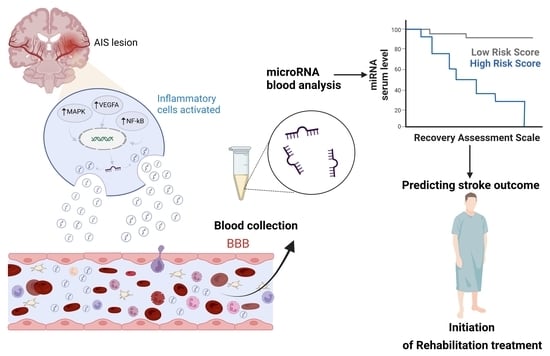
Circulating MicroRNAs and Extracellular Vesicle-Derived MicroRNAs as Predictors of Functional Recovery in Ischemic Stroke Patients: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Included Studies
3.2. Methodological Assessment
3.3. Collection and Profiling of miRNAs-Based Biomarkers
3.4. Prognostic Tools and Prediction of Physical Recovery in IS Patients
3.5. Clinical Utility of miRNA-Based Biomarkers in Prediction of Neurological Recovery after Stroke
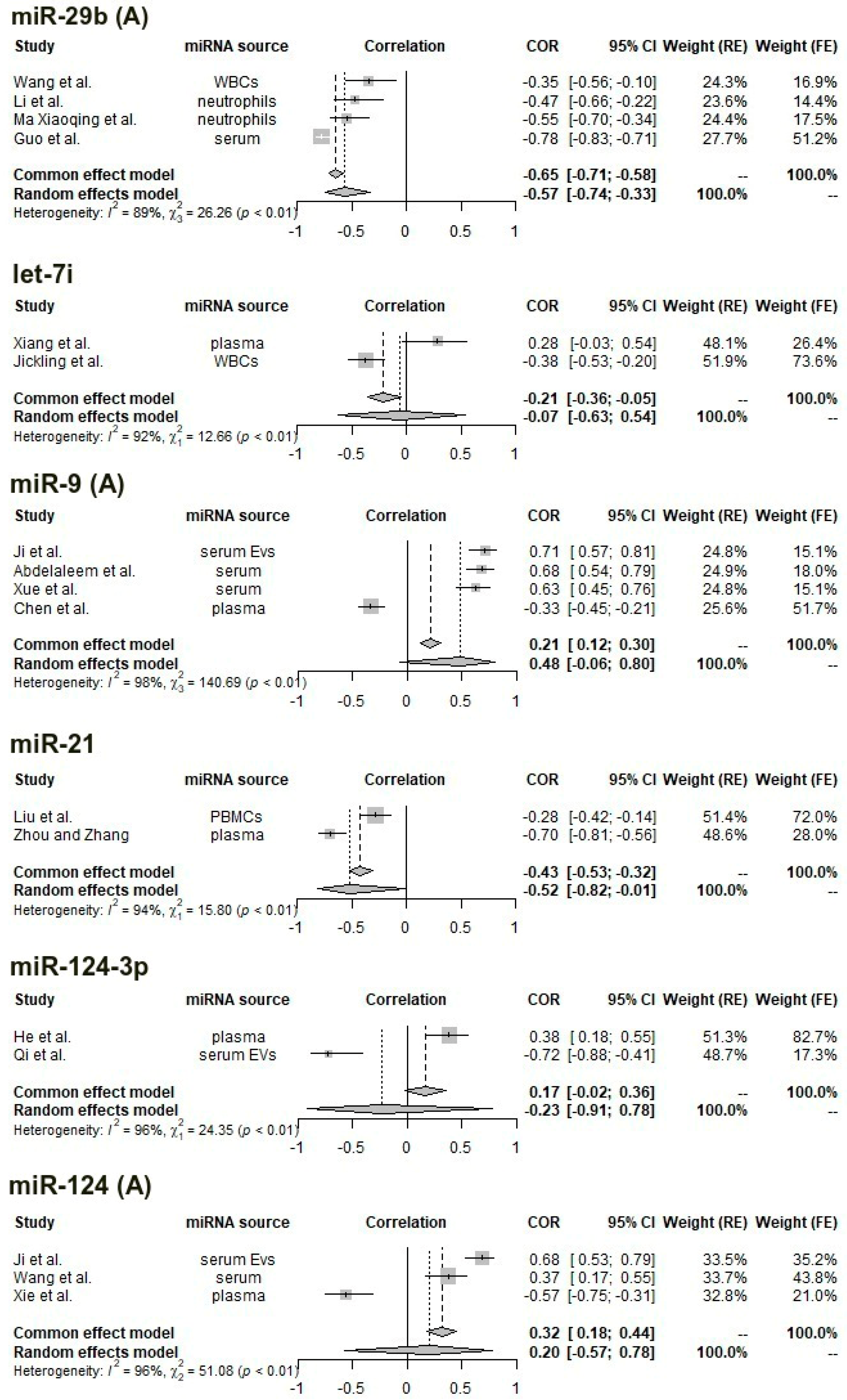
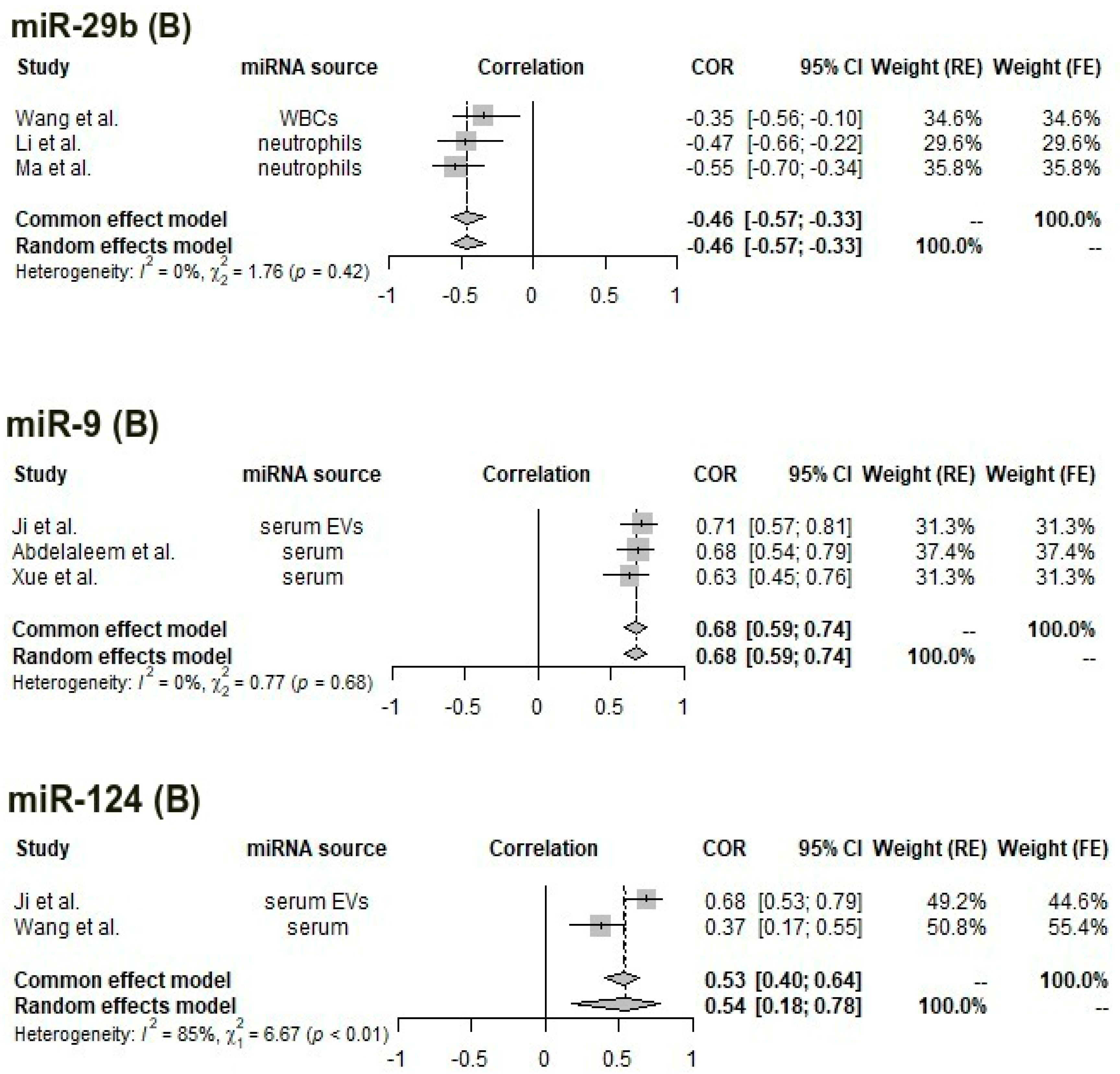
3.6. Meta-Analysis of Correlations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and Regional Mortality from 235 Causes of Death for 20 Age Groups in 1990 and 2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef] [PubMed]
- Elkind, M.S.; Sacco, R.L. Stroke Risk Factors and Stroke Prevention. Semin. Neurol. 1998, 18, 429–440. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diseases and Mental Health Cluster WHO STEPS Stroke Manual: The WHO STEPwise Approach to Stroke Surveillance; World Health Organization: Geneva, Switzerland, 2005.
- Fonarow, G.C.; Zhao, X.; Smith, E.E.; Saver, J.L.; Reeves, M.J.; Bhatt, D.L.; Xian, Y.; Hernandez, A.F.; Peterson, E.D.; Schwamm, L.H. Door-to-Needle Times for Tissue Plasminogen Activator Administration and Clinical Outcomes in Acute Ischemic Stroke before and after a Quality Improvement Initiative. JAMA 2014, 311, 1632–1640. [Google Scholar] [CrossRef]
- Creutzfeldt, C.J.; Holloway, R.G.; Walker, M. Symptomatic and Palliative Care for Stroke Survivors. J. Gen. Intern. Med. 2012, 27, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Hermann, D.M.; Chopp, M. Promoting Neurological Recovery in the Post-Acute Stroke Phase: Benefits and Challenges. Eur. Neurol. 2014, 72, 317–325. [Google Scholar] [CrossRef]
- Lai, Y.-J.; Hanneman, S.K.; Casarez, R.L.; Wang, J.; McCullough, L.D. Blood Biomarkers for Physical Recovery in Ischemic Stroke: A Systematic Review. Am. J. Transl. Res. 2019, 11, 4603–4613. [Google Scholar] [PubMed]
- Schaechter, J.D. Motor Rehabilitation and Brain Plasticity after Hemiparetic Stroke. Prog. Neurobiol. 2004, 73, 61–72. [Google Scholar] [CrossRef]
- Alawieh, A.; Zhao, J.; Feng, W. Factors Affecting Post-Stroke Motor Recovery: Implications on Neurotherapy after Brain Injury. Behav. Brain Res. 2018, 340, 94–101. [Google Scholar] [CrossRef]
- Murie-Fernández, M.; Irimia, P.; Martínez-Vila, E.; John Meyer, M.; Teasell, R. Neuro-Rehabilitation after Stroke. Neurología 2010, 25, 189–196. [Google Scholar] [CrossRef]
- Mureșanu, D.F.; Livinț Popa, L.; Chira, D.; Dăbală, V.; Hapca, E.; Vlad, I.; Văcăraș, V.; Popescu, B.O.; Cherecheș, R.; Strilciuc, Ș.; et al. Role and Impact of Cerebrolysin for Ischemic Stroke Care. J. Clin. Med. 2022, 11, 1273. [Google Scholar] [CrossRef]
- Bolognini, N.; Russo, C.; Edwards, D.J. The Sensory Side of Post-Stroke Motor Rehabilitation. Restor. Neurol. Neurosci. 2016, 34, 571–586. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Lim, S.H.; Kim, K.H.; Kim, K.J.; Kim, Y.R.; Chang, W.N.; Yeom, J.W.; Kim, Y.D.; Hwang, B.Y. Six-Month Functional Recovery of Stroke Patients: A Multi-Time-Point Study. Int. J. Rehabil. Res. 2015, 38, 173–180. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, H.; Li, H.; Zhao, R.; Huang, Q.; Liu, J. Recent Advances in the Development of Neuroprotective Agents and Therapeutic Targets in the Treatment of Cerebral Ischemia. Eur. J. Med. Chem. 2019, 162, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Steliga, A.; Kowiański, P.; Czuba, E.; Waśkow, M.; Moryś, J.; Lietzau, G. Neurovascular Unit as a Source of Ischemic Stroke Biomarkers—Limitations of Experimental Studies and Perspectives for Clinical Application. Transl. Stroke Res. 2020, 11, 553–579. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, S.; Sánchez-Lechuga, B.; Donovan, P.; Halang, L.; Prehn, J.H.M.; Campos-Caro, A.; Byrne, M.M.; López-Tinoco, C. Circulating MiR-330-3p in Late Pregnancy Is Associated with Pregnancy Outcomes Among Lean Women with GDM. Sci. Rep. 2020, 10, 908. [Google Scholar] [CrossRef] [PubMed]
