Cardiac Metabolism and MiRNA Interference
Abstract
1. Introduction
2. Methods
3. Cardiac Metabolism
3.1. Fatty Acid
3.2. Glucose
3.3. Lactate
3.4. Ketone Bodies
3.5. Amino Acids
4. Regulation of Cardiac Metabolism
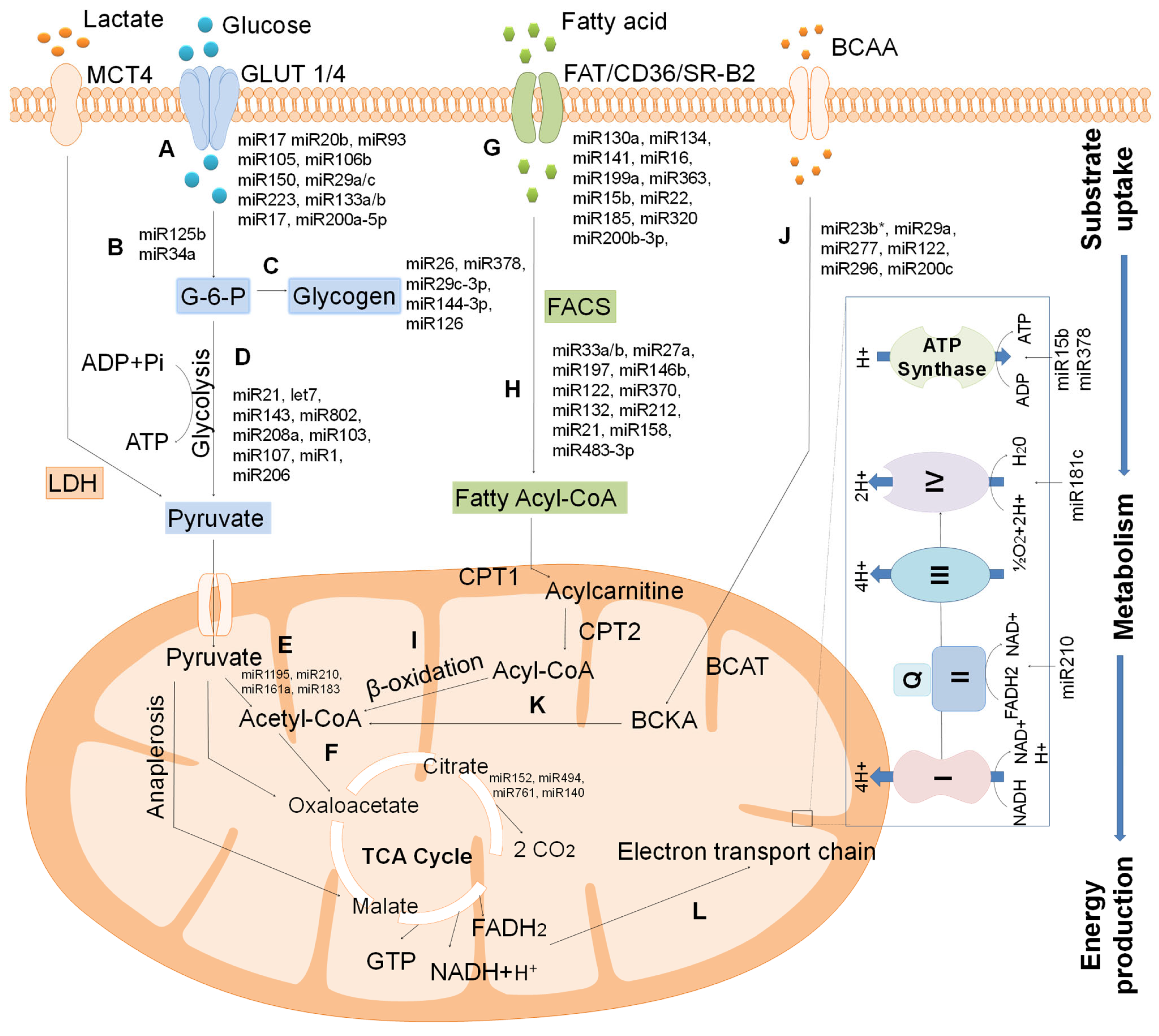
4.1. Regulatory Role of miRNA on Cardiac Metabolism
4.1.1. MiRNA-Regulated Glucose Metabolism
Glucose Uptake: MiRNA Interference
Glucose Metabolism and Glycogen Synthesis: MiRNA Interference
MiRNA Regulated TCA Cycle
4.1.2. MiRNA-Regulated Fatty Acid Metabolism
Fatty Acid Uptake by Cardiomyocytes: MiRNA Interference
Fatty Acid Metabolism and Storage: MiRNA Interference
4.1.3. MiRNA Regulated ETC and ATP Generation
4.1.4. Amino Acid Metabolism Regulated by MiRNA
4.1.5. Polyamine Metabolism Regulated by MiRNA
5. Cardiometabolic Diseases and Their Regulation by MiRNA
6. MiRNA Targeting Therapeutic Approaches
7. Limitations of this Review
8. Future Research Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| miRNA | MicroRNA |
| ATP | Adenosine triphosphate |
| PPAR | Peroxisome proliferator-activated receptors |
| ncRNA | Noncoding RNAs |
| FAO | Fatty acid oxidation |
| DGAT | Diacylglycerol acyltransferases |
| CPT-1 | Carnitine palmitoyltransferase 1 |
| PGC-1 | PPAR γ coactivator- 1 |
| FADH | Flavin adenine dinucleotide |
| NADH2 | Nicotinamide adenine dinucleotide |
| FFA | Free fatty acids |
| FABP | Fatty acid-binding protein |
| GLUT | Glucose transporters |
| G6P | Glucose 6-phosphate |
| PPP | Pentose phosphate pathway |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| HBP | Hexosamine biosynthetic pathway |
| MPC | Mitochondria pyruvate carrier |
| PDH | Pyruvate dehydrogenase |
| MCT4 | Monocarboxylic anion transporter 4 |
| βOHB | d-β-hydroxybutyrate |
| ACAT | Acetoacetyl-CoA thiolase |
| HMGCS | Hydroxymethylglutaryl-CoA synthase |
| HMGCL | HMG-CoA lyase |
| BDH | β-hydroxybutyrate dehydrogenase |
| SCOT | Succinyl-CoA:3-ketoacid-CoA transferase |
| HFrEF | Heart failure with a reduced ejection fraction |
| ETC | Electron transport chain |
| BCAA | Branched-chain amino acid |
| BCAT | Branched-chain aminotransferase |
| BCKA | Branched-chain α-keto acids |
| TCA | Tricarboxylic acid |
| mTOR | Mammalian target of rapamycin |
| GLP-1 | Glucagon-like peptide |
| ERR | Estrogen related receptor |
| Slc2a4 | Solute carrier family 2 member 4 |
| KLF15 | Kruppel-like zinc finger transcription factor 15 |
| Selenoprotein | Selenoproteins |
| IGF-1 | Insulin-like growth factor |
| AS160 | Akt substrate of 160 |
| T2D | Type 2 diabetes |
| NRF2 | Nuclear factor erythroid-2–related factor 2 |
| GSK3β | GSK3β |
| ROS | Reactive oxygen species |
| VLDLR | Very low- density lipoprotein receptor |
| CROT | Carnitine O-octanoyltransferase |
| SREBP | Sterol regulatory element-binding protein family of transcription factors |
| PNPase | Polynucleotide phosphorylase |
| SAT-1 | Spermine N1-acetyltrensferase |
| ODC | Ornithine decarboxylase |
| ABCA1 | ATP binding cassette subfamily A member 1 |
| LNA | Locked nucleic acid |
| 2’-F-2’-MOE | 2’-fluoro-2’-methoxyethyl |
References
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac Metabolism in Heart Failure: Implications beyond ATP Production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Taegtmeyer, H. Cardiac Metabolism as a Target for the Treatment of Heart Failure. Circulation 2004, 110, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Taegtmeyer, H.; Lam, T.; Davogustto, G. Cardiac Metabolism in Perspective. Compr. Physiol. 2016, 6, 1675–1699. [Google Scholar] [PubMed]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Karwi, Q.G.; Uddin, G.M.; Ho, K.L.; Lopaschuk, G.D. Loss of Metabolic Flexibility in the Failing Heart. Front. Cardiovasc. Med. 2018, 5, 68. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Vitale, C. Metabolic Modulation of Cardiac Metabolism in Heart Failure. Card. Fail. Rev. 2018, 4, 99. [Google Scholar] [CrossRef]
- Cotter, D.G.; Schugar, R.C.; Crawford, P.A. Ketone Body Metabolism and Cardiovascular Disease. Am. J. Physiol. Circ. Physiol. 2013, 304, H1060–H1076. [Google Scholar] [CrossRef]
- Ritterhoff, J.; Tian, R. Metabolism in Cardiomyopathy: Every Substrate Matters. Cardiovasc. Res. 2017, 113, 411–421. [Google Scholar] [CrossRef]
- Akki, A.; Smith, K.; Seymour, A.-M.L. Compensated Cardiac Hypertrophy Is Characterised by a Decline in Palmitate Oxidation. Mol. Cell. Biochem. 2008, 311, 215–224. [Google Scholar] [CrossRef]
- Marazzi, G.; Rosanio, S.; Caminiti, G.; Dioguardi, F.S.; Mercuro, G. The Role of Amino Acids in the Modulation of Cardiac Metabolism during Ischemia and Heart Failure. Curr. Pharm. Des. 2008, 14, 2592–2604. [Google Scholar] [CrossRef]
- Ahmed, W.; Ziouzenkova, O.; Brown, J.; Devchand, P.; Francis, S.; Kadakia, M.; Kanda, T.; Orasanu, G.; Sharlach, M.; Zandbergen, F. PPARs and Their Metabolic Modulation: New Mechanisms for Transcriptional Regulation? J. Intern. Med. 2007, 262, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Angin, Y.; Beauloye, C.; Horman, S.; Bertrand, L. Regulation of Carbohydrate Metabolism, Lipid Metabolism, and Protein Metabolism by AMPK. In AMP-Activated Protein Kinase; Springer: Cham, Switzerland, 2016; pp. 23–43. [Google Scholar]
- Pinti, M.V.; Hathaway, Q.A.; Hollander, J.M. Role of MicroRNA in Metabolic Shift during Heart Failure. Am. J. Physiol. Circ. Physiol. 2017, 312, H33–H45. [Google Scholar] [CrossRef] [PubMed]
- Thum, T.; Condorelli, G. Long Noncoding RNAs and MicroRNAs in Cardiovascular Pathophysiology. Circ. Res. 2015, 116, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.E.; Pat, B.M.; Zou, L.; Litovsky, S.H.; Wende, A.R.; Young, M.E.; Chatham, J.C. Novel Role of the ER/SR Ca2+ Sensor STIM1 in the Regulation of Cardiac Metabolism. Am. J. Physiol. Circ. Physiol. 2019, 316, H1014–H1026. [Google Scholar] [CrossRef] [PubMed]
- Erickson, J.R. Mechanisms of CaMKII Activation in the Heart. Front. Pharmacol. 2014, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.F. Cardiac Actions of Protein Kinase C Isoforms. Physiology 2012, 27, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.R.; Jaswal, J.S.; Lopaschuk, G.D. Pyridine Nucleotide Regulation of Cardiac Intermediary Metabolism. Circ. Res. 2012, 111, 628–641. [Google Scholar] [CrossRef]
