Sensitivity of Zea mays and Soil Microorganisms to the Toxic Effect of Chromium (VI)
Abstract
1. Introduction
2. Results
2.1. Zea mays Reaction to Soil Contamination with Cr(VI)
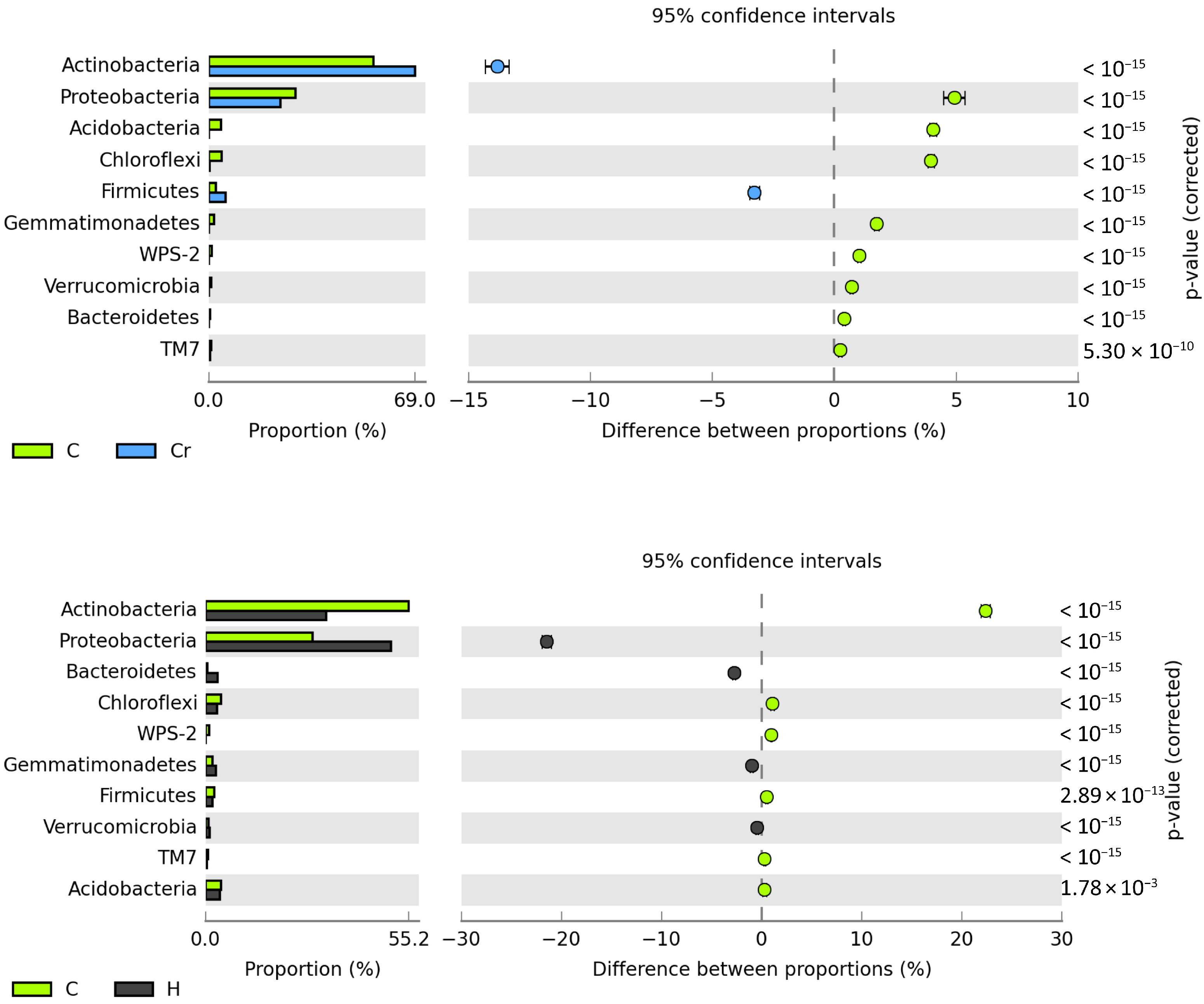
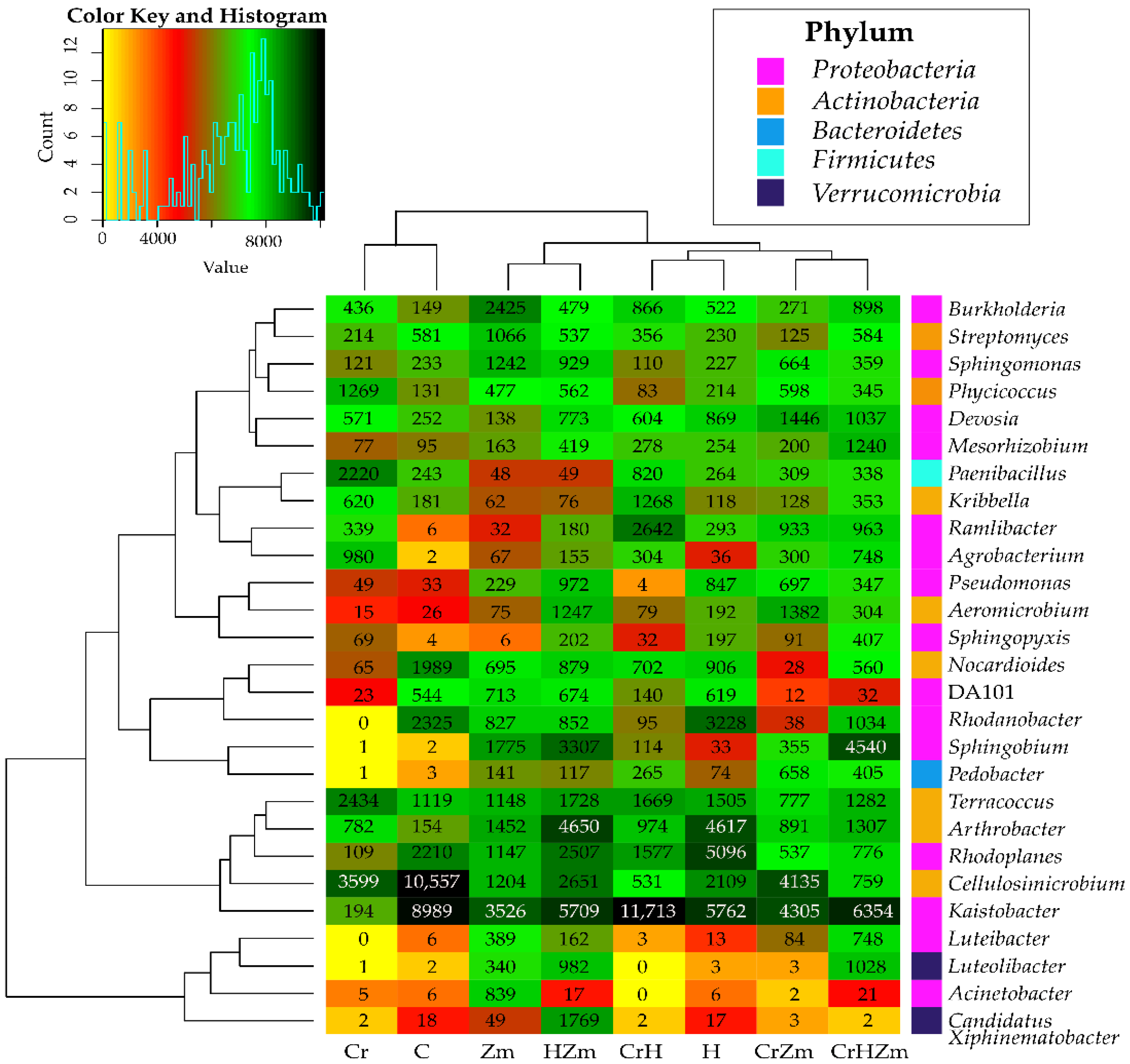
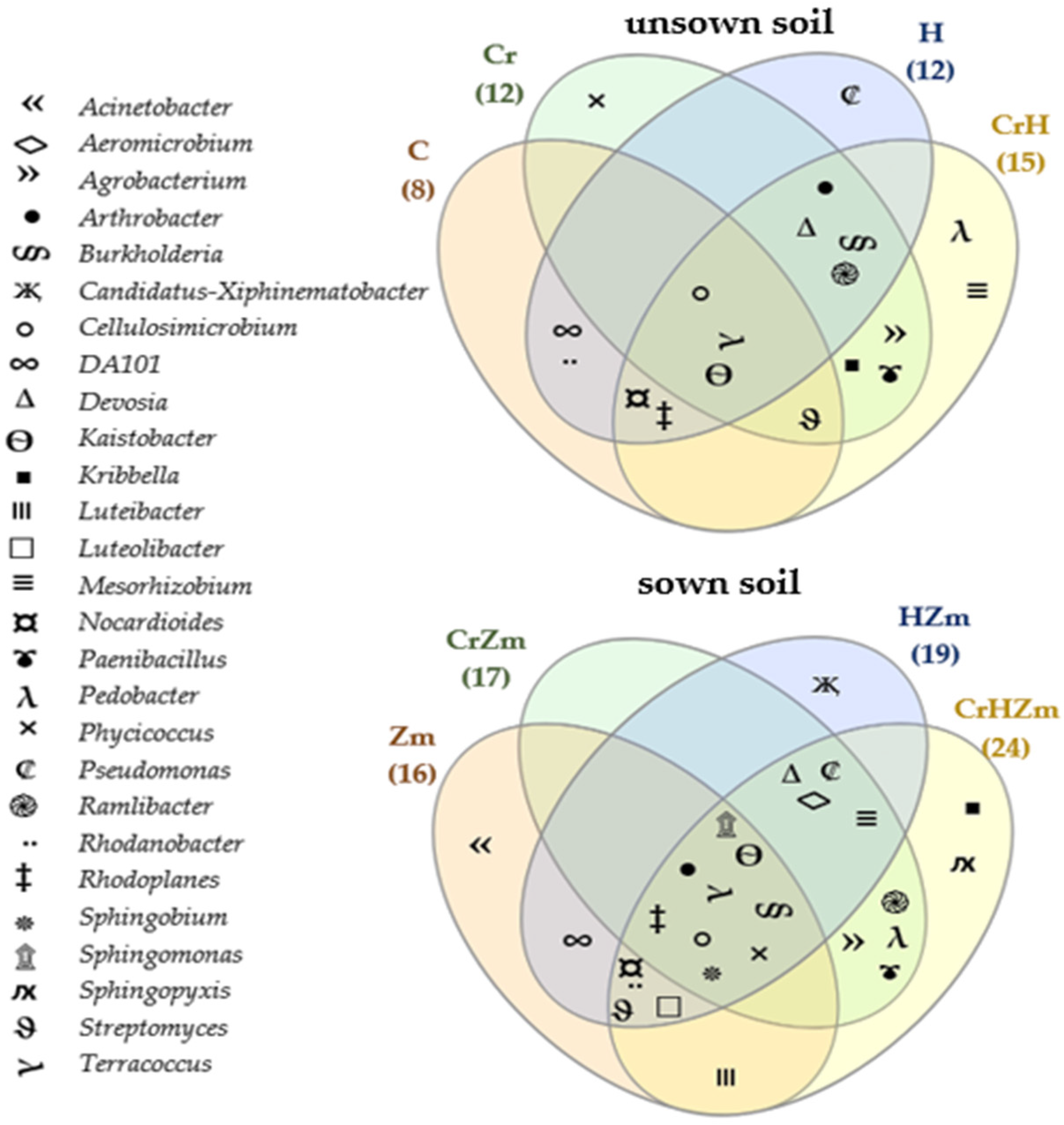
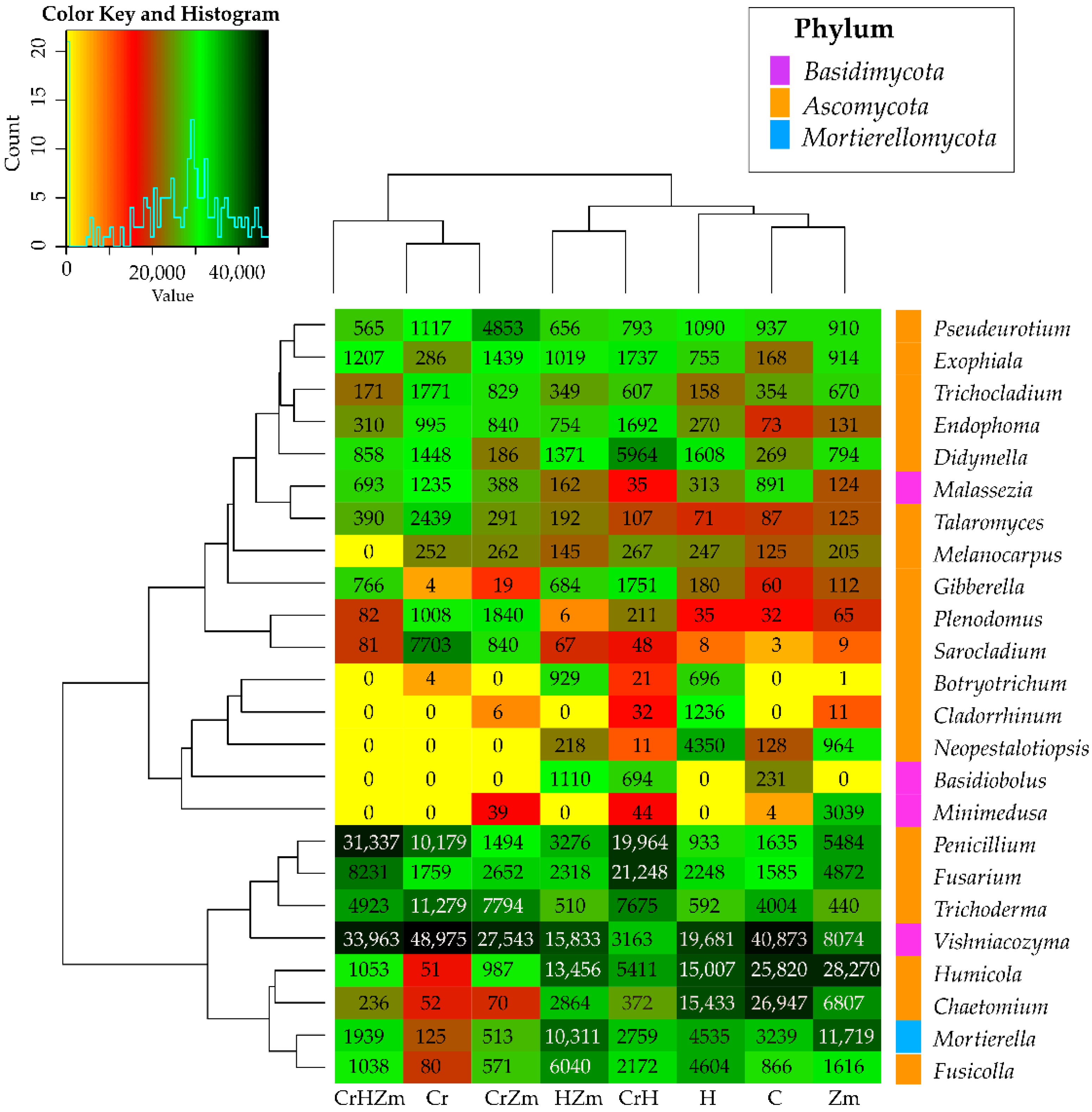
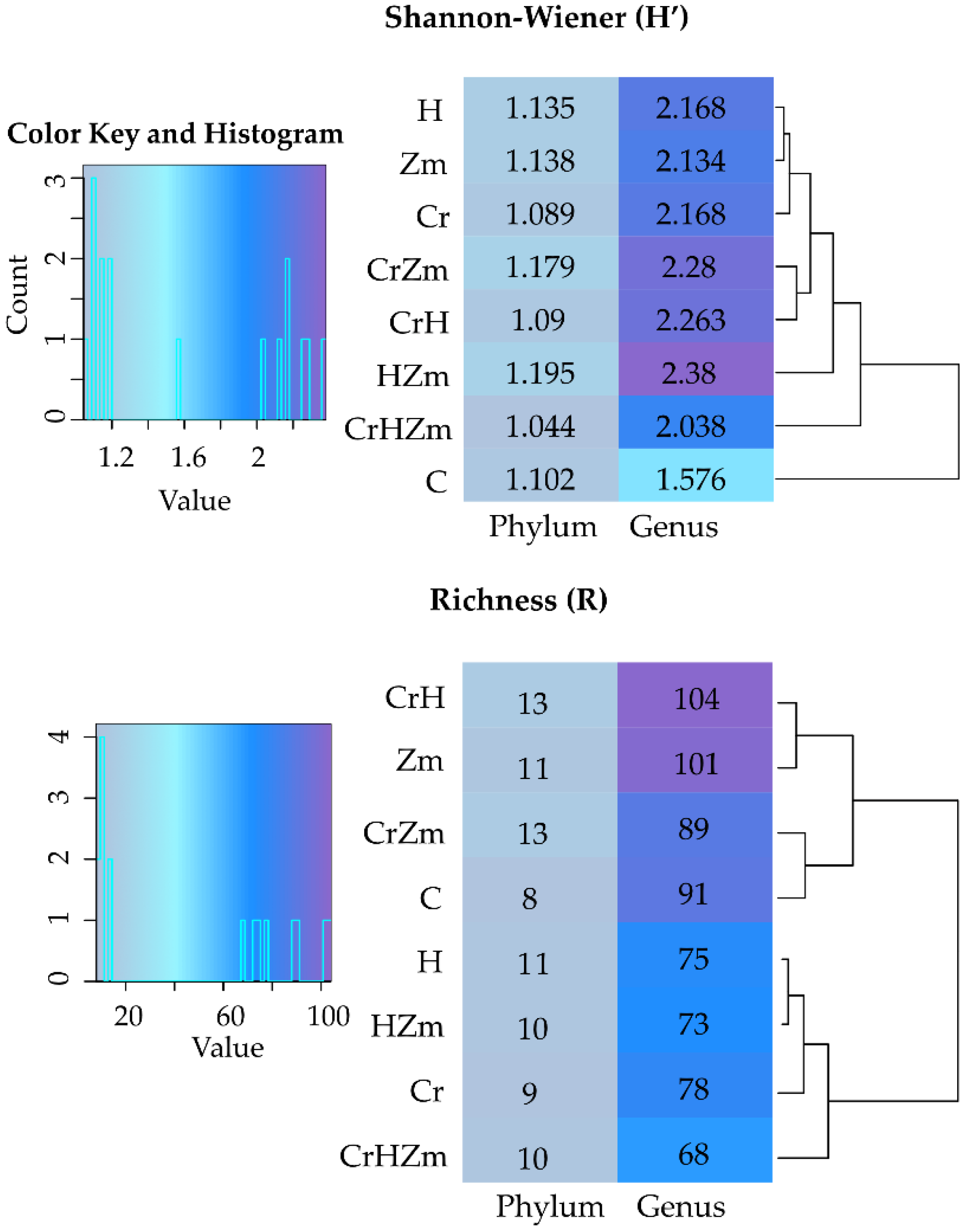
2.2. Reaction of Bacteria and Fungi to Soil Contamination with Cr(VI)
2.2.1. Breeding Microorganisms
Bacteria
Fungi
3. Discussion
3.1. Zea mays Reaction to Soil Contamination with Cr(VI)
3.2. Reaction of Bacteria and Fungi to Soil Contamination with Cr(VI)
3.2.1. Breeding Microorganisms
3.2.2. Bacteria and Fungi Identified by the NGS Method
4. Materials and Methods
4.1. Soil Characteristics
4.2. Study Design
4.3. Methods of Soil Microbiological Analyses
4.4. Physicochemical and Chemical Analyses of Soil
4.5. Data Analysis and Statistical Elaboration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Singh, V.P. The Relative Impact of Toxic Heavy Metals (THMs) (Arsenic (As), Cadmium (Cd), Chromium (Cr)(VI), Mercury (Hg), and Lead (Pb) on the Total Environment: An Overview. Environ. Monit. Assess. 2019, 191, 419. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology; Experientia, Supplementum; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101, pp. 133–164. ISBN 978-3-7643-8339-8. [Google Scholar]
- Prasad, S.; Yadav, K.K.; Kumar, S.; Gupta, N.; Cabral-Pinto, M.M.S.; Rezania, S.; Radwan, N.; Alam, J. Chromium Contamination and Effect on Environmental Health and Its Remediation: A Sustainable Approaches. J. Environ. Manag. 2021, 285, 112174. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, J.J.; Bansal, N.; Chirwa, E.M.N. Chromium in Environment, Its Toxic Effect from Chromite-Mining and Ferrochrome Industries, and Its Possible Bioremediation. Expo. Health 2020, 12, 51–62. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Kucharski, J. Phytoremediation of Soil Contaminated with Nickel, Cadmium and Cobalt. Int. J. Phytoremediation 2021, 23, 252–262. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.