- Larrea, E.; Sole, C.; Manterola, L.; Goicoechea, I.; Armesto, M.; Arestin, M.; Caffarel, M.M.; Araujo, A.M.; Araiz, M.; Fernandez-Mercado, M.; et al. New Concepts in Cancer Biomarkers: Circulating MiRNAs in Liquid Biopsies. Int. J. Mol. Sci. 2016, 17, 627. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M. The Interplay of MicroRNAs in the Inflammatory Mechanisms Following Ischemic Stroke. J. Neuropathol. Exp. Neurol. 2017, 76, 548–561. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Winlow, W.; Farbood, Y.; Moghaddam, H.F.; Farzaneh, M. Emerging Roles of MicroRNAs in Ischemic Stroke: As Possible Therapeutic Agents. J. Stroke 2017, 19, 166–187. [Google Scholar] [CrossRef]
- Salido-Guadarrama, I.; Romero-Cordoba, S.; Peralta-Zaragoza, O.; Hidalgo-Miranda, A.; Rodríguez-Dorantes, M. MicroRNAs Transported by Exosomes in Body Fluids as Mediators of Intercellular Communication in Cancer. OncoTargets Ther. 2014, 7, 1327–1338. [Google Scholar] [CrossRef]
- Raoof, R.; Jimenez-Mateos, E.M.; Bauer, S.; Tackenberg, B.; Rosenow, F.; Lang, J.; Onugoren, M.D.; Hamer, H.; Huchtemann, T.; Körtvélyessy, P.; et al. Cerebrospinal Fluid MicroRNAs Are Potential Biomarkers of Temporal Lobe Epilepsy and Status Epilepticus. Sci. Rep. 2017, 7, 3328. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bejleri, J.; Jirström, E.; Donovan, P.; Williams, D.J.; Pfeiffer, S. Diagnostic and Prognostic Circulating MicroRNA in Acute Stroke: A Systematic and Bioinformatic Analysis of Current Evidence. J. Stroke 2021, 23, 162–182. [Google Scholar] [CrossRef]
- Forró, T.; Bajkó, Z.; Bălașa, A.; Bălașa, R. Dysfunction of the Neurovascular Unit in Ischemic Stroke: Highlights on MicroRNAs and Exosomes as Potential Biomarkers and Therapy. Int. J. Mol. Sci. 2021, 22, 5621. [Google Scholar] [CrossRef]
- Edwardson, M.A.; Zhong, X.; Fiandaca, M.S.; Federoff, H.J.; Cheema, A.K.; Dromerick, A.W. Plasma MicroRNA Markers of Upper Limb Recovery Following Human Stroke. Sci. Rep. 2018, 8, 12558. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; McShane, L.M.; Sauerbrei, W.; Taube, S.E. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and Elaboration. PLoS Med. 2012, 9, e1001216. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; McColgan, P.; Bentley, P.; Edwards, R.J.; Sharma, P. Towards the Identification of Blood Biomarkers for Acute Stroke in Humans: A Comprehensive Systematic Review. Br. J. Clin. Pharm. 2012, 74, 230–240. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, A.R. Table for Conversion of Kendall’S Tau to Spearman’S Rho Within the Context of Measures of Magnitude of Effect for Meta-Analysis. Educ. Psychol. Meas. 1993, 53, 87–92. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Cochran, W.G. Some Methods for Strengthening the Common Χ2 Tests. Biometrics 1954, 10, 417–451. [Google Scholar] [CrossRef]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Statistical Heterogeneity in Systematic Reviews of Clinical Trials: A Critical Appraisal of Guidelines and Practice. J. Health Serv. Res. Policy 2002, 7, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chu, H.; Murad, M.H.; Hong, C.; Qu, Z.; Cole, S.R.; Chen, Y. Empirical Comparison of Publication Bias Tests in Meta-Analysis. J. Gen. Intern. Med. 2018, 33, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Puhan, M.A.; Soesilo, I.; Guyatt, G.H.; Schünemann, H.J. Combining Scores from Different Patient Reported Outcome Measures in Meta-Analyses: When Is It Justified? Health Qual. Life Outcomes 2006, 4, 94. [Google Scholar] [CrossRef] [PubMed]
- Mind Those Outcome Measures! A Guide to Enhanced Critical Appraisal of Outcomes|Cochrane Methods. Available online: https://methods.cochrane.org/news/mind-those-outcome-measures-guide-enhanced-critical-appraisal-outcomes (accessed on 27 September 2022).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Ma, X.; Liao, X.; Liu, J.; Wang, Y.; Wang, X.; Chen, Y.; Yin, X.; Pan, Q. Circulating Endothelial Microvesicles and Their Carried MiR-125a-5p: Potential Biomarkers for Ischaemic Stroke. Stroke Vasc. Neurol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Yuan, Y.; Wu, S.; He, F.; Hu, Y.; Luo, B. MicroRNA Let-7e Is a Potential Circulating Biomarker of Acute Stage Ischemic Stroke. Transl. Stroke Res. 2015, 6, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fan, C.