Neurogenic Hypertension, the Blood–Brain Barrier, and the Potential Role of Targeted Nanotherapeutics
Abstract
1. Neurogenic Hypertension (NH)
2. Pathophysiology and Proposed Mechanisms of NH
2.1. Circumventricular Organs
2.2. The Paraventricular Nucleus (PVN) of the Hypothalamus
2.3. Medullary Structures (Nucleus Tractus Solitarius and the Two Ventrolateral Medullas)
2.3.1. The Nucleus of the Solitary Tract or Nucleus Tractus Solitarius (NTS)
2.3.2. The Caudal Ventrolateral Medulla (CVLM)
2.3.3. The Rostral Ventrolateral Medulla
3. Central Renin–Angiotensin System (RAS) Increases SNA in NH
4. Inflammation and Oxidative Stress in NH
5. Current Management Strategies for NH
5.1. Clinical Management—A Cocktail of Antihypertensive Combination Therapy
5.2. Centrally Acting Agents
5.3. Renal Denervation
5.4. Baroreceptor Reflex Activation
5.5. Other Unconventional Strategies- Drugs Proposed and Drugs under Investigation
6. The Problem of NH Management: The Barriers of the Central Nervous System
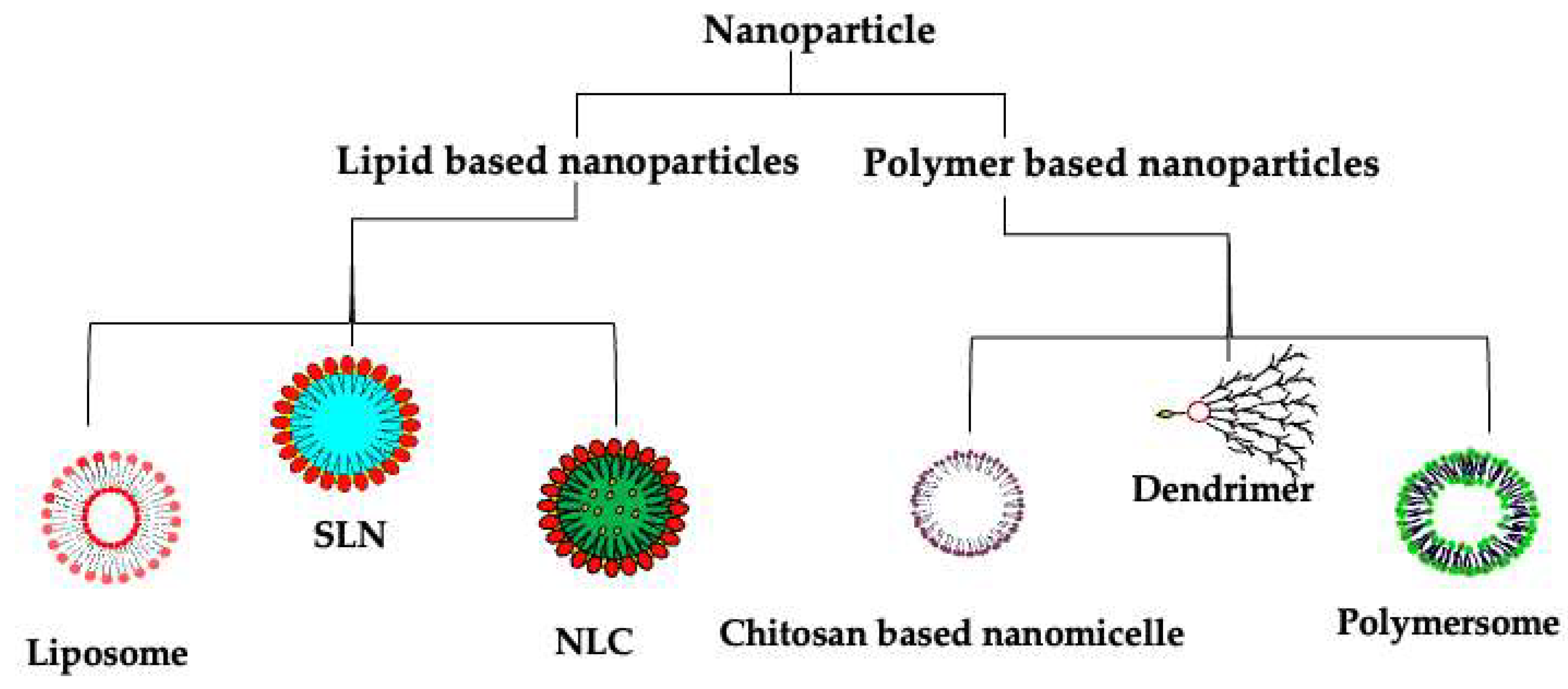
7. Nanotherapeutics: A Potential Solution
7.1. Lipid-Based Nanotherapeutics
7.1.1. Liposomes
7.1.2. Solid Lipid Nanoparticles (SLNs)
7.1.3. Nanostructured Lipid Carriers (NLCs)
7.2. Polymer-Based Nanotherapeutics
7.2.1. Polymersomes
7.2.2. Polymeric Nanoparticles
7.2.3. Dendrimers
8. Considerations for Improving Outcomes with Nanotherapeutics
8.1. Surface Functionalization of Nanotherapeutics
8.2. Bioresponsive Nanoparticles
8.3. Route of Administration
9. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Paton, J.F.; Raizada, M.K. Neurogenic Hypertension; Wiley Online Library: Hoboken, NJ, USA, 2010; pp. 569–571. [Google Scholar]
- Biancardi, V.C.; Sharma, N.M. Connecting sympathetic and renin–angiotensin system overdrive in neurogenic hypertension through miRNA-181a. Hypertens. Res. 2020, 43, 1309–1310. [Google Scholar]
- Kelly, D.M.; Rothwell, P.M. Blood pressure and the brain: The neurology of hypertension. Pract. Neurol. 2020, 20, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.M. Systemic hypertension: An overview of the problem. Semin. Nephrol. 2005, 25, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Saxena, T.; Ali, A.O.; Saxena, M. Pathophysiology of essential hypertension: An update. Expert Rev. Cardiovasc. Ther. 2018, 16, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Staessen, J.A.; Wang, J.; Bianchi, G.; Birkenhäger, W.H. Essential hypertension. Lancet 2003, 361, 1629–1641. [Google Scholar] [CrossRef]
- Mann, S.J. Neurogenic hypertension: Pathophysiology, diagnosis and management. Clin. Auton. Res. 2018, 28, 363–374. [Google Scholar]
- Guyenet, P.G. The sympathetic control of blood pressure. Nat. Rev. Neurosci. 2006, 7, 335–346. [Google Scholar]
- Stocker, S.D.; Kinsman, B.J.; Sved, A.F. Recent advances in neurogenic hypertension: Dietary salt, obesity, and inflammation. Hypertension 2017, 70, 474–478. [Google Scholar]
- Poulter, N.R.; Prabhakaran, D.; Caulfield, M. Hypertension. Lancet 2015, 386, 801–812. [Google Scholar] [CrossRef]
- Paton, J.F.; Wang, S.; Polson, J.W.; Kasparov, S. Signalling across the blood brain barrier by angiotensin II: Novel implications for neurogenic hypertension. J. Mol. Med. 2008, 86, 705–710. [Google Scholar] [CrossRef]
- Reid, I. Actions of angiotensin II on the brain: Mechanisms and physiologic role. Am. J. Physiol.-Ren. Physiol. 1984, 246, F533–F543. [Google Scholar] [CrossRef]
- Schölkens, B.; Jung, W.; Rascher, W.; Dietz, R.; Ganten, D. Intracerebroventricular angiotensin II increases arterial blood pressure in rhesus monkeys by stimulation of pituitary hormones and the sympathetic nervous system. Experientia 1982, 38, 469–470. [Google Scholar] [CrossRef]
- Osborn, J.W.; Fink, G.D.; Sved, A.F.; Toney, G.M.; Raizada, M.K. Circulating angiotensin II and dietary salt: Converging signals for neurogenic hypertension. Curr. Hypertens. Rep. 2007, 9, 228–235. [Google Scholar] [CrossRef]
- Dibona, G.F. Sympathetic nervous system and the kidney in hypertension. Curr. Opin. Nephrol. Hypertens. 2002, 11, 197–200. [Google Scholar] [CrossRef]
- Feng, Y.; Xia, H.; Santos, R.A.; Speth, R.; Lazartigues, E. Angiotensin-converting enzyme 2: A new target for neurogenic hypertension. Exp. Physiol. 2010, 95, 601–606. [Google Scholar] [CrossRef]
- Sharma, N.M.; Haibara, A.S.; Katsurada, K.; Nandi, S.S.; Liu, X.; Zheng, H.; Patel, K.P. Central Ang II (Angiotensin II)-mediated sympathoexcitation: Role for HIF-1α (hypoxia-inducible factor-1α) facilitated glutamatergic tone in the paraventricular nucleus of the hypothalamus. Hypertension 2021, 77, 147–157. [Google Scholar] [CrossRef]
- Iyer, S.N.; Lu, D.; Katovich, M.J.; Raizada, M.K. Chronic control of high blood pressure in the spontaneously hypertensive rat by delivery of angiotensin type 1 receptor antisense. Proc. Natl. Acad. Sci. USA 1996, 93, 9960–9965. [Google Scholar] [CrossRef]
- Sica, D.A. Centrally acting antihypertensive agents: An update. J. Clin. Hypertens. 2007, 9, 399–405. [Google Scholar] [CrossRef]
- Van Zwieten, P.; Thoolen, M.; Timmermans, P. The pharmacology of centrally acting antihypertensive drugs. Br. J. Clin. Pharmacol. 1983, 15 (Suppl. S4), 455S–462S. [Google Scholar] [CrossRef]
- Van Zwieten, P. Centrally acting antihypertensive drugs. Present and future. Clin. Exp. Hypertens. 1999, 21, 859–873. [Google Scholar]
- Dos Santos Rodrigues, B.; Lakkadwala, S.; Kanekiyo, T.; Singh, J. Dual-modified liposome for targeted and enhanced gene delivery into mice brain. J. Pharmacol. Exp. Ther. 2020, 374, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Choi, W.I.; Kim, Y.H.; Tae, G. Brain-targeted delivery of protein using chitosan-and RVG peptide-conjugated, pluronic-based nano-carrier. Biomaterials 2013, 34, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Queiroz, J.F.; Reis, S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J. Nanobiotechnology 2016, 14, 27. [Google Scholar] [CrossRef]
- Anand, A.; Sugumaran, A.; Narayanasamy, D. Brain targeted delivery of anticancer drugs: Prospective approach using solid lipid nanoparticles. IET Nanobiotechnology 2019, 13, 353–362. [Google Scholar] [CrossRef]
- Arora, S.; Singh, J. In vitro and in vivo optimization of liposomal nanoparticles based brain targeted vgf gene therapy. Int. J. Pharm. 2021, 608, 121095. [Google Scholar] [CrossRef] [PubMed]
