Self-Assembled Daunorubicin/Epigallocatechin Gallate Nanocomplex for Synergistic Reversal of Chemoresistance in Leukemia
Abstract
1. Introduction
2. Results and Discussion
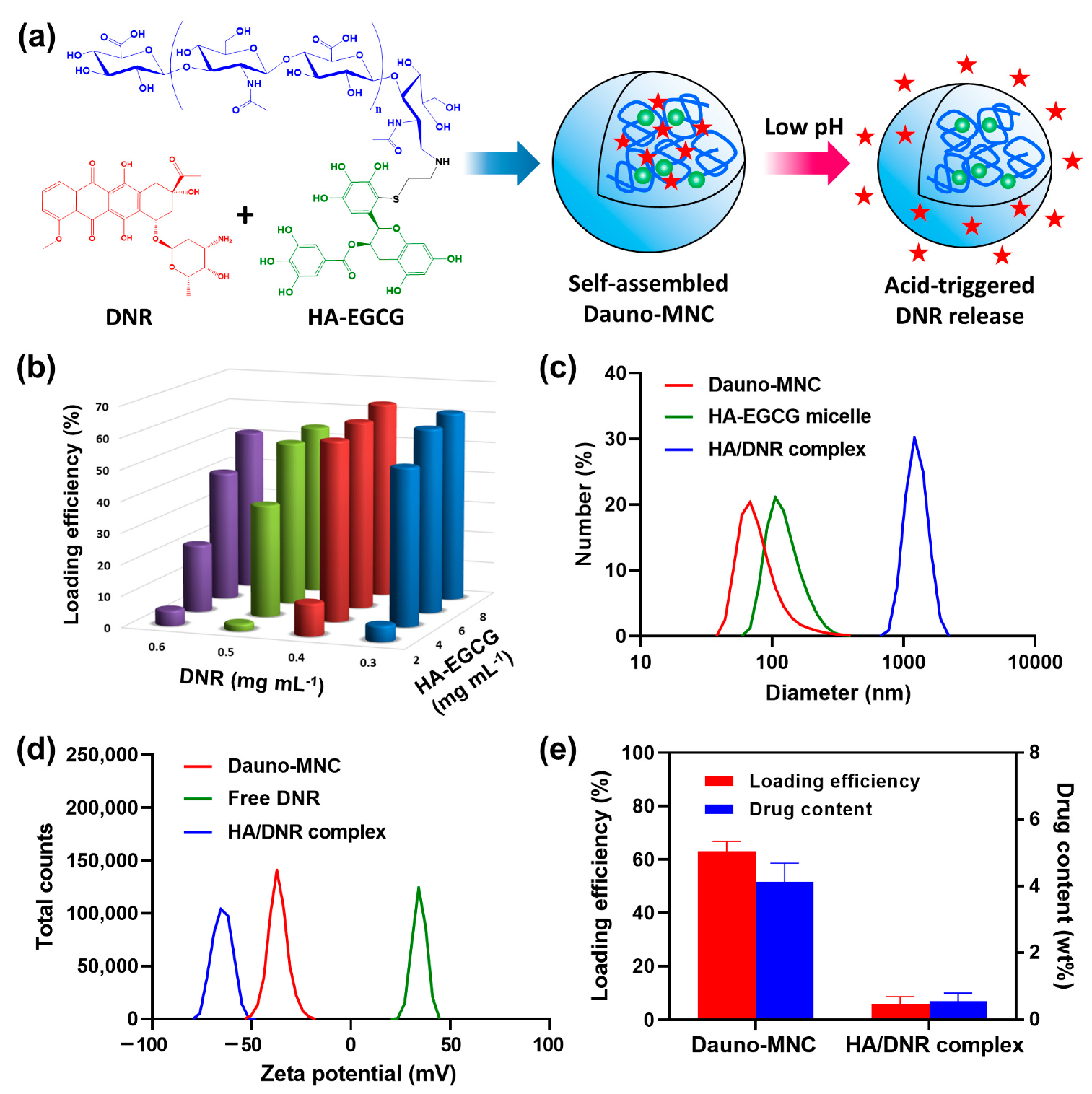
2.1. Production and Characterization of Dauno-MNC
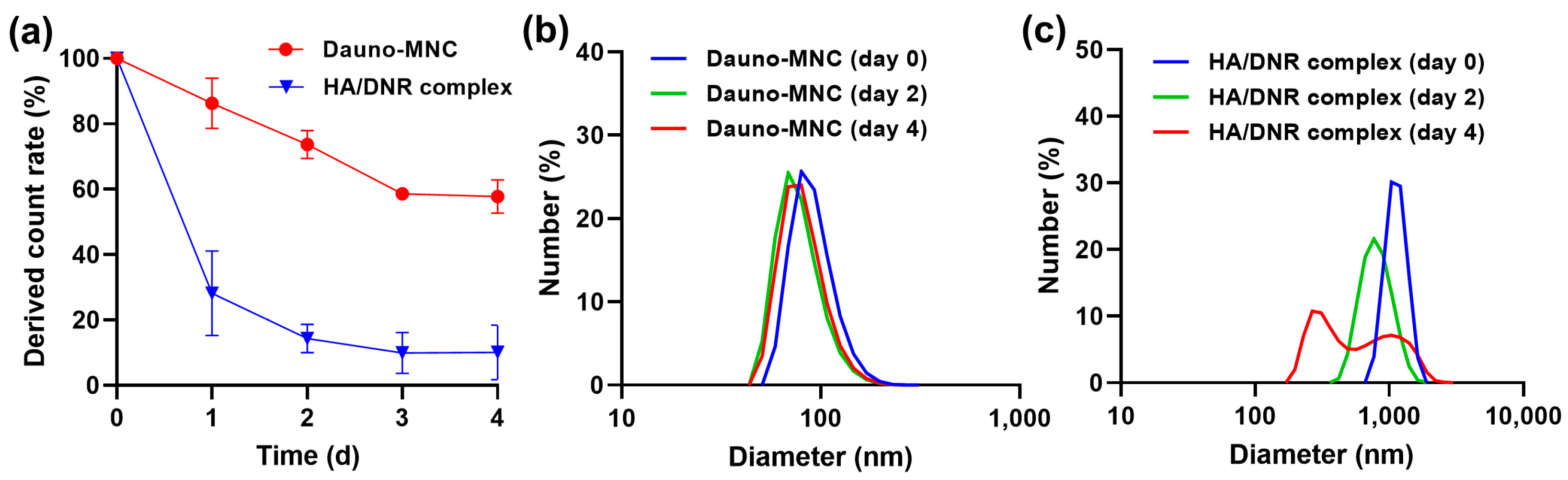
2.2. Assessment of the Particle Stability of Dauno-MNC
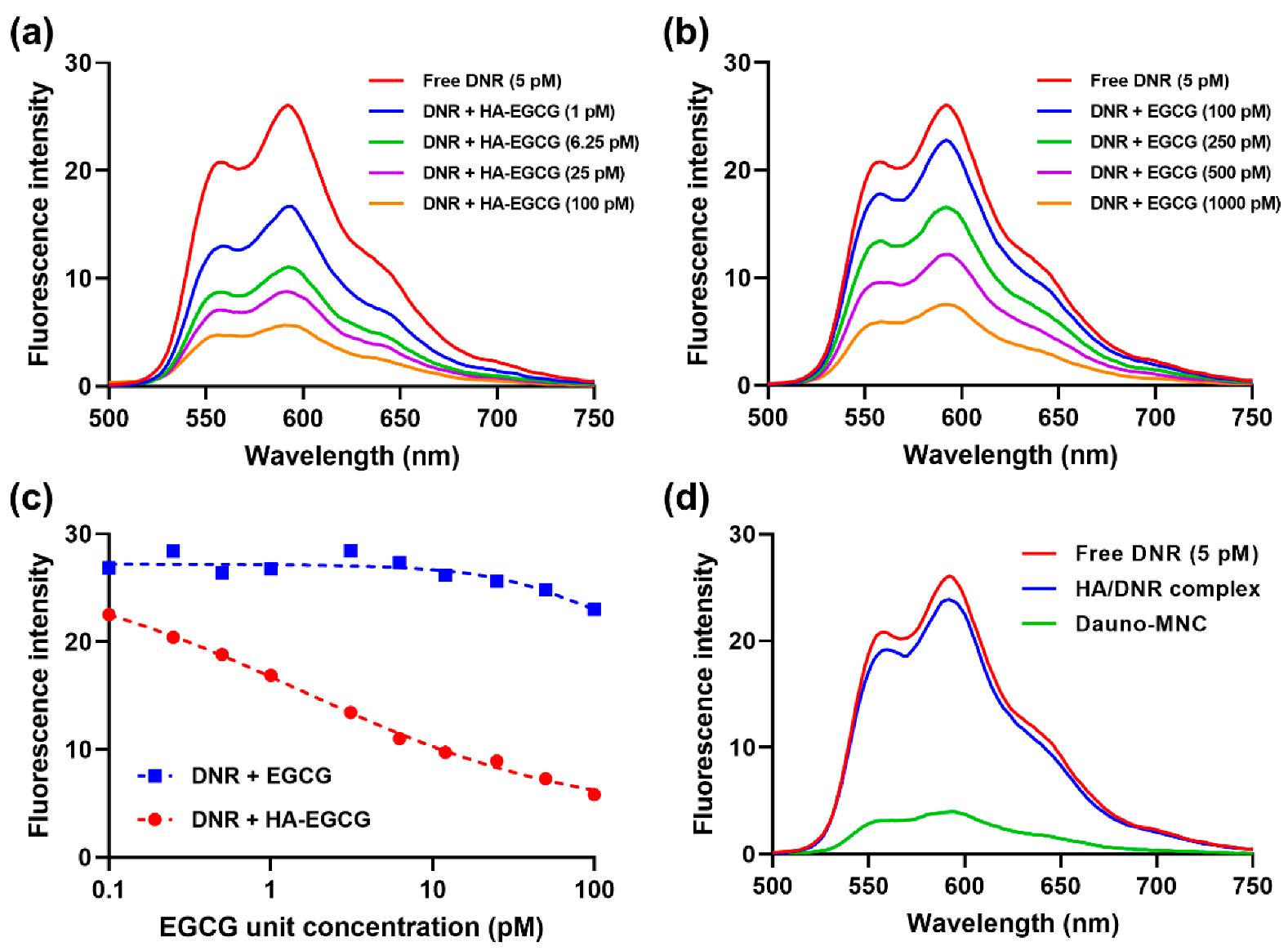
2.3. Fluorescence Spectroscopy Analysis
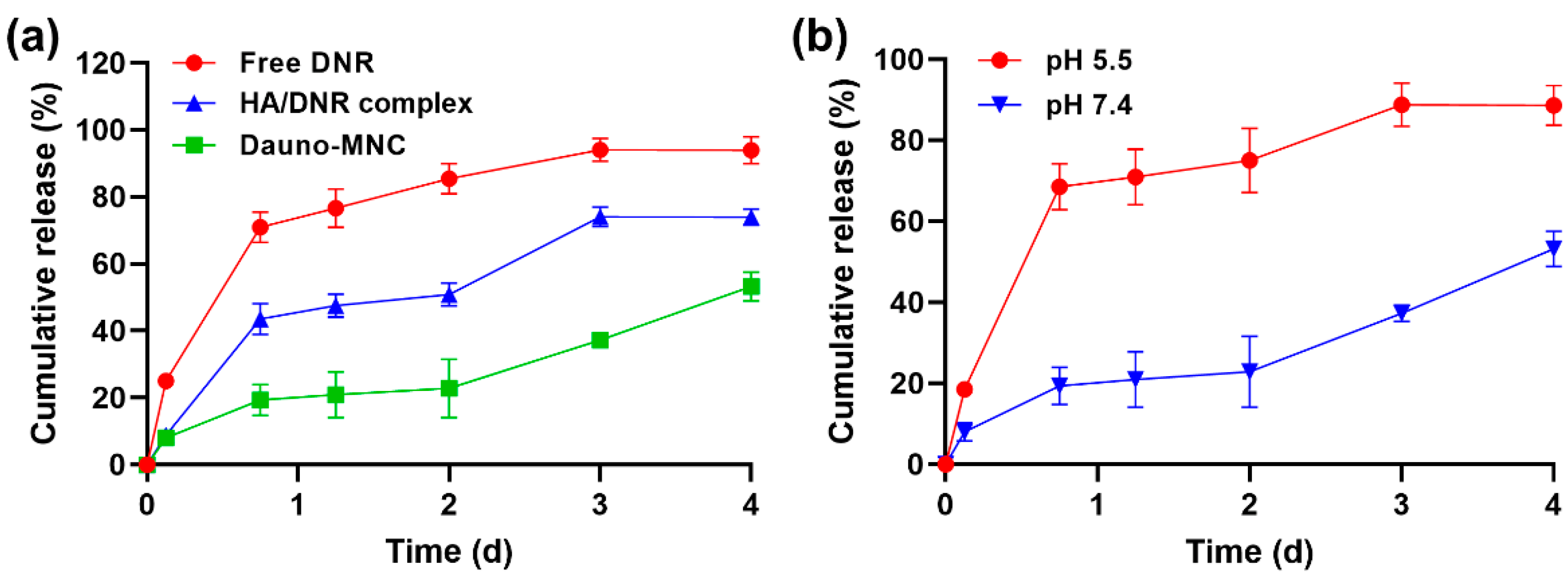
2.4. Drug Release Study
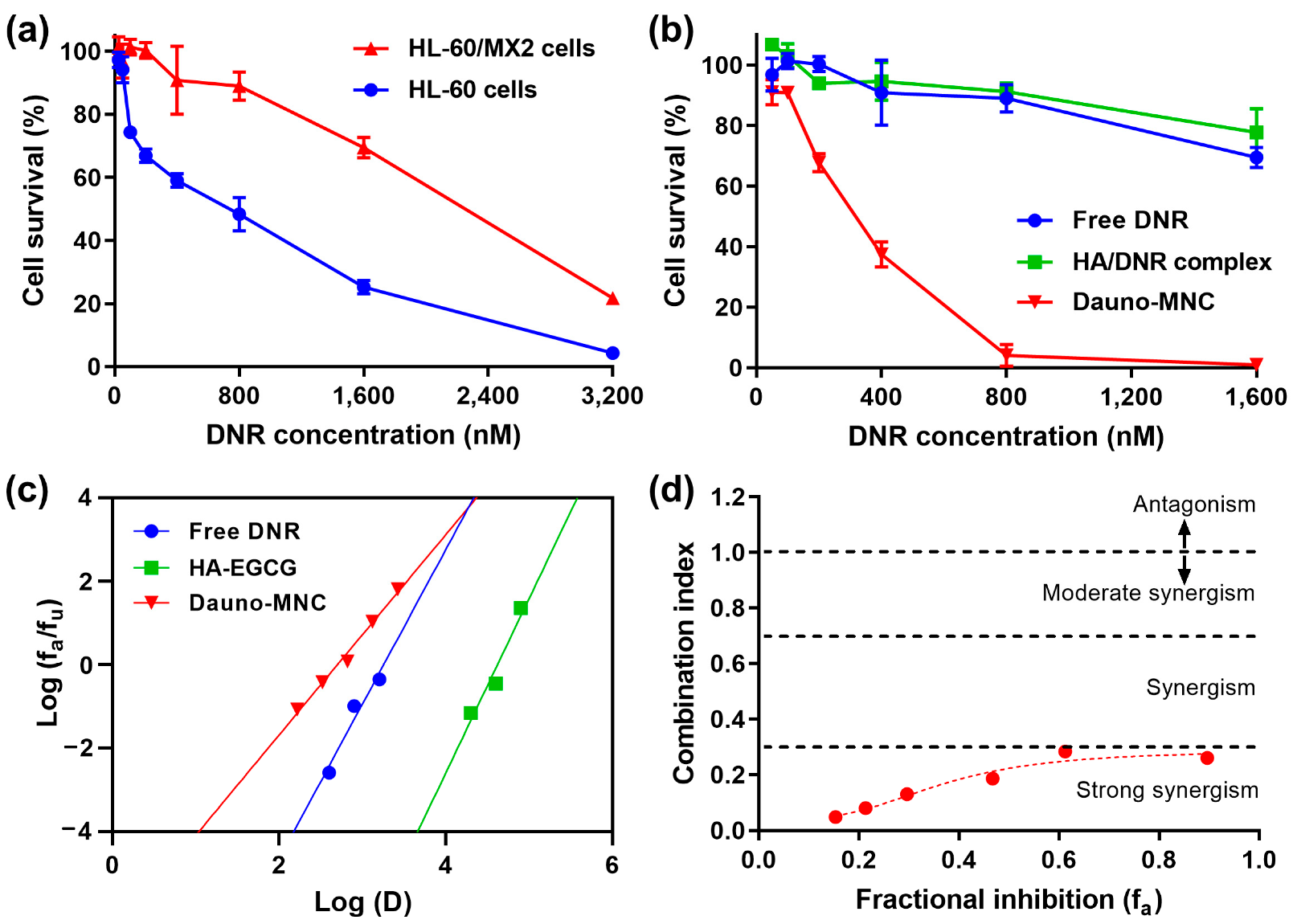
2.5. Cytotoxic Efficacy on Multidrug-Resistant AML Cells
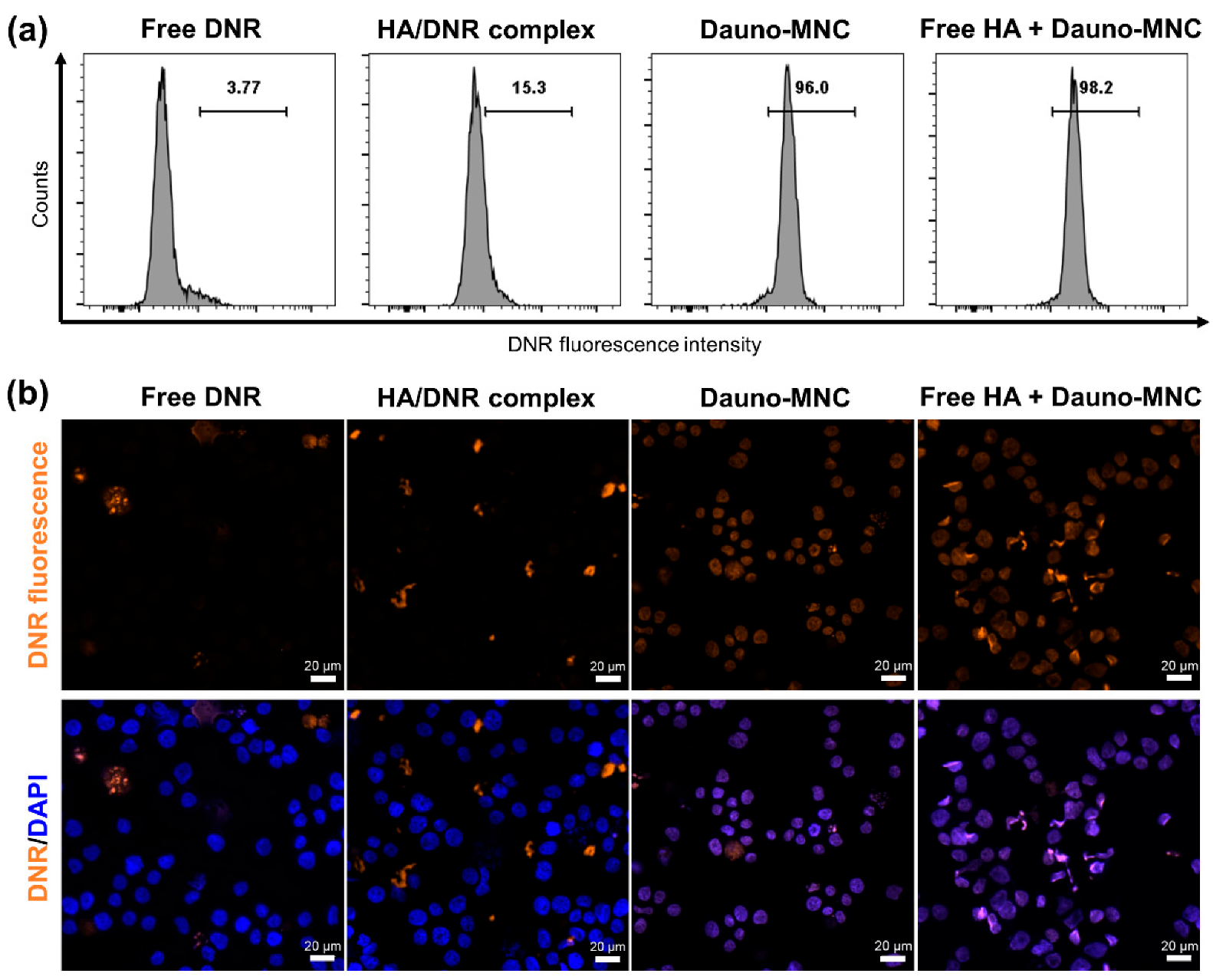
2.6. Cellular Uptake and Nucleus Localization of Dauno-MNC
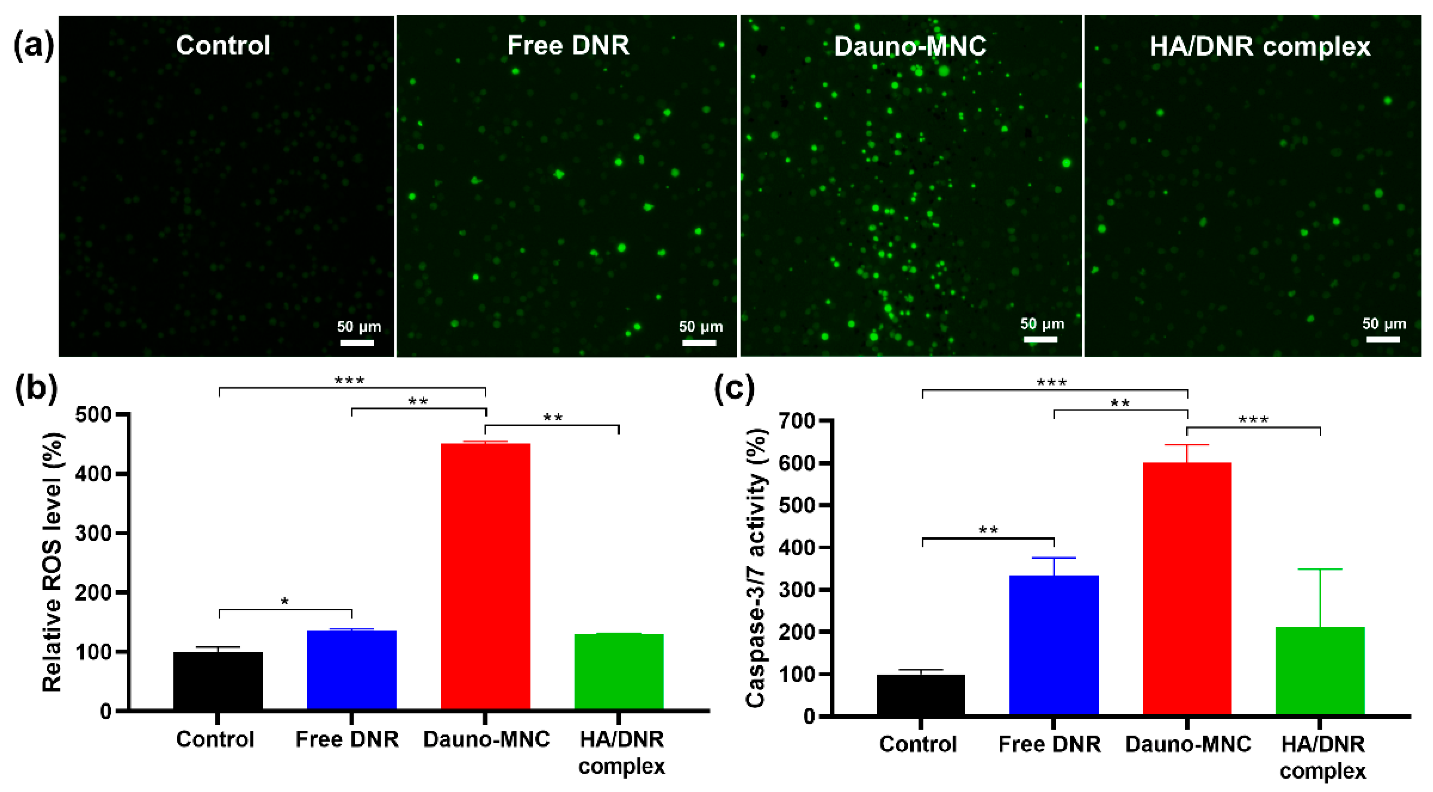
2.7. Intracellular ROS Production and Caspase Activation
3. Materials and Methods
3.1. Materials
3.2. Preparation of Dauno-MNC and HA/DNR Complex
3.3. Characterization of Dauno-MNC
3.4. Fluorescence Spectroscopy
3.5. Drug Release Study
3.6. In Vitro Cytotoxicity Study
3.7. Evaluation of Synergism
3.8. Flow Cytometry and Fluorescence Microscopy
3.9. Detection of Intracellular ROS Generation
3.10. Assessment of Caspase-3/7 Activity
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 310 Diseases and Injuries, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [PubMed]
- Dombret, H.; Gardin, C. An Update of Current Treatments for Adult Acute Myeloid Leukemia. Blood 2016, 127, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Oran, B.; Weisdorf, D.J. Survival for Older Patients with Acute Myeloid Leukemia: A Population-Based Study. Haematologica 2012, 97, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.; Yee, K.W.L. Cytarabine and Daunorubicin for the Treatment of Acute Myeloid Leukemia. Expert Opin. Pharmacother. 