Studying the Impact of Persistent Organic Pollutants Exposure on Human Health by Proteomic Analysis: A Systematic Review
Abstract
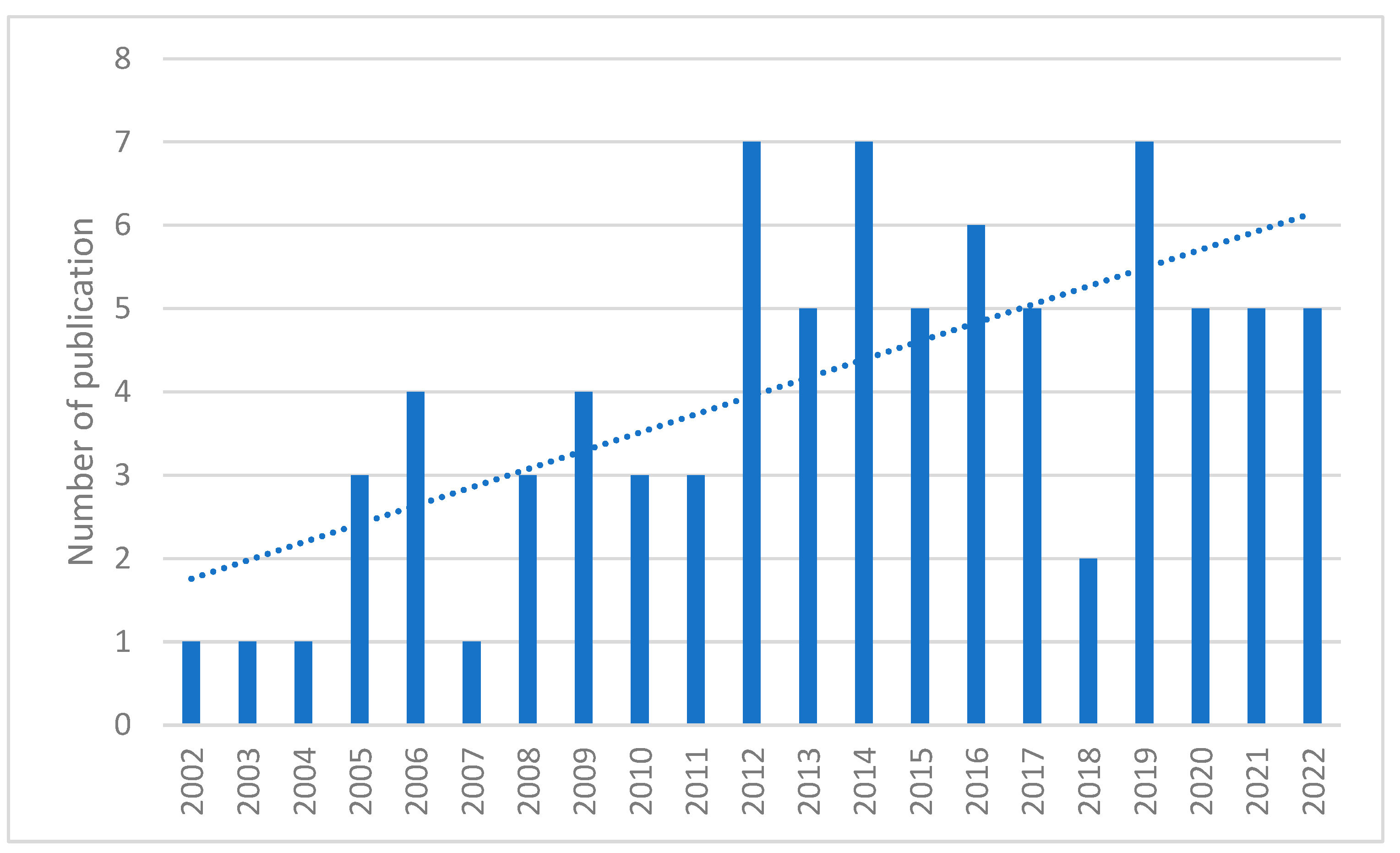
1. Introduction
Proteomic Overview
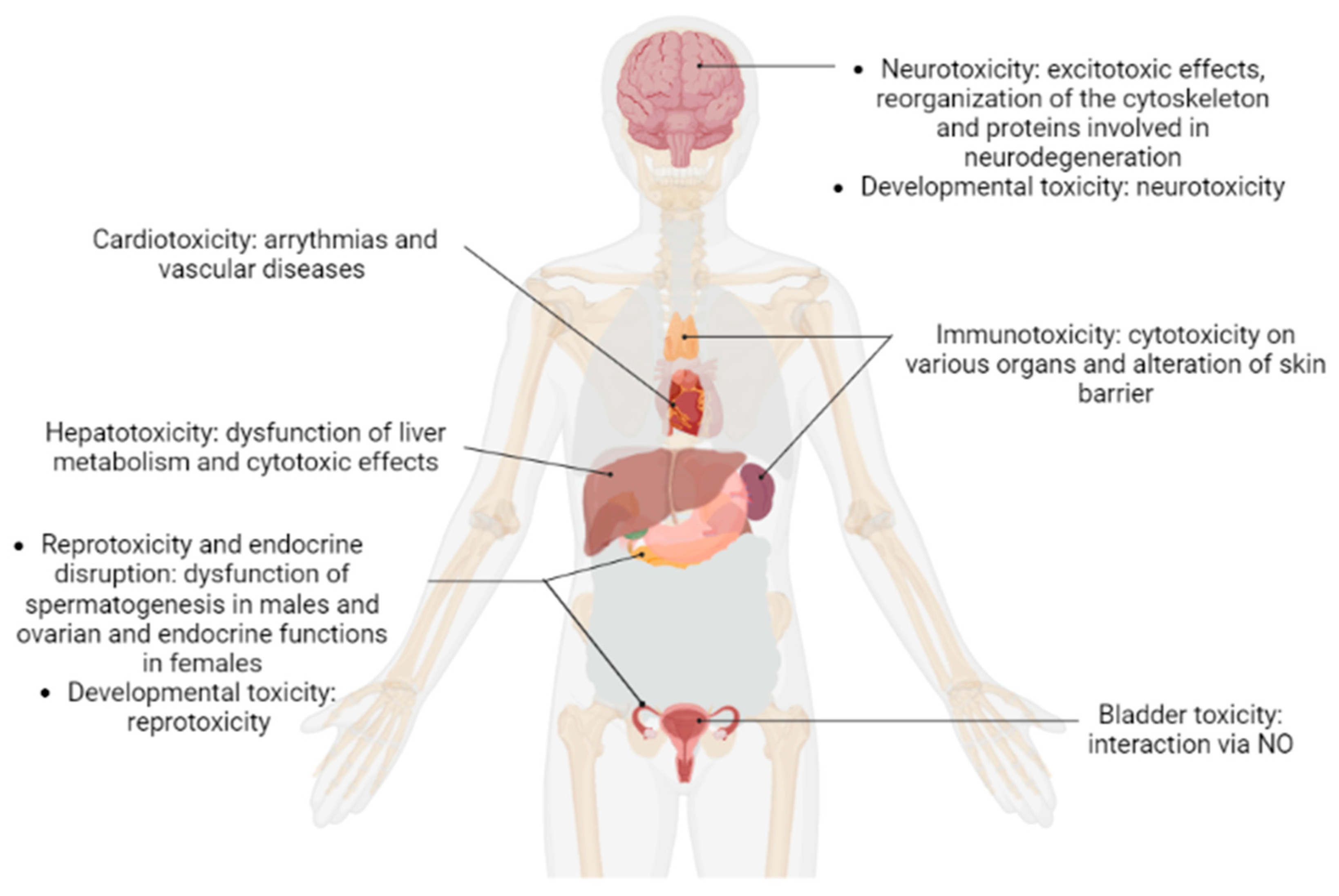
2. Results and Discussion
2.1. Brominated Flame Retardants
2.1.1. BDE-47
2.1.2. BDE-99
2.1.3. BDE-209
2.1.4. HBCD
2.2. Dioxins
2.2.1. PCDD
2.2.2. PCDF
2.3. PCBs
2.4. Pesticides
2.4.1. Chlordane
2.4.2. DDT
2.4.3. Dieldrin
2.4.4. Endosulfan
2.4.5. Lindane
2.4.6. Pentachlorophenol
2.5. Per- and Polyfluoroalkyl Substances
2.5.1. PFOA
2.5.2. PFOS
2.6. Synthesis and Limitations
- -
- The inability to obtain Mesh terms for each POP.
- -
- Physical operator conducted all stages of the screening of articles.
- -
- With inclusion for an unlimited period, different kinds of proteomic analyses were reported, depending on the technologies available during the publication of articles.