-L.; Ma, L.-J.; Liu, T.; Wang, C.; Song, J.-X.; Lv, Q.-S.; Pan, H.; Zhang, C.-N.; Wang, J.-J. Distinctive Expression Signatures of Serum MicroRNAs in Ischaemic Stroke and Transient Ischaemic Attack Patients. Thromb. Haemost. 2017, 117, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Kijpaisalratana, N.; Nimsamer, P.; Khamwut, A.; Payungporn, S.; Pisitkun, T.; Chutinet, A.; Utoomprurkporn, N.; Kerr, S.J.; Vongvasinkul, P.; Suwanwela, N.C. Serum MiRNA125a-5p, MiR-125b-5p, and MiR-433-5p as Biomarkers to Differentiate between Posterior Circulation Stroke and Peripheral Vertigo. BMC Neurol. 2020, 20, 372. [Google Scholar] [CrossRef] [PubMed]
- Kautzky, V. Identifizierung von Einflussfaktoren auf die Konzentrationen der Zirkulierenden microRNAs miR-125a-5p, miR-125b-5p und miR-143-3p Nach Ischämischem Schlaganfall. Text. Ph.D. Thesis, Ludwig-Maximilians-Universität München, München, Germany, 2022. [Google Scholar]
- Zhu, X.; Ding, J.; Wang, B.; Wang, J.; Xu, M. Circular RNA DLGAP4 Is Down-Regulated and Negatively Correlates with Severity, Inflammatory Cytokine Expression and pro-Inflammatory Gene MiR-143 Expression in Acute Ischemic Stroke Patients. Int. J. Clin. Exp. Pathol. 2019, 12, 941–948. [Google Scholar]
- Niu, M.; Li, H.; Li, X.; Yan, X.; Ma, A.; Pan, X.; Zhu, X. Circulating Exosomal MiRNAs as Novel Biomarkers Perform Superior Diagnostic Efficiency Compared with Plasma MiRNAs for Large-Artery Atherosclerosis Stroke. Front. Pharm. 2021, 12, 791644. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ma, X.; Zhao, H.; Fan, J.; Liu, T.; Luo, Y.; Guo, Y. Long Non-Coding RNA H19 Promotes Leukocyte Inflammation in Ischemic Stroke by Targeting the MiR-29b/C1QTNF6 Axis. CNS Neurosci. Ther. 2022, 28, 953–963. [Google Scholar] [CrossRef]
- Kotb, H.G.; Ibrahim, A.H.; Mohamed, E.F.; Ali, O.M.; Hassanein, N.; Badawy, D.; Abdelatty Aly, E. The Expression of MicroRNA 146a in Patients with Ischemic Stroke: An Observational Study. Int. J. Gen. Med. 2019, 12, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, X.; Li, D.; Zhu, Z. Plasma Level of MiR-99b May Serve as Potential Diagnostic and Short-term Prognostic Markers in Patients with Acute Cerebral Infarction. J. Clin. Lab. Anal. 2020, 34, e23093. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, J.; Huang, D.; Zhuo, L.; Liao, S.; Jiang, Z. Serum MiR-132 Is a Risk Marker of Post-Stroke Cognitive Impairment. Neurosci. Lett. 2016, 615, 102–106. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhu, Z.; Li, H.; Zhao, C.; Huang, Y.; Wang, P. MiR-195 and MiR-497 in Acute Stroke and Their Correlations with Post-Stroke Cognitive Impairment. Int. J. Clin. Exp. Pathol. 2020, 13, 3092–3099. [Google Scholar]
- Ma, Q.; Li, G.; Tao, Z.; Wang, J.; Wang, R.; Liu, P.; Luo, Y.; Zhao, H. Blood MicroRNA-93 as an Indicator for Diagnosis and Prediction of Functional Recovery of Acute Stroke Patients. J. Clin. Neurosci. 2019, 62, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, W.; Tao, T.; Zhang, D.; Mao, L.; He, X. Synthetic Role of MiR-411-5p and CT Perfusion Information in Predicting Clinical Outcomes after Thrombolysis in Acute Cerebral Infarction. Acta Neurol. Belg. 2022. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.; Tian, C.; Lin, J.; Wu, X.; Pang, G.; Zhou, L.; Pan, S.; Deng, Z. Plasma Let-7i and MiR-15a Expression Are Associated with the Effect of Recombinant Tissue Plasminogen Activator Treatment in Acute Ischemic Stroke Patients. Thromb. Res. 2017, 158, 121–125. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Li, K.; Huang, J.; Huo, T.-T.; Lv, P.-Y. MicroRNA Let-7i Is a Promising Serum Biomarker for Post-Stroke Cognitive Impairment and Alleviated OGD-Induced Cell Damage in Vitro by Regulating Bcl-2. Front. Neurosci. 2020, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Jickling, G.C.; Ander, B.P.; Shroff, N.; Orantia, M.; Stamova, B.; Dykstra-Aiello, C.; Hull, H.; Zhan, X.; Liu, D.; Sharp, F.R. Leukocyte Response Is Regulated by MicroRNA Let7i in Patients with Acute Ischemic Stroke. Neurology 2016, 87, 2198–2205. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Q.; Ding, J.; Wang, X. Elevated Serum Levels of Brain-Derived Neurotrophic Factor and MiR-124 in Acute Ischemic Stroke Patients and the Molecular Mechanism. 3 Biotech 2019, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Guo, Y.-S.; Zhang, X.-X.; Gao, Z.-K.; Shen, X.-Y.; Han, Y.; Bi, X. Diagnostic Performance of MiR-21, MiR-124, MiR-132, and MiR-200b Serums in Post-Stroke Cognitive Impairment Patients. Folia Neuropathol. 2022, 60, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Qi, L. MiR-124 Is Downregulated in Serum of Acute Cerebral Infarct Patients and Shows Diagnostic and Prognostic Value. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211035446. [Google Scholar] [CrossRef]