- Lee, T.-W.; Bai, K.-J.; Lee, T.-I.; Chao, T.-F.; Kao, Y.-H.; Chen, Y.-J. PPARs Modulate Cardiac Metabolism and Mitochondrial Function in Diabetes. J. Biomed. Sci. 2017, 24, 5. [Google Scholar] [CrossRef]
- Huang, Z.-P.; Wang, D.-Z. MiR-22 in Cardiac Remodeling and Disease. Trends Cardiovasc. Med. 2014, 24, 267–272. [Google Scholar] [CrossRef]
- Bartel, D.P.; Sheng, M.; Lau, L.F.; Greenberg, M.E. Growth Factors and Membrane Depolarization Activate Distinct Programs of Early Response Gene Expression: Dissociation of Fos and Jun Induction. Genes Dev. 1989, 3, 304–313. [Google Scholar] [CrossRef]
- Du, H.; Zhao, Y.; Li, H.; Wang, D.W.; Chen, C. Roles of MicroRNAs in Glucose and Lipid Metabolism in the Heart. Front. Cardiovasc. Med. 2021, 8, 716213. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, G.; Latronico, M.V.G.; Cavarretta, E. MicroRNAs in Cardiovascular Diseases: Current Knowledge and the Road Ahead. J. Am. Coll. Cardiol. 2014, 63, 2177–2187. [Google Scholar] [CrossRef] [PubMed]
- Kumarswamy, R.; Thum, T. Non-Coding RNAs in Cardiac Remodeling and Heart Failure. Circ. Res. 2013, 113, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Dangwal, S.; Thum, T. MicroRNA Therapeutics in Cardiovascular Disease Models. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.J.A.; Olson, E.N. Control of Glucose Homeostasis and Insulin Sensitivity by the Let-7 Family of MicroRNAs. Proc. Natl. Acad. Sci. USA 2011, 108, 21075–21080. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Rahman, S.; Elijovich, F.; Laffer, C.L.; Ertuglu, L.A.; Masenga, S.K.; Kirabo, A. Sox6, A Potential Target for MicroRNAs in Cardiometabolic Disease. Curr. Hypertens. Rep. 2022, 24, 145–156. [Google Scholar] [CrossRef]
- Khan, A.A.; Gupta, V.; Mahapatra, N.R. Key Regulatory MiRNAs in Lipid Homeostasis: Implications for Cardiometabolic Diseases and Development of Novel Therapeutics. Drug Discov. Today 2022, 27, 2170–2180. [Google Scholar] [CrossRef]
- Su, X.; Nie, M.; Zhang, G.; Wang, B. MicroRNA in Cardio-Metabolic Disorders. Clin. Chim. Acta 2021, 518, 134–141. [Google Scholar] [CrossRef]
- Hua, C.-C.; Liu, X.-M.; Liang, L.-R.; Wang, L.-F.; Zhong, J.-C. Targeting the Microrna-34a as a Novel Therapeutic Strategy for Cardiovascular Diseases. Front. Cardiovasc. Med. 2021, 8, 784044. [Google Scholar] [CrossRef]
- Theofilis, P.; Oikonomou, E.; Vogiatzi, G.; Sagris, M.; Antonopoulos, A.S.; Siasos, G.; Iliopoulos, D.C.; Perrea, D.; Vavouranakis, M.; Tsioufis, K. The Role of MicroRNA-126 in Atherosclerotic Cardiovascular Diseases. Curr. Med. Chem. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Rahul, K.; Kumar, S.; Kumar, B.; Chaudhary, V. Circulating MicroRNAs as Potential Novel Biomarkers in Cardiovascular Diseases: Emerging Role, Biogenesis, Current Knowledge, Therapeutics and the Road Ahead. Int. J. Cardiovasc. Acad. 2022, 8, 31. [Google Scholar] [CrossRef]
- Kantor, P.F.; Lopaschuk, G.D.; Opie, L.H. Myocardial Energy Metabolism. In Heart Physiology and Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 543–569. [Google Scholar]
- Rosano, G.; Fini, M.; Caminiti, G.; Barbaro, G. Cardiac Metabolism in Myocardial Ischemia. Curr. Pharm. Des. 2008, 14, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Stanley, W.C.; Recchia, F.A.; Lopaschuk, G.D. Myocardial Substrate Metabolism in the Normal and Failing Heart. Physiol. Rev. 2005, 85, 1093–1129. [Google Scholar] [CrossRef] [PubMed]
- Abozguia, K.; Shivu, G.N.; Ahmed, I.; Phan, T.T.; Frenneaux, M.P. The Heart Metabolism: Pathophysiological Aspects in Ischaemia and Heart Failure. Curr. Pharm. Des. 2009, 15, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, T.; Yoshimura, M.; MC Rosano, G.; Lopaschuk, G.D.; Mochizuki, S. Optimization of Cardiac Metabolism in Heart Failure. Curr. Pharm. Des. 2011, 17, 3846–3853. [Google Scholar] [CrossRef]
- Goodwin, G.W.; Taylor, C.S.; Taegtmeyer, H. Regulation of Energy Metabolism of the Heart during Acute Increase in Heart Work. J. Biol. Chem. 1998, 273, 29530–29539. [Google Scholar] [CrossRef]
- Neubauer, S. The Failing Heart—An Engine out of Fuel. N. Engl. J. Med. 2007, 356, 1140–1151. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.L.; Jaswal, J.S.; Stanley, W.C. Myocardial Fatty Acid Metabolism in Health and Disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef]
- Jaswal, J.; Ussher, J. Myocardial Fatty Acid Utilization as a Determinant of Cardiac Efficiency and Function. Clin. Lipidol. 2009, 4, 379–389. [Google Scholar] [CrossRef]
- Saddik, M.; Gamble, J.; Witters, L.A.; Lopaschuk, G.D. Acetyl-CoA Carboxylase Regulation of Fatty Acid Oxidation in the Heart. J. Biol. Chem. 1993, 268, 25836–25845. [Google Scholar] [CrossRef]
- Grynberg, A.; Demaison, L. Fatty Acid Oxidation in the Heart. J. Cardiovasc. Pharmacol. 1996, 28, 11–17. [Google Scholar]
- Dávila-Román, V.G.; Vedala, G.; Herrero, P.; De Las Fuentes, L.; Rogers, J.G.; Kelly, D.P.; Gropler, R.J. Altered Myocardial Fatty Acid and Glucose Metabolism in Idiopathic Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2002, 40, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Depre, C.; Vanoverschelde, J.-L.J.; Taegtmeyer, H. Glucose for the Heart. Circulation 1999, 99, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Luiken, J.J.F.P.; Coort, S.L.M.; Koonen, D.P.Y.; Van der Horst, D.J.; Bonen, A.; Zorzano, A.; Glatz, J.F.C. Regulation of Cardiac Long-Chain Fatty Acid and Glucose Uptake by Translocation of Substrate Transporters. Pflügers Arch. 2004, 448, 1–15. [Google Scholar] [CrossRef]
- Abel, E.D. Glucose Transport in the Heart. Front. Biosci. 2004, 9, 201–215. [Google Scholar] [CrossRef]
- Tran, D.H.; Wang, Z. V Glucose Metabolism in Cardiac Hypertrophy and Heart Failure. J. Am. Heart Assoc. 2019, 8, e012673. [Google Scholar] [CrossRef]
- Kolwicz, S.C., Jr.; Tian, R. Glucose Metabolism and Cardiac Hypertrophy. Cardiovasc. Res. 2011, 90, 194–201. [Google Scholar] [CrossRef]
- Chatham, J.C. Lactate—The Forgotten Fuel! J. Physiol. 2002, 542, 333. [Google Scholar] [CrossRef]
- Chuo, D.; Lin, D.; Yin, M.; Chen, Y. Genetic Variants of the MIF Gene and Susceptibility of Rectal Cancer. Pharmgenom. Pers. Med. 2021, 14, 55–60. [Google Scholar] [CrossRef]
- Kadir, A.A.; Clarke, K.; Evans, R.D. Cardiac Ketone Body Metabolism. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165739. [Google Scholar] [CrossRef]