3. Methods
3.1. Literature Search
3.2. Selection Criteria
3.3. Data Extraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNEP Stockholm Convention On Persistent Organic Pollutants (POPs). Texts and Annexes. Available online: http://chm.pops.int/TheConvention/Overview/TextoftheConvention/tabid/2232/Default.aspx (accessed on 28 July 2022).
- Mann, M. Origins of Mass Spectrometry-Based Proteomics. Nat. Rev. Mol. Cell Biol. 2016, 17, 678. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.B.; Geyer, P.E.; Colaço, A.R.; Treit, P.V.; Strauss, M.T.; Oroshi, M.; Doll, S.; Virreira Winter, S.; Bader, J.M.; Köhler, N.; et al. The Proteome Landscape of the Kingdoms of Life. Nature 2020, 582, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, M.; Morgan, M.E.; Minden, J.S. Difference Gel Electrophoresis. A Single Gel Method for Detecting Changes in Protein Extracts. Electrophoresis 1997, 18, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, D.; Tarale, P.; Naoghare, P.K.; Bafana, A.; Krishnamurthi, K.; Arrigo, P.; Saravanadevi, S. An Integrated Genomic and Proteomic Approach to Identify Signatures of Endosulfan Exposure in Hepatocellular Carcinoma Cells. Pestic. Biochem. Physiol. 2015, 125, 8–16. [Google Scholar] [CrossRef]
- Gandhi, D.; Tarale, P.; Naoghare, P.K.; Bafana, A.; Kannan, K.; Sivanesan, S. Integrative Genomic and Proteomic Profiling of Human Neuroblastoma SH-SY5Y Cells Reveals Signatures of Endosulfan Exposure. Environ. Toxicol. Pharmacol. 2016, 41, 187–194. [Google Scholar] [CrossRef]
- Pavlikova, N.; Sramek, J.; Jelinek, M.; Halada, P.; Kovar, J. Markers of Acute Toxicity of DDT Exposure in Pancreatic Beta-Cells Determined by a Proteomic Approach. PLoS ONE 2020, 15, e0229430. [Google Scholar] [CrossRef]
- Moulder, R.; Bhosale, S.D.; Goodlett, D.R.; Lahesmaa, R. Analysis of the Plasma Proteome Using ITRAQ and TMT-Based Isobaric Labeling. Mass Spectrom. Rev. 2018, 37, 583–606. [Google Scholar] [CrossRef]
- Cowie, A.M.; Sarty, K.I.; Mercer, A.; Koh, J.; Kidd, K.A.; Martyniuk, C.J. Molecular Networks Related to the Immune System and Mitochondria Are Targets for the Pesticide Dieldrin in the Zebrafish (Danio rerio) Central Nervous System. J. Proteom. 2017, 157, 71–82. [Google Scholar] [CrossRef]
- Ong, S.-E.; Blagoev, B.; Kratchmarova, I.; Kristensen, D.B.; Steen, H.; Pandey, A.; Mann, M. Stable Isotope Labeling by Amino Acids in Cell Culture, SILAC, as a Simple and Accurate Approach to Expression Proteomics. Mol. Cell. Proteom. 2002, 1, 376–386. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Tang, L.-L.; Zheng, B.; Ge, R.-S.; Zhu, D.-Y. Protein Profiles of Cardiomyocyte Differentiation in Murine Embryonic Stem Cells Exposed to Perfluorooctane Sulfonate. J. Appl. Toxicol. 2016, 36, 726–740. [Google Scholar] [CrossRef]
- Lee, H.; Sung, E.J.; Seo, S.; Min, E.K.; Lee, J.-Y.; Shim, I.; Kim, P.; Kim, T.-Y.; Lee, S.; Kim, K.-T. Integrated Multi-Omics Analysis Reveals the Underlying Molecular Mechanism for Developmental Neurotoxicity of Perfluorooctanesulfonic Acid in Zebrafish. Environ. Int. 2021, 157, 106802. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, L.; Zhou, H.; Wang, J.; Zhao, C.; Pang, X. PFOA Regulate Adenosine Receptors and Downstream Concentration-Response CAMP-PKA Pathway Revealed by Integrated Omics and Molecular Dynamics Analyses. Sci. Total. Environ. 2022, 803, 149910. [Google Scholar] [CrossRef]
- Doerr, S.; Harvey, M.J.; Noé, F.; De Fabritiis, G. HTMD: High-Throughput Molecular Dynamics for Molecular Discovery. J. Chem. Theory. Comput. 2016, 12, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Prokopec, S.D.; Lu, A.; Lee, S.C.-E.S.; Yao, C.Q.; Sun, R.X.; Watson, J.D.; Soliymani, R.; de Borja, R.; Wong, A.; Sam, M.; et al. Comparative Toxicoproteogenomics of Mouse and Rat Liver Identifies TCDD-Resistance Genes. Arch. Toxicol. 2019, 93, 2961–2978. [Google Scholar] [CrossRef] [PubMed]
- Morales-Prieto, N.; Abril, N. REDOX Proteomics Reveals Energy Metabolism Alterations in the Liver of M. spretus Mice Exposed to p, p’-DDE. Chemosphere 2017, 186, 848–863. [Google Scholar] [CrossRef]
- Simmons, D.B.D.; Cowie, A.M.; Koh, J.; Sherry, J.P.; Martyniuk, C.J. Label-Free and ITRAQ Proteomics Analysis in the Liver of Zebrafish (Danio rerio) Following Dietary Exposure to the Organochlorine Pesticide Dieldrin. J. Proteom. 2019, 202, 103362. [Google Scholar] [CrossRef]
- Kling, P.; Förlin, L. Proteomic Studies in Zebrafish Liver Cells Exposed to the Brominated Flame Retardants HBCD and TBBPA. Ecotoxicol. Environ. Saf. 2009, 72, 1985–1993. [Google Scholar] [CrossRef]
- Miller, I.; Serchi, T.; Cambier, S.; Diepenbroek, C.; Renaut, J.; Van der Berg, J.H.J.; Kwadijk, C.; Gutleb, A.C.; Rijntjes, E.; Murk, A.J. Hexabromocyclododecane (HBCD) Induced Changes in the Liver Proteome of Eu- and Hypothyroid Female Rats. Toxicol. Lett. 2016, 245, 40–51. [Google Scholar] [CrossRef]
- Miller, I.; Diepenbroek, C.; Rijntjes, E.; Renaut, J.; Teerds, K.J.; Kwadijk, C.; Cambier, S.; Murk, A.J.; Gutleb, A.C.; Serchi, T. Gender Specific Differences in the Liver Proteome of Rats Exposed to Short Term and Low-Concentration Hexabromocyclododecane (HBCD). Toxicol. Res. (Camb) 2016, 5, 1273–1283. [Google Scholar] [CrossRef]
- Fang, Y.; Gao, X.; Zha, J.; Ning, B.; Li, X.; Gao, Z.; Chao, F. Identification of Differential Hepatic Proteins in Rare Minnow (Gobiocypris rarus) Exposed to Pentachlorophenol (PCP) by Proteomic Analysis. Toxicol. Lett. 2010, 199, 69–79. [Google Scholar] [CrossRef]
- Scharmach, E.; Buhrke, T.; Lichtenstein, D.; Lampen, A. Perfluorooctanoic Acid Affects the Activity of the Hepatocyte Nuclear Factor 4 Alpha (HNF4α). Toxicol. Lett. 2012, 212, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, J.; Martin, F.L.; Peng, S.; Tian, M.; Mu, X.; Shen, H. Perfluorooctanoic Acid Induces Apoptosis through the P53-Dependent Mitochondrial Pathway in Human Hepatic Cells: A Proteomic Study. Toxicol. Lett. 2013, 223, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Sun, J.; Yang, J.; Roberts, S.M.; Zhang, X.; Cui, X.; Wei, S.; Ma, L.Q. Molecular Mechanisms of Perfluorooctanoate-Induced Hepatocyte Apoptosis in Mice Using Proteomic Techniques. Environ. Sci. Technol. 2017, 51, 11380–11389. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, H.; Guo, X.; Wang, J.; Dai, J. High Perfluorooctanoic Acid Exposure Induces Autophagy Blockage and Disturbs Intracellular Vesicle Fusion in the Liver. Arch. Toxicol. 2017, 91, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Sun, W.; Zhou, Y.; Griffin, N.; Faulkner, S.; Wang, L. ITRAQ-Based Quantitative Proteomics Analysis of Sprague-Dawley Rats Liver Reveals Perfluorooctanoic Acid-Induced Lipid Metabolism and Urea Cycle Dysfunction. Toxicol. Lett. 2022, 357, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Jin, Y.; Liu, W.; Quan, X.; Chen, J.; Liang, Z. Global Liver Proteome Analysis Using ITRAQ Labeling Quantitative Proteomic Technology to Reveal Biomarkers in Mice Exposed to Perfluorooctane Sulfonate (PFOS). Environ. Sci. Technol. 2012, 46, 12170–12177. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.; Pfohl, M.; Auclair, A.; Jamwal, R.; Barlock, B.J.; Sammoura, F.M.; Goedken, M.; Akhlaghi, F.; Slitt, A.L. Perfluorooctanesulfonic Acid (PFOS) Administration Shifts the Hepatic Proteome and Augments Dietary Outcomes Related to Hepatic Steatosis in Mice. Toxicol. Appl. Pharmacol. 2020, 408, 115250. [Google Scholar] [CrossRef]