2017, 18, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Leith, C.P.; Kopecky, K.J.; Godwin, J.; McConnell, T.; Slovak, M.L.; Chen, I.-M.; Head, D.R.; Appelbaum, F.R.; Willman, C.L. Acute Myeloid Leukemia in the Elderly: Assessment of Multidrug Resistance (MDR1) and Cytogenetics Distinguishes Biologic Subgroups With Remarkably Distinct Responses to Standard Chemotherapy. A Southwest Oncology Group Study. Blood 1997, 89, 3323–3329. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer Prevention by Tea: Animal Studies, Molecular Mechanisms and Human Relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Mao, L.; Hochstetter, D.; Yao, L.; Zhao, Y.; Zhou, J.; Wang, Y.; Xu, P. Green Tea Polyphenol (−)-Epigallocatechin Gallate (EGCG) Attenuates Neuroinflammation in Palmitic Acid-Stimulated BV-2 Microglia and High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2019, 20, 5081. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Barro, L.; Tsai, S.-T.; Feng, T.-W.; Wu, X.-Y.; Chao, C.-W.; Yu, R.-S.; Chin, T.-Y.; Hsieh, M.F. Epigallocatechin-3-Gallate-Loaded Liposomes Favor Anti-Inflammation of Microglia Cells and Promote Neuroprotection. Int. J. Mol. Sci. 2021, 22, 3037. [Google Scholar] [CrossRef]
- Cornwall, S.; Cull, G.; Joske, D.; Ghassemifar, R. Green Tea Polyphenol “Epigallocatechin-3-Gallate”, Differentially Induces Apoptosis in CLL B-and T-Cells but Not in Healthy B-and T-Cells in a Dose Dependant Manner. Leuk. Res. 2016, 51, 56–61. [Google Scholar] [CrossRef]