- Yurista, S.R.; Nguyen, C.T.; Rosenzweig, A.; de Boer, R.A.; Westenbrink, B.D. Ketone Bodies for the Failing Heart: Fuels That Can Fix the Engine? Trends Endocrinol. Metab. 2021, 32, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Crawford, P.A. Multi-Dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and Control of Ketone Body Metabolism: On the Fringe of Lipid Biochemistry. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Sandermann, H., Jr.; McIntyre, J.O.; Fleischer, S. Site-Site Interaction in the Phospholipid Activation of D-Beta-Hydroxybutyrate Dehydrogenase. J. Biol. Chem. 1986, 261, 6201–6208. [Google Scholar] [CrossRef] [PubMed]
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Krüger, M.; Hoppel, C.L. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 2016, 133, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Bedi, K.C., Jr.; Snyder, N.W.; Brandimarto, J.; Aziz, M.; Mesaros, C.; Worth, A.J.; Wang, L.L.; Javaheri, A.; Blair, I.A.; Margulies, K.B. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation 2016, 133, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Drake, K.J.; Sidorov, V.Y.; McGuinness, O.P.; Wasserman, D.H.; Wikswo, J.P. Amino Acids as Metabolic Substrates during Cardiac Ischemia. Exp. Biol. Med. 2012, 237, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Burrage, L.C.; Nagamani, S.C.S.; Campeau, P.M.; Lee, B.H. Branched-Chain Amino Acid Metabolism: From Rare Mendelian Diseases to More Common Disorders. Hum. Mol. Genet. 2014, 23, R1–R8. [Google Scholar] [CrossRef] [PubMed]
- Sweatt, A.J.; Wood, M.; Suryawan, A.; Wallin, R.; Willingham, M.C.; Hutson, S.M. Branched-Chain Amino Acid Catabolism: Unique Segregation of Pathway Enzymes in Organ Systems and Peripheral Nerves. Am. J. Physiol. Metab. 2004, 286, E64–E76. [Google Scholar] [CrossRef]
- Fillmore, N.; Wagg, C.S.; Zhang, L.; Fukushima, A.; Lopaschuk, G.D. Cardiac Branched-Chain Amino Acid Oxidation Is Reduced during Insulin Resistance in the Heart. Am. J. Physiol. Metab. 2018, 315, E1046–E1052. [Google Scholar] [CrossRef]
- Hue, L.; Taegtmeyer, H. The Randle Cycle Revisited: A New Head for an Old Hat. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E578–E591. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.H.; Knuuti, J. The Adrenergic-Fatty Acid Load in Heart Failure. J. Am. Coll. Cardiol. 2009, 54, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Zhang, Y.; Ren, J. IGF-1 Deficiency Resists Cardiac Hypertrophy and Myocardial Contractile Dysfunction: Role of MicroRNA-1 and MicroRNA-133a. J. Cell. Mol. Med. 2012, 16, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Chanon, S.; Durand, C.; Vieille-Marchiset, A.; Robert, M.; Dibner, C.; Simon, C.; Lefai, E. Glucose Uptake Measurement and Response to Insulin Stimulation in in Vitro Cultured Human Primary Myotubes. JoVE 2017, 124, e55743. [Google Scholar] [CrossRef] [PubMed]
- Esteves, J.V.; Enguita, F.J.; Machado, U.F. MicroRNAs-Mediated Regulation of Skeletal Muscle GLUT4 Expression and Translocation in Insulin Resistance. J. Diabetes Res. 2017, 2017, 7267910. [Google Scholar] [CrossRef]
- Ju, J.; Xiao, D.; Shen, N.; Zhou, T.; Che, H.; Li, X.; Zhang, S.; Mokembo, J.N.; Jha, N.K.; Monayo, S.M. MiR-150 Regulates Glucose Utilization through Targeting GLUT4 in Insulin-Resistant Cardiomyocytes. Acta Biochim. Biophys. Sin. 2020, 52, 1111–1119. [Google Scholar] [CrossRef]
- Lu, H.; Buchan, R.J.; Cook, S.A. MicroRNA-223 Regulates Glut4 Expression and Cardiomyocyte Glucose Metabolism. Cardiovasc. Res. 2010, 86, 410–420. [Google Scholar] [CrossRef]
- Horie, T.; Ono, K.; Nishi, H.; Iwanaga, Y.; Nagao, K.; Kinoshita, M.; Kuwabara, Y.; Takanabe, R.; Hasegawa, K.; Kita, T.; et al. MicroRNA-133 Regulates the Expression of GLUT4 by Targeting KLF15 and Is Involved in Metabolic Control in Cardiac Myocytes. Biochem. Biophys. Res. Commun. 2009, 389, 315–320. [Google Scholar] [CrossRef]
- Prosdocimo, D.A.; John, J.E.; Zhang, L.; Efraim, E.S.; Zhang, R.; Liao, X.; Jain, M.K. KLF15 and PPARα Cooperate to Regulate Cardiomyocyte Lipid Gene Expression and Oxidation. PPAR Res. 2015, 2015, 201625. [Google Scholar] [CrossRef]
- Yang, T.; Liu, T.; Cao, C.; Xu, S. MiR-200a-5p Augments Cardiomyocyte Hypertrophy Induced by Glucose Metabolism Disorder via the Regulation of Selenoproteins. J. Cell. Physiol. 2019, 234, 4095–4103. [Google Scholar] [CrossRef] [PubMed]
- Townley-Tilson, W.H.D.; Callis, T.E.; Wang, D. MicroRNAs 1, 133, and 206: Critical Factors of Skeletal and Cardiac Muscle Development, Function, and Disease. Int. J. Biochem. Cell Biol. 2010, 42, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- Guedes, E.C.; França, G.S.; Lino, C.A.; Koyama, F.C.; Moreira, L.D.N.; Alexandre, J.G.; Barreto-Chaves, M.L.M.; Galante, P.A.F.; Diniz, G.P. MicroRNA Expression Signature Is Altered in the Cardiac Remodeling Induced by High Fat Diets. J. Cell. Physiol. 2016, 231, 1771–1783. [Google Scholar] [CrossRef]
- Lopes, M.B.; Freitas, R.C.; Hirata, M.H.; Hirata, R.; Rezende, A.A.; Silbiger, V.N.; Bortolin, R.H.; Luchessi, A.D. MRNA-MiRNA Integrative Analysis of Diabetes-Induced Cardiomyopathy in Rats. Front. Biosci. 2017, 9, 194–229. [Google Scholar] [CrossRef]
- Fan, J.-L.; Zhu, T.-T.; Xue, Z.-Y.; Ren, W.-Q.; Guo, J.-Q.; Zhao, H.-Y.; Zhang, S.-L. LncRNA-XIST Protects the Hypoxia-Induced Cardiomyocyte Injury through Regulating the MiR-125b-Hexokianse 2 Axis. In Vitro Cell. Dev. Biol. Anim. 2020, 56, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-R.; Fang, Q.-J. Inhibiting Glucose Metabolism By MiR-34a and MiR-125b Protects Against Hyperglycemia-Induced Cardiomyocyte Cell Death. Arq. Bras. Cardiol. 2021, 116, 415–422. [Google Scholar] [CrossRef]
- Fomison-Nurse, I.; Saw, E.E.L.; Gandhi, S.; Munasinghe, P.E.; Van Hout, I.; Williams, M.J.A.; Galvin, I.; Bunton, R.; Davis, P.; Cameron, V.; et al. Diabetes Induces the Activation of Pro-Ageing MiR-34a in the Heart, but Has Differential Effects on Cardiomyocytes and Cardiac Progenitor Cells. Cell Death Differ. 2018, 25, 1336–1349. [Google Scholar] [CrossRef]
- Zhu, H.; Shyh-Chang, N.; Segrè, A.V.; Shinoda, G.; Shah, S.P.; Einhorn, W.S.; Takeuchi, A.; Engreitz, J.M.; Hagan, J.P.; Kharas, M.G.; et al. The Lin28/Let-7 Axis Regulates Glucose Metabolism. Cell 2011, 147, 81–94. [Google Scholar] [CrossRef]