- Li, D.; Jiang, L.; Hong, Y.; Cai, Z. Multilayered Glycoproteomic Analysis Reveals the Hepatotoxic Mechanism in Perfluorooctane Sulfonate (PFOS) Exposure Mice. Environ. Pollut. 2021, 268, 115774. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, J.; Peng, S.; Du, M.; Ow, S.; Pu, H.; Pan, C.; Shen, H. Proteomic Analysis of Perfluorooctane Sulfonate-Induced Apoptosis in Human Hepatic Cells Using the ITRAQ Technique. J. Appl. Toxicol. 2014, 34, 1342–1351. [Google Scholar] [CrossRef]
- Cui, R.; Zhang, H.; Guo, X.; Cui, Q.; Wang, J.; Dai, J. Proteomic Analysis of Cell Proliferation in a Human Hepatic Cell Line (HL-7702) Induced by Perfluorooctane Sulfonate Using ITRAQ. J. Hazard. Mater. 2015, 299, 361–370. [Google Scholar] [CrossRef]
- Pfohl, M.; Ingram, L.; Marques, E.; Auclair, A.; Barlock, B.; Jamwal, R.; Anderson, D.; Cummings, B.S.; Slitt, A.L. Perfluorooctanesulfonic Acid and Perfluorohexanesulfonic Acid Alter the Blood Lipidome and the Hepatic Proteome in a Murine Model of Diet-Induced Obesity. Toxicol. Sci. 2020, 178, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Pfohl, M.; Marques, E.; Auclair, A.; Barlock, B.; Jamwal, R.; Goedken, M.; Akhlaghi, F.; Slitt, A.L. An ’Omics Approach to Unraveling the Paradoxical Effect of Diet on Perfluorooctanesulfonic Acid (PFOS) and Perfluorononanoic Acid (PFNA)-Induced Hepatic Steatosis. Toxicol. Sci. 2021, 180, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Rignall, B.; Grote, K.; Gavrilov, A.; Weimer, M.; Kopp-Schneider, A.; Krause, E.; Appel, K.E.; Buchmann, A.; Robertson, L.W.; Lehmler, H.-J.; et al. Biological and Tumor-Promoting Effects of Dioxin-like and Non-Dioxin-like Polychlorinated Biphenyls in Mouse Liver after Single or Combined Treatment. Toxicol. Sci. 2013, 133, 29–41. [Google Scholar] [CrossRef]
- Hardesty, J.E.; Wahlang, B.; Falkner, K.C.; Shi, H.; Jin, J.; Zhou, Y.; Wilkey, D.W.; Merchant, M.L.; Watson, C.T.; Feng, W.; et al. Proteomic Analysis Reveals Novel Mechanisms by Which Polychlorinated Biphenyls Compromise the Liver Promoting Diet-Induced Steatohepatitis. J. Proteome. Res. 2019, 18, 1582–1594. [Google Scholar] [CrossRef]
- Jin, J.; Wahlang, B.; Shi, H.; Hardesty, J.E.; Falkner, K.C.; Head, K.Z.; Srivastava, S.; Merchant, M.L.; Rai, S.N.; Cave, M.C.; et al. Dioxin-like and Non-Dioxin-like PCBs Differentially Regulate the Hepatic Proteome and Modify Diet-Induced Nonalcoholic Fatty Liver Disease Severity. Med. Chem. Res. 2020, 29, 1247–1263. [Google Scholar] [CrossRef]
- Jin, J.; Wahlang, B.; Thapa, M.; Head, K.Z.; Hardesty, J.E.; Srivastava, S.; Merchant, M.L.; Rai, S.N.; Prough, R.A.; Cave, M.C. Proteomics and Metabolic Phenotyping Define Principal Roles for the Aryl Hydrocarbon Receptor in Mouse Liver. Acta Pharm. Sin. B 2021, 11, 3806–3819. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, D.-Y.; Son, W.-K.; Joo, W.-A.; Kim, C.-W. Proteomic Characterization of Rat Liver Exposed to 2,3,7,8-Tetrachlorobenzo-p-Dioxin. J. Proteome Res. 2005, 4, 335–343. [Google Scholar] [CrossRef]
- Sarioglu, H.; Brandner, S.; Haberger, M.; Jacobsen, C.; Lichtmannegger, J.; Wormke, M.; Andrae, U. Analysis of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin-Induced Proteome Changes in 5L Rat Hepatoma Cells Reveals Novel Targets of Dioxin Action Including the Mitochondrial Apoptosis Regulator VDAC2. Mol. Cell. Proteom. 2008, 7, 394–410. [Google Scholar] [CrossRef]
- Bruno, M.E.; Borchers, C.H.; Dial, J.M.; Walker, N.J.; Hartis, J.E.; Wetmore, B.A.; Carl Barrett, J.C.; Tomer, K.B.; Merrick, B.A. Effects of TCDD upon IkappaB and IKK Subunits Localized in Microsomes by Proteomics. Arch. Biochem. Biophys 2002, 406, 153–164. [Google Scholar] [CrossRef]
- Sarioglu, H.; Brandner, S.; Jacobsen, C.; Meindl, T.; Schmidt, A.; Kellermann, J.; Lottspeich, F.; Andrae, U. Quantitative Analysis of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin-Induced Proteome Alterations in 5L Rat Hepatoma Cells Using Isotope-Coded Protein Labels. Proteomics 2006, 6, 2407–2421. [Google Scholar] [CrossRef]
- Oberemm, A.; Meckert, C.; Brandenburger, L.; Herzig, A.; Lindner, Y.; Kalenberg, K.; Krause, E.; Ittrich, C.; Kopp-Schneider, A.; Stahlmann, R.; et al. Differential Signatures of Protein Expression in Marmoset Liver and Thymus Induced by Single-Dose TCDD Treatment. Toxicology 2005, 206, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, S.; Ma, X.; Xu, Y.; Tang, N. Changes in Fibrinopeptide A Peptides in the Sera of Rats Chronically Exposed to Low Doses of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD). Environ. Toxicol. Pharmacol. 2012, 33, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Brandner, S.; Eberhagen, C.; Eckardt-Schupp, F.; Larsen, M.R.; Andrae, U. Quantitative Phosphoproteomic Analysis of Early Alterations in Protein Phosphorylation by 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. J. Proteome Res. 2013, 12, 866–882. [Google Scholar] [CrossRef]
- Kim, J.-H.; In, Y.-J.; Kim, W.K.; Bae, K.-H.; Kang, S.; Lee, S.C. Differential Signatures of Protein Glycosylation and Phosphorylation in Human Chang Liver Cells Induced by TCDD Treatment. Toxicol. Lett. 2008, 178, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, R.; Carpi, D.; Campagna, R.; Airoldi, L.; Pohjanvirta, R.; Viluksela, M.; Hakansson, H.; Boutros, P.C.; Moffat, I.D.; Okey, A.B.; et al. Differential Expression Profiling of the Hepatic Proteome in a Rat Model of Dioxin Resistance: Correlation with Genomic and Transcriptomic Analyses. Mol. Cell. Proteom. 2006, 5, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Lee, D.-Y.; Joo, W.-A.; Kim, C.-W. Plasma Protein Level Changes in Waste Incineration Workers Exposed to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. J. Proteome Res. 2005, 4, 1248–1255. [Google Scholar] [CrossRef]
- Kim, M.K.; Oh, S.; Lee, J.H.; Im, H.; Ryu, Y.M.; Oh, E.; Lee, J.; Lee, E.; Sul, D. Evaluation of Biological Monitoring Markers Using Genomic and Proteomic Analysis for Automobile Emission Inspectors and Waste Incinerating Workers Exposed to Polycyclic Aromatic Hydrocarbons or 2,3,7,8,-Tetracholrodedibenzo-p-Dioxins. Exp. Mol. Med. 2004, 36, 396–410. [Google Scholar] [CrossRef]
- Phark, S.; Park, S.-Y.; Chang, Y.-S.; Choi, S.; Lim, J.; Kim, Y.; Seo, J.B.; Jung, W.-W.; Sul, D. Evaluation of Toxicological Biomarkers in Secreted Proteins of HepG2 Cells Exposed to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin and Their Expressions in the Plasma of Rats and Incineration Workers. Biochim. Biophys Acta 2016, 1864, 584–593. [Google Scholar] [CrossRef]
- Phark, S.; Park, S.-Y.; Choi, S.; Zheng, Z.; Cho, E.; Lee, M.; Lim, J.; Seo, J.B.; Won, N.H.; Jung, W.-W.; et al. Toxicological Biomarkers of 2,3,4,7,8-Pentachlorodibenzofuran in Proteins Secreted by HepG2 Cells. Biochim. Biophys Acta 2012, 1824, 656–666. [Google Scholar] [CrossRef]
