Genetic Regulation Mechanism of Cadmium Accumulation and Its Utilization in Rice Breeding
Abstract
1. Introduction
2. Cd Stress Inhibits the Growth, Yield, and Quality of Rice
2.1. Effects of Cd on Physiological Characteristics of Rice
2.2. Effects of Cd on Rice Yield
2.3. Effects of Cd on Rice Quality
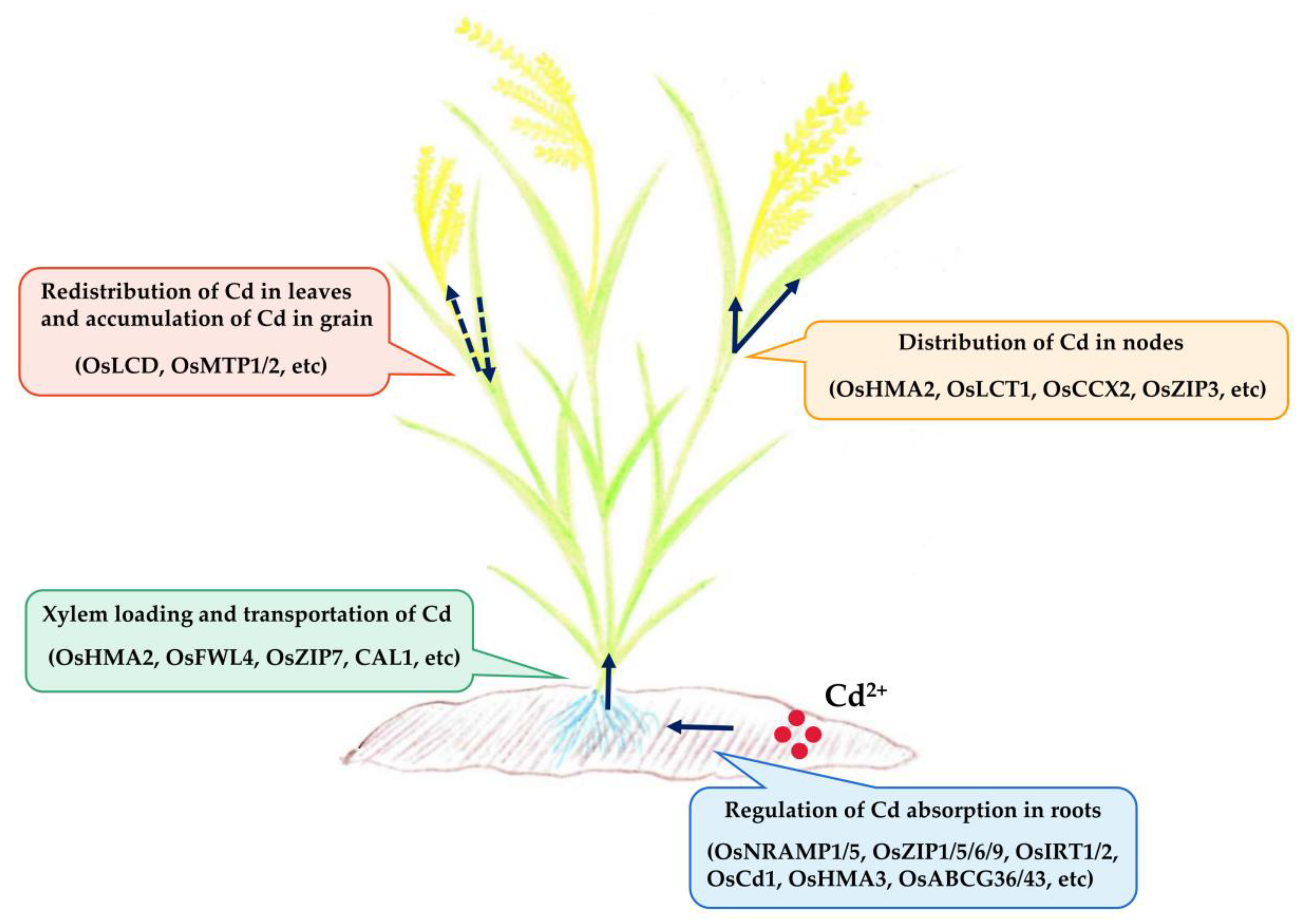
3. Physiological and Molecular Mechanisms of Cd Accumulation in Rice
3.1. Cd Absorption by the Roots
3.2. Loading and Transport of Cd in the Xylem
3.3. Cd Distribution in Stem Nodes
3.4. Redistribution of Cd in Leaves and Cd Accumulation in Grains
4. Regulatory Mechanism of the Cd Stress Response
5. Breeding of Low-Cd Accumulation Rice
5.1. Variety Selection and Cross-Breeding
5.2. Mutation Breeding
5.3. Marker-Assisted Breeding (MAS)
5.4. Genetic Engineering Breeding
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette transporter superfamily |
| CaCA | Cation/calcium superfamily |
| CAL1 | Cadmium accumulation in leaf 1 |
| CCXs | Cation/Ca exchangers |
| Cd | Cadmium |
| CDF | Cation diffusion facilitator |
| CKK | Cho Ko Koku |
| Cu | Copper |
| DVB | Diffuse vascular bundle |
| EVB | Enlarged vascular bundle |
| HMA | Heavy metal ATPase |
| Hsf | Heat shock transcription factor |
| lc5 | Low cadmium 5 |
| LCT | Low-affinity cation transporter |
| lncRNAs | Long non-coding RNAs |
| MAS | Marker-assisted breeding |
| MFS | Major facilitator superfamily |
| miRNAs | microRNAs |
| MPF | Membrane protein family |
| MTPs | Metal tolerance proteins |
| NRAMP | Natural resistance-associated macrophage protein |
| OsHB4 | Homeodomain-containing protein 4 |
| PCB | Parenchyma cell bridge |
| PSI | Photosystem I |
| PSII | Photosystem II |
| TVB | Transit vascular bundle |
| WT | Wild-type |
| Zn | Zinc |
| ZRTs | Zn-regulated transporters |
References
- Cui, J.; Wang, W.; Peng, Y.; Zhou, F.; He, D.; Wang, J.; Chang, Y.; Yang, J.; Zhou, J.; Wang, W.; et al. Effects of simulated Cd deposition on soil Cd availability, microbial response, and crop Cd uptake in the passivation-remediation process of Cd-contaminated purple soil. Sci. Total. Environ. 2019, 683, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Coudon, T.; Hourani, H.; Nguyen, C.; Faure, E.; Mancini, F.; Fervers, B.; Salizzoni, P. Assessment of long-term exposure to airborne dioxin and cadmium concentrations in the Lyon metropolitan area (France). Environ. Int. 2018, 111, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Saifullah; Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef] [PubMed]
- National Bureau of Statistics. China Statistics Yearbook; China Statistics Press: Beijing, China, 2018.
- Zhen, Y.; Cheng, Y.; Pan, G.; Li, L. Cd, Zn and Se content of the polished rice samples from some Chinese open markets and their relevance to food safety. J. Saf. Environ. 2008, 8, 119–122. [Google Scholar]
- Du, Y.; Hu, X.-F.; Wu, X.-H.; Shu, Y.; Jiang, Y.; Yan, X.-J. Affects of mining activities on Cd pollution to the paddy soils and rice grain in Hunan province, Central South China. Environ. Monit. Assess. 2013, 185, 9843–9856. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Chen, C.; Xu, C.; Zhu, Q.; Huang, D. Effects of soil acidification and liming on the phytoavailability of cadmium in paddy soils of central subtropical China. Environ. Pollut. 2016, 219, 99–106. [Google Scholar] [CrossRef]