- Bartman, C.M.; Oyama, Y.; Brodsky, K.; Khailova, L.; Walker, L.; Koeppen, M.; Eckle, T. Intense Light-Elicited Upregulation of MiR-21 Facilitates Glycolysis and Cardioprotection through Per2-Dependent Mechanisms. PLoS ONE 2017, 12, e0176243. [Google Scholar] [CrossRef]
- Chen, C.-H.; Hsu, S.-Y.; Chiu, C.-C.; Leu, S. MicroRNA-21 Mediates the Protective Effect of Cardiomyocyte-Derived Conditioned Medium on Ameliorating Myocardial Infarction in Rats. Cells 2019, 8, 935. [Google Scholar] [CrossRef]
- Ono, K. MicroRNA Links Obesity and Impaired Glucose Metabolism. Cell Res. 2011, 21, 864–866. [Google Scholar] [CrossRef]
- Kornfeld, J.-W.; Baitzel, C.; Könner, A.C.; Nicholls, H.T.; Vogt, M.C.; Herrmanns, K.; Scheja, L.; Haumaitre, C.; Wolf, A.M.; Knippschild, U.; et al. Obesity-Induced Overexpression of MiR-802 Impairs Glucose Metabolism through Silencing of Hnf1b. Nature 2013, 494, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Li, J.; Fu, Y.; Zheng, Y.; Ma, M.; Wang, C. Hypertrophic Adipocyte-Derived Exosomal MiR-802-5p Contributes to Insulin Resistance in Cardiac Myocytes Through Targeting HSP60. Obesity 2020, 28, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Grueter, C.E.; van Rooij, E.; Johnson, B.A.; DeLeon, S.M.; Sutherland, L.B.; Qi, X.; Gautron, L.; Elmquist, J.K.; Bassel-Duby, R.; Olson, E.N. A Cardiac MicroRNA Governs Systemic Energy Homeostasis by Regulation of MED13. Cell 2012, 149, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Trajkovski, M.; Hausser, J.; Soutschek, J.; Bhat, B.; Akin, A.; Zavolan, M.; Heim, M.H.; Stoffel, M. MicroRNAs 103 and 107 Regulate Insulin Sensitivity. Nature 2011, 474, 649–653. [Google Scholar] [CrossRef]
- Rech, M.; Kuhn, A.R.; Lumens, J.; Carai, P.; van Leeuwen, R.; Verhesen, W.; Verjans, R.; Lecomte, J.; Liu, Y.; Luiken, J.J.F.P.; et al. AntagomiR-103 and -107 Treatment Affects Cardiac Function and Metabolism. Mol. Ther. Nucleic Acids 2019, 14, 424–437. [Google Scholar] [CrossRef]
- Singh, A.; Happel, C.; Manna, S.K.; Acquaah-Mensah, G.; Carrerero, J.; Kumar, S.; Nasipuri, P.; Krausz, K.W.; Wakabayashi, N.; Dewi, R.; et al. Transcription Factor NRF2 Regulates MiR-1 and MiR-206 to Drive Tumorigenesis. J. Clin. Investig. 2013, 123, 2921–2934. [Google Scholar] [CrossRef]
- Yang, W.-M.; Jeong, H.-J.; Park, S.-Y.; Lee, W. Saturated Fatty Acid-Induced MiR-195 Impairs Insulin Signaling and Glycogen Metabolism in HepG2 Cells. FEBS Lett. 2014, 588, 3939–3946. [Google Scholar] [CrossRef]
- Li, H.; Fan, J.; Zhao, Y.; Zhang, X.; Dai, B.; Zhan, J.; Yin, Z.; Nie, X.; Fu, X.-D.; Chen, C.; et al. Nuclear MiR-320 Mediates Diabetes-Induced Cardiac Dysfunction by Activating Transcription of Fatty Acid Metabolic Genes to Cause Lipotoxicity in the Heart. Circ. Res. 2019, 125, 1106–1120. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, R.; Liao, X.; Castillero, E.; Kennel, P.J.; Brunjes, D.L.; Franz, M.; Möbius-Winkler, S.; Drosatos, K.; George, I.; et al. MicroRNA-195 Regulates Metabolism in Failing Myocardium Via Alterations in Sirtuin 3 Expression and Mitochondrial Protein Acetylation. Circulation 2018, 137, 2052–2067. [Google Scholar] [CrossRef]
- Song, R.; Dasgupta, C.; Mulder, C.; Zhang, L. MicroRNA-210 Controls Mitochondrial Metabolism and Protects Heart Function in Myocardial Infarction. Circulation 2022, 145, 1140–1153. [Google Scholar] [CrossRef]
- Tanaka, H.; Sasayama, T.; Tanaka, K.; Nakamizo, S.; Nishihara, M.; Mizukawa, K.; Kohta, M.; Koyama, J.; Miyake, S.; Taniguchi, M.; et al. MicroRNA-183 Upregulates HIF-1α by Targeting Isocitrate Dehydrogenase 2 (IDH2) in Glioma Cells. J. Neurooncol. 2013, 111, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Tomasetti, M.; Amati, M.; Santarelli, L.; Neuzil, J. MicroRNA in Metabolic Re-Programming and Their Role in Tumorigenesis. Int. J. Mol. Sci. 2016, 17, 754. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Wang, K.; Li, N.; Murtaza, I.; Xiao, J.-Y.; Fan, Y.-Y.; Liu, C.-Y.; Li, W.-H.; Cheng, Z.; Li, P. MiR-761 Regulates the Mitochondrial Network by Targeting Mitochondrial Fission Factor. Free Radic. Biol. Med. 2013, 65, 371–379. [Google Scholar] [CrossRef]
- Li, Z.; Song, Y.; Liu, L.; Hou, N.; An, X.; Zhan, D.; Li, Y.; Zhou, L.; Li, P.; Yu, L.; et al. MiR-199a Impairs Autophagy and Induces Cardiac Hypertrophy through MTOR Activation. Cell Death Differ. 2017, 24, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, J.; Eyers, F.; Xiang, Y.; Herbert, C.; Tay, H.L.; Foster, P.S.; Yang, M. Identification of the MicroRNA Networks Contributing to Macrophage Differentiation and Function. Oncotarget 2016, 7, 28806–28820. [Google Scholar] [CrossRef] [PubMed]
- Choong, M.L.; Yang, H.H.; McNiece, I. MicroRNA Expression Profiling during Human Cord Blood-Derived CD34 Cell Erythropoiesis. Exp. Hematol. 2007, 35, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Varrone, F.; Gargano, B.; Carullo, P.; Di Silvestre, D.; De Palma, A.; Grasso, L.; Di Somma, C.; Mauri, P.; Benazzi, L.; Franzone, A.; et al. The Circulating Level of FABP3 Is an Indirect Biomarker of MicroRNA-1. J. Am. Coll. Cardiol. 2013, 61, 88–95. [Google Scholar] [CrossRef]
- Xu, L.; Chen, W.; Ma, M.; Chen, A.; Tang, C.; Zhang, C.; Cai, L. Microarray Profiling Analysis Identifies the Mechanism of MiR-200b-3p/MRNA-CD36 Affecting Diabetic Cardiomyopathy via Peroxisome Proliferator Activated Receptor-γ Signaling Pathway. J. Cell. Biochem. 2019, 120, 5193–5206. [Google Scholar] [CrossRef]
- Wan, J.; Lin, S.; Yu, Z.; Song, Z.; Lin, X.; Xu, R.; Du, S. Protective Effects of MicroRNA-200b-3p Encapsulated by Mesenchymal Stem Cells-Secreted Extracellular Vesicles in Myocardial Infarction Via Regulating BCL2L11. J. Am. Heart Assoc. 2022, 11, e024330. [Google Scholar] [CrossRef]
- Rottiers, V.; Najafi-Shoushtari, S.H.; Kristo, F.; Gurumurthy, S.; Zhong, L.; Li, Y.; Cohen, D.E.; Gerszten, R.E.; Bardeesy, N.; Mostoslavsky, R. MicroRNAs in Metabolism and Metabolic Diseases. In Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2011; Volume 76, pp. 225–233. [Google Scholar]
- Novák, J.; Bienertová-Vašků, J.; Kára, T.; Novák, M. MicroRNAs Involved in the Lipid Metabolism and Their Possible Implications for Atherosclerosis Development and Treatment. Mediat. Inflamm. 2014, 2014, 275867. [Google Scholar] [CrossRef]