- Jannetta, P.J.; Segal, R.; Wolfson, S.K., Jr. Neurogenic hypertension: Etiology and surgical treatment. I. Observations in 53 patients. Ann. Surg. 1985, 201, 391. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.F.; Waki, H. Is neurogenic hypertension related to vascular inflammation of the brainstem? Neurosci. Biobehav. Rev. 2009, 33, 89–94. [Google Scholar] [CrossRef]
- Zubcevic, J.; Waki, H.; Raizada, M.K.; Paton, J.F. Autonomic-immune-vascular interaction: An emerging concept for neurogenic hypertension. Hypertension 2011, 57, 1026–1033. [Google Scholar] [CrossRef]
- Waki, H.; Gouraud, S.S. Brain inflammation in neurogenic hypertension. World J. Hypertens. 2014, 4, 1–6. [Google Scholar] [CrossRef]
- Melmed, S.; Polonsky, K.S.; Larsen, P.R.; Kronenberg, H.M. Williams Textbook of Endocrinology E-Book; Elsevier Health Sciences: Philadelphia, PA, USA, 2015. [Google Scholar]
- Ganong, W.F. Circumventricular organs: Definition and role in the regulation of endocrine and autonomic function. Clin. Exp. Pharmacol. Physiol. 2000, 27, 422–427. [Google Scholar] [CrossRef]
- Verheggen, I.; de Jong, J.J.; van Boxtel, M.P.; Postma, A.A.; Verhey, F.R.; Jansen, J.F.; Backes, W.H. Permeability of the windows of the brain: Feasibility of dynamic contrast-enhanced MRI of the circumventricular organs. Fluids Barriers CNS 2020, 17, 66. [Google Scholar] [CrossRef]
- Oldfield, B.J.; McKinley, M.J. Circumventricular organs. In The Rat Nervous System; Elsevier: Amsterdam, The Netherlands, 2015; pp. 315–333. [Google Scholar]
- Johnson, A. Circumventricular organs in Neuroendocrine control. In Encyclopedia of Neuroscience; Elsevier Ltd.: Amsterdam, The Netherlands, 2009; pp. 1003–1006. [Google Scholar]
- Simpson, J.B. The circumventricular organs and the central actions of angiotensin. Neuroendocrinology 1981, 32, 248–256. [Google Scholar] [CrossRef]
- Ganong, W.F.; Murakami, K. The role of angiotensin II in the regulation of ACTH secretion. Ann. N. Y. Acad. Sci. 1987, 512, 176–186. [Google Scholar] [CrossRef]
- Robinson, M.M.; Evered, M.D. Angiotensin II and arterial pressure in the control of thirst. In The Physiology of Thirst and Sodium Appetite; Springer: Berlin/Heidelberg, Germany, 1986; pp. 193–198. [Google Scholar]
- Benarroch, E.E. Circumventricular organs: Receptive and homeostatic functions and clinical implications. Neurology 2011, 77, 1198–1204. [Google Scholar] [CrossRef]
- Ferguson, A.V.; Latchford, K.J.; Samson, W.K. The paraventricular nucleus of the hypothalamus–a potential target for integrative treatment of autonomic dysfunction. Expert Opin. Ther. Targets 2008, 12, 717–727. [Google Scholar] [CrossRef]
- Barson, J.R.; Mack, N.R.; Gao, W.-J. The paraventricular nucleus of the thalamus is an important node in the emotional processing network. Front. Behav. Neurosci. 2020, 14, 598469. [Google Scholar] [CrossRef]
- Savić, B.; Murphy, D.; Japundžić-Žigon, N. The Paraventricular Nucleus of the Hypothalamus in Control of Blood Pressure and Blood Pressure Variability. Front. Physiol. 2022, 13, 858941. [Google Scholar] [CrossRef]
- Qin, C.; Li, J.; Tang, K. The paraventricular nucleus of the hypothalamus: Development, function, and human diseases. Endocrinology 2018, 159, 3458–3472. [Google Scholar] [CrossRef]
- Frahm, K.; Schow, M.; Tobet, S. The vasculature within the paraventricular nucleus of the hypothalamus in mice varies as a function of development, subnuclear location, and GABA signaling. Horm. Metab. Res. 2012, 44, 619–624. [Google Scholar] [CrossRef]
- Cortés-Sol, A.; Lara-Garcia, M.; Alvarado, M.; Hudson, R.; Berbel, P.; Pacheco, P. Inner capillary diameter of hypothalamic paraventricular nucleus of female rat increases during lactation. BMC Neurosci. 2013, 14, 7. [Google Scholar] [CrossRef]
- Braga, V.; Medeiros, I.; Ribeiro, T.; França-Silva, M.; Botelho-Ono, M.; Guimarães, D. Angiotensin-II-induced reactive oxygen species along the SFO-PVN-RVLM pathway: Implications in neurogenic hypertension. Braz. J. Med. Biol. Res. 2011, 44, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.E.; Son, S.; Biancardi, V.C.; Zheng, H.; Sharma, N.; Patel, K.P. Astrocytes contribute to angiotensin II stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension 2016, 68, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Becker, B.K. Shining light on the paraventricular nucleus: The role of glutamatergic PVN neurons in blood pressure control. J. Physiol. 2018, 596, 6127. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-P.; Pan, H.-L. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension 2007, 49, 916–925. [Google Scholar] [CrossRef]
- Basting, T.; Xu, J.; Mukerjee, S.; Epling, J.; Fuchs, R.; Sriramula, S.; Lazartigues, E. Glutamatergic neurons of the paraventricular nucleus are critical contributors to the development of neurogenic hypertension. J. Physiol. 2018, 596, 6235–6248. [Google Scholar] [CrossRef]
- Gao, H.-L.; Yu, X.-J.; Liu, K.-L.; Shi, X.-L.; Qi, J.; Chen, Y.-M.; Zhang, Y.; Bai, J.; Yi, Q.-Y.; Feng, Z.-P. PVN blockade of p44/42 MAPK pathway attenuates salt-induced hypertension through modulating neurotransmitters and attenuating oxidative stress. Sci. Rep. 2017, 7, 43038. [Google Scholar] [CrossRef]
- Basting, T.M.; Lazartigues, E.; Murkerjee, S.; Xu, J. Abstract P386: The Paraventricular Nucleus in Control of Blood Pressure and Its Role in Hypertension. Hypertension 2017, 70, AP386. [Google Scholar] [CrossRef]
- Fernández-Gil, M.A.; Palacios-Bote, R.; Leo-Barahona, M.; Mora-Encinas, J. Anatomy of the brainstem: A gaze into the stem of life. In Seminars in Ultrasound, CT and MRI; Elsevier: Amsterdam, The Netherlands, 2010; pp. 196–219. [Google Scholar]
- Nolte, J. The human brain: An introduction to its functional anatomy. In The Human Brain: An Introduction to Its Functional Anatomy; Mosby Elsevier: Philadelphia, PA, USA, 1981; p. 322. [Google Scholar]
- Jean, A. The nucleus tractus solitarius: Neuroanatomic, neurochemical and functional aspects. Arch. Int. Physiol. Biochim. Biophys. 1991, 99, A3–A52. [Google Scholar]
- Sun, C.; Zubcevic, J.; Polson, J.W.; Potts, J.T.; Diez-Freire, C.; Zhang, Q.; Paton, J.F.; Raizada, M.K. Shift to an Involvement of PI3-Kinase in Angiotensin II Actions on Nucleus Tractus Solitarii Neurons of the Spontaneously Hypertensive Rat. Circ. Res. 2009, 105, 1248. [Google Scholar] [CrossRef]
- Krukoff, T.; Vu, T.; Harris, K.; Aippersbach, S.; Jhamandas, J. Neurons in the rat medulla oblongata containing neuropeptide Y-, angiotensin II-, or galanin-like immunoreactivity project to the parabrachial nucleus. Neuroscience 1992, 47, 175–184. [Google Scholar] [CrossRef]