- Liang, K.; Bae, K.H.; Nambu, A.; Dutta, B.; Chung, J.E.; Osato, M.; Kurisawa, M. A Two-Pronged Anti-Leukemic Agent Based on a Hyaluronic Acid–Green Tea Catechin Conjugate for Inducing Targeted Cell Death and Terminal Differentiation. Biomater. Sci. 2020, 8, 497–505. [Google Scholar] [CrossRef]
- Cheng, T.; Liu, J.; Ren, J.; Huang, F.; Ou, H.; Ding, Y.; Zhang, Y.; Ma, R.; An, Y.; Liu, J.; et al. Green Tea Catechin-Based Complex Micelles Combined with Doxorubicin to Overcome Cardiotoxicity and Multidrug Resistance. Theranostics 2016, 6, 1277–1292. [Google Scholar] [CrossRef]
- Li, H.; Krstin, S.; Wink, M. Modulation of Multidrug Resistant in Cancer Cells by EGCG, Tannic Acid and Curcumin. Phytomedicine 2018, 50, 213–222. [Google Scholar] [CrossRef]
- Vladu, A.F.; Ficai, D.; Ene, A.G.; Ficai, A. Combination Therapy Using Polyphenols: An Efficient Way to Improve Antitumoral Activity and Reduce Resistance. Int. J. Mol. Sci. 2022, 23, 10244. [Google Scholar] [CrossRef]
- Nakazato, T.; Ito, K.; Miyakawa, Y.; Kinjo, K.; Yamada, T.; Hozumi, N.; Ikeda, Y.; Kizaki, M. Catechin, a Green Tea Component, Rapidly Induces Apoptosis of Myeloid Leukemic Cells via Modulation of Reactive Oxygen Species Production In Vitro and Inhibits Tumor Growth In Vivo. Haematologica 2005, 90, 317–325. [Google Scholar]
- Nakazato, T.; Sagawa, M.; Yamato, K.; Xian, M.; Yamamoto, T.; Suematsu, M.; Ikeda, Y.; Kizaki, M. Myeloperoxidase Is a Key Regulator of Oxidative Stress–Mediated Apoptosis in Myeloid Leukemic Cells. Clin. Cancer Res. 2007, 13, 5436–5445. [Google Scholar] [CrossRef]
- Lecumberri, E.; Dupertuis, Y.M.; Miralbell, R.; Pichard, C. Green Tea Polyphenol Epigallocatechin-3-Gallate (EGCG) as Adjuvant in Cancer Therapy. Clin. Nutr. 2013, 32, 894–903. [Google Scholar] [CrossRef]