-V.; Kamyab, H.; Khan, S.A.; Yadav, S.; et al. Hazardous Heavy Metals Contamination of Vegetables and Food Chain: Role of Sustainable Remediation Approaches—A Review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of Heavy Metals in Soils and Their Immobilization at Micro-Scale Interfaces among Diverse Soil Components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Zaborowska, M.; Kucharski, J.; Wyszkowska, J. Biological Activity of Soil Contaminated with Cobalt, Tin, and Molybdenum. Environ. Monit. Assess. 2016, 188, 398. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Kucharski, J. Maintenance of Soil Homeostasis under Exposure to Cadmium. Commun. Soil Sci. Plant Anal. 2015, 46, 2051–2069. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Ayati, A.; Ghanbari, S.; Orooji, Y.; Tanhaei, B.; Karimi, F.; Alizadeh, M.; Rouhi, J.; Fu, L.; Sillanpää, M. Recent Advances in Removal Techniques of Cr(VI) Toxic Ion from Aqueous Solution: A Comprehensive Review. J. Mol. Liq. 2021, 329, 115062. [Google Scholar] [CrossRef]
- Ukhurebor, K.E.; Aigbe, U.O.; Onyancha, R.B.; Nwankwo, W.; Osibote, O.A.; Paumo, H.K.; Ama, O.M.; Adetunji, C.O.; Siloko, I.U. Effect of Hexavalent Chromium on the Environment and Removal Techniques: A Review. J. Environ. Manag. 2021, 280, 111809. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Costa, M. Chapter 8—Chromium. In Handbook on the Toxicology of Metals, 5th ed.; Nordberg, G.F., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 197–220. ISBN 978-0-12-822946-0. [Google Scholar]
- Jobby, R.; Jha, P.; Yadav, A.K.; Desai, N. Biosorption and Biotransformation of Hexavalent Chromium [Cr(VI)]: A Comprehensive Review. Chemosphere 2018, 207, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Namieśnik, J.; Rabajczyk, A. Speciation Analysis of Chromium in Environmental Samples. Crit. Rev. Environ. Sci. Technol. 2012, 42, 327–377. [Google Scholar] [CrossRef]
- Ertani, A.; Mietto, A.; Borin, M.; Nardi, S. Chromium in Agricultural Soils and Crops: A Review. Water Air Soil Pollut. 2017, 228, 190. [Google Scholar] [CrossRef]
- Fendorf, S.; Wielinga, B.W.; Hansel, C.M. Chromium Transformations in Natural Environments: The Role of Biological and Abiological Processes in Chromium(VI) Reduction. Int. Geol. Rev. 2000, 42, 691–701. [Google Scholar] [CrossRef]
- Barnie, S.; Zhang, J.; Obeng, P.A.; Duncan, A.E.; Adenutsi, C.D.; Xu, L.; Chen, H. Mechanism and Multi-Step Kinetic Modelling of Cr(VI) Adsorption, Reduction and Complexation by Humic Acid, Humin and Kerogen from Different Sources. Environ. Sci. Pollut. Res. 2021, 28, 38985–39000. [Google Scholar] [CrossRef]