- Ross, C.A.; Ruggiero, D.A.; Reis, D.J. Projections from the nucleus tractus solitarii to the rostral ventrolateral medulla. J. Comp. Neurol. 1985, 242, 511–534. [Google Scholar] [CrossRef]
- Shan, Z.; Zubcevic, J.; Shi, P.; Jun, J.Y.; Dong, Y.; Murça, T.M.; Lamont, G.J.; Cuadra, A.; Yuan, W.; Qi, Y. Chronic knockdown of the nucleus of the solitary tract AT1 receptors increases blood inflammatory-endothelial progenitor cell ratio and exacerbates hypertension in the spontaneously hypertensive rat. Hypertension 2013, 61, 1328–1333. [Google Scholar] [CrossRef]
- Doba, N.; Reis, D.J. Acute fulminating neurogenic hypertension produced by brainstem lesions in the rat. Circ. Res. 1973, 32, 584–593. [Google Scholar] [CrossRef]
- Colombari, E.; Sato, M.A.; Cravo, S.L.; Bergamaschi, C.T.; Campos, R.R., Jr.; Lopes, O.U. Role of the medulla oblongata in hypertension. Hypertension 2001, 38, 549–554. [Google Scholar] [CrossRef]
- Guyenet, P.G.; Stornetta, R.L.; Souza, G.M.; Abbott, S.B.; Brooks, V.L. Neuronal networks in hypertension: Recent advances. Hypertension 2020, 76, 300–311. [Google Scholar] [CrossRef]
- Cravo, S.L.; Morrison, S.F.; Reis, D.J. Differentiation of two cardiovascular regions within caudal ventrolateral medulla. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1991, 261, R985–R994. [Google Scholar] [CrossRef]
- Dampney, R. Functional organization of central pathways regulating the cardiovascular system. Physiol. Rev. 1994, 74, 323–364. [Google Scholar] [CrossRef]
- Campos, R.; Carillo, B.; Oliveira-Sales, E.; Silva, A.; Silva, N.; Neto, H.F.; Bergamaschi, C. Role of the caudal pressor area in the regulation of sympathetic vasomotor tone. Braz. J. Med. Biol. Res. 2008, 41, 557–562. [Google Scholar] [CrossRef]
- Schreihofer, A.M.; Guyenet, P.G. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J. Comp. Neurol. 1997, 387, 524–536. [Google Scholar] [CrossRef]
- Milner, T.A.; Pickel, V.M.; Abate, C.; Joh, T.H.; Reis, D.J. Ultrastructural characterization of substance P-like immunoreactive neurons in the rostral ventrolateral medulla in relation to neurons containing catecholamine-synthesizing enzymes. J. Comp. Neurol. 1988, 270, 427–445. [Google Scholar] [CrossRef]
- Vincent, S.R. Distributions of tyrosine hydroxylase-, dopamine-β-hydroxylase-, and phenylethanolamine-N-methyltransferase-immunoreactive neurons in the brain of the hamster (Mesocricetus auratus). J. Comp. Neurol. 1988, 268, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-P.; Pan, H.-L. Role of GABAB receptors in autonomic control of systemic blood pressure. Adv. Pharmacol. 2010, 58, 257–286. [Google Scholar] [PubMed]
- Dampney, R.; McAllen, R. Differential control of sympathetic fibres supplying hindlimb skin and muscle by subretrofacial neurones in the cat. J. Physiol. 1988, 395, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Höhle, S.; Blume, A.; Lebrun, C.; Culman, J.; Unger, T. Angiotensin receptors in the brain. Pharmacol. Toxicol. 1995, 77, 306–315. [Google Scholar] [CrossRef]
- Mendelowitz, D. How Does Angiotensin Activate Hypothalamic Neurons Essential for Controlling Sympathetic Activity and Blood Pressure? Hypertension 2016, 68, 1340–1341. [Google Scholar]
- Toney, G.M.; Pedrino, G.R.; Fink, G.D.; Osborn, J.W. Does Enhanced Respiratory–Sympathetic Coupling Contribute to Peripheral Neural Mechanisms of Angiotensin II–Salt Hypertension? Wiley Online Library: Hoboken, NJ, USA, 2010. [Google Scholar]
- Lin, S.-R.; Lin, S.-Y.; Chen, C.-C.; Fu, Y.-S.; Weng, C.-F. Exploring a new natural treating agent for primary hypertension: Recent findings and forthcoming perspectives. J. Clin. Med. 2019, 8, 2003. [Google Scholar] [CrossRef]
- Head, G.A.; Jackson, K.L.; Gueguen, C. Potential therapeutic use of neurosteroids for hypertension. Front. Physiol. 2019, 10, 1477. [Google Scholar] [CrossRef]
- Jackson, K.L.; Head, G.A.; Gueguen, C.; Stevenson, E.R.; Lim, K.; Marques, F.Z. Mechanisms responsible for genetic hypertension in schlager BPH/2 mice. Front. Physiol. 2019, 10, 1311. [Google Scholar] [CrossRef]
- Oyarce, M.P.; Iturriaga, R. Contribution of oxidative stress and inflammation to the neurogenic hypertension induced by intermittent hypoxia. Front. Physiol. 2018, 9, 893. [Google Scholar] [CrossRef]
- Waki, H.; Gouraud, S.S.; Maeda, M.; Paton, J.F. Gene expression profiles of major cytokines in the nucleus tractus solitarii of the spontaneously hypertensive rat. Auton. Neurosci. 2008, 142, 40–44. [Google Scholar] [CrossRef]
- Waki, H.; Gouraud, S.S.; Maeda, M.; Paton, J.F. Specific inflammatory condition in nucleus tractus solitarii of the SHR: Novel insight for neurogenic hypertension? Auton. Neurosci. 2008, 142, 25–31. [Google Scholar] [CrossRef]
- Agarwal, D.; Welsch, M.A.; Keller, J.N.; Francis, J. Chronic exercise modulates RAS components and improves balance between pro-and anti-inflammatory cytokines in the brain of SHR. Basic Res. Cardiol. 2011, 106, 1069–1085. [Google Scholar] [CrossRef]
- Schmid-Schönbein, G.; Seiffge, D.; DeLano, F.A.; Shen, K.; Zweifach, B.W. Leukocyte counts and activation in spontaneously hypertensive and normotensive rats. Hypertension 1991, 17, 323–330. [Google Scholar] [CrossRef]
- Shi, P.; Raizada, M.K.; Sumners, C. Brain cytokines as neuromodulators in cardiovascular control. Clin. Exp. Pharmacol. Physiol. 2010, 37, e52–e57. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. Neuroinflammation and sympathetic overactivity: Mechanisms and implications in hypertension. Auton. Neurosci. 2018, 210, 10–17. [Google Scholar] [CrossRef]
- Liang, B.; Zhao, Y.-N.; Wang, X.; Yu, X.-J.; Li, Y.; Yang, H.-Y.; Su, Q.; Kang, Y.-M.; Yang, Z.-M. Angiotensin-(1-7) attenuates hypertension and cardiac hypertrophy via modulation of nitric oxide and neurotransmitter levels in the paraventricular nucleus in salt-sensitive hypertensive rats. RSC Adv. 2018, 8, 8779–8786. [Google Scholar] [CrossRef]
- Lurbe, E.; Agabiti-Rosei, E.; Cruickshank, J.K.; Dominiczak, A.; Erdine, S.; Hirth, A.; Invitti, C.; Litwin, M.; Mancia, G.; Pall, D. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J. Hypertens. 2016, 34, 1887–1920. [Google Scholar]
- Varon, J.; Marik, P.E. Clinical review: The management of hypertensive crises. Crit. Care 2003, 7, 374–384. [Google Scholar] [CrossRef]