- Bae, K.H.; Tan, S.; Yamashita, A.; Ang, W.X.; Gao, S.J.; Wang, S.; Chung, J.E.; Kurisawa, M. Hyaluronic Acid-Green Tea Catechin Micellar Nanocomplexes: Fail-Safe Cisplatin Nanomedicine for the Treatment of Ovarian Cancer without Off-Target Toxicity. Biomaterials 2017, 148, 41–53. [Google Scholar] [CrossRef]
- Nakai, T.; Hirakura, T.; Sakurai, Y.; Shimoboji, T.; Ishigai, M.; Akiyoshi, K. Injectable Hydrogel for Sustained Protein Release by Salt-Induced Association of Hyaluronic Acid Nanogel. Macromol. Biosci. 2012, 12, 475–483. [Google Scholar] [CrossRef]
- Choi, D.H.; Kang, S.N.; Kim, S.M.; Gobaa, S.; Park, B.J.; Kim, I.H.; Joung, Y.K.; Han, D.K. Growth Factors-Loaded Stents Modified with Hyaluronic Acid and Heparin for Induction of Rapid and Tight Re-Endothelialization. Colloids Surf. B Biointerfaces 2016, 141, 602–610. [Google Scholar] [CrossRef]
- Lee, E.J.; Kang, E.; Kang, S.-W.; Huh, K.M. Thermo-Irreversible Glycol Chitosan/Hyaluronic Acid Blend Hydrogel for Injectable Tissue Engineering. Carbohydr. Polym. 2020, 244, 116432. [Google Scholar] [CrossRef]
- Liang, K.; Chung, J.E.; Gao, S.J.; Yongvongsoontorn, N.; Kurisawa, M. Highly Augmented Drug Loading and Stability of Micellar Nanocomplexes Composed of Doxorubicin and Poly(Ethylene Glycol)-Green Tea Catechin Conjugate for Cancer Therapy. Adv. Mater. 2018, 30, 1706963. [Google Scholar] [CrossRef]
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural Properties of Green Tea Catechins. J. Phys. Chem. B 2015, 119, 12860–12867. [Google Scholar] [CrossRef] [PubMed]
