Dihydromyricetin Attenuates High-Intensity Exercise-Induced Intestinal Barrier Dysfunction Associated with the Modulation of the Phenotype of Intestinal Intraepithelial Lymphocytes
Abstract
1. Introduction
2. Results
2.1. DHM Attenuates the Intestinal Inflammation in Mice Induced by High-Intensity Exercise
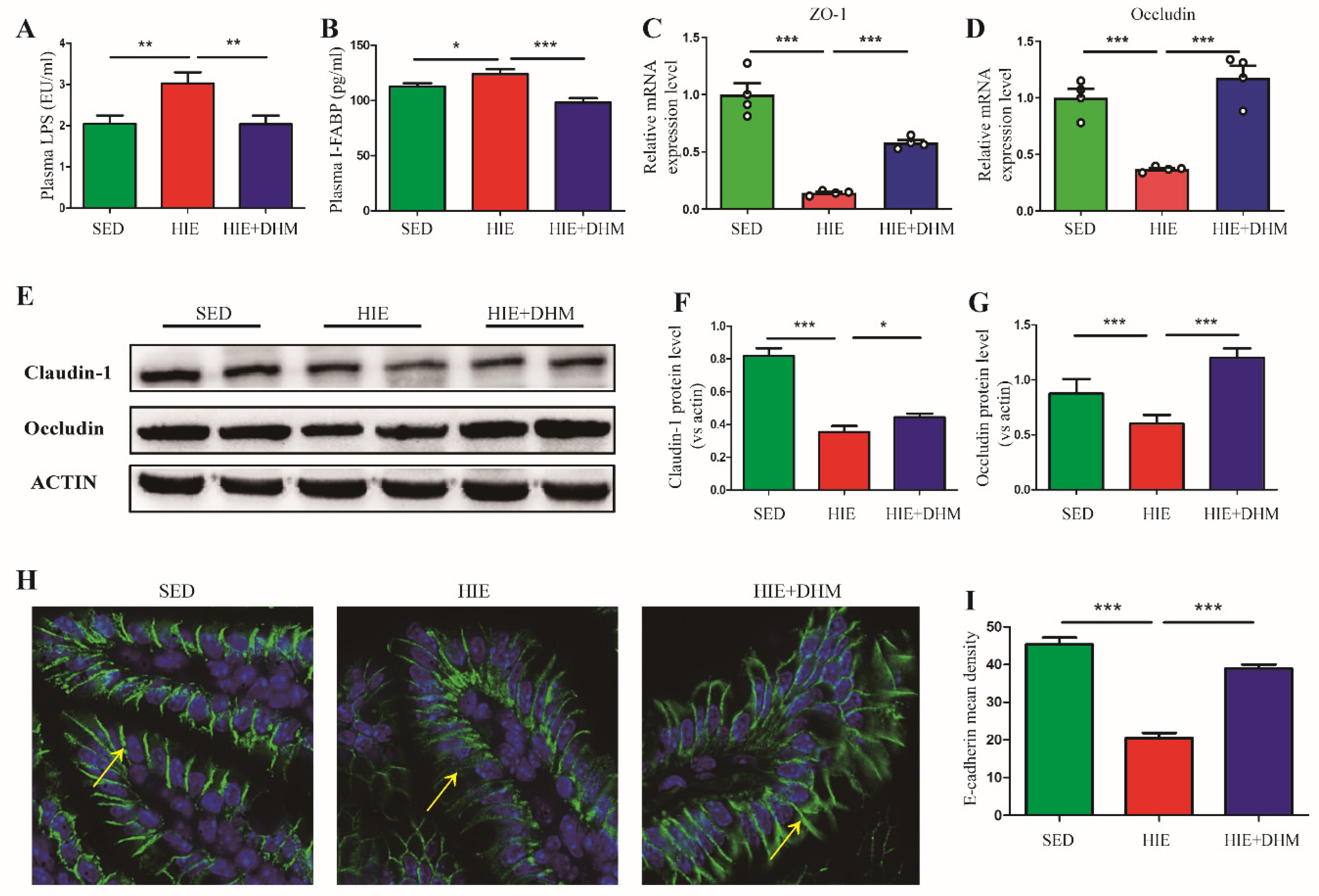
2.2. DHM Maintains the Intestinal Barrier Integrity and Reduces Endotoxemia in Mice following High-Intensity Exercise
2.3. DHM Reduces the Number of IELs and the Frequency of CD8αα+ IELs in Mice following High-Intensity Exercise
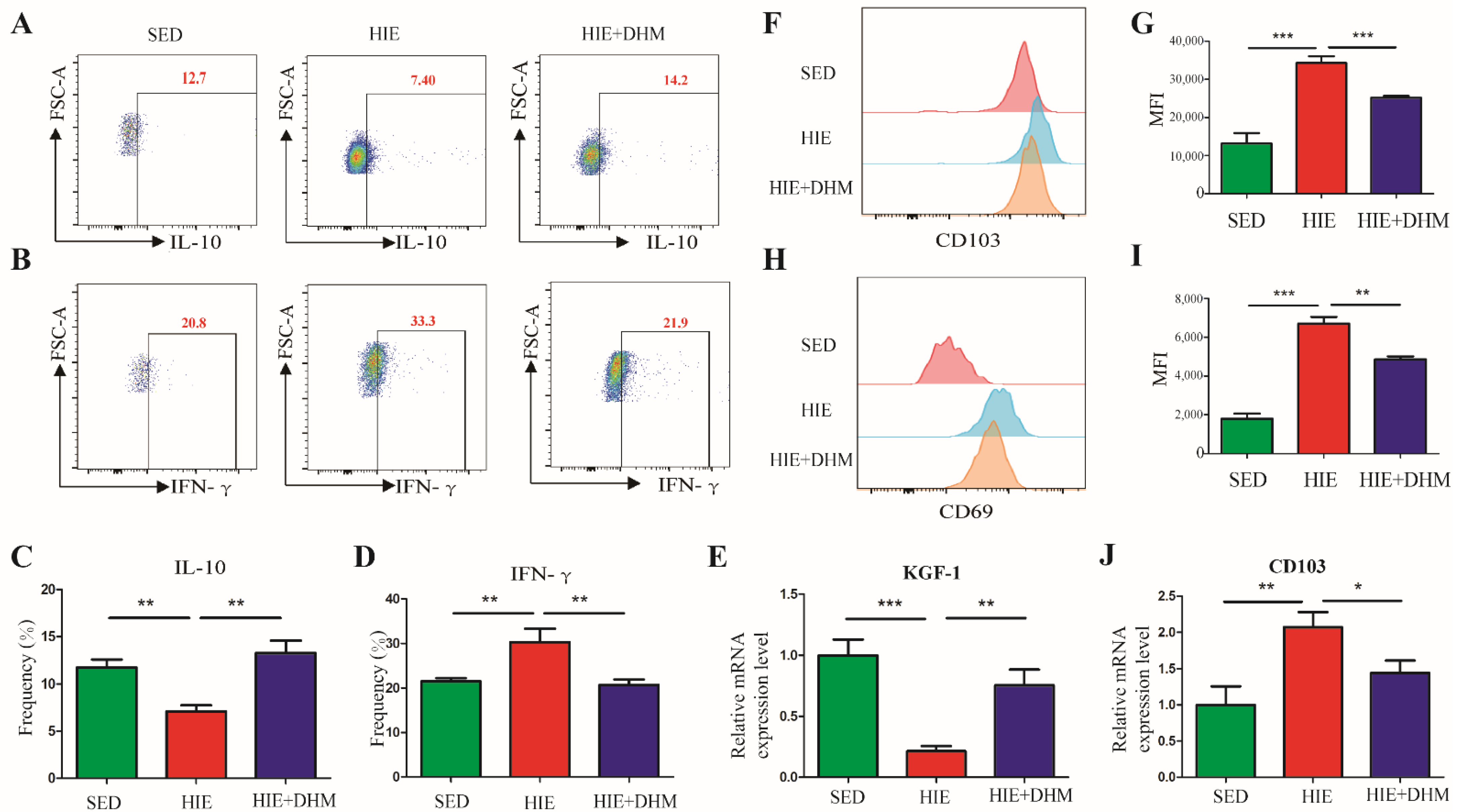
2.4. DHM Modulates the Immune Function of CD8αα+ IELs in Mice Following High-Intensity Exercise
2.5. DHM Attenuates HIE-Induced Intestinal Barrier Dysfunction Associated with the Activation of AhR
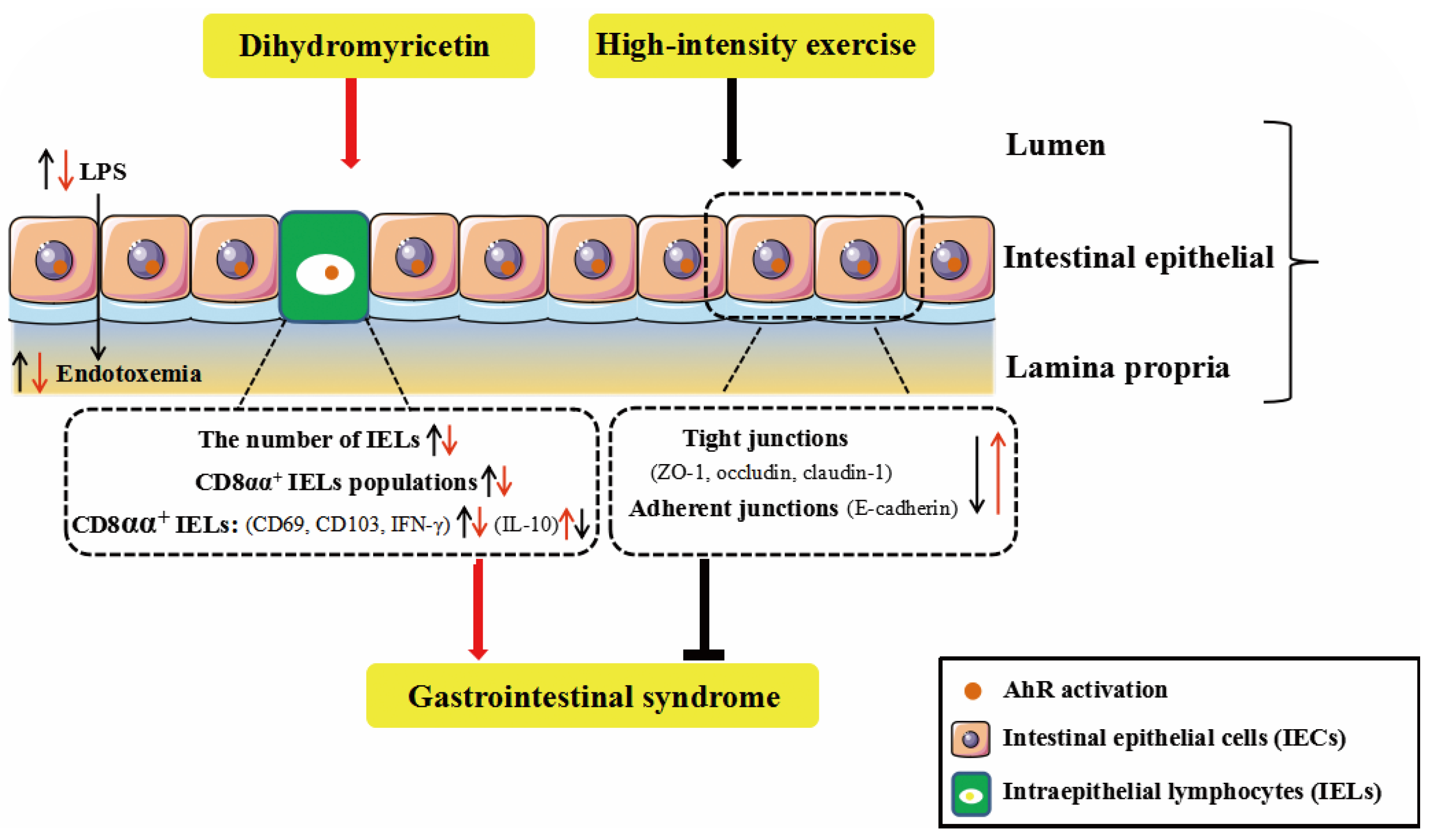
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Protocol
4.2. Biochemical Parameters
4.3. Histological Analysis
4.4. Immunofluorescence Staining
4.5. Isolation of IELs and Flow Cytometry Analysis
4.6. Cell Culture and Treatment
4.7. Cell Viability
4.8. Cells Transfection and Luciferase Reporter Assay
4.9. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.10. Western Blot Analysis
4.11. Molecular Docking
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Phua, L.C.; Wilder-Smith, C.H.; Tan, Y.M.; Gopalakrishnan, T.; Wong, R.K.; Li, X.; Kan, M.E.; Lu, J.; Keshavarzian, A.; Chan, E.C. Gastrointestinal Symptoms and Altered Intestinal Permeability Induced by Combat Training Are Associated with Distinct Metabotypic Changes. J. Proteome Res. 2015, 14, 4734–4742. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.S.; Snipe, R.M.J.; Kitic, C.M.; Gibson, P.R. Systematic review: Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Aliment. Pharmacol. Ther. 2017, 46, 246–265. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.C.; Neumann, M.; Desai, M.S. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat. Rev. Microbiol. 2018, 16, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Mao, X.; Li, R.W.; Hou, E.; Wang, Y.; Xue, C.; Tang, Q. Neoagarotetraose protects mice against intense exercise-induced fatigue damage by modulating gut microbial composition and function. Mol. Nutr. Food Res. 2017, 61, 1600585. [Google Scholar] [CrossRef] [PubMed]
- Cheroutre, H.; Lambolez, F.; Mucida, D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 2011, 11, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Villagomez, D.; Van Kaer, L. Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier. Trends Immunol. 2018, 39, 264–275. [Google Scholar] [CrossRef]
- Troncone, R.; Greco, L.; Mayer, M.; Mazzarella, G.; Maiuri, L.; Congia, M.; Frau, F.; De Virgiliis, S.; Auricchio, S. In siblings of celiac children, rectal gluten challenge reveals gluten sensitization not restricted to celiac HLA. Gastroenterology 1996, 111, 318–324. [Google Scholar] [CrossRef]
- Williams, N.C.; Killer, S.C.; Svendsen, I.S.; Jones, A.W. Immune nutrition and exercise: Narrative review and practical recommendations. Eur. J. Sport Sci. 2019, 19, 49–61. [Google Scholar] [CrossRef]
- Andrade, M.E.; Araujo, R.S.; de Barros, P.A.; Soares, A.D.; Abrantes, F.A.; Generoso Sde, V.; Fernandes, S.O.; Cardoso, V.N. The role of immunomodulators on intestinal barrier homeostasis in experimental models. Clin. Nutr. 2015, 34, 1080–1087. [Google Scholar] [CrossRef]
- Li, H.; Li, Q.; Liu, Z.; Yang, K.; Chen, Z.; Cheng, Q.; Wu, L. The Versatile Effects of Dihydromyricetin in Health. Evid.-Based Complement. Altern. Med. 2017, 2017, 1053617. [Google Scholar] [CrossRef]