- Lilli, M.A.; Nikolaidis, N.P.; Karatzas, G.P.; Kalogerakis, N. Identifying the Controlling Mechanism of Geogenic Origin Chromium Release in Soils. J. Hazard. Mater. 2019, 366, 169–176. [Google Scholar] [CrossRef]
- Xu, T.; Jiang, X.; Tang, Y.; Zeng, Y.; Zhang, W.; Shi, B. Oxidation of Trivalent Chromium Induced by Unsaturated Oils: A Pathway for Hexavalent Chromium Formation in Soil. J. Hazard. Mater. 2021, 405, 124699. [Google Scholar] [CrossRef]
- Bansal, N.; Coetzee, J.J.; Chirwa, E.M.N. In Situ Bioremediation of Hexavalent Chromium in Presence of Iron by Dried Sludge Bacteria Exposed to High Chromium Concentration. Ecotoxicol. Environ. Saf. 2019, 172, 281–289. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Tsadilas, C.D.; Rinklebe, J. A Review of the Distribution Coefficients of Trace Elements in Soils: Influence of Sorption System, Element Characteristics, and Soil Colloidal Properties. Adv. Colloid Interface Sci. 2013, 201–202, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Borah, P.; Singh, P.; Rangan, L.; Karak, T.; Mitra, S. Mobility, Bioavailability and Ecological Risk Assessment of Cadmium and Chromium in Soils Contaminated by Paper Mill Wastes. Ground Sustain. Dev. 2018, 6, 189–199. [Google Scholar] [CrossRef]
- Banks, M.K.; Schwab, A.P.; Henderson, C. Leaching and Reduction of Chromium in Soil as Affected by Soil Organic Content and Plants. Chemosphere 2006, 62, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xiao, C.; Zhou, N.; Chi, R. Speciation, Toxicity, Microbial Remediation and Phytoremediation of Soil Chromium Contamination. Environ. Chem. Lett. 2021, 19, 1413–1431. [Google Scholar] [CrossRef]
- Mukherjee, K.; Saha, R.; Ghosh, A.; Saha, B. Chromium Removal Technologies. Res. Chem. Intermed. 2013, 39, 2267–2286. [Google Scholar] [CrossRef]
- Dhal, B.; Thatoi, H.N.; Das, N.N.; Pandey, B.D. Chemical and Microbial Remediation of Hexavalent Chromium from Contaminated Soil and Mining/Metallurgical Solid Waste: A Review. J. Hazard. Mater. 2013, 250–251, 272–291. [Google Scholar] [CrossRef]
- Yu, X.-Z.; Lu, C.-J.; Tang, S.; Zhang, Q. Transcriptomic Analysis of Cytochrome P450 Genes and Pathways Involved in Chromium Toxicity in Oryza sativa. Ecotoxicology 2020, 29, 503–513. [Google Scholar] [CrossRef]