- Battistini, F.D.; Flores-Martin, J.; Olivera, M.E.; Genti-Raimondi, S.; Manzo, R.H. Hyaluronan as Drug Carrier. The In Vitro Efficacy and Selectivity of Hyaluronan–Doxorubicin Complexes to Affect the Viability of Overexpressing CD44 Receptor Cells. Eur. J. Pharm. Sci. 2014, 65, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.J.; Li, J.; Nation, R.L.; Boyd, B.J. Drug Release from Nanomedicines: Selection of Appropriate Encapsulation and Release Methodology. Drug Deliv. Transl. Res. 2012, 2, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Karukstis, K.K.; Thompson, E.H.Z.; Whiles, J.A.; Rosenfeld, R.J. Deciphering the Fluorescence Signature of Daunomycin and Doxorubicin. Biophys. Chem. 1998, 73, 249–263. [Google Scholar] [CrossRef]
- Bae, K.H.; Lai, F.; Mong, J.; Niibori-Nambu, A.; Chan, K.H.; Her, Z.; Osato, M.; Tan, M.-H.; Chen, Q.; Kurisawa, M. Bone Marrow-Targetable Green Tea Catechin-Based Micellar Nanocomplex for Synergistic Therapy of Acute Myeloid Leukemia. J. Nanobiotechnol. 2022, 20, 481. [Google Scholar] [CrossRef]
- Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Intracellular Drug Release Nanosystems. Mater. Today 2012, 15, 436–442. [Google Scholar] [CrossRef]
- Andrian, T.; Riera, R.; Pujals, S.; Albertazzi, L. Nanoscopy for Endosomal Escape Quantification. Nanoscale Adv. 2021, 3, 10–23. [Google Scholar] [CrossRef]
- Xia, C.Q.; Smith, P.G. Drug Efflux Transporters and Multidrug Resistance in Acute Leukemia: Therapeutic Impact and Novel Approaches to Mediation. Mol. Pharmacol. 2012, 82, 1008–1021. [Google Scholar] [CrossRef]
- Huang, F.; Cheng, R.; Meng, F.; Deng, C.; Zhong, Z. Micelles Based on Acid Degradable Poly(Acetal Urethane): Preparation, PH-Sensitivity, and Triggered Intracellular Drug Release. Biomacromolecules 2015, 16, 2228–2236. [Google Scholar] [CrossRef]