- Song, J.; Li, Z.; He, Y.-T.; Liu, C.-X.; Sun, B.; Zhang, C.-F.; Zeng, J.; Du, P.-L.; Zhang, H.-L.; Yu, Y.; et al. Decabrominated Diphenyl Ether (BDE-209) and/or BDE-47 Exposure Alters Protein Expression in Purified Neural Stem/Progenitor Cells Determined by Proteomics Analysis. Int. J. Dev. Neurosci. 2014, 33, 8–14. [Google Scholar] [CrossRef]
- Fowler, P.A.; Abramovich, D.R.; Haites, N.E.; Cash, P.; Groome, N.P.; Al-Qahtani, A.; Murray, T.J.; Lea, R.G. Human Fetal Testis Leydig Cell Disruption by Exposure to the Pesticide Dieldrin at Low Concentrations. Hum. Reprod. 2007, 22, 2919–2927. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tarazona, S.; Bernabeu, E.; Carmona, H.; Gómez-Giménez, B.; García-Planells, J.; Leonards, P.E.G.; Jung, S.; Conesa, A.; Felipo, V.; Llansola, M. A Multiomics Study To Unravel the Effects of Developmental Exposure to Endosulfan in Rats: Molecular Explanation for Sex-Dependent Effects. ACS Chem. Neurosci. 2019, 10, 4264–4279. [Google Scholar] [CrossRef] [PubMed]
- Rasinger, J.D.; Carroll, T.S.; Lundebye, A.K.; Hogstrand, C. Cross-Omics Gene and Protein Expression Profiling in Juvenile Female Mice Highlights Disruption of Calcium and Zinc Signalling in the Brain Following Dietary Exposure to CB-153, BDE-47, HBCD or TCDD. Toxicology 2014, 321, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rasinger, J.D.; Carroll, T.S.; Maranghi, F.; Tassinari, R.; Moracci, G.; Altieri, I.; Mantovani, A.; Lundebye, A.-K.; Hogstrand, C. Low Dose Exposure to HBCD, CB-153 or TCDD Induces Histopathological and Hormonal Effects and Changes in Brain Protein and Gene Expression in Juvenile Female BALB/c Mice. Reprod. Toxicol. 2018, 80, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Alm, H.; Scholz, B.; Fischer, C.; Kultima, K.; Viberg, H.; Eriksson, P.; Dencker, L.; Stigson, M. Proteomic Evaluation of Neonatal Exposure to 2,2,4,4,5-Pentabromodiphenyl Ether. Environ. Health Perspect. 2006, 114, 254–259. [Google Scholar] [CrossRef]
- Krogenæs, A.K.; Ropstad, E.; Gutleb, A.C.; Hårdnes, N.; Berg, V.; Dahl, E.; Fowler, P.A. In Utero Exposure to Environmentally Relevant Concentrations of PCB 153 and PCB 118 Disrupts Fetal Testis Development in Sheep. J. Toxicol. Environ. Health A 2014, 77, 628–649. [Google Scholar] [CrossRef]
- Brunelli, L.; Llansola, M.; Felipo, V.; Campagna, R.; Airoldi, L.; De Paola, M.; Fanelli, R.; Mariani, A.; Mazzoletti, M.; Pastorelli, R. Insight into the Neuroproteomics Effects of the Food-Contaminant Non-Dioxin like Polychlorinated Biphenyls. J. Proteom. 2012, 75, 2417–2430. [Google Scholar] [CrossRef]
- Campagna, R.; Brunelli, L.; Airoldi, L.; Fanelli, R.; Hakansson, H.; Heimeier, R.A.; De Boever, P.; Boix, J.; Llansola, M.; Felipo, V.; et al. Cerebellum Proteomics Addressing the Cognitive Deficit of Rats Perinatally Exposed to the Food-Relevant Polychlorinated Biphenyl 138. Toxicol. Sci. 2011, 123, 170–179. [Google Scholar] [CrossRef]
- Kodavanti, P.R.S.; Osorio, C.; Royland, J.E.; Ramabhadran, R.; Alzate, O. Aroclor 1254, a Developmental Neurotoxicant, Alters Energy Metabolism- and Intracellular Signaling-Associated Protein Networks in Rat Cerebellum and Hippocampus. Toxicol. Appl. Pharmacol. 2011, 256, 290–299. [Google Scholar] [CrossRef]
- Bruggeman, V.; Van den Bergh, G.; Clerens, S.; Dumez, L.; Onagbesan, O.; Arckens, L.; Decuypere, E. Effect of a Single in Ovo Injection of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin on Protein Expression in Liver and Ovary of the One-Day-Old Chick Analyzed by Fluorescent Two-Dimensional Difference Gel Electrophoresis and Mass Spectrometry. Proteomics 2006, 6, 2576–2585. [Google Scholar] [CrossRef]
- Gin, D.-W.; Kim, H.-R.; Lee, D.-M.; Lee, S.-H.; Seong, A.-R.; Hwang, J.-A.; Park, J.-H. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin up-Regulates Stathmin 1 in the Eye: Suggestive Evidence for the Involvement of Stathmin 1 in Accelerated Eye Opening in Mice Induced by 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Toxicol. Lett. 2010, 196, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Liu, L.; Pu, Y.; Zhang, X.; He, X.; Fu, Y. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin Induces a Proteomic Pattern That Defines Cleft Palate Formation in Mice. Food Chem. Toxicol. 2012, 50, 2270–2274. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.; Garcia, G.R.; La Du, J.K.; Sullivan, C.M.; Dunham, C.L.; Goodale, B.C.; Waters, K.M.; Stanisheuski, S.; Maier, C.S.; Thunga, P.; et al. The Ahr2-Dependent Wfikkn1 Gene Influences Zebrafish Transcriptome, Proteome, and Behavior. Toxicol. Sci. 2022, 187, 325–344. [Google Scholar] [CrossRef]
- Huang, Q.; Luo, L.; Han, X.; Li, F.; Zhang, X.; Tian, M. Low-Dose Perfluorooctanoic Acid Stimulates Steroid Hormone Synthesis in Leydig Cells: Integrated Proteomics and Metabolomics Evidence. J. Hazard. Mater 2022, 424, 127656. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Y.; Luo, B.; Yan, S.; Guo, X.; Dai, J. Proteomic Analysis of Mouse Testis Reveals Perfluorooctanoic Acid-Induced Reproductive Dysfunction via Direct Disturbance of Testicular Steroidogenic Machinery. J. Proteome Res. 2014, 13, 3370–3385. [Google Scholar] [CrossRef] [PubMed]
- Lasserre, J.-P.; Fack, F.; Revets, D.; Planchon, S.; Renaut, J.; Hoffmann, L.; Gutleb, A.C.; Muller, C.P.; Bohn, T. Effects of the Endocrine Disruptors Atrazine and PCB 153 on the Protein Expression of MCF-7 Human Cells. J. Proteome Res. 2009, 8, 5485–5496. [Google Scholar] [CrossRef] [PubMed]
- Lasserre, J.-P.; Fack, F.; Serchi, T.; Revets, D.; Planchon, S.; Renaut, J.; Hoffmann, L.; Gutleb, A.C.; Muller, C.P.; Bohn, T. Atrazine and PCB 153 and Their Effects on the Proteome of Subcellular Fractions of Human MCF-7 Cells. Biochim. Biophys Acta 2012, 1824, 833–841. [Google Scholar] [CrossRef]
- Tremoen, N.H.; Fowler, P.A.; Ropstad, E.; Verhaegen, S.; Krogenæs, A. Exposure to the Three Structurally Different PCB Congeners (PCB 118, 153, and 126) Results in Decreased Protein Expression and Altered Steroidogenesis in the Human Adrenocortical Carcinoma Cell Line H295R. J. Toxicol. Environ. Health A 2014, 77, 516–534. [Google Scholar] [CrossRef]
- Williams, K.E.; Lemieux, G.A.; Hassis, M.E.; Olshen, A.B.; Fisher, S.J.; Werb, Z. Quantitative Proteomic Analyses of Mammary Organoids Reveals Distinct Signatures after Exposure to Environmental Chemicals. Proc. Natl. Acad. Sci. USA 2016, 113, E1343–E1351. [Google Scholar] [CrossRef]
- Orlowska, K.; Swigonska, S.; Sadowska, A.; Ruszkowska, M.; Nynca, A.; Molcan, T.; Ciereszko, R.E. The Effects of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin on the Proteome of Porcine Granulosa Cells. Chemosphere 2018, 212, 170–181. [Google Scholar] [CrossRef]