- Qin, S.; Liu, H.; Nie, Z.; Rengel, Z.; Gao, W.; Li, C.; Zhao, P. Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: A review. Pedosphere 2020, 30, 168–180. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Y.; Wu, D.; Shu, X. Researches of low cadmium accumulation in rice. J. Nucl. Agric. Sci. 2021, 35, 0093–0102. [Google Scholar]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-Ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Rascio, N.; Vecchia, F.D.; La Rocca, N.; Barbato, R.; Pagliano, C.; Raviolo, M.; Gonnelli, C.; Gabbrielli, R. Metal accumulation and damage in rice (cv. Vialone nano) seedlings exposed to cadmium. Environ. Exp. Bot. 2008, 62, 267–278. [Google Scholar] [CrossRef]
- Küpper, H.; Parameswaran, A.; Leitenmaier, B.; Trtílek, M.; Šetlík, I. Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytol. 2010, 175, 655–674. [Google Scholar] [CrossRef]
- Mallicka, N. Use of chlorophyll fluorescence in metal-stress research: A case study with the green microalga Scenedesmus. Ecotoxicol. Environ. Saf. 2003, 55, 64–69. [Google Scholar] [CrossRef]
- Xu, X.; An, P.; Guo, T.; Han, D.; Jia, W.; Huang, W. Research progresses on response mechanisms and control measures of cadmium stress in rice. Chin. J. Rice Sci. 2021, 35, 415–426. [Google Scholar]
- Smiri, M.; Chaoui, A.; El Ferjani, E. Respiratory metabolism in the embryonic axis of germinating pea seed exposed to cadmium. J. Plant Physiol. 2009, 166, 259–269. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Pandey, P.; Rajpoot, R.; Rani, A.; Dubey, R.S. Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 2014, 251, 1047–1065. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, A.; Ansary, M.M.U.; Watanabe, A.; Fujita, M.; Tran, L.-S.P. Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci. Rep. 2015, 5, 14078. [Google Scholar] [CrossRef]
- Roychoudhury, A.; Basu, S.; Sengupta, D.N. Antioxidants and stress-related metabolites in the seedlings of two indica rice varieties exposed to cadmium chloride toxicity. Acta Physiol. Plant. 2011, 34, 835–847. [Google Scholar] [CrossRef]
- Huang, D.; Wang, Z.; Liu, L.; Yang, J. Effect of cadmium on the rice yield and grain quality. Chin. J. Trop. Crops 2010, 31, 19–24. [Google Scholar]
- Li, Y.; Zhou, X.; Jiang, G.; Su, Y.; Yu, D. Influence of irrigation with different concentrations of cadmium solution on rice yield and quality. J. Irrig. Drain. 2012, 31, 120–123. [Google Scholar]
- Ding, Y.; Zong, L.; Xu, X.; Liu, G. Effect of cadmium on the growth and quality of rice (Oryza sativa L.) in different growth period. Ecol. Environ. Sci. 2009, 18, 183–186. [Google Scholar]
- Chen, J. Effects of Cd2+ stress on plant growth and leaves physiological activity of rice. Seed 2009, 28, 38–42. [Google Scholar]
- Liu, C.; Liu, Y.; Luo, S.; Cui, L. Effect of different concentration cadmium on roots activity and grain quality of rice in cold area. J. Anhui Agric. Sci. 2013, 41, 5758–5760. [Google Scholar]
- Ge, C.L. Molecular Mechanism of Heavy Metals Toxicity and Tolerance in Rice(Oryza sativa L.) and Wheat(Triticum aestivum L.); Zhejiang University: Hangzhou, China, 2002. [Google Scholar]
- Wang, K.R.; Gong, H.Q. Effects of cadmium exposures in different stages on plant growth, Cd uptake and Cd concentrations in brown rice of a hybrid and conventional rice variety. Ecol. Environ. Sci. 2006, 156, 1197–1203. [Google Scholar]
- Ministry of Health of the People’s Republic of China. GB 2762-2017 Limits of Pollutants in Food; Standards Press of China: Beijing, China, 2017.
- Wang, N.; Shi, J.; Liu, C.; Run, L. Effects of cadmium on grain quality of several food crops. Agric. Environ. Dev. J. Agric. Resour. Environ. 2008, 25, 114–115. [Google Scholar]
- Uraguchi, S.; Fujiwara, T. Rice breaks ground for cadmium-free cereals. Curr. Opin. Plant Biol. 2013, 16, 328–334. [Google Scholar] [CrossRef]