- Schmieder, R.E. End organ damage in hypertension. Dtsch. Ärzteblatt Int. 2010, 107, 866. [Google Scholar] [CrossRef]
- Kaplan, N.M. Lifestyle modifications for prevention and treatment of hypertension. J. Clin. Hypertens. 2004, 6, 716. [Google Scholar] [CrossRef]
- Yang, M.H.; Kang, S.Y.; Lee, J.A.; Kim, Y.S.; Sung, E.J.; Lee, K.-Y.; Kim, J.-S.; Oh, H.J.; Kang, H.C.; Lee, S.Y. The effect of lifestyle changes on blood pressure control among hypertensive patients. Korean J. Fam. Med. 2017, 38, 173. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, L.; Lu, B.; Chen, M.; Zhang, Y. Evaluation of Bamboo Shoot Peptide Preparation with Angiotensin Converting Enzyme Inhibitory and Antioxidant Abilities from Byproducts of Canned Bamboo Shoots. J. Agric. Food Chem. 2013, 61, 5526–5533. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.; Lu, B.; Xia, D.; Zhang, Y. Evaluation of antihypertensive and antihyperlipidemic effects of bamboo shoot angiotensin converting enzyme inhibitory peptide in vivo. J. Agric. Food Chem. 2012, 60, 11351–11358. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Calhoun, D.A.; Bakris, G.L.; Brook, R.D.; Daugherty, S.L.; Dennison-Himmelfarb, C.R.; Egan, B.M.; Flack, J.M.; Gidding, S.S.; Judd, E. Resistant hypertension: Detection, evaluation, and management: A scientific statement from the American Heart Association. Hypertension 2018, 72, e53–e90. [Google Scholar] [PubMed]
- Doumas, M.; Imprialos, K.P.; Kallistratos, M.S.; Manolis, A.J. Recent advances in understanding and managing resistant/refractory hypertension. F1000Research 2020, 9, 169. [Google Scholar] [CrossRef]
- Krum, H.; Lambert, E.; Windebank, E.; Campbell, D.J.; Esler, M. Effect of angiotensin II receptor blockade on autonomic nervous system function in patients with essential hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H1706–H1712. [Google Scholar] [CrossRef]
- Heusser, K.; Vitkovsky, J.; Raasch, W.; Schmieder, R.E.; Schobel, H.P. Elevation of sympathetic activity by eprosartan in young male subjects. Am. J. Hypertens. 2003, 16, 658–664. [Google Scholar]
- Kasama, S.; Toyama, T.; Kumakura, H.; Takayama, Y.; Ichikawa, S.; Suzuki, T.; Kurabayashi, M. Addition of valsartan to an angiotensin-converting enzyme inhibitor improves cardiac sympathetic nerve activity and left ventricular function in patients with congestive heart failure. J. Nucl. Med. 2003, 44, 884–890. [Google Scholar]
- Doulton, T.W.; He, F.J.; MacGregor, G.A. Systematic review of combined angiotensin-converting enzyme inhibition and angiotensin receptor blockade in hypertension. Hypertension 2005, 45, 880–886. [Google Scholar]
- Sun, Z. Aging, arterial stiffness, and hypertension. Hypertension 2015, 65, 252–256. [Google Scholar] [CrossRef]
- Messerli, F.H.; Bangalore, S.; Bavishi, C.; Rimoldi, S.F. Angiotensin-converting enzyme inhibitors in hypertension: To use or not to use? J. Am. Coll. Cardiol. 2018, 71, 1474–1482. [Google Scholar] [CrossRef]
- Brooks, D.P.; Ruffolo, R.R., Jr. Pharmacological mechanism of angiotensin II receptor antagonists: Implications for the treatment of elevated systolic blood pressure. J. Hypertension. Suppl. Off. J. Int. Soc. Hypertens. 1999, 17, S27–S32. [Google Scholar]
- Neumann, J.; Ligtenberg, G.; Oey, L.; Koomans, H.A.; Blankestijn, P.J. Moxonidine normalizes sympathetic hyperactivity in patients with eprosartan-treated chronic renal failure. J. Am. Soc. Nephrol. 2004, 15, 2902–2907. [Google Scholar] [CrossRef]
- Esler, M.; Lux, A.; Jennings, G.; Hastings, J.; Socratous, F.; Lambert, G. Rilmenidine sympatholytic activity preserves mental stress, orthostatic sympathetic responses and adrenaline secretion. J. Hypertens. 2004, 22, 1529–1534. [Google Scholar] [CrossRef]
- Koldas, L.; Ayan, F.; Ikitimur, B. Short term effects of rilmenidine on left ventricular hypertrophy and systolic and diastolic function in patients with essential hypertension comparison with an angiotensin converting enzyme inhibitor and a calcium antagonist. Jpn. Heart J. 2003, 44, 693–704. [Google Scholar] [CrossRef]
- Fisher, J.P.; Fadel, P.J. Therapeutic Strategies for Targeting Excessive Central Sympathetic Activation in Human Hypertension; Wiley Online Library: Hoboken, NJ, USA, 2010. [Google Scholar]
- Morrissey, D.; Brookes, V.; Cooke, W. Sympathectomy in the treatment of hypertension review of 122 cases. Lancet 1953, 261, 403–408. [Google Scholar] [CrossRef]
- Allen, E.V. Sympathectomy for essential hypertension. Circulation 1952, 6, 131–140. [Google Scholar]
- Grimson, K.S.; Orgain, E.S.; Anderson, B.; D’Angelo, G.J. Total thoracic and partial to total lumbar sympathectomy, splanchnicectomy and celiac ganglionectomy for hypertension. Ann. Surg. 1953, 138, 532. [Google Scholar] [CrossRef]
- CHAPMAN, E.M.; KINSEY, D.; Chapman, W.; SMITHWICK, R.H. Sympathetic Innervation of the Heart in Man: Preliminary Observations of the Effect of Thoracic Sympathectomy on Heart Rate. J. Am. Med. Assoc. 1948, 137, 579–584. [Google Scholar] [CrossRef]
- Al Raisi, S.I.; Pouliopoulos, J.; Swinnen, J.; Thiagalingam, A.; Kovoor, P. Renal artery denervation in resistant hypertension: The good, the bad and the future. Heart Lung Circ. 2020, 29, 94–101. [Google Scholar]
- Singh, R.R.; Denton, K.M. Renal Denervation: A treatment for hypertension and chronic kidney disease. Hypertension 2018, 72, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Krum, H.; Schlaich, M.; Whitbourn, R.; Sobotka, P.A.; Sadowski, J.; Bartus, K.; Kapelak, B.; Walton, A.; Sievert, H.; Thambar, S. Catheter-based renal sympathetic denervation for resistant hypertension: A multicentre safety and proof-of-principle cohort study. Lancet 2009, 373, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Krum, H.; Schlaich, M.P.; Sobotka, P.A.; Böhm, M.; Mahfoud, F.; Rocha-Singh, K.; Katholi, R.; Esler, M.D. Percutaneous renal denervation in patients with treatment-resistant hypertension: Final 3-year report of the Symplicity HTN-1 study. Lancet 2014, 383, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Esler, M. Differentiation in the effects of the angiotensin II receptor blocker class on autonomic function. J. Hypertension. Suppl. Off. J. Int. Soc. Hypertens. 2002, 20, S13–S19. [Google Scholar]
- F.N. RELEASE. FDA Approves New Device to Improve Symptoms in Patients with Advanced Heart Failure. 2019. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-new-device-improve-symptoms-patients-advanced-heart-failure (accessed on 24 October 2022).