- Zou, D.; Chen, K.; Liu, P.; Chang, H.; Zhu, J.; Mi, M. Dihydromyricetin improves physical performance under simulated high altitude. Med. Sci. Sports Exerc. 2014, 46, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Innocentin, S.; Withers, D.R.; Roberts, N.A.; Gallagher, A.R.; Grigorieva, E.F.; Wilhelm, C.; Veldhoen, M. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 2011, 147, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Sheng, B.; Zhang, Z.; Pu, A.; Yin, J.; Wang, Q.; Yang, K.; Sun, L.; Yu, M.; Qiu, Y.; et al. Aryl Hydrocarbon Receptor Activation in Intestinal Obstruction Ameliorates Intestinal Barrier Dysfunction Via Suppression of MLCK-MLC Phosphorylation Pathway. Shock 2016, 46, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.; Falcão, M.A.P.; Rosa, J.G.S.; Disner, G.R.; Lopes-Ferreira, M. Pesticides and Their Impairing Effects on Epithelial Barrier Integrity, Dysbiosis, Disruption of the AhR Signaling Pathway and Development of Immune-Mediated Inflammatory Diseases. Int. J. Mol. Sci. 2022, 23, 12402. [Google Scholar] [CrossRef]
- Imdad, S.; Lim, W.; Kim, J.H.; Kang, C. Intertwined Relationship of Mitochondrial Metabolism, Gut Microbiome and Exercise Potential. Int. J. Mol. Sci. 2022, 23, 2679. [Google Scholar] [CrossRef]
- Costa, R.J.; Snipe, R.; Camoes-Costa, V.; Scheer, V.; Murray, A. The Impact of Gastrointestinal Symptoms and Dermatological Injuries on Nutritional Intake and Hydration Status During Ultramarathon Events. Sports Med. Open 2016, 2, 16. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef]
- Mach, N.; Fuster-Botella, D. Endurance exercise and gut microbiota: A review. J. Sport Health Sci. 2017, 6, 179–197. [Google Scholar] [CrossRef]
- Stuempfle, K.J.; Hoffman, M.D. Gastrointestinal distress is common during a 161-km ultramarathon. J. Sports Sci. 2015, 33, 1814–1821. [Google Scholar] [CrossRef]
- Chen, X.; Yu, J.; Xue, C.; Wang, Y.; Tang, Q.; Mao, X. Mechanism of neoagarotetraose protects against intense exercise-induced liver injury based on molecular ecological network analysis. Biosci. Biotechnol. Biochem. 2019, 83, 1227–1238. [Google Scholar] [CrossRef]