- Li, H.; Luo, N.; Li, Y.W.; Cai, Q.Y.; Mo, C.H.; Wong, M.H. Cadmium in rice: Transport mechanisms, influencing factors, and minimizing measures. Environ. Pollut. 2017, 224, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Ashraf, M.N.; Rahman, S.U.; Abbas, A.; Li, J.; Farooq, M. Cadmium stress in paddy fields: Effects of soil conditions and remediation strategies. Sci. Total. Environ. 2021, 754, 142188. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, P.; Zhang, S.; Dai, J.; Chen, H.-P.; Lombi, E.; Howard, D.L.; van der Ent, A.; Zhao, F.-J.; Kopittke, P.M. Chemical speciation and distribution of cadmium in rice grain and implications for bioavailability to humans. Environ. Sci. Technol. 2020, 54, 12072–12080. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Zhuang, P.; Li, F.; McBride, M.B.; Ren, W.; Li, Y.; Li, Y.; Mo, H.; Fu, H.; Li, Z. Phosphate addition diminishes the efficacy of wollastonite in decreasing Cd uptake by rice (Oryza sativa L.) in paddy soil. Sci. Total Environ. 2019, 687, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, A.; Wang, K.; Li, Q.; Ye, D.; Huang, H.; Zhang, X.; Wang, Y.; Zheng, Z.; Li, T. The role of polysaccharides functional groups in cadmium binding in root cell wall of a cadmium-safe rice line. Ecotoxicol. Environ. Saf. 2021, 226, 112818. [Google Scholar] [CrossRef]
- Lux, A.; Martinka, M.; Vaculík, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2011, 62, 21–37. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Senoura, T.; Shimo, H.; Ishikawa, S.; Arao, T.; Nakanishi, H.; Nishizawa, N.K. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J. Exp. Bot. 2011, 62, 4843–4850. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Takahashi, R.; Bashir, K.; Shimo, H.; Senoura, T.; Sugimoto, K.; Ono, K.; Yano, M.; Ishikawa, S.; Arao, T.; et al. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci. Rep. 2012, 2, 286. [Google Scholar] [CrossRef]
- Liu, X.S.; Feng, S.J.; Zhang, B.Q.; Wang, M.Q.; Cao, H.W.; Rono, J.K.; Chen, X.; Yang, Z.M. OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant Biol. 2019, 19, 283. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Mitani-Ueno, N.; Kashino, M.; Ma, J.F. A node-localized transporter OsZIP3 is responsible for the preferential distribution of Zn to developing tissues in rice. Plant J. 2015, 84, 374–384. [Google Scholar] [CrossRef]
- Tan, L.; Qu, M.; Zhu, Y.; Peng, C.; Wang, J.; Gao, D.; Chen, C. ZINC TRANSPORTER5 and ZINC TRANSPORTER9 function synergistically in zinc/cadmium uptake. Plant Physiol. 2020, 183, 1235–1249. [Google Scholar] [CrossRef]
- Kavitha, P.G.; Kuruvilla, S.; Mathew, M.K. Functional characterization of a transition metal ion transporter, OsZIP6 from rice (Oryza sativa L.). Plant Physiol. Biochem. 2015, 97, 165–174. [Google Scholar]
- Tan, L.; Zhu, Y.; Fan, T.; Peng, C.; Wang, J.; Sun, L.; Chen, C. OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem. Biophys. Res. Commun. 2019, 512, 112–118. [Google Scholar] [CrossRef]
- Ishimaru, Y.; Suzuki, M.; Tsukamoto, T.; Suzuki, K.; Nakazono, M.; Kobayashi, T.; Wada, Y.; Watanabe, S.; Matsuhashi, S.; Takahashi, M.; et al. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J. 2006, 45, 335–346. [Google Scholar] [CrossRef]
- Nakanishi, H.; Ogawa, I.; Ishimaru, Y.; Mori, S.; Nishizawa, N.K. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+transporters OsIRT1 and OsIRT2 in rice. Soil Sci. Plant Nutr. 2006, 52, 464–469. [Google Scholar] [CrossRef]