- Kristensen, A.M.D.; Pareek, M.; Olsen, M.H.; Bhatt, D.L. Baroreflex Activation Therapy for Resistant Hypertension and Heart Failure. J. US Cardiol. Rev. 2020, 13, 83–87. [Google Scholar] [CrossRef]
- Victor, R.G. Carotid baroreflex activation therapy for resistant hypertension. Nat. Rev. Cardiol. 2015, 12, 451–463. [Google Scholar] [CrossRef]
- Duncker, D.; Bauersachs, J. Current and future use of neuromodulation in heart failure. Eur. Heart J. Suppl. 2022, 24, E28–E34. [Google Scholar] [CrossRef]
- Lepori, M.; Sartori, C.; Trueb, L.; Owlya, R.; Nicod, P.; Scherrer, U. Haemodynamic and sympathetic effects of inhibition of nitric oxide synthase by systemic infusion of NG-monomethyl-L-arginine into humans are dose dependent. J. Hypertens. 1998, 16, 519–523. [Google Scholar] [CrossRef]
- Sander, M.; Chavoshan, B.; Victor, R.G. A large blood pressure–raising effect of nitric oxide synthase inhibition in humans. Hypertension 1999, 33, 937–942. [Google Scholar] [CrossRef]
- Rajapakse, N.W.; Mattson, D.L. Role of L-arginine in nitric oxide production in health and hypertension. Clin. Exp. Pharmacol. Physiol. 2008, 36, 249–255. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Tanus-Santos, J.E.; Castro, M.M. The potential of stimulating nitric oxide formation in the treatment of hypertension. Expert Opin. Ther. Targets 2017, 21, 543–556. [Google Scholar] [CrossRef]
- Roberts, J.D., Jr.; Fineman, J.R.; Morin, F.C.; Shaul, P.W.; Rimar, S.; Schreiber, M.D.; Polin, R.A.; Zwass, M.S.; Zayek, M.M.; Gross, I. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N. Engl. J. Med. 1997, 336, 605–610. [Google Scholar] [CrossRef]
- Gastelurrutia, P.; Lupón, J.; De Antonio, M.; Urrutia, A.; Díez, C.; Coll, R.; Altimir, S.; Bayes-Genis, A. Statins in heart failure: The paradox between large randomized clinical trials and real life. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2012; pp. 555–560. [Google Scholar]
- McGown, C.; Brookes, Z. Beneficial effects of statins on the microcirculation during sepsis: The role of nitric oxide. Br. J. Anaesth. 2007, 98, 163–175. [Google Scholar] [CrossRef]
- Greenwood, J.; Mason, J.C. Statins and the vascular endothelial inflammatory response. Trends Immunol. 2007, 28, 88–98. [Google Scholar] [CrossRef]
- Endres, M. Statins: Potential new indications in inflammatory conditions. Atheroscler. Suppl. 2006, 7, 31–35. [Google Scholar] [CrossRef]
- Pliquett, R.U.; Cornish, K.G.; Peuler, J.D.; Zucker, I.H. Simvastatin normalizes autonomic neural control in experimental heart failure. Circulation 2003, 107, 2493–2498. [Google Scholar] [CrossRef]
- Pliquett, R.U.; Cornish, K.G.; Zucker, I.H. Statin therapy restores sympathovagal balance in experimental heart failure. J. Appl. Physiol. 2003, 95, 700–704. [Google Scholar] [CrossRef]
- Kizhakekuttu, T.J.; Widlansky, M.E. Natural antioxidants and hypertension: Promise and challenges. Cardiovasc. Ther. 2010, 28, e20–e32. [Google Scholar] [CrossRef]
- Li, Y.; Qin, C.; Dong, L.; Zhang, X.; Wu, Z.; Liu, L.; Yang, J.; Liu, L. Whole grain benefit: Synergistic effect of oat phenolic compounds and β-glucan on hyperlipidemia via gut microbiota in high-fat-diet mice. Food Funct. 2022, 13, 12686–12696. [Google Scholar] [CrossRef]
- Dong, L.; Qin, C.; Li, Y.; Wu, Z.; Liu, L. Oat phenolic compounds regulate metabolic syndrome in high fat diet-fed mice via gut microbiota. Food Biosci. 2022, 50, 101946. [Google Scholar] [CrossRef]
- Sorriento, D.; De Luca, N.; Trimarco, B.; Iaccarino, G. The antioxidant therapy: New insights in the treatment of hypertension. Front. Physiol. 2018, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A. Fluids and barriers of the CNS: A historical viewpoint. Fluids Barriers CNS 2011, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, M.J. The cerebral circulation. Integr. Syst. Physiol. Mol. Funct. 2009, 1, 1–59. [Google Scholar] [CrossRef] [PubMed]
- Waki, H.; Gouraud, S.S.; Maeda, M.; Raizada, M.K.; Paton, J.F. Contributions of vascular inflammation in the brainstem for neurogenic hypertension. Respir. Physiol. Neurobiol. 2011, 178, 422–428. [Google Scholar] [CrossRef]
- Setiadi, A.; Korim, W.S.; Elsaafien, K.; Yao, S.T. The role of the blood–brain barrier in hypertension. Exp. Physiol. 2018, 103, 337–342. [Google Scholar] [CrossRef]
- Ho, J.K.; Moriarty, F.; Manly, J.J.; Larson, E.B.; Evans, D.A.; Rajan, K.B.; Hudak, E.M.; Hassan, L.; Liu, E.; Sato, N. Blood-brain barrier crossing renin-angiotensin drugs and cognition in the elderly: A meta-analysis. Hypertension 2021, 78, 629–643. [Google Scholar] [CrossRef]
- Kawabe, H.; Kondo, K.; Saruta, T. Effect of the intracerebroventricular injection of dopamine on blood pressure in the spontaneously hypertensive rat. Clin. Exp. Hypertension. Part A Theory Pract. 1983, 5, 1703–1716. [Google Scholar] [CrossRef]
- Nakamura, K.; Sasaki, S.; Moriguchi, J.; Morimoto, S.; Miki, S.; Kawa, T.; Itoh, H.; Nakata, T.; Takeda, K.; Nakagawa, M. Central effects of endothelin and its antagonists on sympathetic and cardiovascular regulation in SHR-SP. J. Cardiovasc. Pharmacol. 1999, 33, 876–882. [Google Scholar] [CrossRef]
- Takenaka, K.; Sasaki, S.; Uchida, A.; Fujita, H.; Nakamura, K.; Ichida, T.; Itoh, H.; Nakata, T.; Takeda, K.; Nakagawa, M. GABAB-ergic stimulation in hypothalamic pressor area induces larger sympathetic and cardiovascular depression in spontaneously hypertensive rats. Am. J. Hypertens. 1996, 9, 964–972. [Google Scholar] [CrossRef]
- Kumar, A.; Roy, S.; Srivastava, A.; Naikwade, M.M.; Purohit, B.; Mahato, K.; Naidu, V.; Chandra, P. Nanotherapeutics: A novel and powerful approach in modern healthcare system. In Nanotechnology in Modern Animal Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–161. [Google Scholar]
- Prasad, M.; Lambe, U.P.; Brar, B.; Shah, I.; Manimegalai, J.; Ranjan, K.; Rao, R.; Kumar, S.; Mahant, S.; Khurana, S.K. Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed. Pharmacother. 2018, 97, 1521–1537. [Google Scholar] [CrossRef]
- Arora, S.; Trivedi, R.; Lamptey, R.N.; Chaulagain, B.; Layek, B.; Singh, J. Smart biopolymers for controlled drug delivery applications. In Tailor-Made and Functionalized Biopolymer Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 53–83. [Google Scholar]
- Croissant, J.G.; Butler, K.S.; Zink, J.I.; Brinker, C.J. Synthetic amorphous silica nanoparticles: Toxicity, biomedical and environmental implications. Nat. Rev. Mater. 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Yi, S.; Zhang, X.; Sangji, M.H.; Liu, Y.; Allen, S.D.; Xiao, B.; Bobbala, S.; Braverman, C.L.; Cai, L.; Hecker, P.I. Surface engineered polymersomes for enhanced modulation of dendritic cells during cardiovascular immunotherapy. Adv. Funct. Mater. 2019, 29, 1904399. [Google Scholar] [CrossRef]
- Mishra, M.; Muthuramu, I.; Kempen, H.; De Geest, B. Administration of apo AI (Milano) nanoparticles reverses pathological remodelling, cardiac dysfunction, and heart failure in a murine model of HFpEF associated with hypertension. Sci. Rep. 2020, 10, 8382. [Google Scholar] [CrossRef]
- Fancher, I.S.; Rubinstein, I.; Levitan, I. Potential strategies to reduce blood pressure in treatment-resistant hypertension using food and drug administration–approved nanodrug delivery platforms. Hypertension 2019, 73, 250–257. [Google Scholar] [CrossRef]
- Horejs, C. Nebulized lipid nanoparticles. Nat. Rev. Mater. 2021, 6, 1077. [Google Scholar] [CrossRef]