- Orlowska, K.; Swigonska, S.; Sadowska, A.; Ruszkowska, M.; Nynca, A.; Molcan, T.; Zmijewska, A.; Ciereszko, R.E. Proteomic Changes of Aryl Hydrocarbon Receptor (AhR)-Silenced Porcine Granulosa Cells Exposed to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD). PLoS ONE 2019, 14, e0223420. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Kim, I.-W.; Hwang, S.-Y.; Shin, B.-J.; Kim, S.-K. Effect of 2,3,7,8-Tetrachlorodibenzo-p-Dioxin on Testicular Spermatogenesis-Related Panels and Serum Sex Hormone Levels in Rats. BJU Int. 2008, 101, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, X.; Ma, S.; Coenraads, P.-J.; Zhang, C.; Liu, J.; Zhao, L.; Sun, M.; Tang, N. Proteomic Analysis of the Rat Ovary Following Chronic Low-Dose Exposure to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD). J. Toxicol. Environ. Health A 2009, 72, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cui, Y.; Guo, X.; Wang, L.; Li, S.; Lu, Y.; Bi, Y.; Huang, X.; Lin, M.; Xia, Y.; et al. 2,2′,4,4′-Tetrabromodiphenyl Ether Disrupts Spermatogenesis, Impairs Mitochondrial Function and Induces Apoptosis of Early Leptotene Spermatocytes in Rats. Reprod. Toxicol. 2015, 51, 114–124. [Google Scholar] [CrossRef]
- Xu, L.; Gao, S.; Zhao, H.; Wang, L.; Cao, Y.; Xi, J.; Zhang, X.; Dong, X.; Luan, Y. Integrated Proteomic and Metabolomic Analysis of the Testes Characterizes BDE-47-Induced Reproductive Toxicity in Mice. Biomolecules 2021, 11, 821. [Google Scholar] [CrossRef]
- López-Merino, E.; Cuartero, M.I.; Esteban, J.A.; Briz, V. Perinatal Exposure to Pesticides Alters Synaptic Plasticity Signaling and Induces Behavioral Deficits Associated with Neurodevelopmental Disorders. Cell Biol. Toxicol. 2022. [Google Scholar] [CrossRef]
- Mudawal, A.; Singh, A.; Yadav, S.; Mishra, M.; Singh, P.K.; Chandravanshi, L.P.; Mishra, J.; Khanna, V.K.; Bandyopadhyay, S.; Parmar, D. Similarities in Lindane Induced Alterations in Protein Expression Profiling in Different Brain Regions with Neurodegenerative Diseases. Proteomics 2015, 15, 3875–3882. [Google Scholar] [CrossRef]
- Srivastava, A.; Srivastava, A.K.; Mishra, M.; Shankar, J.; Agrahari, A.; Kamthan, M.; Singh, P.K.; Yadav, S.; Parmar, D. A Proteomic Approach to Investigate Enhanced Responsiveness in Rechallenged Adult Rats Prenatally Exposed to Lindane. Neurotoxicology 2019, 74, 184–195. [Google Scholar] [CrossRef]
- Alm, H.; Scholz, B.; Kultima, K.; Nilsson, A.; Andrén, P.E.; Savitski, M.M.; Bergman, A.; Stigson, M.; Fex-Svenningsen, A.; Dencker, L. In Vitro Neurotoxicity of PBDE-99: Immediate and Concentration-Dependent Effects on Protein Expression in Cerebral Cortex Cells. J. Proteome Res. 2010, 9, 1226–1235. [Google Scholar] [CrossRef]
- Ji, F.; Sreenivasmurthy, S.G.; Wei, J.; Shao, X.; Luan, H.; Zhu, L.; Song, J.; Liu, L.; Li, M.; Cai, Z. Study of BDE-47 Induced Parkinson’s Disease-like Metabolic Changes in C57BL/6 Mice by Integrated Metabolomic, Lipidomic and Proteomic Analysis. J. Hazard. Mater. 2019, 378, 120738. [Google Scholar] [CrossRef]
- Ji, F.; Zhu, Z.; Zhang, M.; Zhang, H.; Zhu, L.; Cai, X.; Liu, W.; Song, J.; Li, M.; Cai, Z. 6-OH-BDE-47 Exposure-Induced Parkinson’s Disease Pathology in Sprague Dawley Rat. Sci. Total. Environ. 2020, 711, 135184. [Google Scholar] [CrossRef] [PubMed]
- Son, W.-K.; Lee, D.-Y.; Lee, S.-H.; Joo, W.-A.; Kim, C.-W. Analysis of Proteins Expressed in Rat Plasma Exposed to Dioxin Using 2-Dimensional Gel Electrophoresis. Proteomics 2003, 3, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Carpi, D.; Korkalainen, M.; Airoldi, L.; Fanelli, R.; Hakansson, H.; Muhonen, V.; Tuukkanen, J.; Viluksela, M.; Pastorelli, R. Dioxin-Sensitive Proteins in Differentiating Osteoblasts: Effects on Bone Formation in Vitro. Toxicol. Sci. 2009, 108, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Rice, R.H.; Qin, Q.; Phinney, B.S.; Eigenheer, R.A.; Bao, W.; Zhao, B. Proteomic Analysis of Human Keratinocyte Response to 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) Exposure. J. Proteome Res. 2013, 12, 5340–5347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lanham, K.A.; Heideman, W.; Peterson, R.E.; Li, L. Statistically Enhanced Spectral Counting Approach to TCDD Cardiac Toxicity in the Adult Zebrafish Heart. J. Proteome Res. 2013, 12, 3093–3103. [Google Scholar] [CrossRef]
- Ljunggren, S.A.; Helmfrid, I.; Norinder, U.; Fredriksson, M.; Wingren, G.; Karlsson, H.; Lindahl, M. Alterations in High-Density Lipoprotein Proteome and Function Associated with Persistent Organic Pollutants. Environ. Int. 2017, 98, 204–211. [Google Scholar] [CrossRef]
- Felty, Q. Proteomic 2D DIGE Profiling of Human Vascular Endothelial Cells Exposed to Environmentally Relevant Concentration of Endocrine Disruptor PCB153 and Physiological Concentration of 17β-Estradiol. Cell Biol. Toxicol. 2011, 27, 49–68. [Google Scholar] [CrossRef]
- Verma, N.; Pink, M.; Petrat, F.; Rettenmeier, A.W.; Schmitz-Spanke, S. Proteomic Analysis of Human Bladder Epithelial Cells by 2D Blue Native SDS-PAGE Reveals TCDD-Induced Alterations of Calcium and Iron Homeostasis Possibly Mediated by Nitric Oxide. J. Proteome Res. 2015, 14, 202–213. [Google Scholar] [CrossRef]
- Singh, V.; Cortes-Ramirez, J.; Toms, L.-M.; Sooriyagoda, T.; Karatela, S. Effects of Polybrominated Diphenyl Ethers on Hormonal and Reproductive Health in E-Waste-Exposed Population: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 7820. [Google Scholar] [CrossRef]
- Yadav, A.; Verhaegen, S.; Filis, P.; Domanska, D.; Lyle, R.; Sundaram, A.Y.M.; Leithaug, M.; Østby, G.C.; Aleksandersen, M.; Berntsen, H.F.; et al. Exposure to a Human Relevant Mixture of Persistent Organic Pollutants or to Perfluorooctane Sulfonic Acid Alone Dysregulates the Developing Cerebellum of Chicken Embryo. Environ. Int. 2022, 166, 107379. [Google Scholar] [CrossRef]
- DesMarais, V.; Ghosh, M.; Eddy, R.; Condeelis, J. Cofilin Takes the Lead. J. Cell Sci. 2005, 118, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, S.G.; Yuan, P.X.; Wang, M.; Vijayraghavan, S.; Bloom, A.K.; Davis, D.J.; Gobeske, K.T.; Sweatt, J.D.; Manji, H.K.; Arnsten, A.F.T. Protein Kinase C Overactivity Impairs Prefrontal Cortical Regulation of Working Memory. Science 2004, 306, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Rekart, J.L.; Meiri, K.; Routtenberg, A. Hippocampal-Dependent Memory Is Impaired in Heterozygous GAP-43 Knockout Mice. Hippocampus 2005, 15, 1–7. [Google Scholar] [CrossRef]
- González, N.; Domingo, J.L. Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans (PCDD/Fs) in Food and Human Dietary Intake: An Update of the Scientific Literature. Food Chem. Toxicol. 2021, 157, 112585. [Google Scholar] [CrossRef] [PubMed]
- Pazderova-Vejlupková, J.; Nemcova, M.; Píckova, J.; Jirásek, L.; Lukáš, E. The Development and Prognosis of Chronic Intoxication by Tetrachlordibenzo-p-Dioxin in Men. Arch. Environ. Health: Int. J. 1981, 36, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Shankar, P.; McClure, R.S.; Waters, K.M.; Tanguay, R.L. Gene Co-Expression Network Analysis in Zebrafish Reveals Chemical Class Specific Modules. BMC Genom. 2021, 22, 658. [Google Scholar] [CrossRef]
- Visscher, M.O.; Carr, A.N.; Narendran, V. Epidermal Immunity and Function: Origin in Neonatal Skin. Front. Mol. Biosci. 2022, 9, 894496. [Google Scholar] [CrossRef]