- Gill, S.K.; Teixeira, A.; Rama, L.; Prestes, J.; Rosado, F.; Hankey, J.; Scheer, V.; Hemmings, K.; Ansley-Robson, P.; Costa, R.J. Circulatory endotoxin concentration and cytokine profile in response to exertional-heat stress during a multi-stage ultra-marathon competition. Exerc. Immunol. Rev. 2015, 21, 114–128. [Google Scholar] [PubMed]
- Grootjans, J.; Lenaerts, K.; Buurman, W.A.; Dejong, C.H.; Derikx, J.P. Life and death at the mucosal-luminal interface: New perspectives on human intestinal ischemia-reperfusion. World J. Gastroenterol. 2016, 22, 2760–2770. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yang, H. Effects of intraepithelial lymphocyte-derived cytokines on intestinal mucosal barrier function. J. Interferon Cytokine Res. 2013, 33, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Pang, Z.; Shu, W.; Wu, W.; Sun, M.; Cong, Y.; Liu, Z. Anti-TNF Therapy Induces CD4+ T-Cell Production of IL-22 and Promotes Epithelial Repairs in Patients With Crohn’s Disease. Inflamm. Bowel Dis. 2018, 24, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Costes, L.M.M.; Lindenbergh-Kortleve, D.J.; van Berkel, L.A.; Veenbergen, S.; Raatgeep, H.R.C.; Simons-Oosterhuis, Y.; van Haaften, D.H.; Karrich, J.J.; Escher, J.C.; Groeneweg, M.; et al. IL-10 signaling prevents gluten-dependent intraepithelial CD4(+) cytotoxic T lymphocyte infiltration and epithelial damage in the small intestine. Mucosal Immunol. 2019, 12, 479–490. [Google Scholar] [CrossRef]
- Sun, X.; Yang, H.; Nose, K.; Nose, S.; Haxhija, E.Q.; Koga, H.; Feng, Y.; Teitelbaum, D.H. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G139–G147. [Google Scholar] [CrossRef]
- Ter Steege, R.W.; Kolkman, J.J. Review article: The pathophysiology and management of gastrointestinal symptoms during physical exercise, and the role of splanchnic blood flow. Aliment. Pharmacol. Ther. 2012, 35, 516–528. [Google Scholar] [CrossRef]
