About the Transient Effects of Synthetic Amorphous Silica: An In Vitro Study on Macrophages
Abstract
1. Introduction
2. Results
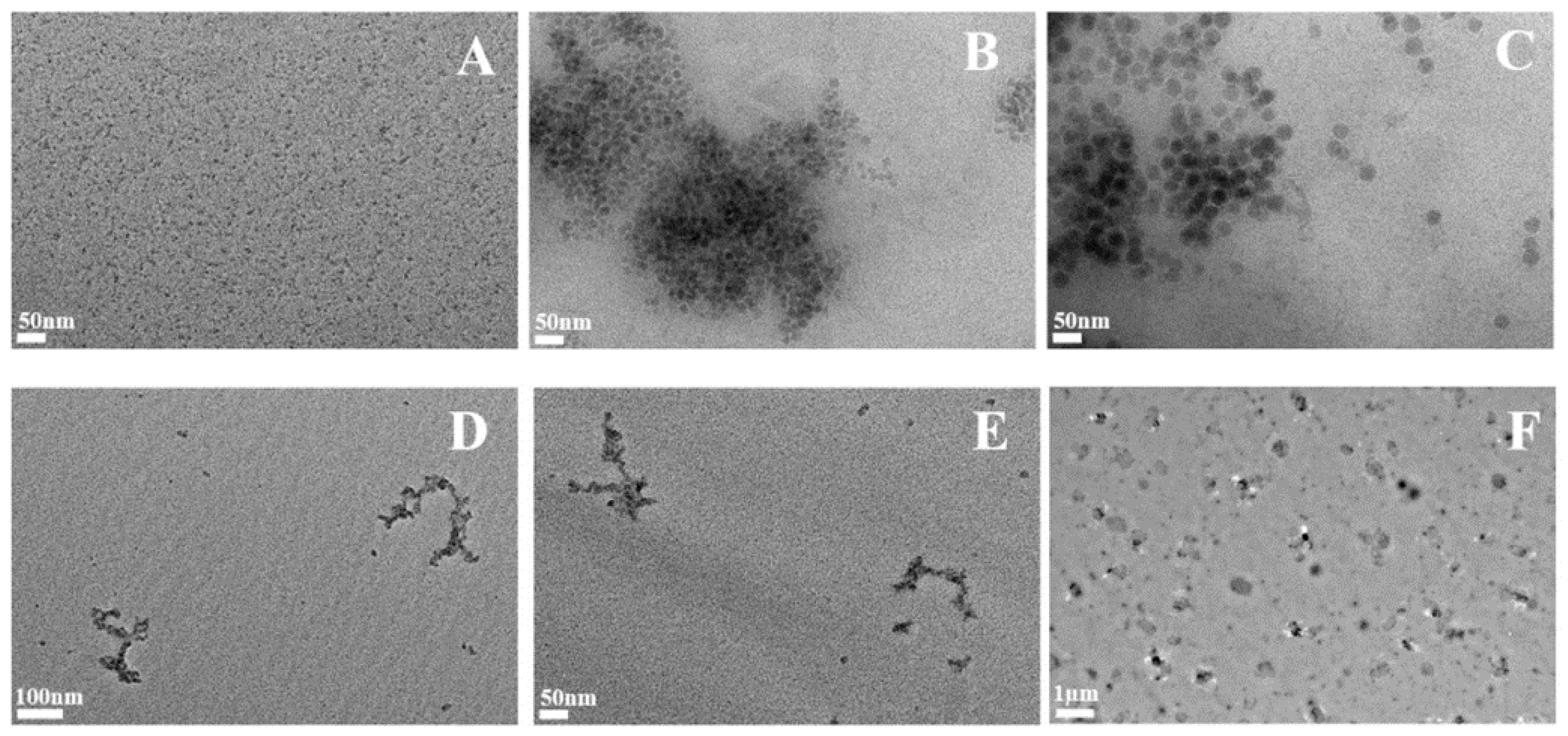
2.1. Synthetic Amorphous Silicas Characterization
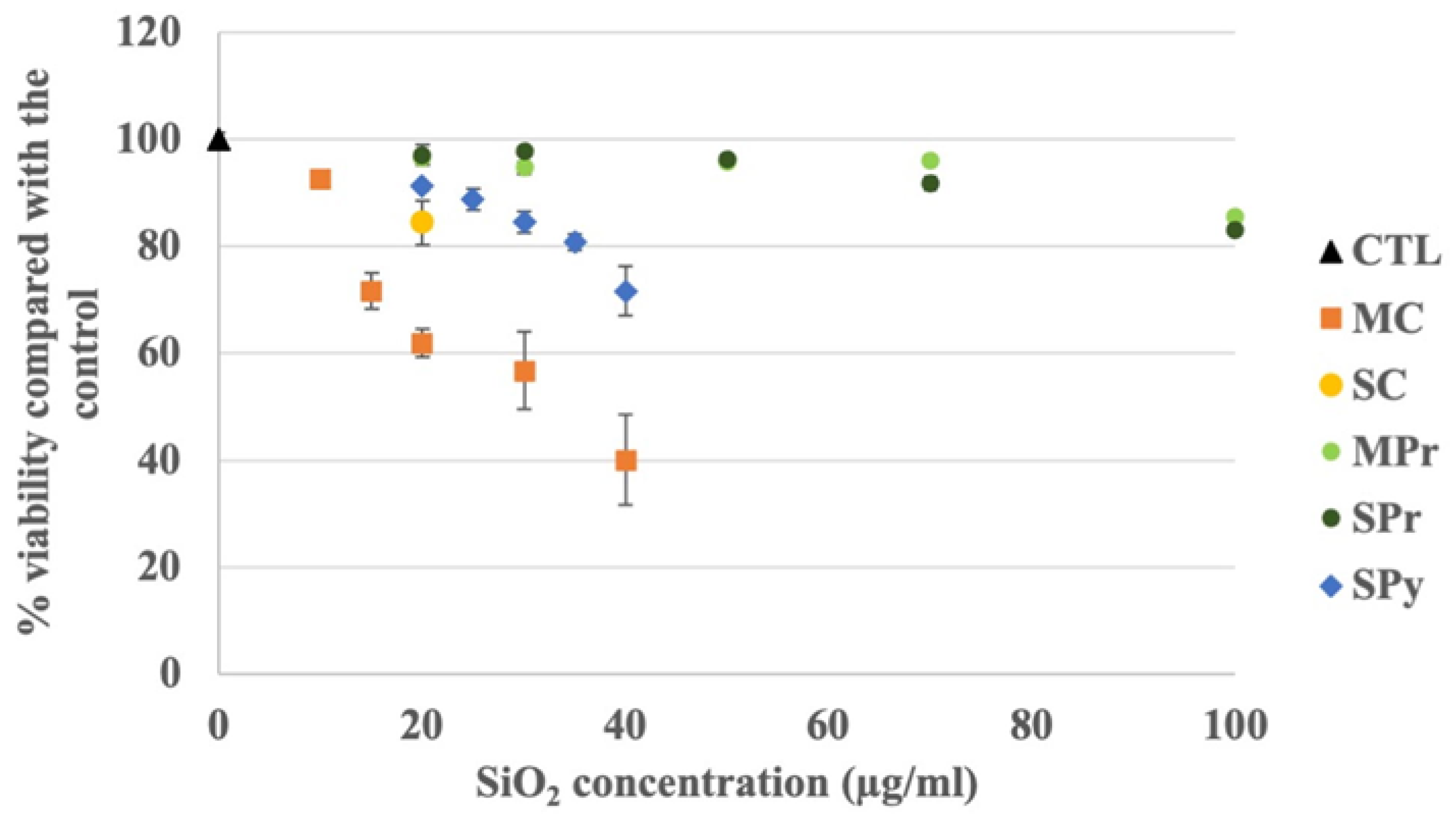
2.2. Viability Studies
2.3. Mineralization Efficiency
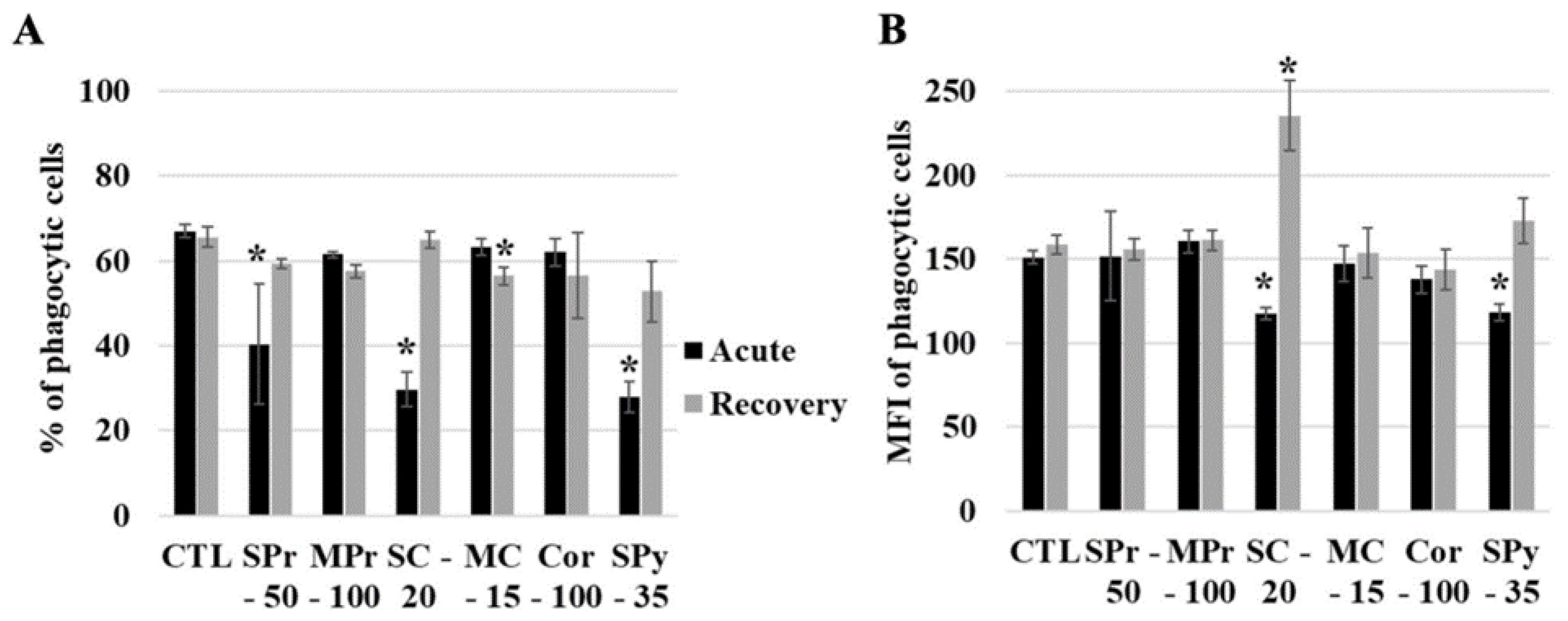
2.4. Silica Uptake by Cells
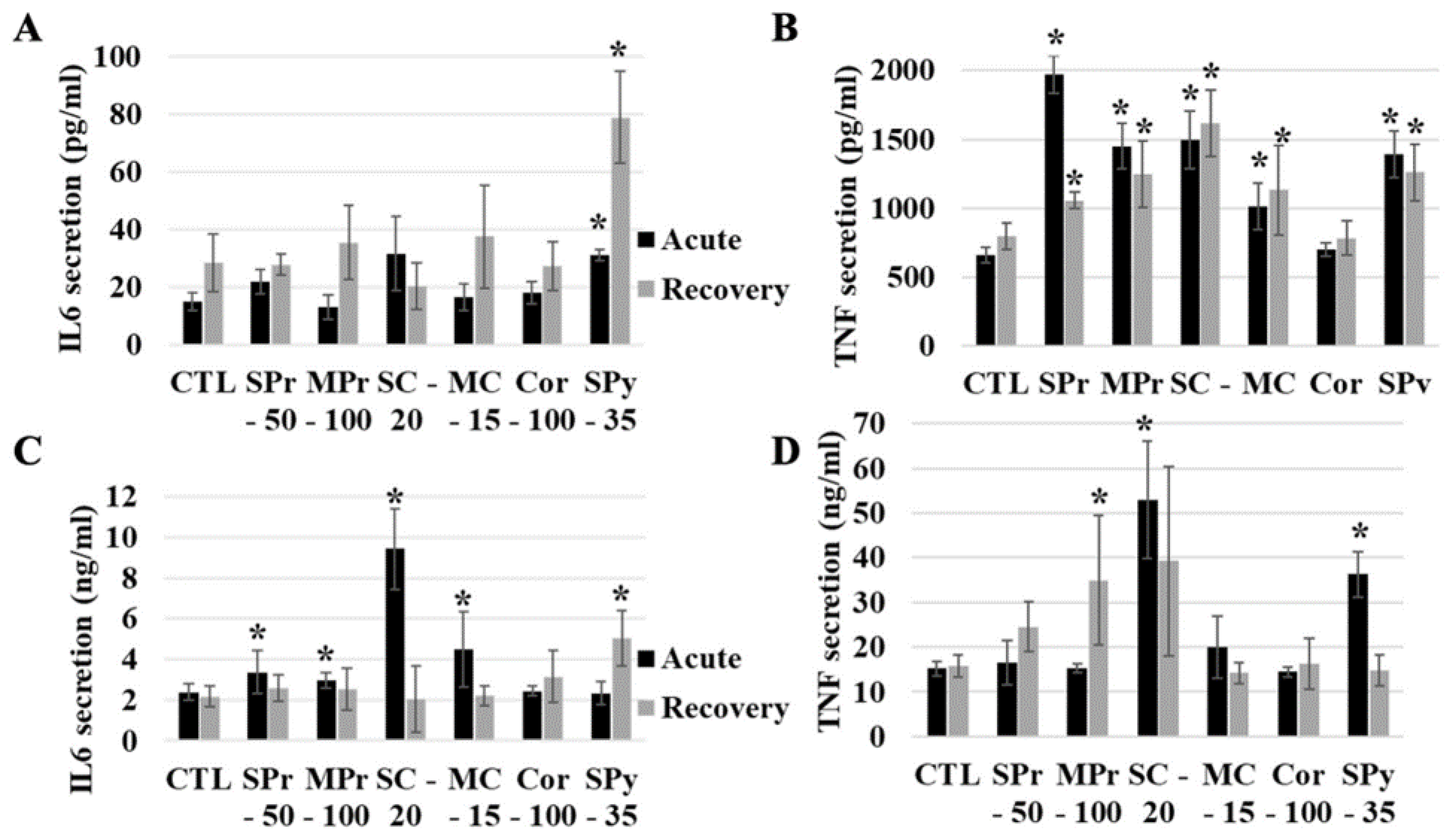
2.5. Functional Effects of Silica on Cells
2.6. Persistence of Silica in Cells
2.7. Synthetic Amorphous Silica Observation after Four Days in Cells
3. Discussion
4. Materials and Methods
4.1. SAS Observation and Characterization
- (i)
- a colloidal silica with small (10 nm) particles, purchased from Sigma-Aldrich (#420808, batch #MKBH8895V, St. Louis, MI, USA), designated as SC—this silica shows a specific area of 220 m2/g;
- (ii)
- a colloidal silica with medium (29 nm) particles, purchased from Sigma-Aldrich (#420778, batch #MKCK1767), designated as MC—this silica shows a specific area of 140 m2/g;
- (iii)
- a precipitated silica with small (13 nm) primary particles, from Solvay Silica (batch #F9C8C4034) designated as SPr—this silica shows a specific area of 220 m2/g;
- (iv)
- a precipitated silica with medium (20 nm) primary particles, from Solvay Silica (batch #F9I21CL032) designated as MPr—this silica shows a specific area of 55 m2/g;
- (v)
- a pyrogenic silica with small (15–20 nm) primary particles, purchased from VWR (#ACRO403731500, batch# A0403808), designated as SPy—this silica shows a specific area of 200 m2/g.