- Jones, K.C.; de Voogt, P. Persistent Organic Pollutants (POPs): State of the Science. Environ. Pollut. 1999, 100, 209–221. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Yang, B.; Wu, Y.; Fan, B.; Zhu, S.; Song, E.; Song, Y. Polychlorinated Biphenyl Quinone Induces Hepatocytes Iron Overload through Up-Regulating Hepcidin Expression. Environ. Int. 2020, 139, 105701. [Google Scholar] [CrossRef]
- Bell, M.R.; Hart, B.G.; Gore, A.C. Two-Hit Exposure to Polychlorinated Biphenyls at Gestational and Juvenile Life Stages: 2. Sex-Specific Neuromolecular Effects in the Brain. Mol. Cell. Endocrinol. 2016, 420, 125–137. [Google Scholar] [CrossRef]
- Peng, L.; Liu, J.; Song, E.; Song, Y. Polychlorinated Biphenyl Quinone Induces Immunotoxicity via Lymphocytes Apoptosis and Th1-Th2 Cell Imbalance in C57BL/6 Mice. Sci. Total. Environ. 2022, 824, 153870. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K.; Geier, D.A.; Homme, K.G.; King, P.G.; Bjørklund, G.; Chirumbolo, S.; Geier, M.R. Developmental Neurotoxicants and the Vulnerable Male Brain: A Systematic Review of Suspected Neurotoxicants That Disproportionally Affect Males. Acta Neurobiol. Exp. (Wars) 2017, 77, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Beale, D.J.; Hillyer, K.; Nilsson, S.; Limpus, D.; Bose, U.; Broadbent, J.A.; Vardy, S. Bioaccumulation and Metabolic Response of PFAS Mixtures in Wild-Caught Freshwater Turtles (Emydura macquarii macquarii) Using Omics-Based Ecosurveillance Techniques. Sci. Total. Environ. 2022, 806, 151264. [Google Scholar] [CrossRef]
- Hoyeck, M.P.; Matteo, G.; MacFarlane, E.M.; Perera, I.; Bruin, J.E. Persistent Organic Pollutants and β-Cell Toxicity: A Comprehensive Review. Am. J. Physiol. Endocrinol. Metab. 2022, 322, E383–E413. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Carrel, D.; Singh, N.K.; Hiester, L.L.; Fanget, I.; Kim, H.; Firestein, B.L. Carboxypeptidase E Independently Changes Microtubule Glutamylation, Dendritic Branching, and Neuronal Migration. ASN Neuro. 2022, 14, 17590914211062764. [Google Scholar] [CrossRef]
- Lv, N.; Yuan, J.; Ji, A.; Shi, L.; Gao, M.; Cui, L.; Jiang, Q. Perfluorooctanoic Acid-Induced Toxicities in Chicken Embryo Primary Cardiomyocytes: Roles of PPAR Alpha and Wnt5a/Frizzled2. Toxicol. Appl. Pharmacol. 2019, 381, 114716. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Jiang, Q.; Geng, M.; Zhu, L.; Xia, Y.; Khanal, A.; Wang, C. The Role of PPAR Alpha in Perfluorooctanoic Acid Induced Developmental Cardiotoxicity and L-Carnitine Mediated Protection-Results of in Ovo Gene Silencing. Environ. Toxicol. Pharmacol. 2017, 56, 136–144. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Inserm. Expertise Collective Inserm Exposition Aux Pesticides et au Chlordécone, Risque de Survenue d’un Cancer de la Prostate; Inserm: Paris, France, 2019. [Google Scholar]
- Krausová, M.; Braun, D.; Buerki-Thurnherr, T.; Gundacker, C.; Schernhammer, E.; Wisgrill, L.; Warth, B. Understanding the Chemical Exposome During Fetal Development and Early Childhood: A Review. Annu. Rev. Pharmacol. Toxicol. 2022. [Google Scholar] [CrossRef]
- Wild, C.P. The Exposome: From Concept to Utility. Int. J. Epidemiol. 2012, 41, 24–32. [Google Scholar] [CrossRef]
- Pristner, M.; Warth, B. Drug-Exposome Interactions: The Next Frontier in Precision Medicine. Trends. Pharmacol. Sci. 2020, 41, 994–1005. [Google Scholar] [CrossRef]
- Marcus, K.; Lelong, C.; Rabilloud, T. What Room for Two-Dimensional Gel-Based Proteomics in a Shotgun Proteomics World? Proteomes 2020, 8, 17. [Google Scholar] [CrossRef]
- Aebersold, R.; Mann, M. Mass-Spectrometric Exploration of Proteome Structure and Function. Nature 2016, 537, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hu, Z.; Sun, C.; Wang, Y.; Li, W.; Peng, Y.; Zheng, J. A Metabolic Activation-Based Chemoproteomic Platform to Profile Adducted Proteins Derived from Furan-Containing Compounds. ACS Chem. Biol. 2022, 17, 873–882. [Google Scholar] [CrossRef]
- Canzler, S.; Schor, J.; Busch, W.; Schubert, K.; Rolle-Kampczyk, U.E.; Seitz, H.; Kamp, H.; von Bergen, M.; Buesen, R.; Hackermüller, J. Prospects and Challenges of Multi-Omics Data Integration in Toxicology. Arch. Toxicol. 2020, 94, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chaudhary, K.; Garmire, L.X. More Is Better: Recent Progress in Multi-Omics Data Integration Methods. Front. Genet. 2017, 8, 84. [Google Scholar] [CrossRef] [PubMed]


| Molecule | Species | Consequences | Technique | Article |
|---|---|---|---|---|
| DDT | Mus spretus (SPRET/EiJ) | Metabolic reprogramming: impact on energy metabolism (glucose and lipid). | 2D-DIGE and MALDI-TOF/TOF | [16] |
| Dieldrin | Danio rerio | Dysregulation of proteins involved in energy metabolism (lipid and glucose), oxidative stress, and drug metabolism. | Label free or iTRAQ labelling and LC-MS/MS | [17] |
| Endosulfan | Homo sapiens cells (HepG2) | Alteration of proteins involved in cellular pathways: apoptosis, immune/inflammatory response, and carbohydrate metabolism. | 2D-DIGE and MALDI-TOF | [5] |
| HBCD | Danio rerio cells (liver) | Dysregulated proteins involved in protein metabolism, cellular energy, and cytoskeleton dynamics. | 2D-DIGE and MALDI-TOF or FT-ICR | [18] |
| Rattus norvegicus (Wistar) | Dysregulated proteins involved in oxidative stress response and energy metabolism (glucose, amino acid, and lipid) in females. | 2D-DIGE and MALDI-TOF/TOF | [19] | |
| Rattus norvegicus (Wistar) | Dysregulated proteins involved in oxidative stress response and energy metabolism (glucose, amino acid, and lipid), depending on sex. | 2D-DIGE and MALDI-TOF/TOF | [20] | |
| Pentachlorophenol | Gobiocypris rarus | Dysregulated proteins involved in transport, metabolism, and oxidative stress response. | 2D-DIGE and MALDI-TOF/TOF | [21] |
| PFOA | Homo sapiens cells (HepG2) | Dysregulated proteins involved in lipid metabolism and cancer, with HNF4 as the key network regulator. | 2D-DIGE and MALDI-TOF | [22] |
| Homo sapiens cells (L-02) | Impact of apoptosis by activation of p53 pathway. | 2D DIGE and MALDI-TOF/TOF | [23] | |
| Mus musculus (BALB/c) | Impact of apoptosis by adenosine receptors (cAMP pathway). | iTRAQ labelling and LC-MS/MS | [24] | |
| Homo sapiens cells (HepG2) | Autophagy blockage and dysregulation of vesicular trafficking. | iTRAQ labelling and LC-MS/MS | [25] | |
| Rattus norvegicus (Sprague-Dawley) | Impact of lipid metabolism mediated by HNF4. | iTRAQ labelling and LC-MS/MS | [26] | |
| PFOS | Mus musculus (Kunming) | Involvement of PPARα mechanism in cellular effects. | iTRAQ labelling and nanoLC-MS/MS | [27] |
| Mus musculus (C57BL/6J) | Activation of PPARα: proteins involved in fatty acid metabolism, lipid synthesis, and xenobiotic metabolism. | SWATH-MS | [28] | |
| Mus musculus (C57BL/6J) | Glycoproteomics: activation of PPARα mechanism and dysregulation of proteins involved in ER stress and neutrophil degranulation. | nanoLC-MS/MS | [29] | |
| Homo sapiens cells (L-02) | Triggering of the apoptotic process by activating of the p53 and c-Myc signalling pathways. | iTRAQ labelling and 2D nanoLC-MS/MS | [30] | |