- Van Wijck, K.; Lenaerts, K.; Grootjans, J.; Wijnands, K.A.; Poeze, M.; van Loon, L.J.; Dejong, C.H.; Buurman, W.A. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: Strategies for evaluation and prevention. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G155–G168. [Google Scholar] [CrossRef]
- Zuhl, M.; Schneider, S.; Lanphere, K.; Conn, C.; Dokladny, K.; Moseley, P. Exercise regulation of intestinal tight junction proteins. Br. J. Sports Med. 2014, 48, 980–986. [Google Scholar] [CrossRef]
- Ji, T.; Xu, C.; Sun, L.; Yu, M.; Peng, K.; Qiu, Y.; Xiao, W.; Yang, H. Aryl Hydrocarbon Receptor Activation Down-Regulates IL-7 and Reduces Inflammation in a Mouse Model of DSS-Induced Colitis. Dig. Dis. Sci. 2015, 60, 1958–1966. [Google Scholar] [CrossRef]
- Hoytema van Konijnenburg, D.P.; Reis, B.S.; Pedicord, V.A.; Farache, J.; Victora, G.D.; Mucida, D. Intestinal Epithelial and Intraepithelial T Cell Crosstalk Mediates a Dynamic Response to Infection. Cell 2017, 171, 783–794.e13. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, X.; Wan, J.; Ran, L.; Qin, Y.; Wang, X.; Gao, Y.; Shu, F.; Zhang, Y.; Liu, P.; et al. Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial. Pharmacol. Res. 2015, 99, 74–81. [Google Scholar] [CrossRef]
- Li, T.; Yan, F.; Meng, X.; Wang, J.; Ting Kam, R.K.; Zeng, X.; Liu, Z.; Zhou, H.; Yang, F.; Ren, R.; et al. Improvement of glucocorticoid-impaired thymus function by dihydromyricetin via up-regulation of PPARgamma-associated fatty acid metabolism. Pharmacol. Res. 2018, 137, 76–88. [Google Scholar] [CrossRef]
- Zeng, X.; Yang, J.; Hu, O.; Huang, J.; Ran, L.; Chen, M.; Zhang, Y.; Zhou, X.; Zhu, J.; Zhang, Q.; et al. Dihydromyricetin Ameliorates Nonalcoholic Fatty Liver Disease by Improving Mitochondrial Respiratory Capacity and Redox Homeostasis Through Modulation of SIRT3 Signaling. Antioxid. Redox Signal. 2019, 30, 163–183. [Google Scholar] [CrossRef]