- Rossi, L.T.R.; Nunes, G.B.; da Silva, C.R.; de Rossi, H.; Dos Santos, P.H.; Nogueira, M.F.G.; Aoki, P.H.B.; Mingoti, G.Z. Use of giant unilamellar lipid vesicles as antioxidant carriers in in vitro culture medium of bovine embryos. Sci. Rep. 2022, 12, 11228. [Google Scholar] [CrossRef]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Editorial. Let’s talk about lipid nanoparticles. Nat. Rev. Mater. 2021, 6, 99. [Google Scholar] [CrossRef]
- Dos Santos Rodrigues, B.; Arora, S.; Kanekiyo, T.; Singh, J. Efficient neuronal targeting and transfection using RVG and transferrin-conjugated liposomes. Brain Res. 2020, 1734, 146738. [Google Scholar] [CrossRef] [PubMed]
- Lakkadwala, S.; Singh, J. Co-delivery of doxorubicin and erlotinib through liposomal nanoparticles for glioblastoma tumor regression using an in vitro brain tumor model. Colloids Surf. B Biointerfaces 2019, 173, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Leonor Pinzon-Daza, M.; Campia, I.; Kopecka, J.; Garzón, R.; Ghigo, D.; Rigant, C. Nanoparticle- and liposome-carried drugs: New strategies for active targeting and drug delivery across blood-brain barrier. Curr. Drug Metab. 2013, 14, 625–640. [Google Scholar] [CrossRef]
- Trivedi, R.; Arora, S.; Lamptey, R.N.; Chaulagain, B.; Singh, J.; Layek, B. A Summarized View of Lipid, Polyplex, Inorganic, and Carbon-Based Nanotherapeutics for Hepatocellular Carcinoma Treatment. In Nanotherapeutics for the Treatment of Hepatocellular Carcinoma; Bentham Science Publishers: Sharjah, United Arab Emirates, 2022; p. 248. [Google Scholar]
- Arora, S.; Layek, B.; Singh, J. Design and validation of liposomal ApoE2 gene delivery system to evade blood–brain barrier for effective treatment of Alzheimer’s disease. Mol. Pharm. 2020, 18, 714–725. [Google Scholar] [CrossRef]
- Gabizon, A.; Martin, F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Drugs 1997, 54, 15–21. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Hodis, H.N.; Amartey, J.K.; Crawford, D.W.; Wickham, E.; Blankenhorn, D.H. In vivo hypertensive arterial wall uptake of radiolabeled liposomes. Hypertension 1990, 15, 600–605. [Google Scholar] [CrossRef]
- Ali El-Basyuni, Y.; Li, Y.; Anand-Srivastava, M.B. Knockdown of Inhibitory Guanine Nucleotide Binding Protein Giα-2 by Antisense Oligodeoxynucleotides Attenuates the Development of Hypertension and Tachycardia in Spontaneously Hypertensive Rats. J. Am. Heart Assoc. 2016, 5, e004594. [Google Scholar] [CrossRef]
- Wielbo, D.; Simon, A.; Phillips, M.I.; Toffolo, S. Inhibition of hypertension by peripheral administration of antisense oligodeoxynucleotides. Hypertension 1996, 28, 147–151. [Google Scholar] [CrossRef]
- Laursen, J.B.; Rajagopalan, S.; Galis, Z.; Tarpey, M.; Freeman, B.A.; Harrison, D.G. Role of superoxide in angiotensin II–induced but not catecholamine-induced hypertension. Circulation 1997, 95, 588–593. [Google Scholar] [CrossRef]
- Dos Santos Rodrigues, B.; Oue, H.; Banerjee, A.; Kanekiyo, T.; Singh, J. Dual functionalized liposome-mediated gene delivery across triple co-culture blood brain barrier model and specific in vivo neuronal transfection. J. Control. Release 2018, 286, 264–278. [Google Scholar] [CrossRef]
- Szebeni, J.; Fontana, J.L.; Wassef, N.M.; Mongan, P.D.; Morse, D.S.; Dobbins, D.E.; Stahl, G.L.; Bünger, R.; Alving, C.R. Hemodynamic changes induced by liposomes and liposome-encapsulated hemoglobin in pigs: A model for pseudoallergic cardiopulmonary reactions to liposomes: Role of complement and inhibition by soluble CR1 and anti-C5a antibody. Circulation 1999, 99, 2302–2309. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Yen, T.-L.; Jan, J.-S.; Tang, R.-D.; Wang, J.-Y.; Taliyan, R.; Yang, C.-H. Solid lipid nanoparticles (SLNs): An advanced drug delivery system targeting brain through BBB. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-based nanoparticles: Application and recent advances in cancer treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Shankar, R.; Joshi, M.; Pathak, K. Lipid nanoparticles: A novel approach for brain targeting. Pharm. Nanotechnol. 2018, 6, 81–93. [Google Scholar] [CrossRef]
- Ju, J.; Huan, M.-L.; Wan, N.; Hou, Y.-L.; Ma, X.-X.; Jia, Y.-Y.; Li, C.; Zhou, S.-Y.; Zhang, B.-L. Cholesterol derived cationic lipids as potential non-viral gene delivery vectors and their serum compatibility. Bioorganic Med. Chem. Lett. 2016, 26, 2401–2407. [Google Scholar] [CrossRef]
- Neves, A.R.; Queiroz, J.F.; Weksler, B.; Romero, I.A.; Couraud, P.-O.; Reis, S. Solid lipid nanoparticles as a vehicle for brain-targeted drug delivery: Two new strategies of functionalization with apolipoprotein E. Nanotechnology 2015, 26, 495103. [Google Scholar] [CrossRef]
- Pandya, N.T.; Jani, P.; Vanza, J.; Tandel, H. Solid lipid nanoparticles as an efficient drug delivery system of olmesartan medoxomil for the treatment of hypertension. Colloids Surf. B Biointerfaces 2018, 165, 37–44. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, F.; Bu, H.; Xiao, J.; Li, Y. Solid lipid nanoparticles loading candesartan cilexetil enhance oral bioavailability: In vitro characteristics and absorption mechanism in rats. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 740–747. [Google Scholar] [CrossRef]
- Dudhipala, N.; Veerabrahma, K. Candesartan cilexetil loaded solid lipid nanoparticles for oral delivery: Characterization, pharmacokinetic and pharmacodynamic evaluation. Drug Deliv. 2016, 23, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Imam, S.S.; Fazil, M.; Khan, S.; Hafeez, A.; Ahmad, F.J.; Ali, A. Formulation and optimization of candesartan cilexetil nano lipid carrier: In vitro and in vivo evaluation. Curr. Drug Deliv. 2017, 14, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Dudhipala, N.; Veerabrahma, K. Pharmacokinetic and pharmacodynamic studies of nisoldipine-loaded solid lipid nanoparticles developed by central composite design. Drug Dev. Ind. Pharm. 2015, 41, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Havanoor, S.M.; Manjunath, K.; Bhagawati, S.T.; Veerapur, V.P. Isradipine loaded solid lipid nanoparticles for better treatment of hypertension–preparation, characterization and in vivo evaluation. Int. J. Biopharm. 2014, 5, 218–224. [Google Scholar]
- Bhalerao, A.; Chaudhari, P.P. Formulation of solid lipid nanoparticles of cilnidipine for the treatment of hypertension. J. Drug Deliv. Ther. 2019, 9, 212–221. [Google Scholar] [CrossRef]
- Sheoran, S.; Arora, S.; Pilli, G. Lipid Based Nanoparticles For Treatment Of Cancer. Heliyon 2022, 8, e09403. [Google Scholar] [CrossRef]
- Elmowafy, M.; Al-Sanea, M.M. Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies. Saudi Pharm. J. 2021, 29, 999–1012. [Google Scholar] [CrossRef]
- Pg, M.; Somasundaram, I. Enhancement Of Bioavilability of Perinodopril Using Lipid Based Nanocarrier Mediated Oral Drug Delivery System. J. Posit. Sch. Psychol. 2022, 6, 1547–1552. [Google Scholar]
- Eleraky, N.E.; Omar, M.M.; Mahmoud, H.A.; Abou-Taleb, H.A. Nanostructured lipid carriers to mediate brain delivery of temazepam: Design and in vivo study. Pharmaceutics 2020, 12, 451. [Google Scholar] [CrossRef]
- Alam, T.; Ansari, M.A.; Baboota, S.; Ali, J. Nanostructured lipid carriers of isradipine for effective management of hypertension and isoproterenol induced myocardial infarction. Drug Deliv. Transl. Res. 2022, 12, 577–588. [Google Scholar] [CrossRef]
- Kaithwas, V.; Dora, C.P.; Kushwah, V.; Jain, S. Nanostructured lipid carriers of olmesartan medoxomil with enhanced oral bioavailability. Colloids Surf. B Biointerfaces 2017, 154, 10–20. [Google Scholar] [CrossRef]