- Yan, H.; Xu, W.; Xie, J.; Gao, Y.; Wu, L.; Sun, L.; Feng, L.; Chen, X.; Zhang, T.; Dai, C.; et al. Variation of a major facilitator superfamily gene contributes to differential cadmium accumulation between rice subspecies. Nat. Commun. 2019, 10, 2562. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, N.; Xia, J.; Mitani-Ueno, N.; Yokosho, K.; Ma, J.F. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol. 2013, 162, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Yamaji, N.; Kono, I.; Huang, C.F.; Ando, T.; Yano, M.; Ma, J.F. Gene limiting cadmium accumulation in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 16500–16505. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Lu, Y.; Zhang, X.; Yang, G.; Chao, D.; Wang, Z.; Shi, M.; Chen, J.; Chao, D.-Y.; Li, R.; et al. The ABC transporter ABCG36 is required for cadmium tolerance in rice. J. Exp. Bot. 2019, 70, 5909–5918. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Otani, M.; Uraguchi, S.; Akihiro, T.; Fujiwara, T. Rice ABCG43 is Cd inducible and confers Cd tolerance on yeast. Biosci. Biotechnol. Biochem. 2011, 75, 1211–1213. [Google Scholar] [CrossRef]
- Xiong, W.T.; Wang, P.; Yan, T.Z.; Cao, B.B.; Xu, J.; Liu, D.F.; Luo, M.Z. The rice “fruit-weight 2.2-like” gene family member OsFWL4 is involved in the translocation of cadmium from roots to shoots. Planta 2018, 247, 1247–1260. [Google Scholar] [CrossRef]
- Luo, J.-S.; Huang, J.; Zeng, D.-L.; Peng, J.-S.; Zhang, G.-B.; Ma, H.-L.; Guan, Y.; Yi, H.-Y.; Fu, Y.-L.; Han, B.; et al. A defensin-like protein drives cadmium efflux and allocation in rice. Nat. Commun. 2018, 9, 645. [Google Scholar] [CrossRef]
- Uraguchi, S.; Kamiya, T.; Sakamoto, T.; Kasai, K.; Sato, Y.; Nagamura, Y.; Yoshida, A.; Kyozuka, J.; Ishikawa, S.; Fujiwara, T. Low-affinity cation transporter ( OsLCT1 ) regulates cadmium transport into rice grains. Proc. Natl. Acad. Sci. USA 2011, 108, 20959–20964. [Google Scholar] [CrossRef]
- Hao, X.; Zeng, M.; Wang, J.; Zeng, Z.; Dai, J.; Xie, Z.; Yang, Y.; Tian, L.; Chen, L.; Li, D. A node-expressed transporter OsCCX2 is involved in grain cadmium accumulation of rice. Front. Plant Sci. 2018, 9, 476. [Google Scholar] [CrossRef]
- Shimo, H.; Ishimaru, Y.; An, G.; Yamakawa, T.; Nakanishi, H.; Nishizawa, N.K. Low cadmium (LCD), a novel gene related to cadmium tolerance and accumulation in rice. J. Exp. Bot. 2011, 62, 5727–5734. [Google Scholar] [CrossRef]
- Yuan, L.; Yang, S.; Liu, B.; Zhang, M.; Wu, K. Molecular characterization of a rice metal tolerance protein, OsMTP1. Plant Cell Rep. 2011, 31, 67–79. [Google Scholar] [CrossRef]
- Liu, B.; Yuan, L.; Wang, J.; Zhang, M. Bioinformatics and expression analysis on metal tolerance protein gene OsMTP2 of rice (Oryza sativa). J. Trop. Subtrop. Bot. 2012, 20, 8–12. [Google Scholar]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Bashir, K.; Senoura, T.; Sugimoto, K.; Ono, K.; Suzui, N.; Kawachi, N.; Ishii, S.; et al. From laboratory to field: OsNRAMP5-knockdown rice is a promising candidate for Cd phytoremediation in paddy fields. PLoS ONE 2014, 9, e98816. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Zhang, L.; Hu, J.; Zhang, X.; Lu, K.; Dong, H.; Wang, D.; Zhao, F.-J.; Huang, C.-F.; et al. OsNRAMP5 contributes to manganese translocation and distribution in rice shoots. J. Exp. Bot. 2014, 65, 4849–4861. [Google Scholar] [CrossRef]
- Yang, C.-H.; Zhang, Y.; Huang, C.-F. Reduction in cadmium accumulation in japonica rice grains by CRISPR/Cas9-mediated editing of OsNRAMP5. J. Integr. Agric. 2019, 18, 688–697. [Google Scholar] [CrossRef]
- Conn, S.; Gilliham, M. Comparative physiology of elemental distributions in plants. Ann. Bot. 2010, 105, 1081–1102. [Google Scholar] [CrossRef]
- Uraguchi, S.; Fujiwara, T. Cadmium transport and tolerance in rice: Perspectives for reducing grain cadmium accumulation. Rice 2012, 5, 5. [Google Scholar] [CrossRef]