- Pei, D.; Buyanova, M. Overcoming Endosomal Entrapment in Drug Delivery. Bioconjug. Chem. 2019, 30, 273–283. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, X.; Nakayama-Ratchford, N.; Dai, H. Supramolecular Chemistry on Water-Soluble Carbon Nanotubes for Drug Loading and Delivery. ACS Nano 2007, 1, 50–56. [Google Scholar] [CrossRef]
- Jang, E.; Lim, E.-K.; Choi, Y.; Kim, E.; Kim, H.-O.; Kim, D.-J.; Suh, J.-S.; Huh, Y.-M.; Haam, S. π-Hyaluronan Nanocarriers for CD44-Targeted and PH-Boosted Aromatic Drug Delivery. J. Mater. Chem. B 2013, 1, 5686. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, L.; Lu, Q.; Fei, Z.; Dyson, P.J. Targeted Delivery and Controlled Release of Doxorubicin to Cancer Cells Using Modified Single Wall Carbon Nanotubes. Biomaterials 2009, 30, 6041–6047. [Google Scholar] [CrossRef]
- Harker, W.G.; Slade, D.L.; Dalton, W.S.; Meltzer, P.S.; Trent, J.M. Multidrug Resistance in Mitoxantrone-Selected HL-60 Leukemia Cells in the Absence of P-Glycoprotein Overexpression. Cancer Res. 1989, 49, 4542–4549. [Google Scholar]
- Chou, T.C. Preclinical versus Clinical Drug Combination Studies. Leuk. Lymphoma 2008, 49, 2059–2080. [Google Scholar] [CrossRef]
- Marin, J.J.G.; Briz, O.; Rodríguez-Macias, G.; Díez-Martín, J.L.; Macias, R.I.R. Role of Drug Transport and Metabolism in the Chemoresistance of Acute Myeloid Leukemia. Blood Rev. 2016, 30, 55–64. [Google Scholar] [CrossRef]
- Tarnowski, B.I.; Spinale, F.G.; Nicholson, J.H. DAPI as a Useful Stain for Nuclear Quantitation. Biotech. Histochem. 1991, 66, 296–302. [Google Scholar] [CrossRef]
- Vidal, R.S.; Quarti, J.; Rumjanek, F.D.; Rumjanek, V.M. Metabolic Reprogramming during Multidrug Resistance in Leukemias. Front. Oncol. 2018, 8, 90. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, H.; Wang, K. Reactive Oxygen Species in Eradicating Acute Myeloid Leukemic Stem Cells. Stem Cell Investig. 2014, 1, 13. [Google Scholar] [CrossRef]