- Ji, Q.; Ji, Y.; Peng, J.; Zhou, X.; Chen, X.; Zhao, H.; Xu, T.; Chen, L.; Xu, Y. Increased Brain-Specific MiR-9 and MiR-124 in the Serum Exosomes of Acute Ischemic Stroke Patients. PLoS ONE 2016, 11, e0163645. [Google Scholar] [CrossRef]
- He, X.-W.; Shi, Y.-H.; Liu, Y.-S.; Li, G.-F.; Zhao, R.; Hu, Y.; Lin, C.-C.; Zhuang, M.-T.; Su, J.-J.; Liu, J.-R. Increased Plasma Levels of MiR-124-3p, MiR-125b-5p and MiR-192-5p Are Associated with Outcomes in Acute Ischaemic Stroke Patients Receiving Thrombolysis. Atherosclerosis 2019, 289, 36–43. [Google Scholar] [CrossRef]
- He, X.-W.; Shi, Y.-H.; Zhao, R.; Liu, Y.-S.; Li, G.-F.; Hu, Y.; Chen, W.; Cui, G.-H.; Su, J.-J.; Liu, J.-R. Plasma Levels of MiR-125b-5p and MiR-206 in Acute Ischemic Stroke Patients After Recanalization Treatment: A Prospective Observational Study. J. Stroke Cereb. Dis. 2019, 28, 1654–1661. [Google Scholar] [CrossRef]
- Qi, Z.; Zhao, Y.; Su, Y.; Cao, B.; Yang, J.-J.; Xing, Q. Serum Extracellular Vesicle–Derived MiR-124-3p as a Diagnostic and Predictive Marker for Early-Stage Acute Ischemic Stroke. Front. Mol. Biosci. 2021, 8, 685088. [Google Scholar] [CrossRef]
- Jin, F.; Xing, J. Circulating Pro-Angiogenic and Anti-Angiogenic MicroRNA Expressions in Patients with Acute Ischemic Stroke and Their Association with Disease Severity. Neurol. Sci. 2017, 38, 2015–2023. [Google Scholar] [CrossRef]
- Jin, F.; Xing, J. Circulating MiR-126 and MiR-130a Levels Correlate with Lower Disease Risk, Disease Severity, and Reduced Inflammatory Cytokine Levels in Acute Ischemic Stroke Patients. Neurol. Sci. 2018, 39, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Su, C.; Huang, S. Long Noncoding RNA HULC in Acute Ischemic Stroke: Association with Disease Risk, Severity, and Recurrence-free Survival and Relation with IL-6, ICAM1, MiR-9, and MiR-195. J. Clin. Lab. Anal. 2020, 34, e23500. [Google Scholar] [CrossRef]
- Yang, G.; Liu, Z.; Wang, L.; Chen, X.; Wang, X.; Dong, Q.; Zhang, D.; Yang, Z.; Zhou, Q.; Sun, J.; et al. MicroRNA-195 Protection against Focal Cerebral Ischemia by Targeting CX3CR1. J. Neurosurg. 2018, 131, 1445–1454. [Google Scholar] [CrossRef]
- Liu, C.; Huang, H.; Li, Y.; Zhao, H. The Relationship of Long Non-Coding RNA Maternally Expressed Gene 3 with MicroRNA-21 and Their Correlation with Acute Ischemic Stroke Risk, Disease Severity and Recurrence Risk. Clin. Neurol. Neurosurg. 2021, 210, 106940. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, J. Identification of MiRNA-21 and MiRNA-24 in Plasma as Potential Early Stage Markers of Acute Cerebral Infarction. Mol. Med. Rep. 2014, 10, 971–976. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Ma, Y.; Tang, G.; Liu, Y.; Chen, X.; Zhang, Z.; Zeng, L.; Wang, Y.; Ouyang, Y.-B.; et al. MicroRNA-29b Is a Therapeutic Target in Cerebral Ischemia Associated with Aquaporin 4. J. Cereb. Blood Flow Metab. 2015, 35, 1977–1984. [Google Scholar] [CrossRef]
- Ma, X.; Yun, H.J.; Elkin, K.; Guo, Y.; Ding, Y.; Li, G. MicroRNA-29b Suppresses Inflammation and Protects Blood-Brain Barrier Integrity in Ischemic Stroke. Mediat. Inflamm. 2022, 2022, e1755416. [Google Scholar] [CrossRef]
- Abdelaleem, O.O.; Shaker, O.G.; Mohamed, M.M.; Ahmed, T.I.; Elkhateeb, A.F.; Abdelghaffar, N.K.; Ahmed, N.A.; Khalefa, A.A.; Hemeda, N.F.; Mahmoud, R.H. Differential Expression of Serum TUG1, LINC00657, MiR-9, and MiR-106a in Diabetic Patients with and Without Ischemic Stroke. Front. Mol. Biosci. 2022, 8, 758742. [Google Scholar] [CrossRef]
- Xue, Y.; Li, M.; Liu, D.; Zhu, Q.; Chen, H. Expression of MiR-9 in the Serum of Patients with Acute Ischemic Stroke and Its Effect on Neuronal Damage. Int. J. Clin. Exp. Pathol. 2018, 11, 5885–5892. [Google Scholar]