- Lee, S.; An, G. Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ. 2010, 32, 408–416. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Nakanishi, H.; Nishizawa, N.K. Role of the iron transporter OsNRAMP1 in cadmium uptake and accumulation in rice. Plant Signal. Behav. 2011, 6, 1813–1816. [Google Scholar] [CrossRef]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.; Takahashi, H.; et al. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Ma, J.F. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J. Exp. Bot. 2014, 65, 6013–6021. [Google Scholar] [CrossRef]
- Liu, C.; Gao, Z.; Shang, L.; Yang, C.; Ruan, B.; Zeng, D.; Guo, L.; Zhao, F.; Huang, C.; Qian, Q. Natural variation in the promoter of OsHMA3 contributes to differential grain cadmium accumulation between Indica and Japonica rice. J. Integr. Plant Biol. 2019, 62, 314–329. [Google Scholar] [CrossRef]
- Uraguchi, S.; Mori, S.; Kuramata, M.; Kawasaki, A.; Arao, T.; Ishikawa, S. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J. Exp. Bot. 2009, 60, 2677–2688. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Suzui, N.; Ito-Tanabata, S.; Ishii, S.; Igura, M.; Abe, T.; Kuramata, M.; Kawachi, N.; Fujimaki, S. Real-time imaging and analysis of differences in cadmium dynamics in rice cultivars (Oryza sativa) using positron-emitting 107Cd tracer. BMC Plant Biol. 2011, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant, Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.-J.; Huang, X.-Y. Cadmium phytoremediation: Call rice CAL1. Mol. Plant 2018, 11, 640–642. [Google Scholar] [CrossRef]
- Fujimaki, S.; Suzui, N.; Ishioka, N.S.; Kawachi, N.; Ito, S.; Chino, M.; Nakamura, S.-I. Tracing cadmium from culture to spikelet: Noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice plant. Plant Physiol. 2010, 152, 1796–1806. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F. The node, a hub for mineral nutrient distribution in graminaceous plants. Trends Plant Sci. 2014, 19, 556–563. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F. Node-controlled allocation of mineral elements in Poaceae. Curr. Opin. Plant Biol. 2017, 39, 18–24. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, L.; Li, X. Arabidopsis and rice showed a distinct pattern in ZIPs genes expression profile in response to Cd stress. Bot. Stud. 2018, 59, 22. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, M.; Mou, R.; Cao, Z.; Zhang, W.; Lin, X. Advances in research of cadmium metabolism and control in rice plants. Sci. Agric. Sin. 2014, 47, 3633–3640. [Google Scholar]
- Kashiwagi, T.; Shindoh, K.; Hirotsu, N.; Ishimaru, K. Evidence for separate translocation pathways in determining cadmium accumulation in grain and aerial plant parts in rice. BMC Plant Biol. 2009, 9, 8. [Google Scholar] [CrossRef]
- Rodda, M.S.; Li, G.; Reid, R.J. The timing of grain Cd accumulation in rice plants: The relative importance of remobilisation within the plant and root Cd uptake post-flowering. Plant Soil 2011, 347, 105–114. [Google Scholar] [CrossRef]
- Kato, M.; Ishikawa, S.; Inagaki, K.; Chiba, K.; Hayashi, H.; Yanagisawa, S.; Yoneyama, T. Possible chemical forms of cadmium and varietal differences in cadmium concentrations in the phloem sap of rice plants (Oryza sativa L.). Soil Sci. Plant Nutr. 2010, 56, 839–847. [Google Scholar] [CrossRef]
- Hao, X.; Dai, J.; Ji, W.; Huang, D.; Li, D.; Tian, L. Screening and identification of LCD-interacting proteins in rice. Biotechnol. Bull. 2020, 36, 21–29. [Google Scholar]
- Sharma, S.S.; Dietz, K.-J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef]