4.2. Nanoparticles
4.3. Cell Culture
4.4. Viability Assay
4.5. Phagocytic Activity Assay
4.6. Cytokine Secretion Dosages
4.7. SAS Quantification by ICP-AES
4.7.1. Sample Preparation
4.7.2. ICP-AES Dosage
4.8. TEM Analysis of Internalized Silica
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Silicosis and Silicate Disease Committee Diseases Associated with Exposure to Silica and Nonfibrous Silicate Minerals. Arch. Pathol. Lab. Med. 1988, 112, 673–720.
- Adverse effects of crystalline silica exposure. American Thoracic Society Committee of the Scientific Assembly on Environmental and Occupational Health. Am. J. Respir. Crit. Care Med. 1997, 155, 761–768. [CrossRef] [PubMed]
- Norboo, T.; Angchuk, P.T.; Yahya, M.; Kamat, S.R.; Pooley, F.D.; Corrin, B.; Kerr, I.H.; Bruce, N.; Ball, K.P. Silicosis in a Himalayan village population: Role of environmental dust. Thorax 1991, 46, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Napierska, D.; Thomassen, L.C.J.; Lison, D.; Martens, J.A.; Hoet, P.H. The nanosilica hazard: Another variable entity. Part. Fibre Toxicol. 2010, 7, 39. [Google Scholar] [CrossRef]
- Fruijtier-Polloth, C. The Toxicological Mode of Action and the Safety of Synthetic Amorphous Silica—A Nanostructured Material. Toxicology 2012, 294, 61–79. [Google Scholar] [CrossRef]
- Warheit, D.B.; A McHugh, T.; A Hartsky, M. Differential pulmonary responses in rats inhaling crystalline, colloidal or amorphous silica dusts. Scand. J. Work. Environ. Health 1995, 21, 19–21. [Google Scholar]
- Johnston, C.J.; Driscoll, K.E.; Finkelstein, J.N.; Baggs, R.; O’Reilly, M.A.; Carter, J.; Gelein, R.; Oberdörster, G. Pulmonary Chemokine and Mutagenic Responses in Rats after Subchronic Inhalation of Amorphous and Crystalline Silica. Toxicol. Sci. 2000, 56, 405–413. [Google Scholar] [CrossRef]
- Sayes, C.; Reed, K.L.; Warheit, D.B. Assessing Toxicity of Fine and Nanoparticles: Comparing In Vitro Measurements to In Vivo Pulmonary Toxicity Profiles. Toxicol. Sci. 2007, 97, 163–180. [Google Scholar] [CrossRef]
- Arts, J.H.; Muijser, H.; Duistermaat, E.; Junker, K.; Kuper, F. Five-day inhalation toxicity study of three types of synthetic amorphous silicas in Wistar rats and post-exposure evaluations for up to 3 months. Food Chem. Toxicol. 2007, 45, 1856–1867. [Google Scholar] [CrossRef]
- Costantini, L.M.; Gilberti, R.M.; Knecht, D.A. The Phagocytosis and Toxicity of Amorphous Silica. PLoS ONE 2011, 6, e14647. [Google Scholar] [CrossRef]
- Breznan, D.; Das, D.D.; O’Brien, J.S.; MacKinnon-Roy, C.; Nimesh, S.; Vuong, N.Q.; Bernatchez, S.; DeSilva, N.; Hill, M.; Kumarathasan, P.; et al. Differential cytotoxic and inflammatory potency of amorphous silicon dioxide nanoparticles of similar size in multiple cell lines. Nanotoxicology 2017, 11, 223–235. [Google Scholar] [CrossRef]
- Park, M.V.D.Z.; Lynch, I.; Ramírez-García, S.; Dawson, K.A.; De La Fonteyne, L.; Gremmer, E.; Slob, W.; Briedé, J.J.; Elsaesser, A.; Howard, C.V.; et al. In vitro evaluation of cytotoxic and inflammatory properties of silica nanoparticles of different sizes in murine RAW 264.7 macrophages. J. Nanoparticle Res. 2011, 13, 6775–6787. [Google Scholar] [CrossRef]