- Colbey, C.; Cox, A.J.; Pyne, D.B.; Zhang, P.; Cripps, A.W.; West, N.P. Upper Respiratory Symptoms, Gut Health and Mucosal Immunity in Athletes. Sports Med. 2018, 48 (Suppl. S1), 65–77. [Google Scholar] [CrossRef]
- Mazzucchelli, R.I.; Riva, A.; Durum, S.K. The human IL-7 receptor gene: Deletions, polymorphisms and mutations. Semin. Immunol. 2012, 24, 225–230. [Google Scholar] [CrossRef]
- Laky, K.; Lefrancois, L.; Lingenheld, E.G.; Ishikawa, H.; Lewis, J.M.; Olson, S.; Suzuki, K.; Tigelaar, R.E.; Puddington, L. Enterocyte expression of interleukin 7 induces development of gammadelta T cells and Peyer’s patches. J. Exp. Med. 2000, 191, 1569–1580. [Google Scholar] [CrossRef]
- Abadie, V.; Jabri, B. IL-15: A central regulator of celiac disease immunopathology. Immunol. Rev. 2014, 260, 221–234. [Google Scholar] [CrossRef]
- Villella, V.R.; Venerando, A.; Cozza, G.; Esposito, S.; Ferrari, E.; Monzani, R.; Spinella, M.C.; Oikonomou, V.; Renga, G.; Tosco, A.; et al. A pathogenic role for cystic fibrosis transmembrane conductance regulator in celiac disease. EMBO J. 2019, 38, e100101. [Google Scholar] [CrossRef]
- Cai, Y.; Wang, W.; Liang, H.; Sun, L.; Teitelbaum, D.H.; Yang, H. Keratinocyte growth factor improves epithelial structure and function in a mouse model of intestinal ischemia/reperfusion. PLoS ONE 2012, 7, e44772. [Google Scholar] [CrossRef]
- Lamas, B.; Natividad, J.M.; Sokol, H. Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol. 2018, 11, 1024–1038. [Google Scholar] [CrossRef]
- Koch, D.C.; Jang, H.S.; O’Donnell, E.F.; Punj, S.; Kopparapu, P.R.; Bisson, W.H.; Kerkvliet, N.I.; Kolluri, S.K. Anti-androgen flutamide suppresses hepatocellular carcinoma cell proliferation via the aryl hydrocarbon receptor mediated induction of transforming growth factor-beta1. Oncogene 2015, 34, 6092–6104. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Q.; Ma, Y.; Li, L.; Yu, K.; Zhang, Z.; Chen, G.; Li, X.; Xiao, W.; Xu, P.; et al. Aryl Hydrocarbon Receptor Activation Modulates Intestinal Epithelial Barrier Function by Maintaining Tight Junction Integrity. Int. J. Biol. Sci. 2018, 14, 69–77. [Google Scholar] [CrossRef]