- Xie, Z.; Liu, B.; Zhou, M.; Chen, Y. Expression and clinical significance of plasma miR-124 in acute ischemic stroke. J. Pract. Med. 2019, 24, 343–345. [Google Scholar]
- Guo, C.; Zhong, C.; Li, Q.; Gao, Y.; Li, W.; Ou, Y. Expressions and neural function prognostic evaluation of serum microRNA-24 and microRNA-29b in elderly patients with acute ischemic stroke. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020, 32, 78–82. [Google Scholar] [CrossRef]
- Yuan, M.; Yuan, H.; Zhou, C.; Liu, F.; Lin, C.; Tang, Y. The Significance of Low Plasma MiR-335 Level in Patients with Acute Cerebral Infarction May Be Associated with the Loss of Control of CALM1 Expression. Int. J. Clin. Exp. Med. 2016, 9, 19595–19601. [Google Scholar]
- Yuan, M.; Tang, Y.; Zhou, C.; Liu, F.; Chen, L.; Yuan, H. Elevated Plasma CaM Expression in Patients with Acute Cerebral Infarction Predicts Poor Outcomes and Is Inversely Associated with MiR-26b Expression. Int. J. Neurosci. 2016, 126, 408–414. [Google Scholar] [CrossRef]
- Zhao, B.; Zhu, Z.; Hao, J.; Wan, Z.; Guo, X. Decreased Plasma MiR-335 Expression in Patients with Acute Ischemic Stroke and Its Association with Calmodulin Expression. J. Int. Med. Res. 2016, 44, 1331–1338. [Google Scholar] [CrossRef]
- Zhong, C.; Yin, C.; Niu, G.; Ning, L.; Pan, J. MicroRNA MiR-497 Is Closely Associated with Poor Prognosis in Patients with Cerebral Ischemic Stroke. Bioengineered 2021, 12, 2851–2862. [Google Scholar] [CrossRef]
- Song, X.-D.; Li, S.-X.; Zhu, M. Plasma MiR-409-3p Promotes Acute Cerebral Infarction via Suppressing CTRP3. Kaohsiung J. Med. Sci. 2021, 37, 324–333. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Qiu, W.; Pan, Q.; Chen, Y.; Chen, Y.; Ma, X. Plasma Endothelial Microvesicles and Their Carrying MiRNA-155 Serve as Biomarkers for Ischemic Stroke. J. Neurosci. Res. 2020, 98, 2290–2301. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, J.; Wang, Y.; Wang, L.; Weng, S.; Chen, S.; Yang, G.-Y. Cocktail Blood Biomarkers: Prediction of Clinical Outcomes in Patients with Acute Ischemic Stroke. Eur. Neurol. 2013, 69, 68–75. [Google Scholar] [CrossRef]
- Yang, S.; Zhan, X.; He, M.; Wang, J.; Qiu, X. MiR-135b Levels in the Peripheral Blood Serve as a Marker Associated with Acute Ischemic Stroke. Exp. Ther. Med. 2020, 19, 3551–3558. [Google Scholar] [CrossRef]
- Liu, P.; Han, Z.; Ma, Q.; Liu, T.; Wang, R.; Tao, Z.; Li, G.; Li, F.; Zhang, S.; Li, L.; et al. Upregulation of MicroRNA-128 in the Peripheral Blood of Acute Ischemic Stroke Patients Is Correlated with Stroke Severity Partially through Inhibition of Neuronal Cell Cycle Reentry. Cell Transpl. 2019, 28, 839–850. [Google Scholar] [CrossRef]
- Zhao, H.; Li, G.; Wang, R.; Tao, Z.; Ma, Q.; Zhang, S.; Han, Z.; Yan, F.; Li, F.; Liu, P.; et al. Silencing of MicroRNA-494 Inhibits the Neurotoxic Th1 Shift via Regulating HDAC2-STAT4 Cascade in Ischaemic Stroke. Br. J. Pharmacol. 2020, 177, 128–144. [Google Scholar] [CrossRef]
- Sheikhbahaei, S.; Manizheh, D.; Mohammad, S.; Hasan, T.M.; Saman, N.; Laleh, R.; Mahsa, M.; Sanaz, A.K.; Shaghayegh, H.J. Can MiR-503 Be Used as a Marker in Diabetic Patients with Ischemic Stroke? BMC Endocr. Disord. 2019, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yao, Y.; Li, Q.; Gao, Y.; Cao, H. Expression and Clinical Value of MiR-185 and MiR-424 in Patients with Acute Ischemic Stroke. Int. J. Gen. Med. 2022, 15, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Chen, S.; Cai, N.; Liu, Z.; Wang, P.; Zhao, J. Potential Neuroprotective Effect of MiR-451 Against Cerebral Ischemia/Reperfusion Injury in Stroke Patients and a Mouse Model. World Neurosurg. 2019, 130, e54–e61. [Google Scholar] [CrossRef]
- Ye, Z.; Hu, J.; Xu, H.; Sun, B.; Jin, Y.; Zhang, Y.; Zhang, J. Serum Exosomal MicroRNA-27-3p Aggravates Cerebral Injury and Inflammation in Patients with Acute Cerebral Infarction by Targeting PPARγ. Inflammation 2021, 44, 1035–1048. [Google Scholar] [CrossRef]