- Panas, A.; Marquardt, C.; Nalcaci, O.; Bockhorn, H.; Baumann, W.; Paur, H.-R.; Mülhopt, S.; Diabaté, S.; Weiss, C. Screening of different metal oxide nanoparticles reveals selective toxicity and inflammatory potential of silica nanoparticles in lung epithelial cells and macrophages. Nanotoxicology 2012, 7, 259–273. [Google Scholar] [CrossRef]
- Wang, X.; Sun, B.B.; Liu, S.J.; Xia, T. Structure Activity Relationships of Engineered Nanomaterials in Inducing NLRP3 In-flammasome Activation and Chronic Lung Fibrosis. Nanoimpact 2017, 6, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, W.J.; Lag, M.; Holme, J.A.; Friede, B.; Gualtieri, M.; Kruszewski, M.; Schwarze, P.E.; Skuland, T.; Refsnes, M. Comparison of Non-Crystalline Silica Nanoparticles in IL-1 Beta Release from Macrophages. Part. Fibre Toxicol. 2012, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Fritsch-Decker, S.; Marquardt, C.; Stoeger, T.; Diabaté, S.; Weiss, C. Revisiting the stress paradigm for silica nanoparticles: Decoupling of the anti-oxidative defense, pro-inflammatory response and cytotoxicity. Arch. Toxicol. 2018, 92, 2163–2174. [Google Scholar] [CrossRef] [PubMed]
- Uboldi, C.; Giudetti, G.; Broggi, F.; Gilliland, D.; Ponti, J.; Rossi, F. Amorphous silica nanoparticles do not induce cytotoxicity, cell transformation or genotoxicity in Balb/3T3 mouse fibroblasts. Mutat. Res. Toxicol. Environ. Mutagen. 2012, 745, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dunphy, D.R.; Jiang, X.; Meng, H.; Sun, B.; Tarn, D.; Xue, M.; Wang, X.; Lin, S.; Ji, Z.; et al. Processing Pathway Dependence of Amorphous Silica Nanoparticle Toxicity: Colloidal vs Pyrolytic. J. Am. Chem. Soc. 2012, 134, 15790–15804. [Google Scholar] [CrossRef]
- Di Cristo, L.; Movia, D.; Bianchi, M.G.; Allegri, M.; Mohamed, B.M.; Bell, A.P.; Moore, C.; Pinelli, S.; Rasmussen, K.; Riego-Sintes, J.; et al. Proinflammatory Effects of Pyrogenic and Precipitated Amorphous Silica Nanoparticles in Innate Immunity Cells. Toxicol. Sci. 2015, 150, 40–53. [Google Scholar] [CrossRef]
- Sun, B.; Wang, X.; Liao, Y.-P.; Ji, Z.; Chang, C.H.; Pokhrel, S.; Ku, J.; Liu, X.; Wang, M.; Dunphy, D.R.; et al. Repetitive Dosing of Fumed Silica Leads to Profibrogenic Effects through Unique Structure–Activity Relationships and Biopersistence in the Lung. ACS Nano 2016, 10, 8054–8066. [Google Scholar] [CrossRef]
- Fubini, B.; Zanetti, G.; Altilia, S.; Tiozzo, R.; Lison, D.; Saffiotti, U. Relationship between Surface Properties and Cellular Responses to Crystalline Silica: Studies with Heat-Treated Cristobalite. Chem. Res. Toxicol. 1999, 12, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Ghiazza, M.; Polimeni, M.; Fenoglio, I.; Gazzano, E.; Ghigo, D.; Fubini, B. Does Vitreous Silica Contradict the Toxicity of the Crystalline Silica Paradigm? Chem. Res. Toxicol. 2010, 23, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Turci, F.; Pavan, C.; Leinardi, R.; Tomatis, M.; Pastero, L.; Garry, D.; Anguissola, S.; Lison, D.; Fubini, B. Revisiting the paradigm of silica pathogenicity with synthetic quartz crystals: The role of crystallinity and surface disorder. Part. Fibre Toxicol. 2015, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Pavan, C.; Santalucia, R.; Leinardi, R.; Fabbiani, M.; Yakoub, Y.; Uwambayinema, F.; Ugliengo, P.; Tomatis, M.; Martra, G.; Turci, F.; et al. Nearly free surface silanols are the critical molecular moieties that initiate the toxicity of silica particles. Proc. Natl. Acad. Sci. USA 2020, 117, 27836–27846. [Google Scholar] [CrossRef] [PubMed]