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur Assimilation in Photosynthetic Organisms: Molecular Functions and Regulations of Transporters and Assimilatory Enzymes. Annu. Rev. Plant Biol. 2011, 62, 157–184. [Google Scholar] [CrossRef]
- López-Bucio, J.S.; Ravelo-Ortega, G.; López-Bucio, J. Chromium in Plant Growth and Development: Toxicity, Tolerance and Hormesis. Environ. Pollut. 2022, 312, 120084. [Google Scholar] [CrossRef]
- Terzi, H.; Yıldız, M. Proteomic Analysis Reveals the Role of Exogenous Cysteine in Alleviating Chromium Stress in Maize Seedlings. Ecotoxicol. Environ. Saf. 2021, 209, 111784. [Google Scholar] [CrossRef]
- Kotaś, J.; Stasicka, Z. Chromium Occurrence in the Environment and Methods of Its Speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Nandi, R.; Saha, B. Sources and Toxicity of Hexavalent Chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Bielicka, A.; Bojanowska, I.; Wiśniewski, A. Two Faces of Chromium-Pollutant and Bioelement. Pol. J. Environ. Stud. 2005, 14, 5–10. [Google Scholar]
- Fuge, R. Anthropogenic Sources. In Essentials of Medical Geology: Revised Edition; Selinus, O., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 59–74. ISBN 978-94-007-4375-5. [Google Scholar]
- Wu, Q.; Mo, W.; Liu, J.; Peng, S.; Li, Q.; Wan, R. Remediation of High-Concentration Cr(VI)-Contaminated Soils with FeSO4 Combined with Biostimulation: Cr(VI) Transformation and Stabilization. J. Hazard. Mater. Adv. 2022, 8, 100161. [Google Scholar] [CrossRef]
- Murthy, M.K.; Khandayataray, P.; Padhiary, S.; Samal, D. A Review on Chromium Health Hazards and Molecular Mechanism of Chromium Bioremediation. Rev. Environ. Health 2022. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, M.I.; Dar, M.I.; Khan, F.A.; Raghib, F.; Rajakaruna, N. Trophic transfer and bioaccumulation of lead along soil–plant–aphid–ladybird food chain. Environ. Sci. Pollut. Res. 2019, 26, 23460–23470. [Google Scholar] [CrossRef]
- Ao, M.; Chen, X.; Deng, T.; Sun, S.; Tang, Y.; Morel, J.L.; Qiu, R.; Wang, S. Chromium Biogeochemical Behaviour in Soil-Plant Systems and Remediation Strategies: A Critical Review. J. Hazard. Mater. 2022, 424, 127233. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42; IARC: Lyon, France, 1987; ISBN 978-92-832-1411-3. [Google Scholar]
- Agency for Toxic Substances and Disease Registry, USA. Substance Priority List|ATSDR. Available online: https://www.atsdr.cdc.gov/spl/index.html (accessed on 25 September 2022).