| Homo sapiens cells (HL-7702) | Upregulation of proteins driving cells into the cell cycle from the G1 stage. | iTRAQ labelling and LC-MS/MS | [31] | |
| Mus musculus (C57BL/6J) | Lipidomic analysis: activation of PPARα mechanism. | SWATH-MS | [32] | |
| Mus musculus (C57BL/6J) | Transcriptomic and proteomic integrative analysis: activation of PPARα mechanism. | SWATH-MS | [33] | |
| PCB | Mus musculus (C3H/N) | Dose-dependent increases in Cyp1a1 and Cyp2b10. | 2D-DIGE and MALDI-TOF/TOF | [34] |
| Mus musculus (C57BL/6J) | Dysregulation of transcription factors involved in liver function. | LC-MS/MS | [35] | |
| Mus musculus (C57BL/6J) | Overall impact on liver metabolism by activating AhR. | TMT labelling and nanoLC-MS/MS | [36] | |
| Mus musculus (C57BL/6J) | Ahr-dependent mechanism: dysregulation of proteins involved in liver metabolism and bioenergetics and endocrine response and function. | TMT labelling and nanoLC-MS/MS | [37] | |
| PCDD | Rattus norvegicus (Sprague-Dawley) | Short-term exposure: increase of apolipoprotein A-IV. | 2D-DIGE and nanoLC-MS/MS | [38] |
| Rattus norvegicus cells (H4IIEC3) | Dysregulated proteins involved in oxidative stress and mitochondrial function. | 2D-DIGE and MALDI-TOF/TOF | [39] | |
| Rattus norvegicus (Sprague-Dawley) | Dysregulation of proteins involved in the NF-κB pathway, which may increase oxidative stress. | 2D DIGE and MALDI-TOF | [40] | |
| Rattus norvegicus cells (H4IIEC3) | Dysregulated proteins involved in cell cycle regulation, growth factor signalling, and control of apoptosis. | ICPL labelling and 1-D nano-LC and MALDI-TOF/TOF | [41] | |
| Callithrix jacchus | Dysregulation of proteins involved in the immune response and downregulation of thymidine phosphorylase. | 2D DIGE and LC-MS/MS | [42] | |
| Rattus norvegicus (Sprague-Dawley) | Fibrinopeptide A levels correlated with liver fatty degeneration and necrosis. | magnetic bead separation and MALDI-TOF/TOF and nanoLC-MS/MS | [43] | |
| Rattus norvegicus cells (H4IIEC3) | Phosphoproteomics: dysregulation of transcription factors (ARNT) and coregulators and regulators of small GTPases. | SILAC labelling and LC-MS/MS | [44] | |
| Homo sapiens cells (Chang liver) | Dysregulated proteins involved in cellular protein folding and turnover, energy metabolism, cytoskeletal network, and vesicular trafficking. | 2D-DIGE and MALDI-TOF/TOF | [45] | |
| Rattus norvegicus (Long evans and Han/Wistar) | Role of AhR in major differences in response between strains of rats. | 2D DIGE and LC-MS/MS | [46] | |
| Mus musculus (C57BL/6 and DBA/2 J) | Three altered proteins (SNRK, IGTP, and IMPA2) showing consistent strain-dependent changes. | LC-MS (HDMS) | [15] | |
| Homo sapiens | Seven upregulated proteins: AFP, fibronectin, pre-albumin, fibrinogen gamma A chain precursor, XAP-5, human rab GDI, and follistatin. One downregulated protein: albumin. | 2D-DIGE and MALDI-TOF/TOF | [47] | |
| Homo sapiens | Upregulation of proteins involved in oxidative stress. | 2D-DIGE and MALDI-TOF | [48] | |
| Homo sapiens cells (HepG2) | Validation of eight biomarkers (GLO 1, HGD, PRX 1, PSMB 5 and 6, UDPGlcDH, HADH, and STF). | 2D-DIGE and nanoLC-MS/MS | [49] | |
| PCDF | Homo sapiens cells (HepG2) | Potential biomarkers: protein DJ-1, proteasome activator complex subunit 1, and plasminogen activator inhibitor-3. | 2D-DIGE and nanoLC-MS/MS | [50] |
| Molecule | Species | Consequences | Technique | Article |
|---|---|---|---|---|
| BDE-209 | Rattus norvegicus cells (neural stem culture) | Decrease in proteins involved in cytoskeletal maintenance. | 2D-DIGE and MALDI-TOF | [51] |
| Dieldrin | Homo sapiens culture (fetal testis) | Dysregulation of proteins involved in the incidence of abnormal cell development: antagonization of LH effects. | 2D-DIGE and MALDI-TOF | [52] |
| Endosulfan | Rattus norvegicus (Wistar) | In males: alteration of motor coordination through phosphatidylinositol pathways. In females: up-regulation of GAD. | TMT LC-MS3 | [53] |
| HBCD | Mus musculus (BALB/c) | Dysregulated proteins involved in the disruption of cellular calcium homeostasis. | 2D-DIGE and LC-MS/MS | [54] |
| Mus musculus (BALB/c) | Alteration of T/E2 ratios through calcium dysfunction, responsible for oxidative stress and lipid metabolism. | 2D-DIGE and LC-MS/MS | [55] | |
| BDE-99 | Mus musculus (NMRI) | Altered proteins in the striatum involved in neurodegeneration and neuroplasticity; in the hippocampus, proteins involved in metabolism and energy production were affected. | 2D-DIGE and MALDI-TOF-MS | [56] |
| PCB | Ovis aries (Norwegian White) | Subtle effects on the developing fetal testis: changes in cellular processes as stress response and cytoskeleton regulation. | 2D-DIGE and LC-MS/MS | [57] |
| Rattus norvegicus neurons (Wistar) | Dysregulation of proteins involved in calcium homeostasis, cytoskeletal process, and trafficking of proteins. | LC-MS/MS | [58] | |
| Rattus norvegicus (Wistar) | Dysregulation of proteins involved in calcium homeostasis and phosphoinositol signalling pathway. | 1D-DIGE and LC-MS/MS | [59] | |
| Rattus norvegicus (Long-Evans) | Dysregulation of proteins involved in calcium homeostasis. | 2D-DIGE and MALDI-TOF/TOF | [60] | |
| Mus musculus (BALB/c) | Dysregulation of proteins involved in calcium homeostasis and lipid metabolism. | 2D-DIGE and LC-MS/MS | [54] | |
| Mus musculus (BALB/c) | Dysregulation of proteins involved in calcium homeostasis and energy metabolism. | 2D-DIGE and LC-MS/MS | [55] | |
| PCDD | Gallus gallus domesticus (chick) | Dysregulated proteins involved in blood clotting, oxidative stress, electron transport, and calcium regulation. | 2D-DIGE and LC-MS | [61] |
| Mus musculus (BALB/c) | Probable proliferation of corneal epithelial cells before eye opening: increase in stathmin 1. | 2D-DIGE and MALDI-TOF/TOF | [62] | |
| Mus musculus (C57BL/6J) | Association peroxiredoxin-1 and cleft palate formation. | 2D-DIGE and MALDI-TOF/TOF | [63] | |
| Mus musculus (BALB/c) | Dysregulation of proteins involved in calcium homeostasis and lipid metabolism. | 2D-DIGE and LC-MS/MS | [54] | |
| Mus musculus (BALB/c) | Dysregulation of proteins involved in calcium homeostasis and energy metabolism. | 2D-DIGE and LC-MS/MS | [55] | |
| Danio rerio | Role in neurological pathway: WFIKKN1 involved in skeletal muscle development. | LC-MS | [64] | |
| BDE-47 | Rattus norvegicus cells (neural stem culture) | Decrease in proteins involved in cytoskeletal maintenance. | 2D DIGE and MALDI-TOF-MS | [51] |
| Mus musculus (BALB/c) | Dysregulation of proteins involved in calcium homeostasis and lipid metabolism. | 2D-DIGE and LC-MS/MS | [54] | |
| PFOS | Mus musculus cells (embryonic stem) | Cardiovascular dysfunction: dysregulation of proteins involved in xenobiotic metabolism. | SILAC labelling and LC-MS/MS | [11] |
| Danio rerio | Abnormal neurological development: dysregulation of Rab proteins and proteins involved in calcium homeostasis and axonal formation. | TMT labelling and LC-HR-MS | [12] |
| Molecule | Species | Consequences | Technique | Article |
|---|---|---|---|---|