- Montanini, B.; Blaudez, D.; Jeandroz, S.; Sanders, D.; Chalot, M. Phylogenetic and functional analysis of the Cation Diffusion Facilitator (CDF) family: Improved signature and prediction of substrate specificity. BMC Genom. 2007, 8, 107–123. [Google Scholar] [CrossRef]
- Liang, C.; Shilai, S.; Ninfei, J.; Hira, K.; Mustafa, W.G.; Changlan, Z. Genome-wide analysis of long non-coding RNAs affecting roots development at an early stage in the rice response to cadmium stress. BMC Genom. 2018, 19, 460–469. [Google Scholar]
- He, F.; Liu, Q.; Zheng, L.; Cui, Y.; Shen, Z.; Zheng, L. RNA-Seq analysis of rice roots reveals the involvement of post-transcriptional regulation in response to cadmium stress. Front. Plant Sci. 2015, 6, 1136. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Z.; Zhu, C. Microarray-based analysis of cadmium-responsive microRNAs in rice (Oryza sativa L). J. Exp. Bot. 2011, 62, 3563–3573. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Y.; Jiang, Z.; Wang, F.; Jiang, Q.; Sun, J.; Chen, Z.; Zhu, C. MicroRNA268 overexpression affects rice seedling growth under cadmium stress. J. Agric. Food Chem. 2017, 65, 5860–5867. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ye, Y.; Jiang, Z.; Wang, Y.; Zhu, C. MicroRNA390 is involved in cadmium tolerance and accumulation in rice. Front. Plant Sci. 2016, 7, 235. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.F.; Gong, S.H.; Wang, Y.; Wang, F.J.; Bao, H.X.G.D.L.; Sun, J.W.; Cai, C.; Yi, K.K.; Chen, Z.X.; Zhu, C. MicroRNA166 modulates cadmium tolerance and accumulation in rice. Plant Physiol. 2018, 177, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wu, N.; Fu, J.; Wang, S.; Li, X.; Xiao, J.; Xiong, L. A GH3 family member, OsGH3-2, modulates auxin and methylation and chromatin patterning abscisic acid levels and differentially affects drought and cold tolerance in rice. J. Exp. Bot. 2012, 63, 6467–6480. [Google Scholar] [CrossRef]
- Meng, J.G.; Zhang, X.D.; Tan, S.K.; Zhao, K.X.; Yang, Z.M. Genome-wide identification of Cd-responsive NRAMP transporter genes and analyzing expression of NRAMP 1 mediated by miR167 in Brassica napus. Biometals 2017, 30, 917–931. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, Y.-C.; Shen, C.; He, C.-T.; Yuan, J.-G.; Yang, Z.-Y. Comparative analysis between low- and high-cadmium-accumulating cultivars of Brassica parachinensis to identify difference of cadmium-induced microRNA and their targets. Plant Soil 2017, 420, 223–237. [Google Scholar] [CrossRef]
- Tanaka, N.; Uraguchi, S.; Kajikawa, M.; Saito, A.; Ohmori, Y.; Fujiwara, T. A rice PHD-finger protein OsTITANIA, is a growth regulator that functions through elevating expression of transporter genes for multiple metals. Plant J. 2018, 96, 997–1006. [Google Scholar] [CrossRef]
- Wang, B.; Tan, M.; Liu, Y.; Xu, D.; Zheng, Q.; Zhao, H.; Zhang, J. Application of rice transcription factor OsNAC3 in improving cadmium tolerance of plants. Chinese Patent CN111041035A, 21 April 2020. [Google Scholar]
- Hu, S.; Shinwari, K.; Song, Y.; Xia, J.; Xu, H.; Du, B.; Luo, L.; Zheng, L. OsNAC300 positively regulates cadmium stress responses and tolerance in rice roots. Agronomy 2021, 11, 95. [Google Scholar] [CrossRef]
- Shim, D.; Hwang, J.-U.; Lee, J.; Lee, S.; Choi, Y.; An, G.; Martinoia, E.; Lee, Y. Orthologs of the class A4 heat shock transcription factor HsfA4a confer cadmium tolerance in wheat and rice. Plant Cell 2009, 21, 4031–4043. [Google Scholar] [CrossRef]
- Hu, S.; Yu, Y.; Chen, Q.; Mu, G.; Shen, Z.; Zheng, L. OsMYB45 plays an important role in rice resistance to cadmium stress. Plant Sci. 2017, 264, 1–8. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Peng, X.; Bai, N.; Wang, H. Isolation and expression profiles of cadmium stress-responsive rice WRKY15 transcription factor gene. Chin. J. Rice Sci. 2018, 32, 103–110. [Google Scholar]