- Arshad, M.; Khan, A.H.A.; Hussain, I.; Badar-uz-Zaman; Anees, M.; Iqbal, M.; Soja, G.; Linde, C.; Yousaf, S. The Reduction of Chromium (VI) Phytotoxicity and Phytoavailability to Wheat (Triticum aestivum L.) Using Biochar and Bacteria. Appl. Soil Ecol. 2017, 114, 90–98. [Google Scholar] [CrossRef]
- Katsayal, B.S.; Sallau, A.B.; Muhammad, A. Kinetics and Thermodynamics of Cr (VI) Reduction by Tamarindus Indica Methanol Leaves Extract under Optimized Reaction Conditions. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 50. [Google Scholar] [CrossRef]
- Zanganeh, F.; Heidari, A.; Sepehr, A.; Rohani, A. Bioaugmentation and Bioaugmentation–Assisted Phytoremediation of Heavy Metal Contaminated Soil by a Synergistic Effect of Cyanobacteria Inoculation, Biochar, and Purslane (Portulaca oleracea L.). Environ. Sci. Pollut. Res. 2022, 29, 6040–6059. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Evaluation of the Usefulness of Sorbents in the Remediation of Soil Exposed to the Pressure of Cadmium and Cobalt. Materials 2022, 15, 5738. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Mitigation of the Adverse Impact of Copper, Nickel, and Zinc on Soil Microorganisms and Enzymes by Mineral Sorbents. Materials 2022, 15, 5198. [Google Scholar] [CrossRef] [PubMed]
- Azeez, N.A.; Dash, S.S.; Gummadi, S.N.; Deepa, V.S. Nano-Remediation of Toxic Heavy Metal Contamination: Hexavalent Chromium [Cr(VI)]. Chemosphere 2021, 266, 129204. [Google Scholar] [CrossRef] [PubMed]
- Zaborowska, M.; Wyszkowska, J.; Kucharski, J. Biochemical Activity of Soil Contaminated with BPS, Bioaugmented with a Mould Fungi Consortium and a Bacteria Consortium. Environ. Sci. Pollut. Res. 2019, 26, 37054–37069. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Boros-Lajszner, E.; Borowik, A.; Kucharski, J. The Role of Cellulose in Microbial Diversity Changes in the Soil Contaminated with Cadmium. Sustainability 2022, 14, 14242. [Google Scholar] [CrossRef]
- Ju, L.; Jiao, Z.; Ge, S.; Zhan, W.; Liu, Y.; Ren, Q.; Liao, Q.; Yang, Z.; Wang, Y. Formation, Stability and Mobility of Soluble Cr(III) during Cr(VI) Reduction by Pannonibacter phragmitetus BB. Environ. Technol. Innov. 2022, 27, 102496. [Google Scholar] [CrossRef]
- Galani, A.; Mamais, D.; Noutsopoulos, C.; Anastopoulou, P.; Varouxaki, A. Biotic and Abiotic Biostimulation for the Reduction of Hexavalent Chromium in Contaminated Aquifers. Water 2022, 14, 89. [Google Scholar] [CrossRef]
- Erisman, J.W.; van Eekeren, N.; De Wit, J.; Koopmans, C.J.; Cuijpers, W.J.M.; Oerlemans, N.; Koks, B. Agriculture and Biodiversity: A Better Balance Benefits Both. AIMS Agric. Food 2016, 1, 157–174. [Google Scholar] [CrossRef]
- Nowicka, B. Heavy metal–induced stress in eukaryotic algae—mechanisms of heavy metal toxicity and tolerance with particular emphasis on oxidative stress in exposed cells and the role of antioxidant response. Environ. Sci. Pollut. Res. 2022, 29, 16860–16911. [Google Scholar] [CrossRef]
- Mohammed, B.; Mohammed, T.; M’hammed, E.; Tarik, A. Physiological and Physico-Chemical Study of the Effect of Chromium VI on the Nutritional Quality of Maize (Zea mays L). Procedia Comput. Sci. 2021, 191, 463–468. [Google Scholar] [CrossRef]
- Polti, M.A.; Atjián, M.C.; Amoroso, M.J.; Abate, C.M. Soil Chromium Bioremediation: Synergic Activity of Actinobacteria and Plants. Int. Biodeterior. Biodegrad. 2011, 65, 1175–1181. [Google Scholar] [CrossRef]
- Yang, S.; Ulhassan, Z.; Shah, A.M.; Khan, A.R.; Azhar, W.; Hamid, Y.; Hussain, S.; Sheteiwy, M.S.; Salam, A.; Zhou, W. Salicylic Acid Underpins Silicon in Ameliorating Chromium Toxicity in Rice by Modulating Antioxidant Defense, Ion Homeostasis and Cellular Ultrastructure. Plant Physiol. Biochem. 2021, 166, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Xu, M. Chromium Morpho-Phytotoxicity. Plants 2020, 9, 564. [Google Scholar] [CrossRef] [PubMed]
- Stambulska, U.Y.; Bayliak, M.M.; Lushchak, V.I. Chromium(VI) Toxicity in Legume Plants: Modulation Effects of Rhizobial Symbiosis. BioMed Res. Int. 2018, 2018, e8031213. [Google Scholar] [CrossRef]
- Sharma, D.C.; Sharma, C.P.; Tripathi, R.D. Phytotoxic Lesions of Chromium in Maize. Chemosphere 2003, 51, 63–68. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Khan, I.; Hussain, M.; Khan, A.R.; Hamid, Y.; Hussain, S.; Allakhverdiev, S.I.; Zhou, W. Efficacy of Metallic Nanoparticles in Attenuating the Accumulation and Toxicity of Chromium in Plants: Current Knowledge and Future Perspectives. Environ. Pollut. 2022, 315, 120390. [Google Scholar] [CrossRef]
- Rodriguez, E.; Santos, C.; Azevedo, R.; Moutinho-Pereira, J.; Correia, C.; Dias, M.C. Chromium (VI) Induces Toxicity at Different Photosynthetic Levels in Pea. Plant Physiol. Biochem. 2012, 53, 94–100. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium Bioaccumulation and Its Impacts on Plants: An Overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium Toxicity and Tolerance in Plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Hamilton, E.M.; Young, S.D.; Bailey, E.H.; Humphrey, O.S.; Watts, M.J. Assessment of Chromium Species Dynamics in Root Solutions Using Isotope Tracers. J. Trace Elem. Med. Biol. 2020, 61, 126514. [Google Scholar] [CrossRef]
- Afshan, S.; Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Abbas, F.; Ibrahim, M.; Mehmood, M.A.; Abbasi, G.H. Citric Acid Enhances the Phytoextraction of Chromium, Plant Growth, and Photosynthesis by Alleviating the Oxidative Damages in Brassica napus L. Environ Sci. Pollut. Res. 2015, 22, 11679–11689. [Google Scholar] [CrossRef]
- Gondar, D.; López, R.; Fiol, S.; Antelo, J.M.; Arce, F. Cadmium, Lead, and Copper Binding to Humic Acid and Fulvic Acid Extracted from an Ombrotrophic Peat Bog. Geoderma 2006, 135, 196–203. [Google Scholar] [CrossRef]
- Garcia-Mina, J.M. Stability, Solubility and Maximum Metal Binding Capacity in Metal–Humic Complexes Involving Humic Substances Extracted from Peat and Organic Compost. Org. Geochem. 2006, 37, 1960–1972. [Google Scholar] [CrossRef]
- Tang, X.; Huang, Y.; Li, Y.; Wang, L.; Pei, X.; Zhou, D.; He, P.; Hughes, S.S. Study on Detoxification and Removal Mechanisms of Hexavalent Chromium by Microorganisms. Ecotoxicol. Environ. Saf. 2021, 208, 111699. [Google Scholar] [CrossRef]
- Lowe, K.L.; Straube, W.; Little, B.; Jones-Meehan, J. Aerobic and Anaerobic Reduction of Cr(VI) by Shewanella Oneidensis Effects of Cationic Metals, Sorbing Agents and Mixed Microbial Cultures. Acta Biotechnol. 2003, 23, 161–178. [Google Scholar] [CrossRef]
- Singh, P.; Itankar, N.; Patil, Y. Biomanagement of Hexavalent Chromium: Current Trends and Promising Perspectives. J. Environ. Manag. 2021, 279, 111547. [Google Scholar] [CrossRef]
- Villacís-García, M.; Villalobos, M.; Gutiérrez-Ruiz, M. Optimizing the Use of Natural and Synthetic Magnetites with Very Small Amounts of Coarse Fe(0) Particles for Reduction of Aqueous Cr(VI). J. Hazard. Mater. 2015, 281, 77–86. [Google Scholar] [CrossRef]
- Uddin, M.J.; Jeong, Y.-K.; Lee, W. Microbial Fuel Cells for Bioelectricity Generation through Reduction of Hexavalent Chromium in Wastewater: A Review. Int. J. Hydrogen Energy 2021, 46, 11458–11481. [Google Scholar] [CrossRef]