- Liang, H.; Dai, Z.; Liu, N.; Ji, Y.; Chen, J.; Zhang, Y.; Yang, Y.; Li, J.; Wu, Z.; Wu, G. Dietary L-Tryptophan Modulates the Structural and Functional Composition of the Intestinal Microbiome in Weaned Piglets. Front. Microbiol. 2018, 9, 1736. [Google Scholar] [CrossRef]
- Bostikova, Z.; Moserova, M.; Pavek, P.; Stiborova, M.; Hodek, P. Role of dihydromyricetin in cytochrome P450-mediated metabolism and carcinogen activation. Neuro Endocrinol. Lett. 2015, 36 (Suppl. S1), 46–52. [Google Scholar]
- Zhou, X.; Yu, L.; Zhou, M.; Hou, P.; Yi, L.; Mi, M. Dihydromyricetin ameliorates liver fibrosis via inhibition of hepatic stellate cells by inducing autophagy and natural killer cell-mediated killing effect. Nutr. Metab. 2021, 18, 64. [Google Scholar] [CrossRef]
- Furumatsu, K.; Nishiumi, S.; Kawano, Y.; Ooi, M.; Yoshie, T.; Shiomi, Y.; Kutsumi, H.; Ashida, H.; Fujii-Kuriyama, Y.; Azuma, T.; et al. A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig. Dis. Sci. 2011, 56, 2532–2544. [Google Scholar] [CrossRef]
- Schefer, V.; Talan, M.I. Oxygen consumption in adult and AGED C57BL/6J mice during acute treadmill exercise of different intensity. Exp. Gerontol. 1996, 31, 387–392. [Google Scholar] [CrossRef]



| Genes | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| β-actin | CTACCTCATGAAGATCCTGACC | CACAGCTTCTCTTTGATGTCAC |
| ZO-1 | CTGGTGAAGTCTCGGAAAAATG | CATCTCTTGCTGCCAAACTATC |
| Occludin | TGCTTCATCGCTTCCTTAGTAA | GGGTTCACTCCCATTATGTACA |
| TNF-a | ATGTCTCAGCCTCTTCTCATTC | GCTTGTCACTCGAATTTTGAGA |
| IFN-γ | CTTGAAAGACAATCAGGCCATC | CTTGGCAATACTCATGAATGCA |
| IL-10 | ACATTTAGAGACTTGCTCTTGCAC | CTGAGCCAGGCATGATGGAG |
| IL-15 | TCTCCTGGAATTGCAGGTTATT | GCCAGATTCTGCTACATTCTTG |
| IL-7 | GGAAGCTGCTTTTCTAAATCGT | TGTGCCTTGTGATACTGTTAGT |