- Reuzel, P.; Bruijntjes, J.; Feron, V.; Woutersen, R. Subchronic inhalation toxicity of amorphous silicas and quartz dust in rats. Food Chem. Toxicol. 1991, 29, 341–354. [Google Scholar] [CrossRef]
- Dussert, F.; Arthaud, P.-A.; Arnal, M.-E.; Dalzon, B.; Torres, A.; Douki, T.; Herlin, N.; Rabilloud, T.; Carriere, M. Toxicity to RAW264.7 Macrophages of Silica Nanoparticles and the E551 Food Additive, in Combination with Genotoxic Agents. Nanomaterials 2020, 10, 1418. [Google Scholar] [CrossRef]
- Torres, A.; Dalzon, B.; Collin-Faure, V.; Diemer, H.; Fenel, D.; Schoehn, G.; Cianférani, S.; Carrière, M.; Rabilloud, T. How Reversible Are the Effects of Fumed Silica on Macrophages? A Proteomics-Informed View. Nanomaterials 2020, 10, 1939. [Google Scholar] [CrossRef]
- Rabolli, V.; Thomassen, L.C.J.; Princen, C.; Napierska, D.; Gonzalez, L.; Kirsch-Volders, M.; Hoet, P.H.; Huaux, F.; Kirschhock, C.E.A.; Martens, J.A.; et al. Influence of size, surface area and microporosity on the in vitro cytotoxic activity of amorphous silica nanoparticles in different cell types. Nanotoxicology 2010, 4, 307–318. [Google Scholar] [CrossRef]
- Decan, N.; Wu, D.; Williams, A.; Bernatchez, S.; Johnston, M.; Hill, M.; Halappanavar, S. Characterization of in vitro genotoxic, cytotoxic and transcriptomic responses following exposures to amorphous silica of different sizes. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 796, 8–22. [Google Scholar] [CrossRef]
- Murugadoss, S.; Brule, S.V.D.; Brassinne, F.; Sebaihi, N.; Mejia, J.; Lucas, S.; Petry, J.; Godderis, L.; Mast, J.; Lison, D.; et al. Is aggregated synthetic amorphous silica toxicologically relevant? Part. Fibre Toxicol. 2020, 17, 1. [Google Scholar] [CrossRef]
- Dalzon, B.; Torres, A.; Devcic, J.; Fenel, D.; Sergent, J.-A.; Rabilloud, T. A Low-Serum Culture System for Prolonged in Vitro Toxicology Experiments on a Macrophage System. Front. Toxicol. 2021, 3, 780778. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Collin-Faure, V.; Diemer, H.; Moriscot, C.; Fenel, D.; Gallet, B.; Cianférani, S.; Sergent, J.-A.; Rabilloud, T. Repeated Exposure of Macrophages to Synthetic Amorphous Silica Induces Adaptive Proteome Changes and a Moderate Cell Activation. Nanomaterials 2022, 12, 1424. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G.; Holzwarth, U.; Schleh, C.; Hirn, S.; Wenk, A.; Schäffler, M.; Haberl, N.; Semmler-Behnke, M.; Gibson, N. Quantitative biokinetics over a 28 day period of freshly generated, pristine, 20 nm titanium dioxide nanoparticle aerosols in healthy adult rats after a single two-hour inhalation exposure. Part. Fibre Toxicol. 2019, 16, 29. [Google Scholar] [CrossRef]
- Russell, D.; VanderVen, B.C.; Glennie, S.; Mwandumba, H.; Heyderman, R.S. The macrophage marches on its phagosome: Dynamic assays of phagosome function. Nat. Rev. Immunol. 2009, 9, 594–600. [Google Scholar] [CrossRef]