- Otero-Ortega, L.; Alonso-López, E.; Pérez-Mato, M.; Laso-García, F.; Gómez-de Frutos, M.C.; Diekhorst, L.; García-Bermejo, M.L.; Conde-Moreno, E.; Fuentes, B.; de Leciñana, M.A.; et al. Circulating Extracellular Vesicle Proteins and MicroRNA Profiles in Subcortical and Cortical-Subcortical Ischaemic Stroke. Biomedicines 2021, 9, 786. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-B.; Li, T.-B.; Zhang, Z.; Ren, K.-D.; Zheng, Z.-F.; Peng, J.; Luo, X.-J. The Diagnostic Value of Circulating Brain-Specific MicroRNAs for Ischemic Stroke. Intern. Med. 2016, 55, 1279–1286. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, L.; Chen, B.; Huang, S.; Zeng, C.; Wu, H.; Chen, C.; Long, F. Increased Serum Exosomal MiR-134 Expression in the Acute Ischemic Stroke Patients. BMC Neurol. 2018, 18, 198. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, K.; Huang, J.; Zheng, G.; Lv, Y.; Luo, N.; Liang, M.; Huang, L. Upregulated Serum MiR-146b Serves as a Biomarker for Acute Ischemic Stroke. Cell Physiol. Biochem. 2018, 45, 397–405. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Huang, J.; Qu, M.; Zhang, Y.; Geng, J.; Zhang, Z.; Liu, J.; Yang, G.-Y. Increased Circulating Exosomal MiRNA-223 Is Associated with Acute Ischemic Stroke. Front. Neurol. 2017, 8, 57. [Google Scholar] [CrossRef]
- Liang, T.; Lou, J. Increased Expression of Mir-34a-5p and Clinical Association in Acute Ischemic Stroke Patients and in a Rat Model. Med. Sci. Monit. 2016, 22, 2950–2955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Huang, J.; Chen, X.; Gu, X.; Wang, Y.; Zeng, L.; Yang, G.-Y. Increase of Circulating MiR-223 and Insulin-like Growth Factor-1 Is Associated with the Pathogenesis of Acute Ischemic Stroke in Patients. BMC Neurol. 2014, 14, 77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, F.; Fu, F.; Liu, J.; Sun, W.; Chen, Y. Diagnostic and Prognostic Value of Serum MiR-9-5p and MiR-128-3p Levels in Early-Stage Acute Ischemic Stroke. Clinics 2021, 76, e2958. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Hao, F.; Wang, W.; Qu, Y. Circulating MiR-145 Is Associated with Plasma High-Sensitivity C-Reactive Protein in Acute Ischemic Stroke Patients. Cell Biochem. Funct. 2015, 33, 314–319. [Google Scholar] [CrossRef]
- Liang, H.-B.; He, J.-R.; Tu, X.-Q.; Ding, K.-Q.; Yang, G.-Y.; Zhang, Y.; Zeng, L.-L. MicroRNA-140-5p: A Novel Circulating Biomarker for Early Warning of Late-Onset Post-Stroke Depression. J. Psychiatr. Res. 2019, 115, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhou, W.; Zhou, Z.; Yang, Q.; Xu, J.; Dong, W. MiR-22 and Cerebral Microbleeds in Brainstem and Deep Area Are Associated with Depression One Month after Ischemic Stroke. Braz. J. Med. Biol. Res. 2020, 53, e9162. [Google Scholar] [CrossRef]
- Cui, Y.; Ma, G.; Kong, F.; Song, L. Diagnostic Values of MiR-221-3p in Serum and Cerebrospinal Fluid for Post-Stroke Depression and Analysis of Risk Factors. Iran. J. Public Health 2021, 50, 1241. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, J.; Wang, Y.; Wang, L.; Weng, S.; Tang, Y.; Zheng, C.; Cheng, Q.; Chen, S.; Yang, G.-Y. MicroRNA-210 as a Novel Blood Biomarker in Acute Cerebral Ischemia. Front. Biosci. 2011, 3, 1265–1272. [Google Scholar] [CrossRef]
- Bang, O.Y.; Park, H.Y.; Yoon, J.H.; Yeo, S.H.; Kim, J.W.; Lee, M.A.; Park, M.H.; Lee, P.H.; Joo, I.S.; Huh, K. Predicting the Long-Term Outcome after Subacute Stroke within the Middle Cerebral Artery Territory. J. Clin. Neurol. 2005, 1, 148–158. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 978-0-203-77158-7. [Google Scholar]
- Mompeón, A.; Ortega-Paz, L.; Vidal-Gómez, X.; Costa, T.J.; Pérez-Cremades, D.; Garcia-Blas, S.; Brugaletta, S.; Sanchis, J.; Sabate, M.; Novella, S.; et al. Disparate MiRNA Expression in Serum and Plasma of Patients with Acute Myocardial Infarction: A Systematic and Paired Comparative Analysis. Sci. Rep. 2020, 10, 5373. [Google Scholar] [CrossRef]
- Zhou, B.; Li, B.; Feng, P.; Wang, X.; Gao, H.; Xu, L.; Wang, T.; Guo, X. Identification of a MiRNA Biomarker for the Large Artery Atherosclerosis Subtype of Acute Ischemic Stroke. Folia Neuropathol. 2022, 60, 210–220. [Google Scholar] [CrossRef]