| PFOA | Mus musculus cells (MLTC-1 Leydig) | Stimulation of steroidogenesis by accelerating fatty acid metabolism and steroidogenic processes. | nanoLC-MS/MS | [65] |
| Homo sapiens cells (SKBr3) | Dysregulation of the cAMP pathway by adenosine receptors. | TMT labelling and nanoLC-MS/MS | [13] | |
| Mus musculus (BALB/c) | Inhibition of proteins involved in steroidogenesis (CYP11A1, INSL3) in a dose-dependent manner. | iTRAQ labelling and LC-ESI-MS/MS | [66] | |
| PCB | Homo sapiens cells (MCF7) | Endocrine disruption properties: dysregulated proteins involved in stress response and cytoskeleton process. | 2D-DIGE and MALDI-TOF/TOF | [67] |
| Homo sapiens cells (MCF7) | Endocrine disruption properties: dysregulated proteins involved in stress response and cytoskeleton process in subcellular fractions. | 2D-DIGE and MALDI-TOF/TOF | [68] | |
| Homo sapiens cells (H295R) | Perturbation of steroidogenesis: dysregulated proteins involved in protein synthesis, cortisol synthesis, stress response, and apoptosis. | 2D-DIGE and LC-MS/MS | [69] | |
| Mus musculus gland organoids (FVB/n) | Oestrogen-independent effects: dysregulation of proteins involved in apoptosis, cell adhesion, and proliferation. | iTRAQ labelling and LC-MS/MS | [70] | |
| PCDD | Sus domesticus cells (granulosa from AVG-16 line) | Affects ovarian follicle fate by reorganising the cytoskeleton and extracellular matrix and modulating proteins important for the cellular response to stress. | 2D-DIGE and MALDI-TOF/TOF | [71] |
| Sus domesticus cells (granulosa from AVG-16 line) | Alteration of three proteins by an AhR-independent mechanism: PDI, ATPbeta, and AnxA5. | 2D-DIGE and MALDI-TOF/TOF | [72] | |
| Rattus norvegicus (Sprague-Dawley) | Upregulation of six testicular proteins involved in stress response and downregulation of SP22, a potential biomarker to diagnose human infertility in dioxin-exposed men. | 2D-DIGE and MALDI-TOF/TOF | [73] | |
| Rattus norvegicus (Sprague-Dawley) | Disruption of ovarian and endocrine functions by dysregulated proteins involved in stress response. | 2D-DIGE and MALDI-TOF/TOF | [74] | |
| BDE-47 | Rattus norvegicus (Sprague-Dawley) | Disruption of spermatogenesis: dysregulated proteins involved in apoptosis and mitochondrial function. | 2D-DIGE and MALDI-TOF/TOF | [75] |
| Mus musculus (C57BL/6J) | Affects male reproduction: dysregulation of proteins involved in stress response, cell maintenance, and inflammatory response. | iTRAQ labelling and nanoLC-MS/MS | [76] | |
| DDT | Homo sapiens cells (NES2Y) | Dysregulated proteins involved in stress response, mitochondrial process, cytoskeletal process, and cell maintenance. | 2D-DIGE and MALDI-TOF/TOF | [7] |
| Molecule | Species | Consequences | Technique | Article |
|---|---|---|---|---|
| Chlordane | Rattus norvegicus (Wistar) | Dysregulation in the hippocampus of proteins involved in brain development and synaptic signalling. | LC–MS/MS | [77] |
| Dieldrin | Danio rerio | Dysregulated proteins involved in Parkinson’s and Huntington’s disease and mitochondria function. | iTRAQ and LC-MS/MS | [9] |
| Endosulfan | Homo sapiens cells (SH-SY5Y) | Dysregulated proteins involved in regulation of neuronal development, cell adhesion and related processes, apoptosis, signal transduction, and regulation of transmission of nerve impulses. | 2D-DIGE and MALDI-TOF | [6] |
| Lindane | Rattus norvegicus (Wistar) | Accumulation of lewy body and beta-amyloid fibrils in the brain, dysfunction of neurotransmission, and dysregulated proteins involved in energy metabolism and oxidative stress. | 2D DIGE and MALDI-TOF/TOF | [78] |
| Rattus norvegicus (Wistar) | Dysregulation of proteins related to energy metabolism and oxidative stress. | 2D DIGE and MALDI-TOF/TOF | [79] | |
| BDE-99 | Rattus norvegicus culture cells (Sprague-Dawley) | Disruption of neurite growth: dysregulation of cytoskeletal proteins and Gap43. | 2D-DIGE and nanoLC-MS/MS | [80] |
| BDE-47 | Mus musculus (C57BL/6J) | Dysregulation of proteins involved in the Parkinson’s disease pathology. | iTRAQ and nano LC-MS | [81] |
| Rattus norvegicus (Sprague-Dawley) | Affection of protein degradation pathways: neurotransmitter system disturbance, increased formation of a-synuclein aggregate, and induction of motor defect, all hallmarks of Parkinson’s disease. | LC-MS/MS | [82] |
| Molecule | Species | Consequences | Technique | Article |
|---|---|---|---|---|
| PCDD | Rattus norvegicus (Sprague-Dawley) | Stimulation of immune system: increase in cytokeratin 8 polypeptide, Ig lambda-1 chain C region, and Ig lambda-2 chain C region. | 2D-DIGE and MALDI-TOF or nanoLC-MS/MS | [83] |
| Callithrix jacchus | Dysregulation of proteins involved in oxidative stress and cytoskeleton maintenance. | 2D-DIGE and LC-MS/MS | [42] | |
| Rattus norvegicus cells (MSC) | Affects osteoblast differentiation by altering their cell architecture, adhesive properties, and calcium homeostasis. | 2D-DIGE and LC-MS/MS | [84] | |
| Homo sapiens cells (hEpB, hEpE and SIK) | Decreases in the differentiation keratinocyte markers: filaggrin, keratin 1, and keratin 10. | 2D-DIGE and LC-MS/MS | [85] |
| Molecule | Species | Consequences | Technique | Article |
|---|---|---|---|---|
| PCDD | Danio rerio | Cardiac hypertrophy and heart failure: alterations of proteins involved in calcium handling, energy metabolism, and cellular redox state. | 2D-DIGE and LC-MS/MS | [86] |
| PCB | Homo sapiens | Positive association of PCB exposure: proteins involved in lipid metabolism. Negative association: proteins involved in inflammation/immunity response and platelet degranulation. | nanoLC-MS/MS | [87] |
| Homo sapiens cells (HMVEC) | Vascular wall damage by a central network focused on c-Myc and TGFB1. | 2D-DIGE and MALDI-TOF/TOF | [88] |
| Molecule | Species | Consequences | Technique | Article |
|---|---|---|---|---|
| PCDD | Homo sapiens cells (RT4) | PCDD exposure: interaction between calcium and iron via NO upon PCDD exposure. | 2D-DIGE and MALDI-TOF | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillotin, S.; Delcourt, N. Studying the Impact of Persistent Organic Pollutants Exposure on Human Health by Proteomic Analysis: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 14271. https://doi.org/10.3390/ijms232214271
Guillotin S, Delcourt N. Studying the Impact of Persistent Organic Pollutants Exposure on Human Health by Proteomic Analysis: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(22):14271. https://doi.org/10.3390/ijms232214271
Chicago/Turabian StyleGuillotin, Sophie, and Nicolas Delcourt. 2022. "Studying the Impact of Persistent Organic Pollutants Exposure on Human Health by Proteomic Analysis: A Systematic Review" International Journal of Molecular Sciences 23, no. 22: 14271. https://doi.org/10.3390/ijms232214271
APA StyleGuillotin, S., & Delcourt, N. (2022). Studying the Impact of Persistent Organic Pollutants Exposure on Human Health by Proteomic Analysis: A Systematic Review. International Journal of Molecular Sciences, 23(22), 14271. https://doi.org/10.3390/ijms232214271