- Xu, T.; Nan, F.; Jiang, X.; Tang, Y.; Zeng, Y.; Zhang, W.; Shi, B. Effect of Soil PH on the Transport, Fractionation, and Oxidation of Chromium(III). Ecotoxicol. Environ. Saf. 2020, 195, 110459. [Google Scholar] [CrossRef]
- Löv, Å.; Sjöstedt, C.; Larsbo, M.; Persson, I.; Gustafsson, J.P.; Cornelis, G.; Kleja, D.B. Solubility and Transport of Cr(III) in a Historically Contaminated Soil—Evidence of a Rapidly Reacting Dimeric Cr(III) Organic Matter Complex. Chemosphere 2017, 189, 709–716. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Kucharski, J.; Jastrzębska, E.; Hłasko, A. The Biological Properties of Soil as Influenced by Chromium Contamination. Pol. J. Environ. Stud. 2001, 10, 37–42. [Google Scholar]
- Mushtaq, Z.; Liaquat, M.; Nazir, A.; Liaquat, R.; Iftikhar, H.; Anwar, W.; Itrat, N. Potential of Plant Growth Promoting Rhizobacteria to Mitigate Chromium Contamination. Environ. Technol. Innov. 2022, 28, 102826. [Google Scholar] [CrossRef]
- Wang, C.; Cui, Y. Recognition of a New Cr(VI)-Reducing Strain and Study of the Potential Capacity for Reduction of Cr(VI) of the Strain. BioMed Res. 2019, 2019, e5135017. [Google Scholar] [CrossRef]
- Ramírez-Díaz, M.I.; Díaz-Pérez, C.; Vargas, E.; Riveros-Rosas, H.; Campos-García, J.; Cervantes, C. Mechanisms of Bacterial Resistance to Chromium Compounds. Biometals 2008, 21, 321–332. [Google Scholar] [CrossRef]
- Castro, C.; Urbieta, M.S.; Plaza Cazón, J.; Donati, E.R. Metal Biorecovery and Bioremediation: Whether or Not Thermophilic Are Better than Mesophilic Microorganisms. Bioresour. Technol. 2019, 279, 317–326. [Google Scholar] [CrossRef]
- Kathiravan, M.N.; Karthick, R.; Muthukumar, K. Ex Situ Bioremediation of Cr(VI) Contaminated Soil by Bacillus Sp.: Batch and Continuous Studies. Chem. Eng. J. 2011, 169, 107–115. [Google Scholar] [CrossRef]
- Kalola, V.; Desai, C. Biosorption of Cr(VI) by Halomonas Sp. DK4, a Halotolerant Bacterium Isolated from Chrome Electroplating Sludge. Environ. Sci. Pollut. Res. Int. 2020, 27, 27330–27344. [Google Scholar] [CrossRef]
- Jobby, R.; Jha, P.; Gupta, A.; Gupte, A.; Desai, N. Biotransformation of Chromium by Root Nodule Bacteria Sinorhizobium Sp. SAR1. PLoS ONE 2019, 14, e0219387. [Google Scholar] [CrossRef]
- Campitelli, P.A.; Velasco, M.I.; Ceppi, S.B. Chemical and Physicochemical Characteristics of Humic Acids Extracted from Compost, Soil and Amended Soil. Talanta 2006, 69, 1234–1239. [Google Scholar] [CrossRef]
- Borges, F.; Guimarães, C.; Lima, J.L.F.C.; Pinto, I.; Reis, S. Potentiometric Studies on the Complexation of Copper(II) by Phenolic Acids as Discrete Ligand Models of Humic Substances. Talanta 2005, 66, 670–673. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Kucharski, J.; Baćmaga, M.; Tomkiel, M.; Boros-Lajszner, E. The Effect of Organic Fertilizers on the Biochemical Properties of Soil Contaminated with Zinc. Plant Soil Environ. 2013, 59, 500–504. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Effect of Separate and Combined Toxicity of Bisphenol A and Zinc on the Soil Microbiome. Int. J. Mol. Sci. 2022, 23, 5937. [Google Scholar] [CrossRef]
- Polti, M.A.; García, R.O.; Amoroso, M.J.; Abate, C.M. Bioremediation of Chromium(VI) Contaminated Soil by Streptomyces Sp. MC1. J. Basic Microbiol. 2009, 49, 285–292. [Google Scholar] [CrossRef]
- Laxman, R.S.; More, S. Reduction of Hexavalent Chromium by Streptomyces Griseus. Miner. Eng. 2002, 15, 831–837. [Google Scholar] [CrossRef]
- Polti, M.A.; Amoroso, M.J.; Abate, C.M. Intracellular Chromium Accumulation by Streptomyces Sp. MC1. Water Air Soil Pollut. 2011, 214, 49–57. [Google Scholar] [CrossRef]
- Fernández, P.M.; Viñarta, S.C.; Bernal, A.R.; Cruz, E.L.; Figueroa, L.I.C. Bioremediation Strategies for Chromium Removal: Current Research, Scale-up Approach and Future Perspectives. Chemosphere 2018, 208, 139–148. [Google Scholar] [CrossRef]
- Vimala, R.; Das, N. Mechanism of Cd(II) Adsorption by Macrofungus Pleurotus platypus. J. Environ. Sci. 2011, 23, 288–293. [Google Scholar] [CrossRef]
- Canton, G.C.; Bertolazi, A.A.; Cogo, A.J.D.; Eutrópio, F.J.; Melo, J.; de Souza, S.B.; Krohling, C.A.; Campostrini, E.; da Silva, A.G.; Façanha, A.R.; et al. Biochemical and Ecophysiological Responses to Manganese Stress by Ectomycorrhizal Fungus Pisolithus Tinctorius and in Association with Eucalyptus Grandis. Mycorrhiza 2016, 26, 475–487. [Google Scholar] [CrossRef]
- Salam, M.; Varma, A. Bacterial Community Structure in Soils Contaminated with Electronic Waste Pollutants from Delhi NCR, India. Electron. J. Biotechnol. 2019, 41, 72–80. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A Global Atlas of the Dominant Bacteria Found in Soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A. Soil Microbiome Response to Contamination with Bisphenol A, Bisphenol F and Bisphenol S. Int. J. Mol. Sci. 2020, 21, 3529. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, Y.; Huang, H.; Mou, L.; Ru, J.; Zhao, J.; Xiao, S. Long-Term and High-Concentration Heavy-Metal Contamination Strongly Influences the Microbiome and Functional Genes in Yellow River Sediments. Sci. Total Environ. 2018, 637–638, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Thatoi, H.; Das, S.; Mishra, J.; Rath, B.P.; Das, N. Bacterial Chromate Reductase, a Potential Enzyme for Bioremediation of Hexavalent Chromium: A Review. J. Environ. Manag. 2014, 146, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Karthik, C.; Barathi, S.; Pugazhendhi, A.; Ramkumar, V.S.; Thi, N.B.D.; Arulselvi, P.I. Evaluation of Cr(VI) Reduction Mechanism and Removal by Cellulosimicrobium Funkei Strain AR8, a Novel Haloalkaliphilic Bacterium. J. Hazard. Mater. 2017, 333, 42–53. [Google Scholar] [CrossRef]
- Bharagava, R.N.; Mishra, S. Hexavalent Chromium Reduction Potential of Cellulosimicrobium Sp. Isolated from Common Effluent Treatment Plant of Tannery Industries. Ecotoxicol. Environ. Saf. 2018, 147, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, K.; Murugan, K.; Benelli, G.; Higuchi, A.; Rajasekar, A. Bioreduction of Hexavalent Chromium by Pseudomonas stutzeri L1 and Acinetobacter naumannii L2. Ann. Microbiol. 2017, 67, 91–98. [Google Scholar] [CrossRef]
- Banerjee, S.; Kamila, B.; Barman, S.; Joshi, S.R.; Mandal, T.; Halder, G. Interlining Cr(VI) Remediation Mechanism by a Novel Bacterium Pseudomonas Brenneri Isolated from Coalmine Wastewater. J. Environ. Manag. 2019, 233, 271–282. [Google Scholar] [CrossRef]
- Zhang, Q.; Amor, K.; Galer, S.J.G.; Thompson, I.; Porcelli, D. Using Stable Isotope Fractionation Factors to Identify Cr(VI) Reduction Pathways: Metal-Mineral-Microbe Interactions. Water Res. 2019, 151, 98–109. [Google Scholar] [CrossRef]
- Chen, J.M.; Hao, O.J. Microbial Chromium (VI) Reduction. Crit. Rev. Environ. Sci. Technol. 1998, 28, 219–251. [Google Scholar] [CrossRef]
- He, Y.; Dong, L.; Zhou, S.; Jia, Y.; Gu, R.; Bai, Q.; Gao, J.; Li, Y.; Xiao, H. Chromium Resistance Characteristics of Cr(VI) Resistance Genes ChrA and ChrB in Serratia Sp. S2. Ecotoxicol. Environ. Saf. 2018, 157, 417–423. [Google Scholar] [CrossRef]