- Legchenko, E.; Chouvarine, P.; Borchert, P.; Fernandez-Gonzalez, A.; Snay, E.; Meier, M.; Maegel, L.; Mitsialis, S.A.; Rog-Zielinska, E.A.; Kourembanas, S.; et al. PPARγ Agonist Pioglitazone Reverses Pulmonary Hypertension and Prevents Right Heart Failure via Fatty Acid Oxidation. Sci. Transl. Med. 2018, 10, eaao0303. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, D.; Drosatos, K.; Hiyama, Y.; Goldberg, I.J.; Zannis, V.I. MicroRNA-370 Controls the Expression of MicroRNA-122 and Cpt1alpha and Affects Lipid Metabolism. J. Lipid Res. 2010, 51, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Soni, M.S.; Rabaglia, M.E.; Bhatnagar, S.; Shang, J.; Ilkayeva, O.; Mynatt, R.; Zhou, Y.-P.; Schadt, E.E.; Thornberry, N.A.; Muoio, D.M.; et al. Downregulation of Carnitine Acyl-Carnitine Translocase by MiRNAs 132 and 212 Amplifies Glucose-Stimulated Insulin Secretion. Diabetes 2014, 63, 3805–3814. [Google Scholar] [CrossRef] [PubMed]
- Soh, J.; Iqbal, J.; Queiroz, J.; Fernandez-Hernando, C.; Hussain, M.M. MicroRNA-30c Reduces Hyperlipidemia and Atherosclerosis in Mice by Decreasing Lipid Synthesis and Lipoprotein Secretion. Nat. Med. 2013, 19, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhao, Y.; He, M.; Li, H.; Fan, J.; Nie, X.; Yan, M.; Chen, C.; Wang, D.W. MiR-30c/PGC-1β Protects against Diabetic Cardiomyopathy via PPARα. Cardiovasc. Diabetol. 2019, 18, 7. [Google Scholar] [CrossRef]
- Aoi, W.; Naito, Y.; Mizushima, K.; Takanami, Y.; Kawai, Y.; Ichikawa, H.; Yoshikawa, T. The MicroRNA MiR-696 Regulates PGC-1{alpha} in Mouse Skeletal Muscle in Response to Physical Activity. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E799–E806. [Google Scholar] [CrossRef]
- Yang, Z.; Cappello, T.; Wang, L. Emerging Role of MicroRNAs in Lipid Metabolism. Acta Pharm. Sin. B 2015, 5, 145–150. [Google Scholar] [CrossRef]
- Ramirez, C.M.; Dávalos, A.; Goedeke, L.; Salerno, A.G.; Warrier, N.; Cirera-Salinas, D.; Suárez, Y.; Fernández-Hernando, C. MicroRNA-758 Regulates Cholesterol Efflux through Posttranscriptional Repression of ATP-Binding Cassette Transporter A1. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2707–2714. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lee, D.S.; Choong, O.K.; Chang, S.-K.; Hsu, T.; Nicholson, M.W.; Liu, L.-W.; Lin, P.-J.; Ruan, S.-C.; Lin, S.-W.; et al. Cardiac-Specific MicroRNA-125b Deficiency Induces Perinatal Death and Cardiac Hypertrophy. Sci. Rep. 2021, 11, 2377. [Google Scholar] [CrossRef]
- Ferland-McCollough, D.; Fernandez-Twinn, D.S.; Cannell, I.G.; David, H.; Warner, M.; Vaag, A.A.; Bork-Jensen, J.; Brøns, C.; Gant, T.W.; Willis, A.E.; et al. Programming of Adipose Tissue MiR-483-3p and GDF-3 Expression by Maternal Diet in Type 2 Diabetes. Cell Death Differ. 2012, 19, 1003–1012. [Google Scholar] [CrossRef]
- Nishi, H.; Ono, K.; Iwanaga, Y.; Horie, T.; Nagao, K.; Takemura, G.; Kinoshita, M.; Kuwabara, Y.; Mori, R.T.; Hasegawa, K.; et al. MicroRNA-15b Modulates Cellular ATP Levels and Degenerates Mitochondria via Arl2 in Neonatal Rat Cardiac Myocytes. J. Biol. Chem. 2010, 285, 4920–4930. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Zhang, Y.-Y.; Hemann, C.; Mahoney, C.E.; Zweier, J.L.; Loscalzo, J. MicroRNA-210 Controls Mitochondrial Metabolism during Hypoxia by Repressing the Iron-Sulfur Cluster Assembly Proteins ISCU1/2. Cell Metab. 2009, 10, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Baseler, W.A.; Thapa, D.; Jagannathan, R.; Dabkowski, E.R.; Croston, T.L.; Hollander, J.M. MiR-141 as a Regulator of the Mitochondrial Phosphate Carrier (Slc25a3) in the Type 1 Diabetic Heart. Am. J. Physiol. Cell Physiol. 2012, 303, C1244–C1251. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, Q.A.; Pinti, M.V.; Durr, A.J.; Waris, S.; Shepherd, D.L.; Hollander, J.M. Regulating MicroRNA Expression: At the Heart of Diabetes Mellitus and the Mitochondrion. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H293–H310. [Google Scholar] [CrossRef]
- Demkes, C.J.; van Rooij, E. MicroRNA-146a as a Regulator of Cardiac Energy Metabolism. Circulation 2017, 136, 762–764. [Google Scholar] [CrossRef]
- Das, S.; Ferlito, M.; Kent, O.A.; Fox-Talbot, K.; Wang, R.; Liu, D.; Raghavachari, N.; Yang, Y.; Wheelan, S.J.; Murphy, E.; et al. Nuclear MiRNA Regulates the Mitochondrial Genome in the Heart. Circ. Res. 2012, 110, 1596–1603. [Google Scholar] [CrossRef]
- Das, S.; Kohr, M.; Dunkerly-Eyring, B.; Lee, D.I.; Bedja, D.; Kent, O.A.; Leung, A.K.L.; Henao-Mejia, J.; Flavell, R.A.; Steenbergen, C. Divergent Effects of MiR-181 Family Members on Myocardial Function Through Protective Cytosolic and Detrimental Mitochondrial MicroRNA Targets. J. Am. Heart Assoc. 2017, 6, e004694. [Google Scholar] [CrossRef]
- Chen, B.; Li, H.; Zeng, X.; Yang, P.; Liu, X.; Zhao, X.; Liang, S. Roles of MicroRNA on Cancer Cell Metabolism. J. Transl. Med. 2012, 10, 228. [Google Scholar] [CrossRef]
- Mersey, B.D.; Jin, P.; Danner, D.J. Human MicroRNA (MiR29b) Expression Controls the Amount of Branched Chain Alpha-Ketoacid Dehydrogenase Complex in a Cell. Hum. Mol. Genet. 2005, 14, 3371–3377. [Google Scholar] [CrossRef]
- Chang, J.; Nicolas, E.; Marks, D.; Sander, C.; Lerro, A.; Buendia, M.A.; Xu, C.; Mason, W.S.; Moloshok, T.; Bort, R.; et al. MiR-122, a Mammalian Liver-Specific MicroRNA, Is Processed from Hcr MRNA and May Downregulate the High Affinity Cationic Amino Acid Transporter CAT-1. RNA Biol. 2004, 1, 106–113. [Google Scholar] [CrossRef]
- Liu, F.; Li, Y.; Liu, G. MicroRNA-200c Exacerbates the Ischemia/Reperfusion Injury of Heart through Targeting the Glutaminase (GLS)-Mediated Glutamine Metabolism. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 3282–3289. [Google Scholar] [PubMed]
- Uemura, T.; Stringer, D.E.; Blohm-Mangone, K.A.; Gerner, E.W. Polyamine Transport Is Mediated by Both Endocytic and Solute Carrier Transport Mechanisms in the Gastrointestinal Tract. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G517–G522. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, N.N.; Van den Haute, C.; Vanhoutte, R.; Sannerud, R.; Azfar, M.; Mayer, R.; Cortés Calabuig, Á.; Swinnen, J.V.; Agostinis, P.; Baekelandt, V.; et al. ATP13A3 Is a Major Component of the Enigmatic Mammalian Polyamine Transport System. J. Biol. Chem. 2021, 296, 100182. [Google Scholar] [CrossRef]
