ-induced β-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J. Immunol. 2007, 179, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Roulet, V.; Satie, A.P.; Ruffault, A.; Le Tortorec, A.; Denis, H.; Guist’hau, O.; Patard, J.-J.; Rioux-Leclerq, N.; Gicquel, J.; Jegou, B.; et al. Susceptibility of human testis to human immunodeficiency virus-1 infection in situ and in vitro. Am. J. Pathol. 2006, 169, 2094–2103. [Google Scholar] [CrossRef]
- Zeng, X.; Blancett, C.D.; Koistinen, K.A.; Schellhase, C.W.; Bearss, J.J.; Radoshitzky, S.R.; Honnold, S.P.; Chance, T.B.; Warren, T.K.; Froude, J.W.; et al. Identification and pathological characterization of persistent asymptomatic Ebola virus infection in rhesus monkeys. Nat. Microbiol. 2017, 2, 17113. [Google Scholar] [CrossRef]
- Wijayarathna, R.; Pasalic, A.; Nicolas, N.; Biniwale, S.; Ravinthiran, R.; Genovese, R.; Muir, J.A.; Loveland, K.L.; Meinhardt, A.; Fijak, M.; et al. Region-specific immune responses to autoimmune epididymitis in the murine reproductive tract. Cell Tissue Res. 2020, 381, 351–360. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, C.; He, C.; Mei, C.; Liao, A.; Huang, D. The Immune Characteristics of the Epididymis and the Immune Pathway of the Epididymitis Caused by Different Pathogens. Front. Immunol. 2020, 11, 2115. [Google Scholar] [CrossRef]
| Location | Cell Type | Classification | Markers | References | |
|---|---|---|---|---|---|
| Human | Mice | ||||
| Blood | Monocytes | Classical Monocytes | CD14++CD16− | Ly6Chi CX3CR1int CCR2+ CD62L+ CD43low | [15,17] |
| Nonclassical Monocytes | CD14+CD16++ | Ly6Clow CX3CR1hi CCR2low CD62L− | |||
| Intermediate monocytes | CD14++CD16+ | Ly6C++ CD43++ | [15] | ||
| Testes | DC | cDC | CD1C+ | CD11b+ CD172a+ (cDC2) | [16,30] |
| CD141+ | CD8α+ CD103+ (cDC1) | ||||
| Macrophage | Peritubular macrophages | CD68+ CD163+ | M-CSFRlo CD64lo MHCII+ | [20,24,26,74,112] | |
| Interstitial macrophages | M-CSFR+ CD64hi MHCII− | ||||
| M1 phenotype (infiltrating TMs) | MHC II+ CD68+ CD80+ CD86+ | CD68+ CD163− (rat) | [18,20] | ||
| M2 phenotype (resident TMs) | CD68− CD163+ (rat) | ||||
| Intermediate TMs | CD68+ CD163+ (rat) | ||||
| Epididymis | DC | cDC1 | CD11c+ IL-23p19+ | CD64− CD24+ CD11c+ MHC II+ CD11b− CD103+ | [53,80] |
| cDC2 | CD64− CD24+ CD11c+ MHC II+ CD11b+ CD103− | ||||
| pDC | Lin-MHC II+ CD303(BDCA-2)+ CD304 (BDCA-4)+ | [15,53,101] | |||
| Macrophage | F4/80 or CD11b+ CD64 + MHC II | [83] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Zhao, H.; Kang, Y.; Dong, X.; Yu, C.; Xie, Q.; Duan, Y.; Liao, A.; Huang, D. The Role of Mononuclear Phagocytes in the Testes and Epididymis. Int. J. Mol. Sci. 2023, 24, 53. https://doi.org/10.3390/ijms24010053
Shi X, Zhao H, Kang Y, Dong X, Yu C, Xie Q, Duan Y, Liao A, Huang D. The Role of Mononuclear Phagocytes in the Testes and Epididymis. International Journal of Molecular Sciences. 2023; 24(1):53. https://doi.org/10.3390/ijms24010053
Chicago/Turabian StyleShi, Xu, Hu Zhao, Yafei Kang, Xinyi Dong, Caiqian Yu, Qinying Xie, Yonggang Duan, Aihua Liao, and Donghui Huang. 2023. "The Role of Mononuclear Phagocytes in the Testes and Epididymis" International Journal of Molecular Sciences 24, no. 1: 53. https://doi.org/10.3390/ijms24010053
APA StyleShi, X., Zhao, H., Kang, Y., Dong, X., Yu, C., Xie, Q., Duan, Y., Liao, A., & Huang, D. (2023). The Role of Mononuclear Phagocytes in the Testes and Epididymis. International Journal of Molecular Sciences, 24(1), 53. https://doi.org/10.3390/ijms24010053