- Modak, J.M.; Roy-O’Reilly, M.; Zhu, L.; Staff, I.; McCullough, L.D. Differential MicroRibonucleic Acid Expression in Cardioembolic Stroke. J. Stroke Cereb. Dis. 2019, 28, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.; Armugam, A.; Sepramaniam, S.; Lim, K.Y.; Setyowati, K.D.; Wang, C.W.; Jeyaseelan, K. Expression Profile of MicroRNAs in Young Stroke Patients. PLoS ONE 2009, 4, e7689. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Huang, P.; Zhang, F.; Chen, T. Association of MicroRNAs with Risk of Stroke: A Meta-Analysis. Front. Neurol. 2022, 13, 865265. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yuan, Y.; Cho, J.-H.; McClarty, S.; Baxter, D.; Galas, D.J. Comparing the MicroRNA Spectrum between Serum and Plasma. PLoS ONE 2012, 7, e41561. [Google Scholar] [CrossRef]
- Glinge, C.; Clauss, S.; Boddum, K.; Jabbari, R.; Jabbari, J.; Risgaard, B.; Tomsits, P.; Hildebrand, B.; Kääb, S.; Wakili, R.; et al. Stability of Circulating Blood-Based MicroRNAs—Pre-Analytic Methodological Considerations. PLoS ONE 2017, 12, e0167969. [Google Scholar] [CrossRef]
- Faraldi, M.; Gomarasca, M.; Sansoni, V.; Perego, S.; Banfi, G.; Lombardi, G. Normalization Strategies Differently Affect Circulating MiRNA Profile Associated with the Training Status. Sci. Rep. 2019, 9, 1584. [Google Scholar] [CrossRef]



| Study and Author | Country | N | Featured miRNAs | MiRNA Source | miRNA Sample Timing | Clinical Outcome Measured |
|---|---|---|---|---|---|---|
| Ma et al., 2022 [40] | China | 72/56 | EMV-miR-125a-5p | Plasma | <24 h | NIHSS |
| Peng et al., 2015 [41] | China | 72/52 | miR-let-7e | Serum | <24 h | NIHSS |
| Wu et al., 2017 [42] | China | 131/50 | miR-23b-3p, -29b-3p, -181a-5p, -21-5p | Serum | <24 h | NIHSS, BI, mRS |
| Kijpaisalratana et al., 2020 [43] | Thailand | 23/35 | miR-125a-5p, -125b-5p, -433-5p | Serum | <72 h | NIHSS |
| Kautzky et al., 2022 [44] | Germany | 40/40 | miR-125a-5p, miR-125b-5p | Plasma | <24 h | NIHSS |
| Zhu et al., 2019 [45] | China | 170/170 | miR-143 | PBMC | < 24 h | NIHSS |
| Niu et al., 2021 [46] | China | 453/143 | Exosomal-miR-369-3p, -493-3p, -379-5p, -1296-5p | Plasma | <72 h | NIHSS |
| Li et al., 2022 [47] | China | 50/42 | miR-29b | Leukocytes | <6 h | NIHSS |
| Kotb et al., 2019 [48] | Lebanon | 44/22 | miR-146a | Serum | <24 h | GCS |
| Wu et al., 2020 [49] | China | 112/112 | miR-99b | Plasma | NR | GOS |
| Huang et al., 2016 [50] | China | 76/38 | miR-132 | Serum | Chronic | MoCA |
| Zhai et al., [51] | China | 108/76 | miR-195, -497 | Serum | <72 h | NIHSS, MoCA |
| Ma et al., 2019 [52] | China | 33/20 | miR-93 | Neutrophils and plasma | <6 h | NIHSS, BI, mRS |
| Lin et al., 2022 [53] | China | 96 | miR-411-5p | Serum | Before rt-PA, 24 h after rt-PA, and at 3 months | NIHSS |
| Xiang et al., 2017 [54] | China | 40/46 | let-7i | Plasma | 24 h after thrombolysis | NIHSS |
| Wang et al., 2020 [55] | China | 76 | let-7i | Serum | Chronic (within 1 year) | MoCA |
| Jickling et al., 2016 [56] | USA | 106/106 | let-7i | Circulating leukocytes | <72 h | NIHSS |
| Wang et al., 2019 [57] | China | 40/40 | miR-124 | Serum | <24 h | NIHSS |
| Yuan et al., 2022 [58] | China | 77 | miR-21, -132, -200b | Serum | 14 days | MMSE |
| Zhou and Qi, 2021 [59] | China | 108/108 | miR-124 | Serum | At 24, 48, and 72 h | GOS |
| Ji et al., 2016 [60] | China | 65/66 | Exosomal-miR-9, -124 | Serum | Mean 16.5 h | NIHSS |
| He et al., 2019 [61] | China | 94 | miR-124-3p, -125b-5p, -192-5p, -125b-5p | Plasma | 24 h after thrombolysis | NIHSS |
| He et al., 2019 [62] | China | 84 | miR-125b-5p, -206 | Plasma | 24 h after thrombolysis | NIHSS |
| Qi et al., 2021 [63] | China | 10/10 | Exosomal-miR-124-3p | Serum | At 2, 4, 6 h | NIHSS |
| Jin and Xing, 2017 [64] | China | 106/110 | miR-126, -130a, -185, -221, -222, -218, -378, -19a, -296, -101, -206 | Plasma | <24 h (after thrombolysis) | NIHSS |
| Jin and Xing, 2018 [65] | China | 148/148 | miR-126, -218, -130a, -185, -222 | Plasma | <24 h | NIHSS |
| Chen et al., 2020 [66] | China | 215/215 | miR-9, -195 | Plasma | <24 h | NIHSS |
| Yang et al., 2018 [67] | China | 96/45 | miR-195 | Plasma | <72 h | NIHSS |
| Liu et al., 2021 [68] | China | 170/100 | miR-21 | PBMCs + serum | <24 h | NIHSS |
| Zhou and Zhang, 2014 [69] | China | 68/21 | miR-21 | Plasma | <24 h | NIHSS |
| Wang et al., 2015 [70] | China | 58/59 | miR-29b | WBCs | <72 h | NIHSS |
| Ma et al., 2020 [71] | China | 60/40 | miR-29b | Neutrophils | <6 h | NIHSS |