- Viti, C.; Marchi, E.; Decorosi, F.; Giovannetti, L. Molecular Mechanisms of Cr(VI) Resistance in Bacteria and Fungi. FEMS Microbiol. Rev. 2014, 38, 633–659. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhou, S.; He, Y.; Jia, Y.; Bai, Q.; Deng, P.; Gao, J.; Li, Y.; Xiao, H. Analysis of the Genome and Chromium Metabolism-Related Genes of Serratia Sp. S2. Appl. Biochem. Biotechnol. 2018, 185, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yin, Q.; Gu, R.; Li, M.; Yan, J.; Liu, Y.; Qiu, Y.; Bai, Q.; Li, Y.; Ji, Y.; et al. Effects of Exogenous Sulfate on the Chromium(VI) Metabolism of Chromium(VI)-Resistant Engineered Strains. Ecotoxicol. Environ. Saf. 2021, 228, 112984. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.R.; Dussan, J. Transcriptional Analysis and Molecular Dynamics Simulations Reveal the Mechanism of Toxic Metals Removal and Efflux Pumps in Lysinibacillus Sphaericus OT4b.31. Int. Biodeterior. Biodegrad. 2018, 127, 46–61. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Fengying, Y.; Bao, J.; Hu, Z.; Zhu, W.; Zhao, Y.; Lin, Z.; Dong, Q. Chromium (VI) Detoxification by Oxidation and Flocculation of Exopolysaccharides from Arthrobacter Sp. B4. Int. J. Biol. Macromol. 2015, 81, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.; Li, A.; Qiu, J.; Feng, L.; Zhou, L.; Zhao, H.-P.; Ma, F. Enhanced Recovery of Hexavalent Chromium by Remodeling Extracellular Polymeric Substances through Engineering Agrobacterium Tumefaciens F2. J. Clean. Prod. 2021, 279, 123829. [Google Scholar] [CrossRef]
- Yu, C.; Tang, X.; Li, L.-S.; Chai, X.-L.; Xiao, R.; Wu, D.; Tang, C.-J.; Chai, L.-Y. The Long-Term Effects of Hexavalent Chromium on Anaerobic Ammonium Oxidation Process: Performance Inhibition, Hexavalent Chromium Reduction and Unexpected Nitrite Oxidation. Bioresour. Technol. 2019, 283, 138–147. [Google Scholar] [CrossRef]
- Şahin, Y.; Öztürk, A. Biosorption of Chromium(VI) Ions from Aqueous Solution by the Bacterium Bacillus thuringiensis. Proc. Biochem. 2005, 40, 1895–1901. [Google Scholar] [CrossRef]
- Kiliç, N.K.; Stensballe, A.; Otzen, D.E.; Dönmez, G. Proteomic Changes in Response to Chromium(VI) Toxicity in Pseudomonas aeruginosa. Bioresour. Technol. 2010, 101, 2134–2140. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, S.; Li, W.; Tang, Q.; Luo, H. Effects of Chromium Stress on the Rhizosphere Microbial Community Composition of Cyperus alternifolius. Ecotoxicol. Environ. Saf. 2021, 218, 112253. [Google Scholar] [CrossRef]
- Benila Smily, J.R.M.; Sumithra, P.A. Optimization of Chromium Biosorption by Fungal Adsorbent, Trichoderma Sp. BSCR02 and Its Desorption Studies. HAYATI J. Biosci. 2017, 24, 65–71. [Google Scholar] [CrossRef]
- Saranya, N.; Suganya, E.; Selvaraju, N.; Senthilkumar, S.; Sivasubramanian, V.; Sivakumar, P.; Raja, S. 3-Level Box–Behnkenoptimization of Hexavalent Chromium Reduction by Chromate Resistant Trichoderma asperellum Cells from Simulated and Industrial Effluent. Environ. Technol. Innov. 2020, 19, 101024. [Google Scholar] [CrossRef]
- Vankar, P.S.; Bajpai, D. Phyto-Remediation of Chrome-VI of Tannery Effluent by Trichoderma Species. Desalination 2008, 222, 255–262. [Google Scholar] [CrossRef]
- Arévalo-Rangel, D.L.; Cárdenas-González, J.F.; Martínez-Juárez, V.M.; Acosta-Rodríguez, I. Hexavalent Chromate Reductase Activity in Cell Free Extracts of Penicillium Sp. Bioinorg. Chem. Appl. 2013, 2013, e909412. [Google Scholar] [CrossRef]
- Ahemad, M. Bacterial Mechanisms for Cr(VI) Resistance and Reduction: An Overview and Recent Advances. Folia Microbiol. 2014, 59, 321–332. [Google Scholar] [CrossRef]
- Bao, S.; Mu, J.; Yin, P.; Chen, H.; Zhou, S. Exploration of Anti-Chromium Mechanism of Marine Penicillium janthinellum P1 through Combinatorial Transcriptomic Analysis and WGCNA. Ecotoxicol. Environ. Saf. 2022, 233, 113326. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- OECD-FAO Agricultural Outlook 2021–2030; OECD Publishing: Paris, France, 2021; ISBN 978-92-64-43607-7.
- Erenstein, O.; Chamberlin, J.; Sonder, K. Estimating the Global Number and Distribution of Maize and Wheat Farms. Glob. Food Secur. 2021, 30, 100558. [Google Scholar] [CrossRef]
- AL-Huqail, A.A.; El-Bondkly, A.M.A. Improvement of Zea mays L. Growth Parameters under Chromium and Arsenic Stress by the Heavy Metal-Resistant Streptomyces Sp. NRC21696. Int. J. Environ. Sci. Technol. 2022, 19, 5301–5322. [Google Scholar] [CrossRef]
- Meers, E.; Van Slycken, S.; Adriaensen, K.; Ruttens, A.; Vangronsveld, J.; Du Laing, G.; Witters, N.; Thewys, T.; Tack, F.M.G. The Use of Bio-Energy Crops (Zea mays) for ‘Phytoattenuation’ of Heavy Metals on Moderately Contaminated Soils: A Field Experiment. Chemosphere 2010, 78, 35–41. [Google Scholar] [CrossRef]
- Morales-Máximo, C.N.; López-Sosa, L.B.; Rutiaga-Quiñones, J.G.; Corral-Huacuz, J.C.; Aguilera-Mandujano, A.; Pintor-Ibarra, L.F.; López-Miranda, A.; Delgado-Domínguez, S.N.; Rodríguez-Magallón, M.d.C.; Morales-Máximo, M. Characterization of Agricultural Residues of Zea mays for Their Application as Solid Biofuel: Case Study in San Francisco Pichátaro, Michoacán, Mexico. Energies 2022, 15, 6870. [Google Scholar] [CrossRef]
- Boros-Lajszner, E.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Energetic Value of Elymus elongatus L. and Zea mays L. Grown on Soil Polluted with Ni2+, Co2+, Cd2+, and Sensitivity of Rhizospheric Bacteria to Heavy Metals. Energies 2021, 14, 4903. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. 2019, 2019, e6730305. [Google Scholar] [CrossRef]
- Fu, Z.; Guo, W.; Dang, Z.; Hu, Q.; Wu, F.; Feng, C.; Zhao, X.; Meng, W.; Xing, B.; Giesy, J.P. Refocusing on Nonpriority Toxic Metals in the Aquatic Environment in China. Environ. Sci. Technol. 2017, 51, 3117–3118. [Google Scholar] [CrossRef] [PubMed]
- Bunt, J.S.; Rovira, A.D. Microbiological Studies of Some Subantarctic Soils. J. Soil Sci. 1955, 6, 119–128. [Google Scholar] [CrossRef]
- Parkinson, D.; Gray, T.R.G.; Williams, S.T. Methods for Studying the Ecology of Soil Microorganisms; IBP Handbook; Blackwell Scientific Publications [for the] International Biological Programme: Oxford, UK, 1971; ISBN 978-0-632-08260-5. [Google Scholar]
- Martin, J.P. Use of Acid, Rose Bengal, and Streptomycin in the Plate Method for Estimating Soil Fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, M.; Kucharski, J. Implications of Soil Pollution with Diesel Oil and BP Petroleum with ACTIVE Technology for Soil Health. Int. J. Environ. Res. Public Health 2019, 16, 2474. [Google Scholar] [CrossRef]
- Ferris, M.J.; Muyzer, G.; Ward, D.M. Denaturing Gradient Gel Electrophoresis Profiles of 16S RRNA-Defined Populations Inhabiting a Hot Spring Microbial Mat Community. Appl. Environ. Microbiol. 1996, 62, 340–346. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Kucharski, J. The Role of Grass Compost and Zea Mays in Alleviating Toxic Effects of Tetracycline on the Soil Bacteria Community. Int. J. Environ. Res. Public Health 2022, 19, 7357. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Wyszkowski, M. Resistance of Aerobic Microorganisms and Soil Enzyme Response to Soil Contamination with Ekodiesel Ultra Fuel. Environ. Sci. Pollut. Res. 2017, 24, 24346–24363. [Google Scholar] [CrossRef]
- PN ISO 11047:2001; Soil Quality—Determination of Cadmium, Chromium, Cobalt, Copper, Lead, Manganese, Nickel and Zinc in Aqua Regia Extracts of Soil—Flame and Electrothermal Atomic Absorption Spectrometric Methods. Polish Committee for Standardization: Warsaw, Poland, 2013.