- Bachur, N.R.; Gordon, S.L.; Gee, M.V. A General Mechanism for Microsomal Activation of Quinone Anticancer Agents to Free Radicals. Cancer Res. 1978, 38, 1745–1750. [Google Scholar] [PubMed]
- Kalghatgi, S.; Kelly, C.M.; Cerchar, E.; Torabi, B.; Alekseev, O.; Fridman, A.; Friedman, G.; Azizkhan-Clifford, J. Effects of Non-Thermal Plasma on Mammalian Cells. PLoS ONE 2011, 6, e16270. [Google Scholar] [CrossRef] [PubMed]
- Pourmohammadi, K.; Abedi, E. Enzymatic Modifications of Gluten Protein: Oxidative Enzymes. Food Chem. 2021, 356, 129679. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Trombetti, S.; Cesaro, E.; Catapano, R.; Sessa, R.; Lo Bianco, A.; Izzo, P.; Grosso, M. Oxidative Stress and ROS-Mediated Signaling in Leukemia: Novel Promising Perspectives to Eradicate Chemoresistant Cells in Myeloid Leukemia. Int. J. Mol. Sci. 2021, 22, 2470. [Google Scholar] [CrossRef]
- Kwon, S.; Ko, H.; You, D.G.; Kataoka, K.; Park, J.H. Nanomedicines for Reactive Oxygen Species Mediated Approach: An Emerging Paradigm for Cancer Treatment. Acc. Chem. Res. 2019, 52, 1771–1782. [Google Scholar] [CrossRef]
- Earnshaw, W.C.; Martins, L.M.; Kaufmann, S.H. Mammalian Caspases: Structure, Activation, Substrates, and Functions during Apoptosis. Annu. Rev. Biochem. 1999, 68, 383–424. [Google Scholar] [CrossRef]
- Kitagawa, S.; Nabekura, T.; Kamiyama, S. Inhibition of P-Glycoprotein Function by Tea Catechins in KB-C2 Cells. J. Pharm. Pharmacol. 2010, 56, 1001–1005. [Google Scholar] [CrossRef]