- Tomasi, M.L.; Cossu, C.; Spissu, Y.; Floris, A.; Ryoo, M.; Iglesias-Ara, A.; Wang, Q.; Pandol, S.J.; Bhowmick, N.A.; Seki, E.; et al. S-Adenosylmethionine and Methylthioadenosine Inhibit Cancer Metastasis by Targeting MicroRNA 34a/b-Methionine Adenosyltransferase 2A/2B Axis. Oncotarget 2017, 8, 78851–78869. [Google Scholar] [CrossRef]
- Yang, H.; Cho, M.E.; Li, T.W.H.; Peng, H.; Ko, K.S.; Mato, J.M.; Lu, S.C. MicroRNAs Regulate Methionine Adenosyltransferase 1A Expression in Hepatocellular Carcinoma. J. Clin. Investig. 2013, 123, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Greco, S.; Fasanaro, P.; Castelvecchio, S.; D’Alessandra, Y.; Arcelli, D.; Di Donato, M.; Malavazos, A.; Capogrossi, M.C.; Menicanti, L.; Martelli, F. MicroRNA Dysregulation in Diabetic Ischemic Heart Failure Patients. Diabetes 2012, 61, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Kambis, T.N.; Tofilau, H.M.N.; Gawargi, F.I.; Chandra, S.; Mishra, P.K. Regulating Polyamine Metabolism by MiRNAs in Diabetic Cardiomyopathy. Curr. Diab. Rep. 2021, 21, 52. [Google Scholar] [CrossRef] [PubMed]
- Kirk, E.P.; Klein, S. Pathogenesis and Pathophysiology of the Cardiometabolic Syndrome. J. Clin. Hypertens. 2009, 11, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Ndisang, J.F.; Rastogi, S. Cardiometabolic Diseases and Related Complications: Current Status and Future Perspective. BioMed Res. Int. 2013, 2013, 467682. [Google Scholar] [CrossRef]
- Gerin, I.; Clerbaux, L.-A.; Haumont, O.; Lanthier, N.; Das, A.K.; Burant, C.F.; Leclercq, I.A.; MacDougald, O.A.; Bommer, G.T. Expression of MiR-33 from an SREBP2 Intron Inhibits Cholesterol Export and Fatty Acid Oxidation. J. Biol. Chem. 2010, 285, 33652–33661. [Google Scholar] [CrossRef]
- Rayner, K.J.; Sheedy, F.J.; Esau, C.C.; Hussain, F.N.; Temel, R.E.; Parathath, S.; Van Gils, J.M.; Rayner, A.J.; Chang, A.N.; Suarez, Y. Antagonism of MiR-33 in Mice Promotes Reverse Cholesterol Transport and Regression of Atherosclerosis. J. Clin. Investig. 2011, 121, 2921–2931. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Goedeke, L.; Smibert, P.; Ramírez, C.M.; Warrier, N.P.; Andreo, U.; Cirera-Salinas, D.; Rayner, K.; Suresh, U.; Pastor-Pareja, J.C. MiR-33a/b Contribute to the Regulation of Fatty Acid Metabolism and Insulin Signaling. Proc. Natl. Acad. Sci. USA 2011, 108, 9232–9237. [Google Scholar] [CrossRef] [PubMed]
- Elmen, J.; Lindow, M.; Silahtaroglu, A.; Bak, M.; Christensen, M.; Lind-Thomsen, A.; Hedtjärn, M.; Hansen, J.B.; Hansen, H.F.; Straarup, E.M. Antagonism of MicroRNA-122 in Mice by Systemically Administered LNA-AntimiR Leads to up-Regulation of a Large Set of Predicted Target MRNAs in the Liver. Nucleic Acids Res. 2008, 36, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Elmén, J.; Lindow, M.; Schütz, S.; Lawrence, M.; Petri, A.; Obad, S.; Lindholm, M.; Hedtjärn, M.; Hansen, H.F.; Berger, U. LNA-Mediated MicroRNA Silencing in Non-Human Primates. Nature 2008, 452, 896–899. [Google Scholar] [CrossRef]
- Goedeke, L.; Rotllan, N.; Canfrán-Duque, A.; Aranda, J.F.; Ramírez, C.M.; Araldi, E.; Lin, C.-S.; Anderson, N.N.; Wagschal, A.; De Cabo, R. MicroRNA-148a Regulates LDL Receptor and ABCA1 Expression to Control Circulating Lipoprotein Levels. Nat. Med. 2015, 21, 1280–1289. [Google Scholar] [CrossRef]
- Wagschal, A.; Najafi-Shoushtari, S.H.; Wang, L.; Goedeke, L.; Sinha, S.; Delemos, A.S.; Black, J.C.; Ramírez, C.M.; Li, Y.; Tewhey, R. Genome-Wide Identification of MicroRNAs Regulating Cholesterol and Triglyceride Homeostasis. Nat. Med. 2015, 21, 1290–1297. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, J.; Gong, Y.; Chen, M.; Chen, J.; Zhao, W.; Tan, S. Hsa-MiR-140-5p down-Regulates LDL Receptor and Attenuates LDL-C Uptake in Human Hepatocytes. Atherosclerosis 2020, 297, 111–119. [Google Scholar] [CrossRef]
- Vickers, K.C.; Shoucri, B.M.; Levin, M.G.; Wu, H.; Pearson, D.S.; Osei-Hwedieh, D.; Collins, F.S.; Remaley, A.T.; Sethupathy, P. MicroRNA-27b Is a Regulatory Hub in Lipid Metabolism and Is Altered in Dyslipidemia. Hepatology 2013, 57, 533–542. [Google Scholar] [CrossRef]
- Kurtz, C.L.; Peck, B.C.E.; Fannin, E.E.; Beysen, C.; Miao, J.; Landstreet, S.R.; Ding, S.; Turaga, V.; Lund, P.K.; Turner, S. MicroRNA-29 Fine-Tunes the Expression of Key FOXA2-Activated Lipid Metabolism Genes and Is Dysregulated in Animal Models of Insulin Resistance and Diabetes. Diabetes 2014, 63, 3141–3148. [Google Scholar] [CrossRef]
- Fu, X.; Dong, B.; Tian, Y.; Lefebvre, P.; Meng, Z.; Wang, X.; Pattou, F.; Han, W.; Wang, X.; Lou, F. MicroRNA-26a Regulates Insulin Sensitivity and Metabolism of Glucose and Lipids. J. Clin. Investig. 2015, 125, 2497–2509. [Google Scholar] [CrossRef]
- Shantikumar, S.; Caporali, A.; Emanueli, C. Role of MicroRNAs in Diabetes and Its Cardiovascular Complications. Cardiovasc. Res. 2012, 93, 583–593. [Google Scholar] [CrossRef] [PubMed]
- López-Pastor, A.R.; Infante-Menéndez, J.; Escribano, Ó.; Gómez-Hernández, A. MiRNA Dysregulation in the Development of Non-Alcoholic Fatty Liver Disease and the Related Disorders Type 2 Diabetes Mellitus and Cardiovascular Disease. Front. Med. 2020, 7, 527059. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Huang, F.; Liu, X.; Xiao, X.; Yang, M.; Hu, G.; Liu, H.; Liao, L. MiR-21 Regulates Triglyceride and Cholesterol Metabolism in Non-Alcoholic Fatty Liver Disease by Targeting HMGCR. Int. J. Mol. Med. 2015, 35, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-C.; Hsu, S.-D.; Hsu, C.-S.; Lai, T.-C.; Chen, S.-J.; Shen, R.; Huang, Y.; Chen, H.-C.; Lee, C.-H.; Tsai, T.-F. MicroRNA-122 Plays a Critical Role in Liver Homeostasis and Hepatocarcinogenesis. J. Clin. Investig. 2012, 122, 2884–2897. [Google Scholar] [CrossRef]
- Van Rooij, E.; Marshall, W.S.; Olson, E.N. Toward Microrna–Based Therapeutics for Heart Disease: The Sense in Antisense. Circ. Res. 2008, 103, 919–928. [Google Scholar] [CrossRef]
- Krützfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of MicroRNAs In Vivo with ‘Antagomirs’. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef]
- Xiao, J.; Yang, B.; Lin, H.; Lu, Y.; Luo, X.; Wang, Z. Retracted: Novel Approaches for Gene-specific Interference via Manipulating Actions of MicroRNAs: Examination on the Pacemaker Channel Genes HCN2 and HCN4. J. Cell. Physiol. 2007, 212, 285–292. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; Rebhan, M.A.E.; Crivelli, S.E.M.; Denzler, R.; Stoffel, M.; Hall, J. Miravirsen (SPC3649) Can Inhibit the Biogenesis of MiR-122. Nucleic Acids Res. 2014, 42, 609–621. [Google Scholar] [CrossRef]