- PN-ISO-11466:2002; Polish Committee for Standardization. Soil Quality—Extraction of Trace Elements Soluble in Aqua Regia. Polish Committee for Standardization: Warsaw, Poland, 2002.
- De Leij, F.A.A.M.; Whipps, J.M.; Lynch, J.M. The Use of Colony Development for the Characterization of Bacterial Communities in Soil and on Roots. Microb. Ecol. 1993, 27, 81–97. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Oszust, K. Functional Diversity of Fungal Communities in Soil Contaminated with Diesel Oil. Front. Microbiol. 2017, 8, 1862. [Google Scholar] [CrossRef] [PubMed]
- TIBCO Software Inc Statistica (Data Analysis Software System), Version 13. 2017. Available online: http://statistica.io (accessed on 23 November 2022).
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 18 November 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.r-project.org/ (accessed on 18 November 2022).
- Warnes, G.R.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Huber, W.; Liaw, A.; Lumley, T.; Maechler, M.; Magnusson, M.; Moeller, S.; et al. Gplots: Various R Programming Tools for Plotting Data. R Package Version 2.17.0. 2020. Available online: https://cran.r-project.org/package=gplots (accessed on 18 November 2022).
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical Analysis of Taxonomic and Functional Profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef] [PubMed]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A Web-Based Tool for the Analysis of Sets through Venn Diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]






| Dose of Cr(VI), mg kg−1 d.m. of Soil | Yield, (d.m. g Pot−1) | SPAD BBCH Zea mays | Plant Height, cm | ||
|---|---|---|---|---|---|
| Aerial Parts | Roots | 14 | 19 | ||
| Control | |||||
| 0 | 44.923 b | 16.324 a | 32.394 a | 27.129 b | 138.800 b |
| 60 | 4.363 c | 1.359 c | 17.156 c | 19.446 c | 70.500 c |
| HumiAgra | |||||
| 0 | 52.567 a | 15.242 ab | 32.598 a | 27.838 b | 162.818 a |
| 60 | 49.624 a | 14.088 b | 32.481 a | 33.136 a | 146.750 b |
| Dose of Cr(VI), mg kg−1 d.m. of Soil | Soil Use | Aerial Parts | Roots | |
|---|---|---|---|---|
| Unsown | Sown with Zea mays | |||
| Control | ||||
| 0 | 12.375 d | 15.075 d | 0.830 c | 3.170 c |
| 60 | 69.825 b | 72.525 b | 1.040 a | 17.670 a |
| HumiAgra | ||||
| 0 | 19.500 c | 18.900 c | 0.660 d | 1.230 d |
| 60 | 76.950 a | 76.350 a | 0.950 b | 8.010 b |
| Dose of Cr(VI), mg kg−1 d.m. of Soil | D µg pot−1 | TI | TF | BFA | BFR | BF |
|---|---|---|---|---|---|---|
| Control | ||||||
| 0 | 89.033 b | - | 0.262 b | 0.055 a | 0.210 b | 0.265 a |
| 60 | 28.549 d | 0.093 | 0.059 d | 0.014 c | 0.244 a | 0.258 a |
| HumiAgra | ||||||
| 0 | 53.441 c | - | 0.537 a | 0.035 b | 0.065 d | 0.100 c |
| 60 | 159.989 a | 0.940 | 0.119 c | 0.012 c | 0.105 c | 0.117 b |
| Location | University of Warmia and Mazury in Olsztyn, Poland | ||
|---|---|---|---|
| An experimental plant | Zea mays L. variety LG 32.58 (variety registered in European Union), eight seeds were sown in a pot; after emergence, four plants were left in the pot | ||
| Soil | Sandy loam: clay < 0.002 mm–3.71%, silt 0.02–0.05 mm–32.68% and sand–0.0–2.0 mm–63.61%. | ||
| Physicochemical properties: | |||
| pHKCl–4.40 HAC–26.10 EBC–63.60 CEC–89.70 |  | mmol (+) kg−1 d.m. | |
| ACS–70.90% | |||
| Exchangeable cations: | |||
| K+ 168.00 Ca2+ 1190.50 Na+ 10.00 Mg2+ 82.10 |  | mg kg−1 d.m. | |
| Chemical properties per 1 kg d.m: Ntot 0.83 g, Corg 10.00 g, Pav 81.10 mg, Kav 145.25 mg, Mgav 71.00 mg, Crtot 12.37 mg | |||
| Mineral fertilisation | mg kg−1 of d.m. soil: N 140 [CO(NH2)2], P 50 [KH2PO4], K 140 [ KH2PO4 + KCl], Mg 20 [MgSO4 × 7H2O] The form of fertilizer is given in parentheses | ||
| Soil contamination with Cr(VI) | 60 mg Cr(VI) kg−1 d.m. of soil in form of K2Cr2O7 | ||
| Use of biostimulation | HumiAgra (AgraPlant, Kielce, Poland) is an ecological product, contains 90% humic acids (50% humic acids and 50% fulvic acids). Dark brown powder, pH 8–10. Contains 8% K2O and 3% S. HumiAgra was used in the amount of 3 g C kg−1 of d.m. soil | ||
| The duration of the experiment | Total: 50 days | ||
| Number repetitions | Vases were with five repetitions per treatment, arranged in random, with complete blocks on tables in the same vegetation hall | ||
| Conditions in the vegetation hall | June–July 2021: day length ranged from 15 h 5 min to 17 h 5 min; the average temperature was 16.5 °C, and the average humidity was 77.5%. Natural light was used, watering up to 60% m.w.c. deionized water | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Sensitivity of Zea mays and Soil Microorganisms to the Toxic Effect of Chromium (VI). Int. J. Mol. Sci. 2023, 24, 178. https://doi.org/10.3390/ijms24010178
Wyszkowska J, Borowik A, Zaborowska M, Kucharski J. Sensitivity of Zea mays and Soil Microorganisms to the Toxic Effect of Chromium (VI). International Journal of Molecular Sciences. 2023; 24(1):178. https://doi.org/10.3390/ijms24010178
Chicago/Turabian StyleWyszkowska, Jadwiga, Agata Borowik, Magdalena Zaborowska, and Jan Kucharski. 2023. "Sensitivity of Zea mays and Soil Microorganisms to the Toxic Effect of Chromium (VI)" International Journal of Molecular Sciences 24, no. 1: 178. https://doi.org/10.3390/ijms24010178
APA StyleWyszkowska, J., Borowik, A., Zaborowska, M., & Kucharski, J. (2023). Sensitivity of Zea mays and Soil Microorganisms to the Toxic Effect of Chromium (VI). International Journal of Molecular Sciences, 24(1), 178. https://doi.org/10.3390/ijms24010178