- Kataria, D.; Zafar, A.; Ali, J.; Khatoon, K.; Khan, S.; Imam, S.S.; Yasir, M.; Ali, A. Formulation of Lipid-Based Nanocarriers of Lacidipine for Improvement of Oral Delivery: Box-Behnken Design Optimization, In Vitro, Ex Vivo, and Preclinical Assessment. ASSAY Drug Dev. Technol. 2022, 20, 5–21. [Google Scholar] [CrossRef]
- Poonia, N.; Kharb, R.; Lather, V.; Pandita, D. Nanostructured lipid carriers: Versatile oral delivery vehicle. Future Sci. OA 2016, 2, FSO135. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, D. Improvement of oral bioavailability of lovastatin by using nanostructured lipid carriers. Drug Des. Dev. Ther. 2015, 9, 5269. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Layek, B.; Singh, J. Amino acid grafted chitosan for high performance gene delivery: Comparison of amino acid hydrophobicity on vector and polyplex characteristics. Biomacromolecules 2013, 14, 485–494. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Liu, Y.-S. Nanoparticles in the diagnosis and treatment of vascular aging and related diseases. Signal Transduct. Target. Ther. 2022, 7, 231. [Google Scholar] [CrossRef]
- Leong, J.; Teo, J.Y.; Aakalu, V.K.; Yang, Y.Y.; Kong, H. Engineering polymersomes for diagnostics and therapy. Adv. Healthc. Mater. 2018, 7, 1701276. [Google Scholar] [CrossRef]
- Danafar, H.; Manjili, H.; Najafi, M. Study of copolymer composition on drug loading efficiency of enalapril in polymersomes and cytotoxicity of drug loaded nanoparticles. Drug Res. 2016, 66, 495–504. [Google Scholar] [CrossRef]
- Xia, W.; Liu, P.; Zhang, J.; Chen, J. Biological activities of chitosan and chitooligosaccharides. Food Hydrocoll. 2011, 25, 170–179. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Gothwal, A.; Trivedi, R.; Arora, S.; Singh, J. Synthesis and Characterization of Fatty Acid Grafted Chitosan Polymeric Micelles for Improved Gene Delivery of VGF to the Brain through Intranasal Route. Biomedicines 2022, 10, 493. [Google Scholar] [CrossRef] [PubMed]
- Chadha, R.; Bhandari, S.; Kataria, D.; Gupta, S. Exploring lecithin/chitosan nanoparticles of ramipril for improved antihypertensive efficacy. J. Nanopharm. Drug Deliv. 2013, 1, 173–181. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, R.; Jain, D.K. Preparation, characterization and evaluation of nebivolol loaded chitosan nanoparticles. J. Drug Deliv. Ther. 2018, 8, 118–122. [Google Scholar] [CrossRef]
- Auwal, S.M.; Zarei, M.; Tan, C.P.; Basri, M.; Saari, N. Improved in vivo efficacy of anti-hypertensive biopeptides encapsulated in chitosan nanoparticles fabricated by ionotropic gelation on spontaneously hypertensive rats. Nanomaterials 2017, 7, 421. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Dutta, N.K.; Baek, M.-W.; Kim, D.-J.; Na, Y.-R.; Seok, S.-H.; Lee, B.-H.; Cho, J.-E.; Cho, G.-S.; Park, J.-H. NaCl plus chitosan as a dietary salt to prevent the development of hypertension in spontaneously hypertensive rats. J. Vet. Sci. 2009, 10, 141–146. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, P.; Liu, Y.; Wu, Z.; Wang, L.; Liu, L. Citrus pectin research advances: Derived as a biomaterial in the construction and applications of micro/nano-delivery systems. Food Hydrocoll. 2022, 133, 107910. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, M.; Li, Y.; Li, Y.; Ji, H.; Hu, Q. Cardiovascular protective effects of plant polysaccharides: A review. Front. Pharmacol. 2021, 12, 783641. [Google Scholar] [CrossRef]
- Xie, M.; Tao, W.; Wu, F.; Wu, K.; Huang, X.; Ling, G.; Zhao, C.; Lv, Q.; Wang, Q.; Zhou, X. Anti-hypertensive and cardioprotective activities of traditional Chinese medicine-derived polysaccharides: A review. Int. J. Biol. Macromol. 2021, 185, 917–934. [Google Scholar] [CrossRef]
- Li, Y.; Dong, L.; Mu, Z.; Liu, L.; Yang, J.; Wu, Z.; Pan, D.; Liu, L. Research Advances of Lactoferrin in Electrostatic Spinning, Nano Self-Assembly, and Immune and Gut Microbiota Regulation. J. Agric. Food Chem. 2022, 70, 10075–10089. [Google Scholar] [CrossRef]
- Fillebeen, C.; Descamps, L.; Dehouck, M.-P.; Fenart, L.; Benaıssa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef]
- Sabra, S.; Agwa, M.M. Lactoferrin, a unique molecule with diverse therapeutical and nanotechnological applications. Int. J. Biol. Macromol. 2020, 164, 1046–1060. [Google Scholar] [CrossRef]
- Kalomiraki, M.; Thermos, K.; Chaniotakis, N.A. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 2016, 11, 1–12. [Google Scholar]
- Caminade, A.-M.; Turrin, C.-O. Dendrimers for drug delivery. J. Mater. Chem. B 2014, 2, 4055–4066. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for drug delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Liu, M.; Fréchet, J.M. Designing dendrimers for drug delivery. Pharm. Sci. Technol. Today 1999, 2, 393–401. [Google Scholar] [CrossRef]
- Spataro, G.; Malecaze, F.; Turrin, C.-O.; Soler, V.; Duhayon, C.; Elena, P.-P.; Majoral, J.-P.; Caminade, A.-M. Designing dendrimers for ocular drug delivery. Eur. J. Med. Chem. 2010, 45, 326–334. [Google Scholar] [CrossRef]
- Mignani, S.; El Kazzouli, S.; Bousmina, M.; Majoral, J.-P. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: A concise overview. Adv. Drug Deliv. Rev. 2013, 65, 1316–1330. [Google Scholar] [CrossRef]
- Singh, M.K.; Pooja, D.; Kulhari, H.; Jain, S.K.; Sistla, R.; Chauhan, A.S. Poly (amidoamine) dendrimer-mediated hybrid formulation for combination therapy of ramipril and hydrochlorothiazide. Eur. J. Pharm. Sci. 2017, 96, 84–92. [Google Scholar] [CrossRef]
- Jose, S.; Sowmya, S.; Cinu, T.; Aleykutty, N.; Thomas, S.; Souto, E. Surface modified PLGA nanoparticles for brain targeting of Bacoside-A. Eur. J. Pharm. Sci. 2014, 63, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle surface functionalization: How to improve biocompatibility and cellular internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Maleki Dizaj, S.; Yari Khosroushahi, A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol. Int. 2015, 39, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Li, C.; Li, L.; Guo, J.; Zhang, J. Bioresponsive drug delivery systems for the treatment of inflammatory diseases. J. Control. Release Off. J. Control. Release Soc. 2020, 327, 641–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, L.; Liu, L.; Wu, Z.; Pan, D.; Liu, L. Recent Advances of Stimuli-Responsive Polysaccharide Hydrogels in Delivery Systems: A Review. J. Agric. Food Chem. 2022, 70, 6300–6316. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: A narrative review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef]
- Chang, B.; Chen, D.; Wang, Y.; Chen, Y.; Jiao, Y.; Sha, X.; Yang, W. Bioresponsive controlled drug release based on mesoporous silica nanoparticles coated with reductively sheddable polymer shell. Chem. Mater. 2013, 25, 574–585. [Google Scholar] [CrossRef]
- Xie, J.; Gonzalez-Carter, D.; Tockary, T.A.; Nakamura, N.; Xue, Y.; Nakakido, M.; Akiba, H.; Dirisala, A.; Liu, X.; Toh, K. Dual-sensitive nanomicelles enhancing systemic delivery of therapeutically active antibodies specifically into the brain. ACS Nano 2020, 14, 6729–6742. [Google Scholar] [CrossRef]
- Trevino, J.; Quispe, R.; Khan, F.; Novak, V. Non-invasive strategies for nose-to-brain drug delivery. J. Clin. Trials 2020, 10, 439. [Google Scholar]
- Wu, H.; Hu, K.; Jiang, X. From nose to brain: Understanding transport capacity and transport rate of drugs. Expert Opin. Drug Deliv. 2008, 5, 1159–1168. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, G.; Tsang, W.C.; Schätzlein, A.G.; Uchegbu, I.F. Nose-to-brain delivery. J. Pharmacol. Exp. Ther. 2019, 370, 593–601. [Google Scholar] [CrossRef]