- Hydbring, P.; Badalian-Very, G. Clinical Applications of MicroRNAs. F1000Research 2013, 2, 136. [Google Scholar] [CrossRef]
- Natarelli, L.; Geißler, C.; Csaba, G.; Wei, Y.; Zhu, M.; di Francesco, A.; Hartmann, P.; Zimmer, R.; Schober, A. MiR-103 Promotes Endothelial Maladaptation by Targeting LncWDR59. Nat. Commun. 2018, 9, 2645. [Google Scholar] [CrossRef]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for MicroRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef] [PubMed]
- Hamburg, N.M.; Leeper, N.J. Therapeutic Potential of Modulating MicroRNA in Peripheral Artery Disease. Curr. Vasc. Pharmacol. 2015, 13, 316–323. [Google Scholar] [CrossRef] [PubMed]
- van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L. Safety and Activity of MicroRNA-Loaded Minicells in Patients with Recurrent Malignant Pleural Mesothelioma: A First-in-Man, Phase 1, Open-Label, Dose-Escalation Study. Lancet Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Gallant-Behm, C.L.; Piper, J.; Lynch, J.M.; Seto, A.G.; Hong, S.J.; Mustoe, T.A.; Maari, C.; Pestano, L.A.; Dalby, C.M.; Jackson, A.L. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. J. Investig. Dermatol. 2019, 139, 1073–1081. [Google Scholar] [CrossRef]
- Valencia, T.; Schairer, A.; Kersjes, K.; Li, J.; Davis, S.; Flaten, A.; Patel, V.; Lee, E. Preclinical Evaluation of RGLS4326 for the Treatment of Autosomal Dominant Polycystic Kidney Diseases. Nephrol. Dial. Transplant. 2018, 33, i623. [Google Scholar] [CrossRef]
- Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Bär, C.; Borchert, T.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D. CDR132L Improves Systolic and Diastolic Function in a Large Animal Model of Chronic Heart Failure. Eur. Heart J. 2021, 42, 192–201. [Google Scholar] [CrossRef]
- Sassi, Y.; Avramopoulos, P.; Ramanujam, D.; Grüter, L.; Werfel, S.; Giosele, S.; Brunner, A.-D.; Esfandyari, D.; Papadopoulou, A.S.; De Strooper, B. Cardiac Myocyte MiR-29 Promotes Pathological Remodeling of the Heart by Activating Wnt Signaling. Nat. Commun. 2017, 8, 1614. [Google Scholar] [CrossRef]
- Carrer, M.; Liu, N.; Grueter, C.E.; Williams, A.H.; Frisard, M.I.; Hulver, M.W.; Bassel-Duby, R.; Olson, E.N. Control of Mitochondrial Metabolism and Systemic Energy Homeostasis by MicroRNAs 378 and 378. Proc. Natl. Acad. Sci. USA 2012, 109, 15330–15335. [Google Scholar] [CrossRef]
- Deng, Y.; Campbell, F.; Han, K.; Theodore, D.; Deeg, M.; Huang, M.; Hamatake, R.; Lahiri, S.; Chen, S.; Horvath, G. Randomized Clinical Trials towards a Single-visit Cure for Chronic Hepatitis C: Oral GSK2878175 and Injectable RG-101 in Chronic Hepatitis C Patients and Long-acting Injectable GSK2878175 in Healthy Participants. J. Viral Hepat. 2020, 27, 699–708. [Google Scholar] [CrossRef]
- Parisi, C.; Arisi, I.; D’Ambrosi, N.; Storti, A.E.; Brandi, R.; D’Onofrio, M.; Volonte, C. Dysregulated MicroRNAs in Amyotrophic Lateral Sclerosis Microglia Modulate Genes Linked to Neuroinflammation. Cell Death Dis. 2013, 4, e959. [Google Scholar] [CrossRef]
- Bonauer, A.; Carmona, G.; Iwasaki, M.; Mione, M.; Koyanagi, M.; Fischer, A.; Burchfield, J.; Fox, H.; Doebele, C.; Ohtani, K. MicroRNA-92a Controls Angiogenesis and Functional Recovery of Ischemic Tissues in Mice. Science 2009, 324, 1710–1713. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.L.; Hullinger, T.G.; Semus, H.M.; Dickinson, B.A.; Seto, A.G.; Lynch, J.M.; Stack, C.; Latimer, P.A.; Olson, E.N.; Van Rooij, E. Therapeutic Inhibition of MiR-208a Improves Cardiac Function and Survival during Heart Failure. Circulation 2011, 124, 1537–1547. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Seto, A.G.; Beatty, X.; Hermreck, M.; Gilles, M.-E.; Stroopinsky, D.; Pinter-Brown, L.C.; Pestano, L.; Marchese, C.; Avigan, D. Cobomarsen, an Oligonucleotide Inhibitor of MiR-155, Slows DLBCL Tumor Cell Growth In Vitro and In VivoCobomarsen, a MiRNA-Based Compound for DLBCL Treatment. Clin. Cancer Res. 2021, 27, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhao, S.-P.; Zhao, Y.-H. MicroRNA-143/-145 in Cardiovascular Diseases. BioMed Res. Int. 2015, 2015, 531740. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Kao, S.C.; Pavlakis, N.; Brahmbhatt, H.; MacDiarmid, J.; Clarke, S.; Boyer, M.; van Zandwijk, N. Clinical Development of TargomiRs, a MiRNA Mimic-Based Treatment for Patients with Recurrent Thoracic Cancer. Epigenomics 2016, 8, 1079–1085. [Google Scholar] [CrossRef]
- Kashtan, C.E.; Gross, O. Clinical Practice Recommendations for the Diagnosis and Management of Alport Syndrome in Children, Adolescents, and Young Adults—An Update for 2020. Pediatr. Nephrol. 2021, 36, 711–719. [Google Scholar] [CrossRef]
- Najafi-Shoushtari, S.H. MicroRNAs in Cardiometabolic Disease. Curr. Atheroscler. Rep. 2011, 13, 202–207. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, H.; Xu, H.; Tan, Y.; Zhang, C.; Zeng, Q.; Liu, L.; Qu, S. Targeting the MicroRNAs in Exosome: A Potential Therapeutic Strategy for Alleviation of Diabetes-Related Cardiovascular Complication. Pharmacol. Res. 2021, 173, 105868. [Google Scholar] [CrossRef]
- Van Rooij, E.; Olson, E.N. MicroRNA Therapeutics for Cardiovascular Disease: Opportunities and Obstacles. Nat. Rev. Drug Discov. 2012, 11, 860–872. [Google Scholar] [CrossRef]
| Cardiometabolic Complications/Diseases | miRNA/Host Gene | ∆ Expression | Target Transcript | Mechanism of Dysregulated Cardiac Metabolism | Types of Model Studied and Pathological Features | Reference |
|---|---|---|---|---|---|---|
| Atherosclerosis (lipid dyshomeostasis- mediated) | miR-33a/ SREBP2, miR-33b/SREBP1 | Upregulation | CROT, CPTA1α, HADHB | Downregulates fatty acid β-oxidation enzymes | HepG2 cells, THP1 cells, Y1 cells | [133] |
| ABCA1 | Downregulates the cholesterol transporter ABC transporter Decreases the plasma high-density lipoprotein (HDL) levels | Anti-miR33-treated mice/ Increases atherosclerotic plaques, inflammatory marker gene expression | [134] | |||
| CROT, CPTA1α, HADHB, AMPKα, IRS2, SIRT6 | Reduces fatty acid oxidation and insulin signaling | Huh7 cells/ Increased fatty acid accumulation | [135] | |||