| CD132 | CAGTGCGAATGAAGACATCAAA | GGAGAACAAATAGTGACTGCAC |
| CD103 | GTACATCTACAACGGACACTCA | GGGGTAAAGGTCATAGATACGG |
| KGF-1 | TGGGCACTATATCTCTAGCTTGC | GGGTGCGACAGAACAGTCT |
| AhR | CATCGACATAACGGACGAAATC | CTGTTGCTGTTGCTCTAGTTG |
| CYP1A1 (mouse) | ACCCTTACAAGTATTTGGTCGT | GTCATCATGGTCATAACGTTGG |
| CYP1A1 (human) | CGTTGTGTCTTTGTAAACCAGT | ACTTAACACCTTGTCGATAGCA |
| β-actin (human) | CTACCTCATGAAGATCCTCACC | AGTTGAAGGTAGTTTCGTGGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, P.; Wang, D.; Lang, H.; Yao, Y.; Zhou, J.; Zhou, M.; Zhu, J.; Yi, L.; Mi, M. Dihydromyricetin Attenuates High-Intensity Exercise-Induced Intestinal Barrier Dysfunction Associated with the Modulation of the Phenotype of Intestinal Intraepithelial Lymphocytes. Int. J. Mol. Sci. 2023, 24, 221. https://doi.org/10.3390/ijms24010221
Hou P, Wang D, Lang H, Yao Y, Zhou J, Zhou M, Zhu J, Yi L, Mi M. Dihydromyricetin Attenuates High-Intensity Exercise-Induced Intestinal Barrier Dysfunction Associated with the Modulation of the Phenotype of Intestinal Intraepithelial Lymphocytes. International Journal of Molecular Sciences. 2023; 24(1):221. https://doi.org/10.3390/ijms24010221
Chicago/Turabian StyleHou, Pengfei, Dawei Wang, Hedong Lang, Yu Yao, Jie Zhou, Min Zhou, Jundong Zhu, Long Yi, and Mantian Mi. 2023. "Dihydromyricetin Attenuates High-Intensity Exercise-Induced Intestinal Barrier Dysfunction Associated with the Modulation of the Phenotype of Intestinal Intraepithelial Lymphocytes" International Journal of Molecular Sciences 24, no. 1: 221. https://doi.org/10.3390/ijms24010221
APA StyleHou, P., Wang, D., Lang, H., Yao, Y., Zhou, J., Zhou, M., Zhu, J., Yi, L., & Mi, M. (2023). Dihydromyricetin Attenuates High-Intensity Exercise-Induced Intestinal Barrier Dysfunction Associated with the Modulation of the Phenotype of Intestinal Intraepithelial Lymphocytes. International Journal of Molecular Sciences, 24(1), 221. https://doi.org/10.3390/ijms24010221