| Abdelaleem et al., 2022 [72] | Egypt | 77/71 | miR-9, -106a | Serum | At diagnosis | NIHSS |
| Xue et al., 2018 [73] | China | 65/55 | miR-9 | Serum | At diagnosis | NIHSS |
| Xie et al., 2019 [74] | China | 40 IS + 40 Controls | miR-124 | Plasma | NR | NIHSS |
| Guo et al., 2020 [75] | China | 170/65 | miR-24, -29b | Serum | NR | NIHSS |
| Yuan et al., 2016 [76] | China | 152/136 | miR-335 | Plasma | <24 h | NIHSS |
| Yuan et al., 2016 [77] | China | 152/136 | miR-26b | Plasma | <24 h | NIHSS |
| Zhaou et al., 2016 [78] | China | 168/104 | miR-335 | Plasma | <24 h | NIHSS |
| Zhong et al., 2021 [79] | China | 89/39 | miR-497 | Serum | At admission (<24 h) and at discharge | NIHSS |
| Song et al., 2021 [80] | China | 80/30 | miR-409-3p | Serum | <9 h | NIHSS |
| Zhang et al., 2020 [81] | China | 93/70 | EMVs-miR-155 | Plasma | (<24 h) | NIHSS |
| Zeng et al., 2013 [82] | China | 105 | miR-210 | Leukocytes | <72 h | NIHSS, mRS |
| Yang et al., 2020 [83] | China | 79/60 | miR-135b | Serum | <24 h and 14 days after admission | NIHSS |
| Liu et al., 2019 [84] | China | 40/25 | miR-128 | Lymphocytes | <72 h | NIHSS, mRS |
| Zhao et al., 2020 [85] | China | 43/26 | miR-494 | Lymphocytes | <6 h | |
| Sheikhbahaei et al., 2019 [86] | Iran | 33/17 | miR-503 | Serum | <72 h + 3 months | NIHSS, mRS |
| Guo et al., 2022 [87] | China | 142/50 | miR-185, -424 | Serum | <24 h | NIHSS, mRS |
| Fu et al., 2019 [88] | China | 108/97 | miR-451 | Whole blood | <12 h | NIHSS |
| Ye et al., 2021 [89] | China | 43/43 | Exosomal-miR-27-3p | Serum | On admission | NIHSS |
| Otero-Ortega et al., 2021 [90] | Spain | 81/22 | miR-100-5p | Serum | <24h | NIHSS, mRS |
| Yang et al., 2016 [91] | China | 114/58 | miR-107, -128b, -153 | Plasma | <24 h | NIHSS |
| Zhou et al., 2018 [92] | China | 50/50 | Exosomal-miR-134 | Serum | 24-, 48- and 72 h | NIHSS |
| Chen et al., 2018 [93] | China | 128/102 | miR-146b | Serum | < 24 h | NIHSS |
| Chen et al., 2017 [94] | China | 50/33 | Exosomal-miR-223 | Serum | < 72 h | NIHSS |
| Liang et al., 2016 [95] | China | 102/97 | miR-34a-5p | Plasma | <12 h | NIHSS |
| Wang et al., 2014 [96] | China | 79/75 | miR-223 | Leucocytes | At 24, 48, 72 h | NIHSS |
| Wang et al., 2021 [97] | China | 88/88 | miR-9-5p, -128-3p | Serum | <6 h | mRS |
| Jia et al., 2015 [98] | China | 146/96 | miR-145 | Serum | <24 h | NIHSS |
| Liang et al., 2019 [99] | China | 62/62 | miR-140-5p | Plasma | <24 h | HAMD |
| Hu et al., 2020 [100] | China | 257 | miR-22 | Plasma | <7 days | HAMD |
| Cui et al., 2021 [101] | China | 136 | miR-221-3p | Serum | Chronic | NIHSS, HAMD |
| Zeng et al., 2011 [102] | China | 112/60 | miR-210 | Leucocytes | <3 days, 7 days, 14 days | NIHSS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burlacu, C.-C.; Ciobanu, D.; Badulescu, A.-V.; Chelaru, V.-F.; Mitre, A.-O.; Capitanescu, B.; Hermann, D.M.; Popa-Wagner, A. Circulating MicroRNAs and Extracellular Vesicle-Derived MicroRNAs as Predictors of Functional Recovery in Ischemic Stroke Patients: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 251. https://doi.org/10.3390/ijms24010251
Burlacu C-C, Ciobanu D, Badulescu A-V, Chelaru V-F, Mitre A-O, Capitanescu B, Hermann DM, Popa-Wagner A. Circulating MicroRNAs and Extracellular Vesicle-Derived MicroRNAs as Predictors of Functional Recovery in Ischemic Stroke Patients: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2023; 24(1):251. https://doi.org/10.3390/ijms24010251
Chicago/Turabian StyleBurlacu, Codrin-Constantin, Daniela Ciobanu, Andrei-Vlad Badulescu, Vlad-Florin Chelaru, Andrei-Otto Mitre, Bogdan Capitanescu, Dirk M. Hermann, and Aurel Popa-Wagner. 2023. "Circulating MicroRNAs and Extracellular Vesicle-Derived MicroRNAs as Predictors of Functional Recovery in Ischemic Stroke Patients: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 24, no. 1: 251. https://doi.org/10.3390/ijms24010251
APA StyleBurlacu, C.-C., Ciobanu, D., Badulescu, A.-V., Chelaru, V.-F., Mitre, A.-O., Capitanescu, B., Hermann, D. M., & Popa-Wagner, A. (2023). Circulating MicroRNAs and Extracellular Vesicle-Derived MicroRNAs as Predictors of Functional Recovery in Ischemic Stroke Patients: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 24(1), 251. https://doi.org/10.3390/ijms24010251