| miR-370, miR-122 | Upregulation | CPTA1α, DGAT2 | Downregulates CPTA1α expression Decreases the rate of β-oxidation Affects FAS and ACC-1 expression | HepG2 cells | [105] | |
| miR-122 | Upregulation | CPTA1α | Downregulates the enzymes of lipid metabolism including HMGCS, HMGCR, and 7-dehydrocholesterol reductase (DHCR7) | Locked nucleic acid (LNA)- antimiR-122 treated African green monkey and mice | [136,137] | |
| miR-30 | Downregulation | LPGAT1, MTP | Reduces lipid synthesis, triglyceride secretion | APOE knockout mice/Decreases atherosclerotic plaques | [107] | |
| Hyperlipidemia | miR-148a/ R3HDM1 | Upregulation | ABCA1 | Downregulates the expression of nuclear receptors (PPARs, PGC-1α), LDLR, AMPK and ABCA1 essential for fatty acid metabolism by SREBP1 mediated pathway Increases circulating level of LDL-C | miR-148a-deficient mice, APOE- knockout mice | [138] |
| miR-128 | Upregulation | LDLR | Represses the sirtuin 1 (SIRT1) gene expression Increases circulating level of LDL-C | miR-128-deficient mice | [139] | |
| miR-30 | Downregulation | LPGAT1, MTP | Inhibits microsomal transfer protein (MTP) Decreases lipid synthesis and lipoprotein secretion Decreases serum level of triglycerides and LDL-C | Anti-miR-30c treated mice | [107] | |
| miR-140-5p | Upregulation | LDLR | Deceases LDLR expression Reduces LDL-C uptake | Anti-miR-104-5p (Simvastatin) transfected HepG2 cells | [140] | |
| miR-27a miR-27b | Upregulation | ABCA1 PPARγ | Reduces lipid metabolism by downregulating the lipid metabolic genes (Fatty acid synthase, SREBP-1, SREBP-2, PPARα, PPARγ) and ApoA1, ApoB100 and ApoE3 | 293T cells | [103] | |
| miR-378/PGC-1β | Upregulation | CRAT, MED13, ERRγ, GABPA, IGF1R, ABCG1 | Counterbalances the metabolic functions of PGC-1β | miR-378 knockout mice | [110] | |
| Dyslipidemia | miR-27b | Upregulation | GPAM, ANGPTL3 | Downregulates lipid metabolism by repressing lipid metabolic genes such as PPARγ, GPAM, and ANGPTL3 Increases levels of plasma lipid | APOE knockout mice | [141] |
| Type II diabetes mellitus and obesity | miR-29 | Upregulation | FOXA2 | Inhibits the activation of lipid metabolism genes including PPARGC1A, HMGCS2, and ABHD5 Inhibits the lipid metabolism | Zucker diabetic fatty (fa/fa) rats | [142] |
| miR-26a | Downregulation | Acc1, Acc2, Acly, Dgat2, Fasn, Lipc, Srebf1 | Improves insulin sensitivity Decreases glucose production and fatty acid synthesis Protects from obesity-induced metabolic complications | miR-26a transgenic obese mice | [143] | |
| miR-375 | Upregulation | Insulin, Mtpn | Downregulates glucose-stimulated insulin secretion Downregulates phosphoinositide-dependent protein kinase-1 Reduces the activation of AKT and GSK3 | miR-375 knockout mice, ob/ob mice (obese, insulin resistant, T2DM) | [144] | |
| Let-7 | Upregulation | INSR, IRS2 | Represses the gene expression of INSR, IRS2 Impairs global glucose homeostasis Blocks insulin signaling | Diet-induced obese mice | [145] | |
| miR-33/SREBP2 | Upregulation | ABCA1 | Translational repression of ABCA1 to reduce cellular cholesterol export Downregulates fatty acid β-oxidation enzymes | Drosophila melanogaster | [133] | |
| Non-alcoholic fatty liver disease (NAFLD) | miR-21 | Upregulation | HMGCR | Downregulates the lipid and triglyceride metabolism Increases serum level of mRNA and protein HMGCR | Palmitic acid and oleic acid treated HepG2 | [146] |
| miR-122 | Upregulation | FASN, ACC, SCD1, SREBP | Enhances the serum levels of lipid and triglyceride Decreases fatty acid oxidation | miR-122 knockout mice | [147] |
| Therapeutic Drug | Target MiRNA | Investigating Diseases | Stage of Trials | References |
|---|---|---|---|---|
| Miravirsen (SPC3649) | miR-122 | Hepatitis C virus infection | Phase II clinical trials | [151] |
| MGN-1374 | miR-15 and miR-195 | Post-myocardial infraction | Preclinical stage | [152] |
| AZD4076 | miR-103/107-3p | Type II diabetes with non-alcoholic fatty liver disease Type II diabetes with non-alcoholic steatohepatitis | Phase I/IIa | [153] |
| MRX34 | miR-34a | Different types of cancer | Phase I clinical trials | [154] |
| MGN-6114 | miR-92 | Peripheral arterial disease | Preclinical stage | [155] |
| MesomiR-1 | miR-16 | Lung cancer, Mesothelioma | Phase I clinical trial | [156] |
| Remlarsan (MRG-201) | miR-29 | Fibrosis | Phase I clinical trial | [157] |
| RGLS4326 | miR-17-5p | Polycystic kidney disease (PKD) | Phase I clinical trials | [158] |
| CDR132L | miR-132-3p | Stable heart failure | Phase I clinical trial | [159] |
| MGN-4220 | miR-29 | Cardiac fibrosis | Preclinical stage | [160] |
| MGN-5804 | miR-378 | Cardiometabolic disease | Preclinical stage | [161] |
| RG-101 | miR-122 | Virus infection | Phase IB clinical trials | [162] |
| MRG-107 | miR-155 | Amyotrophic lateral sclerosis (ALS) | Entering in clinical trial | [163] |
| MRG-110 | miR-92a | Ischemia | Phase I clinical trial | [164] |
| MGN-9103 | miR-208 | Chronic heart failure | Preclinical stage | [165] |
| Cobomarsen (MRG-106) | miR-155 | Cutaneous T-cell lymphoma (CTCL) | Phase I clinical trials | [166] |
| MGN-2677 | miR-143/145 | Vascular disease | Preclinical stage | [167] |
| TargomiR | miR16-5p | Malignant pleural mesothelioma | Phase I clinical trial | [168] |
| RG-012 Lademirsen | miR-21-5p | Nephropathy | Preclinical stage | [169] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumaiya, K.; Ponnusamy, T.; Natarajaseenivasan, K.; Shanmughapriya, S. Cardiac Metabolism and MiRNA Interference. Int. J. Mol. Sci. 2023, 24, 50. https://doi.org/10.3390/ijms24010050
Sumaiya K, Ponnusamy T, Natarajaseenivasan K, Shanmughapriya S. Cardiac Metabolism and MiRNA Interference. International Journal of Molecular Sciences. 2023; 24(1):50. https://doi.org/10.3390/ijms24010050
Chicago/Turabian StyleSumaiya, Krishnamoorthi, Thiruvelselvan Ponnusamy, Kalimuthusamy Natarajaseenivasan, and Santhanam Shanmughapriya. 2023. "Cardiac Metabolism and MiRNA Interference" International Journal of Molecular Sciences 24, no. 1: 50. https://doi.org/10.3390/ijms24010050
APA StyleSumaiya, K., Ponnusamy, T., Natarajaseenivasan, K., & Shanmughapriya, S. (2023). Cardiac Metabolism and MiRNA Interference. International Journal of Molecular Sciences, 24(1), 50. https://doi.org/10.3390/ijms24010050