- Hu, Y.; Cheng, H.; Tao, S. The challenges and solutions for cadmium-contaminated rice in China: A critical review. Environ. Int. 2016, 92, 515–532. [Google Scholar] [CrossRef]
- Irshad, M.K.; Chen, C.; Noman, A.; Ibrahim, M.; Adeel, M.; Shang, J. Goethite-modified biochar restricts the mobility and transfer of cadmium in soil-rice system. Chemosphere 2019, 242, 125152. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, Y.Q.; Liang, C.H. Evaluation of phosphate fertilizers for the immobilization of Cd in contaminated soils. PLoS ONE 2015, 10, e0124022. [Google Scholar] [CrossRef]
- Hu, P.; Ouyang, Y.; Wu, L.; Shen, L.; Luo, Y.; Christie, P. Effects of water management on arsenic and cadmium speciation and accumulation in an upland rice cultivar. J. Environ. Sci. 2015, 27, 225–231. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, J.; Huang, Q.; Su, D.; Jiang, R.; Li, H. Application of a rotation system to oilseed rape and rice fields in Cd-contaminated agricultural land to ensure food safety. Ecotoxicol. Environ. Saf. 2014, 108, 287–293. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Rizwan, M.; Ali, S.; Parveen, A.; Malik, Z.; Wang, X. Cadmium uptake and translocation: Selenium and silicon roles in Cd detoxification for the production of low Cd crops: A critical review. Chemosphere 2021, 273, 129690. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Kao, C.H. Role of abscisic acid in cadmium tolerance of rice (Oryza sativa L.) seedlings. Plant Cell Environ. 2003, 26, 867–874. [Google Scholar] [CrossRef]
- Takahashi, R.; Ito, M.; Kawamoto, T. The road to practical application of cadmium phytoremediation using rice. Plants 2021, 10, 1926. [Google Scholar] [CrossRef]
- Dixit, R.; Wasiullah Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; Lade, H.; et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, W.; Zhang, S.; Yang, T.; Liu, Q.; Dong, J.; Fu, H.; Mao, X.; Liu, B. Genome-wide association study and candidate gene analysis of rice cadmium accumulation in grain in a diverse rice collection. Rice 2018, 11, 61. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, H. Genotypic differences in concentrations of plumbum, cadmium and arsenicum in polished rice grains. J. Yunnan Norm. Univ. 2002, 22, 37–40. [Google Scholar]
- Huang, D.; Zhu, Q.; Zhu, H.; Xu, C.; Liu, S. Advances and prospects of safety agro-utilization of heavy metal contaminated farmland soil. Res. Agric. Mod. 2018, 39, 1030–1043. [Google Scholar]
- Huang, X.; Jiang, H.; Zhang, J.; Tang, W.; Xiao, Y. Improvement of blast resistance and low cadmium accumulation of dual-purpose genic male sterile rice line chuang 5S by molecular marker-assisted selection. Hybrid Rice 2019, 34, 51–56. [Google Scholar]
- Ishikawa, S.; Ishimaru, Y.; Igura, M.; Kuramata, M.; Abe, T.; Senoura, T.; Hase, Y.; Arao, T.; Nishizawa, N.K.; Nakanishi, H. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc. Natl. Acad. Sci. USA 2012, 109, 19166–19171. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.R. Absorption, Transport, and Accumulation of Cadmium in Rice Cultivars and Identification of Low-Cadmium Rice Mutant; University of Science and Technology of China: Hefei, China, 2019. [Google Scholar]
- Ishikawa, S. Mechanisms of cadmium accumulation in rice grains and molecular breeding for its reduction. Soil Sci. Plant Nutr. 2020, 66, 28–33. [Google Scholar] [CrossRef]
- Chen, C.; Tang, W. A perspective on the selection and breeding of low-Cd rice. Res. Agric. Mod. 2018, 39, 1044–1051. [Google Scholar]