- Wong, C.; Pratiwi, F.W.; Chen, P.; Mou, C.; Hsu, S. Revealing the Phagosomal pH Regulation and Inflammation of Macrophages after Endocytosing Polyurethane Nanoparticles by a Ratiometric pH Nanosensor. Adv. Biol. 2020, 5, e2000200. [Google Scholar] [CrossRef] [PubMed]
- Canton, J.; Khezri, R.; Glogauer, M.; Grinstein, S. Contrasting phagosome pH regulation and maturation in human M1 and M2 macrophages. Mol. Biol. Cell 2014, 25, 3330–3341. [Google Scholar] [CrossRef] [PubMed]
- Jablonski, K.A.; Amici, S.A.; Webb, L.M.; Ruiz-Rosado, J.D.D.; Popovich, P.G.; Partida-Sanchez, S.; Guerau-De-Arellano, M. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS ONE 2015, 10, e0145342. [Google Scholar] [CrossRef]
- Beck-Speier, I.; Kreyling, W.G.; Maier, K.L.; Dayal, N.; Schladweiler, M.C.; Mayer, P.; Semmler-Behnke, M.; Kodavanti, U.P. Soluble iron modulates iron oxide particle-induced inflammatory responses via prostaglandin E2 synthesis: In vitro and in vivo studies. Part. Fibre Toxicol. 2009, 6, 34. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Holzwarth, U.; Hirn, S.; Schleh, C.; Wenk, A.; Schäffler, M.; Haberl, N.; Gibson, N. Quantitative biokinetics over a 28 day period of freshly generated, pristine, 20 nm silver nanoparticle aerosols in healthy adult rats after a single 1½-hour inhalation exposure. Part. Fibre Toxicol. 2020, 17, 21. [Google Scholar] [CrossRef]
- Semmler-Behnke, M.; Takenaka, S.; Fertsch, S.; Wenk, A.; Seitz, J.; Mayer, P.; Oberdörster, G.; Kreyling, W. Efficient Elimination of Inhaled Nanoparticles from the Alveolar Region: Evidence for Interstitial Uptake and Subsequent Reentrainment onto Airways Epithelium. Environ. Health Perspect. 2007, 115, 728–733. [Google Scholar] [CrossRef]
- Semmler, M.; Seitz, J.; Erbe, F.; Mayer, P.; Heyder, J.; Oberdörster, G.; Kreyling, W.G. Long-Term Clearance Kinetics of Inhaled Ultrafine Insoluble Iridium Particles from the Rat Lung, Including Transient Translocation into Secondary Organs. Inhal. Toxicol. 2004, 16, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Rabilloud, T. Optimization of the Cydex Blue Assay: A One-Step Colorimetric Protein Assay Using Cyclodextrins and Com-patible with Detergents and Reducers. PLoS ONE 2018, 13, e0195755. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Godin, B.; Tanaka, T.; Lee, S.-Y.; Chiappini, C.; Liu, X.; Ferrari, M. Size and shape effects in the biodistribution of intravascularly injected particles. J. Control. Release 2010, 141, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Viveros, R.D.; Liberman, A.; Trogler, W.C.; Kummel, A.C. Alkaline and ultrasonic dissolution of biological materials for trace silicon determination. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2015, 33, 031803. [Google Scholar] [CrossRef]
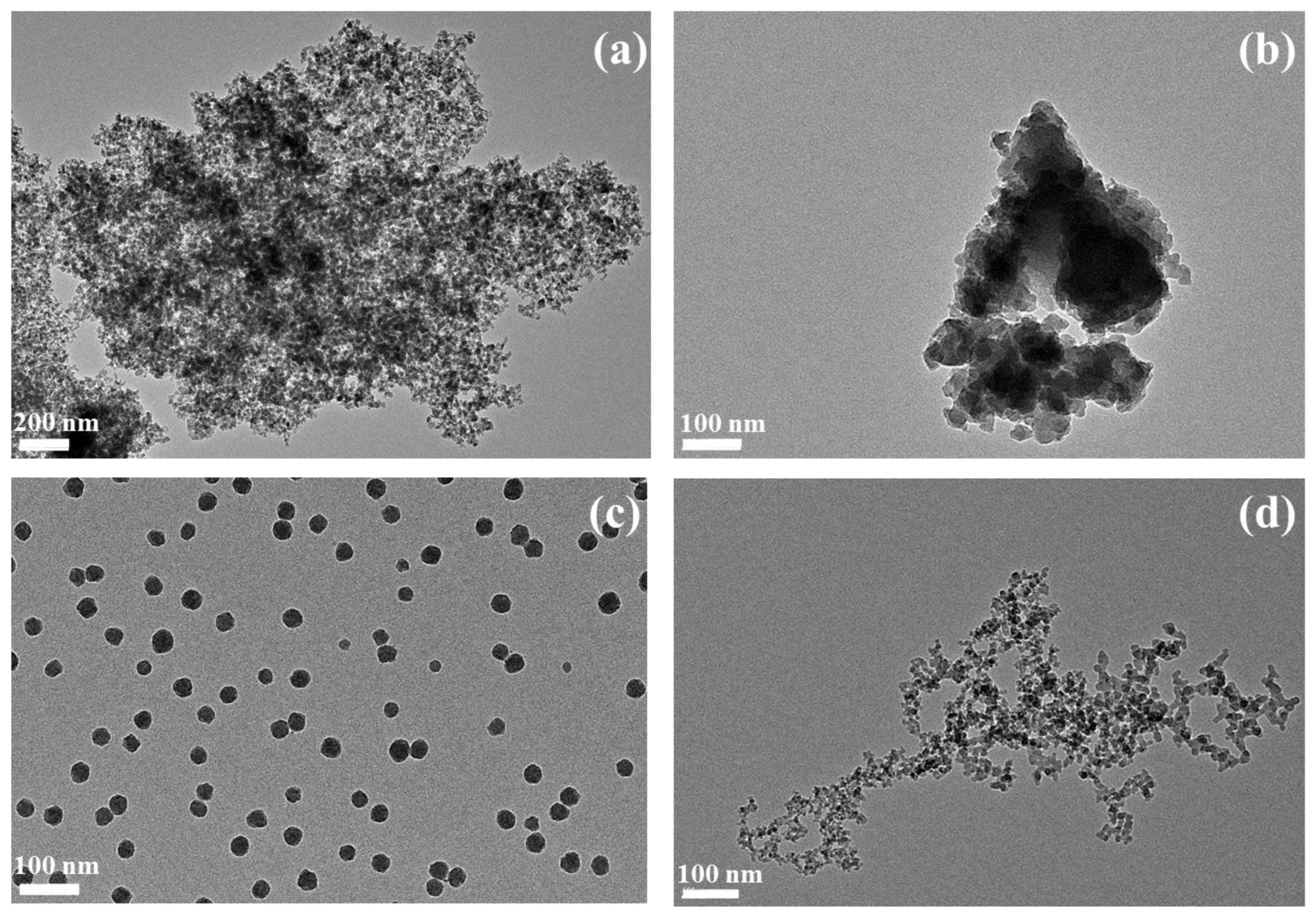
| Condition | Amount Measured in Well (µg) | Measured Amount/Well (µg) (Mean ± SD) | Mineralization Yield | Mean Mineralization Yield |
|---|---|---|---|---|
| CTL | 1.531 | 0.988 ± 0.496 | ||
| 0.558 | ||||
| 0.876 | ||||
| SC—20 | 21.760 | 16.213 ± 4.918 | 0.544 | 0.405 |
| 12.384 | 0.310 | |||
| 14.496 | 0.362 | |||
| MC—15 | 14.560 | 14.357 ± 0.526 | 0.485 | 0.479 |
| 14.752 | 0.492 | |||
| 13.760 | 0.459 | |||
| SPr—100 | 54.368 | 53.461 ± 2.939 | 0.272 | 0.267 |
| 50.176 | 0.251 | |||
| 55.840 | 0.279 | |||
| MPr—100 | 29.152 | 37.92 ± 9.267 | 0.146 | 0.190 |