- Tóth, E.; Erdődi, F.; Kiss, A. Activation of Myosin Phosphatase by Epigallocatechin-Gallate Sensitizes THP-1 Leukemic Cells to Daunorubicin. Anticancer. Agents Med. Chem. 2021, 21, 1092–1098. [Google Scholar] [CrossRef]
- Zhou, H.; Fu, L.; Li, L.; Chen, Y.; Zhu, H.; Zhou, J.; Lv, M.; Gan, R.; Zhang, X.; Liang, G. The Epigallocatechin Gallate Derivative Y6 Reduces the Cardiotoxicity and Enhances the Efficacy of Daunorubicin against Human Hepatocellular Carcinoma by Inhibiting Carbonyl Reductase 1 Expression. J. Ethnopharmacol. 2020, 261, 113118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, K.H.; Lai, F.; Oruc, B.; Osato, M.; Chen, Q.; Kurisawa, M. Self-Assembled Daunorubicin/Epigallocatechin Gallate Nanocomplex for Synergistic Reversal of Chemoresistance in Leukemia. Int. J. Mol. Sci. 2023, 24, 381. https://doi.org/10.3390/ijms24010381
Bae KH, Lai F, Oruc B, Osato M, Chen Q, Kurisawa M. Self-Assembled Daunorubicin/Epigallocatechin Gallate Nanocomplex for Synergistic Reversal of Chemoresistance in Leukemia. International Journal of Molecular Sciences. 2023; 24(1):381. https://doi.org/10.3390/ijms24010381
Chicago/Turabian StyleBae, Ki Hyun, Fritz Lai, Betul Oruc, Motomi Osato, Qingfeng Chen, and Motoichi Kurisawa. 2023. "Self-Assembled Daunorubicin/Epigallocatechin Gallate Nanocomplex for Synergistic Reversal of Chemoresistance in Leukemia" International Journal of Molecular Sciences 24, no. 1: 381. https://doi.org/10.3390/ijms24010381
APA StyleBae, K. H., Lai, F., Oruc, B., Osato, M., Chen, Q., & Kurisawa, M. (2023). Self-Assembled Daunorubicin/Epigallocatechin Gallate Nanocomplex for Synergistic Reversal of Chemoresistance in Leukemia. International Journal of Molecular Sciences, 24(1), 381. https://doi.org/10.3390/ijms24010381