- Jiao, L.; Yang, Y.; Yu, W.; Zhao, Y.; Long, H.; Gao, J.; Ding, K.; Ma, C.; Li, J.; Zhao, S. The olfactory route is a potential way for SARS-CoV-2 to invade the central nervous system of rhesus monkeys. Signal Transduct. Target. Ther. 2021, 6, 169. [Google Scholar] [CrossRef]
- Nampoothiri, S.; Sauve, F.; Ternier, G.; Fernandois, D.; Coelho, C.; Imbernon, M.; Deligia, E.; Perbet, R.; Florent, V.; Baroncini, M. The hypothalamus as a hub for SARS-CoV-2 brain infection and pathogenesis. BioRxiv 2020. [Google Scholar] [CrossRef]

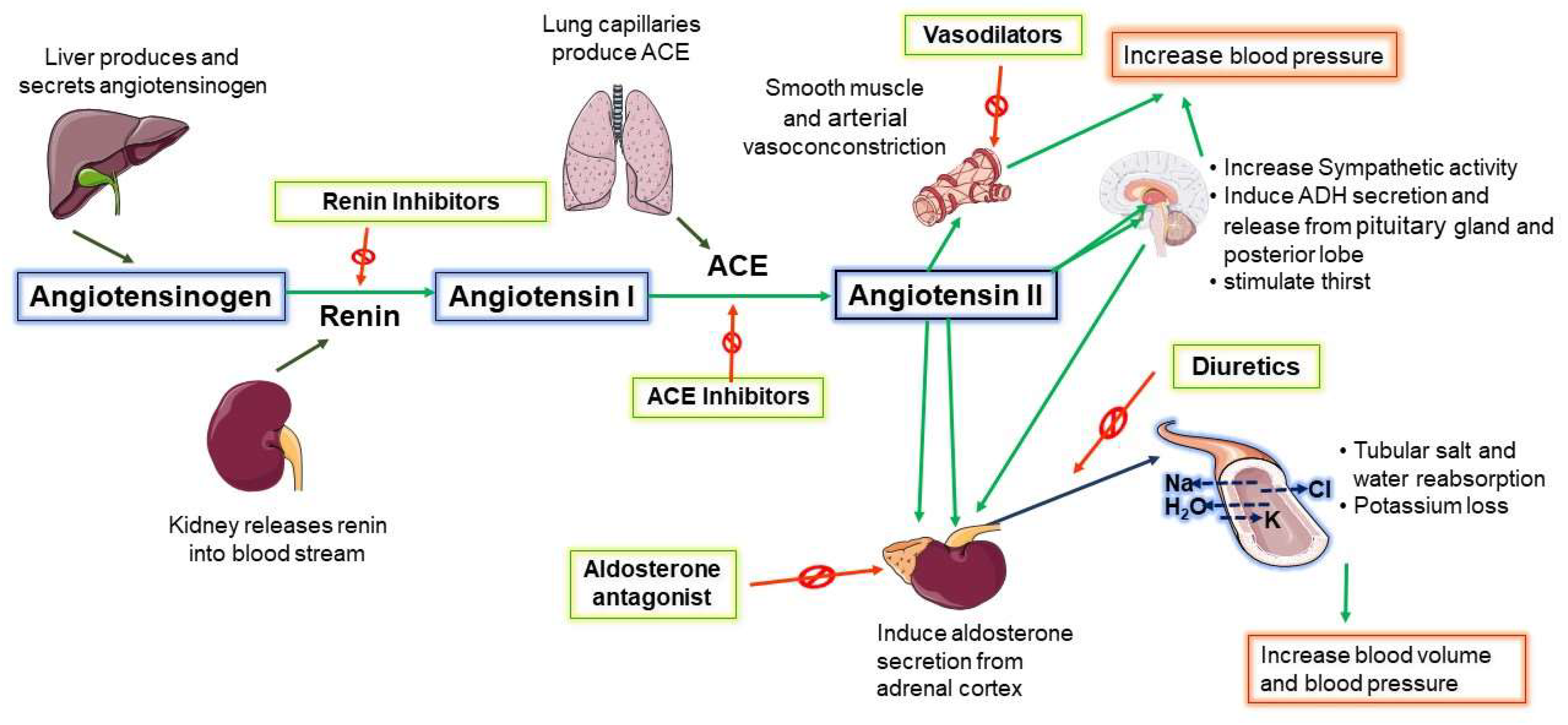
| Drug Class | Mechanism | Examples | Special Notes |
|---|---|---|---|
| ACE inhibitors | Block the action of ACE to prevent the conversion of Ang I to Ang II | Benazepril, captopril, enalapril, fosinopril, lisinopril, moexipril, perindopril, quinapril, ramipril, trandolapril | Used in combination with other therapeutic agents [99]. |
| ARBs | Reduce the action of Ang II at its receptor by blocking it, causing vasodilation. | Azilsartan, eprosartan, losartan, irbesartan, olmesartan, valsartan, telmisartan, candesartan | Eprosartan possesses sympathoinhibitory [100]. |
| Calcium channel blockers | Prevents calcium influx into heart and arterial cells by blocking the calcium channels. | Nifedipine, amlodipine, felodipine, nicardipine, isradipine, nisoldipine, diltiazem, verapamil | |
| Diuretics | Induce excretion of body salt and water by aiding in the movement of sodium into the urine. | Chlorothiazide,# indapamide,# metolazone,# chlorthalidone,# hydrochlorothiazide,# bumetanide,* ethacrynic acid,* furosemide,* torsemide,* spironolactone,& amiloride,& eplerenone,& triamterene& | They can be used as monotherapy or in combination. Three types of diuretics are in clinical use: thiazide,# loop,* and potassium sparing& |
| Aldosterone antagonist | Block the action of aldosterone resulting in salt and water loss. | Spironolactone, eplerenone, finerenone | |
| α-blockers | Partially block alpha-adrenergic receptor activity. | Doxazosin, prazosin, terazosin | They are used in combination therapies. Improves urine flow in older men with prostate problems. |
| Vasodilators | Improve blood flow by relaxing blood vessels. | Hydralazine, minoxidil | |
| β-blockers | Block beta receptors | Atenolol, pindolol, metoprolol, propranolol, bisoprolol, timolol, labetalol, carvedilol, acebutolol | Not generally recommended as first-line drugs |
| Renin-inhibitors | Inhibit the enzyme renin from triggering a process that helps regulate BP. | Aliskiren | Have an additive effect when used with diuretics |
| Centrally acting antihypertensives (α-2 agonist) | Stimulate presynaptic alpha2-adrenergic receptors in the brain stem, which reduces SNA | Methyldopa, clonidine, guanfacine |
| Study Title | Status | Interventions | Primary Measured Outcome | Clinicaltrials.gov Identifier |
|---|---|---|---|---|
| Efficacy and safety of renal sympathetic denervation from the adventitia on resistant hypertension | Unknown | Renal denervation via radiofrequency ablation instruments | Change in 24-hour average systolic BP | NCT03758196 |
| A pragmatic randomized clinical evaluation of renal denervation for treatment resistant hypertension | Terminated | Renal denervation | Average systolic 24-hour ambulatory BP | NCT01895140 |
| Renablate feasibility study cs156 (EC12-02) study of catheter based renal denervation to treat resistant hypertension | Completed | Renal denervation using celsius® thermocool® | Incidence of major cardiovascular and/or renal adverse events related to the renal denervation procedure that occurred within 30 days post-procedure. | NCT01756300 |
| The effect of baton bp and sympathetic function in resistant hypertension (the Nordic BAT study) (BAT) | Active | Baroreflex activation therapy | Change in systolic ambulatory BP in response to bat therapy | NCT02572024 |
| Renal denervation in treatment resistant hypertension (ReSET-2) | Terminated | Ablation of the renal arteries | Change from baseline in daytime systolic BP (24-hour ambulatory bp measurement | NCT01762488 |
| Treatment of resistant hypertension using renal denervation in china (REDUCE-HTN-CN) | Terminated | Percutaneous renal denervation with the vessix™ renal denervation system | The mean reduction of systolic bp measured using office-based BP assessment | NCT02027012 |
| Treatment of resistant hypertension using a radiofrequency percutaneous transluminal angioplasty catheter (REDUCE-HTN) | Completed | Renal denervation | Change in systolic and diastolic bp at six (6) months as measured using office-based BP assessment | NCT01541865 |
| Rapid renal sympathetic denervation for resistant hypertension ii (RAPID II) | Withdrawn | Renal denervation using one-shot™ renal denervation system) | Major adverse event (MAE) rate through 30 days post-randomization | NCT01939392 |
| Renal sympathetic denervation in patients with drug-resistant hypertension and symptomatic atrial fibrillation | Unknown | Renal sympathetic denervation | Change in atrial fibrillation burden | NCT01713270 |
| Feasibility study of renal denervation for the treatment of resistant hypertension | Unknown | Ultrasound-based renal denervation | Major adverse events | NCT01865591 |
| Renal denervation for management of drug-resistant hypertension (INSPiRED) | Completed | Renal denervation | Change in systolic BP from baseline to 6 months on 24-h ambulatory measurement | NCT01505010 |
| Randomized controlled trial of renal denervation for resistant hypertension | Unknown | Renal denervation using radiofrequency ablation catheter with drug treatment: amlodipine, losartan potassium and hydrochlorothiazide | Change in average 24-hour systolic BP using ambulatory bp monitoring from baseline | NCT02900729 |
| Renal denervation in treatment resistant hypertension | Completed | Renal denervation using symplicity catheter system | Change in office BP from baseline to 6 months post-renal denervation | NCT01687725 |
| Effects of renal sympathetic denervation on the cardiac and renal functions in patients with drug-resistant hypertension through mri evaluation (RDN) | Unknown | Renal denervation (enligHTN™) with the enligHTN™ renal denervation system. | Cardiac function (evaluated using MRI) | NCT02164435 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamptey, R.N.L.; Sun, C.; Layek, B.; Singh, J. Neurogenic Hypertension, the Blood–Brain Barrier, and the Potential Role of Targeted Nanotherapeutics. Int. J. Mol. Sci. 2023, 24, 2213. https://doi.org/10.3390/ijms24032213
Lamptey RNL, Sun C, Layek B, Singh J. Neurogenic Hypertension, the Blood–Brain Barrier, and the Potential Role of Targeted Nanotherapeutics. International Journal of Molecular Sciences. 2023; 24(3):2213. https://doi.org/10.3390/ijms24032213
Chicago/Turabian StyleLamptey, Richard Nii Lante, Chengwen Sun, Buddhadev Layek, and Jagdish Singh. 2023. "Neurogenic Hypertension, the Blood–Brain Barrier, and the Potential Role of Targeted Nanotherapeutics" International Journal of Molecular Sciences 24, no. 3: 2213. https://doi.org/10.3390/ijms24032213
APA StyleLamptey, R. N. L., Sun, C., Layek, B., & Singh, J. (2023). Neurogenic Hypertension, the Blood–Brain Barrier, and the Potential Role of Targeted Nanotherapeutics. International Journal of Molecular Sciences, 24(3), 2213. https://doi.org/10.3390/ijms24032213