| 36.992 | 0.185 | |||
| 47.616 | 0.238 | |||
| SPy—35 | 28.480 | 27.563 ± 1.989 | 0.407 | 0.394 |
| 25.280 | 0.361 | |||
| 28.928 | 0.413 |
| Cell Lysate (Acute) | ||||||
|---|---|---|---|---|---|---|
| Condition | Amount Measured in Well | Corrected Amount/Well * | Amount/Well (Mean ± SD) | ng Si/µg Prot | ng Si/µg Prot (Mean ± SD) | Fraction Silica Incorporated in Cells |
| CTL | 0.208 | 0.21 | 0.27 ± 0.30 | 0.01 | 0.013 ± 0.01 | N/A |
| 0.598 | 0.60 | 0.03 | ||||
| 0.018 | 0.02 | 0.00 | ||||
| SC—20 | 6.944 | 12.76 | 13.95 ± 1.08 | 9.78 | 10.09 ± 0.94 | 0.35 |
| 4.603 | 14.87 | 11.15 | ||||
| 5.157 | 14.23 | 9.34 | ||||
| MC—15 | 6.368 | 13.12 | 12.16 ± 1.72 | 8.07 | 6.67 ± 1.38 | 0.41 |
| 5.003 | 10.17 | 5.31 | ||||
| 6.050 | 13.19 | 6.64 | ||||
| SPr—100 | 13.328 | 49.03 | 43.65 ± 11.09 | 38.61 | 41.74 ± 3.02 | 0.22 |
| 12.800 | 51.02 | 44.63 | ||||
| 8.624 | 30.89 | 41.98 | ||||
| MPr—100 | 13.440 | 92.21 | 85.04 ± 14.81 | 66.98 | 55.94 ± 13.40 | 0.43 |
| 17.552 | 94.90 | 59.82 | ||||
| 16.192 | 68.01 | 41.03 | ||||
| SPy—35 | 5.864 | 14.41 | 12.18 ± 4.95 | 8.86 | 6.77 ± 3.04 | 0.17 |
| 5.643 | 15.63 | 8.16 | ||||
| 2.690 | 6.51 | 3.28 |
| Cell Lysate (Recovery) | ||||||
|---|---|---|---|---|---|---|
| Condition | Amount Measured in Well | Yield Corrected Amount/Well * | Amount/Well (Mean ± SD) | ngSi/µg Protein (Mean ± SD) | Fraction Silica Incorporated in Cells | Ratio D4/D1 |
| CTL | 0.831 | 0.831 | 0.63 ± 0.45 | 0.31 ± 0.22 | ||
| 0.947 | 0.947 | |||||
| 0.118 | 0.118 | |||||
| SCc20 | 5.051 | 13.444 | 4.89 ± 7.43 | 2.09 ± 3.06 | 0.12 | 0.35 |
| 0.003 | 0.009 | |||||
| 0.456 | 1.214 | |||||
| MC—15 | 3.662 | 9.747 | 5.34 ± 3.99 | 3.70 ± 3.01 | 0.18 | 0.44 |
| 1.616 | 4.301 | |||||
| 0.745 | 1.984 | |||||
| SPr—100 | 5.418 | 14.419 | 13.18 ± 1.60 | 5.47 ± 0.99 | 0.07 | 0.30 |
| 4.275 | 11.378 | |||||
| 5.162 | 13.737 | |||||
| MPr—100 | 42.592 | 113.357 | 97.36 ± 15.74 | 44.29 ± 7.90 | 0.49 | 1.14 |
| 30.768 | 81.888 | |||||
| 36.384 | 96.835 | |||||
| SPy—35 | 2.270 | 6.043 | 5.84 ± 1.87 | 2.88 ± 0.61 | 0.08 | 0.48 |
| 2.859 | 7.610 | |||||
| 1.457 | 3.878 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, A.; Collin-Faure, V.; Fenel, D.; Sergent, J.-A.; Rabilloud, T. About the Transient Effects of Synthetic Amorphous Silica: An In Vitro Study on Macrophages. Int. J. Mol. Sci. 2023, 24, 220. https://doi.org/10.3390/ijms24010220
Torres A, Collin-Faure V, Fenel D, Sergent J-A, Rabilloud T. About the Transient Effects of Synthetic Amorphous Silica: An In Vitro Study on Macrophages. International Journal of Molecular Sciences. 2023; 24(1):220. https://doi.org/10.3390/ijms24010220
Chicago/Turabian StyleTorres, Anaëlle, Véronique Collin-Faure, Daphna Fenel, Jacques-Aurélien Sergent, and Thierry Rabilloud. 2023. "About the Transient Effects of Synthetic Amorphous Silica: An In Vitro Study on Macrophages" International Journal of Molecular Sciences 24, no. 1: 220. https://doi.org/10.3390/ijms24010220
APA StyleTorres, A., Collin-Faure, V., Fenel, D., Sergent, J.-A., & Rabilloud, T. (2023). About the Transient Effects of Synthetic Amorphous Silica: An In Vitro Study on Macrophages. International Journal of Molecular Sciences, 24(1), 220. https://doi.org/10.3390/ijms24010220