- Wang, T.; Li, Y.; Song, S.; Fu, Y.; Yu, Y.; Bai, L.; Li, L. Excavation of rice resources with low cadmium accumulation in grains and development of new materials. Hybrid Rice 2021, 36, 68–74. [Google Scholar]
- Sebastian, A.; Prasad, M.N.V. Cadmium minimization in rice. A review. Agron. Sustain. Dev. 2014, 34, 155–173. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, J.; Liu, Y.; Meng, J.; Xu, S.; Tan, Y.; Li, Y.; Shu, Q.; Huang, J. Characterization and evaluation of OsLCT1 and OsNramp5 mutants generated through CRISPR/Cas9-mediated mutagenesis for breeding low Cd rice. Rice Sci. 2019, 26, 88–97. [Google Scholar] [CrossRef]
- Syu, C.-H.; Nieh, T.-I.; Hsieh, M.-T.; Lo, Y.-C.; Du, P.-R.; Lin, Y.-W.; Wu, D.-H. Uncovering the genetic of cadmium accumulation in the rice 3K panel. Plants 2022, 11, 2813. [Google Scholar] [CrossRef]
- Tang, L.; Mao, B.; Li, Y.; Lv, Q.; Zhang, L.; Chen, C.; He, H.; Wang, W.; Zeng, X.; Shao, Y.; et al. Knockout of OsNramp5 using the CRISPR/Cas9 system produces low Cd-accumulating indica rice without compromising yield. Sci. Rep. 2017, 7, 14438. [Google Scholar] [CrossRef]
- Ai, H.; Wu, D.; Li, C.; Hou, M. Advances in molecular mechanisms underlying cadmium uptake and translocation in rice. Front. Plant Sci. 2022, 13, 1003953. [Google Scholar] [CrossRef]

| Gene Name | Gene ID | Main Expression Tissues | Subcellular Localization | Reference |
|---|---|---|---|---|
| OsNRAMP1 | LOC_Os07g15460 | Root tissues | Plasma membrane | [35] |
| OsNRAMP5 | LOC_Os07g15370 | Root epidermis, exodermis, lateral cortex and tissues around xylem in the vascular bundle | Plasma membrane | [36] |
| OsZIP1 | LOC_Os01g74110 | Root tissues | Plasma membrane | [37] |
| OsZIP3 | LOC_Os04g52310 | Parenchyma cells around enlarged vascular bundles in stem nodes | Plasma membrane | [38] |
| OsZIP5 | LOC_Os05g39560 | Root epidermis and parenchyma cells around xylem, parenchyma cells around xylem tissue in enlarged vascular bundle in stem nodes, parenchyma cells around xylem, and phloem of diffuse vascular bundles | Plasma membrane | [39] |
| OsZIP6 | LOC_Os05g07210 | Root and above-ground tissues | Plasma membrane | [40] |
| OsZIP7 | LOC_Os05g10940 | Parenchyma cells in root stelar, parenchyma cells around the enlarged vascular bundle in stem nodes | Plasma membrane | [41] |
| OsZIP9 | LOC_Os05g39540 | Root epidermis, parenchyma cells around the xylem in enlarged vascular bundle, parenchyma cells around xylem and phloem in diffuse vascular bundle in stem nodes | Plasma membrane | [39] |
| OsIRT1 | LOC_Os03g46470 | Epidermis and exodermis in the root elongation zone, cortex of mature root areas, companion cells in phloem of root stelar, phloem in stem nodes | Plasma membrane | [42,43] |
| OsIRT2 | LOC_Os03g46454 | Root tissues | Plasma membrane | [43] |
| OsCd1 | LOC_Os03g02380 | All root tissues | Plasma membrane | [44] |
| OsHMA2 | LOC_Os06g48720 | Root pericycle, phloem of enlarged vascular bundle, and diffuse vascular bundle in stem nodes | Plasma membrane | [45] |
| OsHMA3 | LOC_Os07g12900 | All root tissues | Tonoplast membrane | [46] |
| OsABCG36 (OsPDR9) | LOC_Os01g42380 | All root tissues except epidermis | Plasma membrane | [47] |
| OsABCG43 (OsPDR5) | LOC_Os07g33780 | Root and aboveground tissues | Plasma membrane | [48] |
| OsFWL4 | LOC_Os03g61440 | Root, flag leaf, and leaf sheath tissues | \ | [49] |
| CAL1 | LOC_Os02g41904 | Root exodermis, parenchyma cells around xylem in the root and leaf sheath | \ | [50] |
| OsLCT1 | LOC_Os06g38120 | Leaf, parenchyma cells around the enlarged vascular bundle and diffuse vascular bundle in Node I | Plasma membrane | [51] |
| OsCCX2 | LOC_Os03g45370 | Parenchyma cells in enlarged vascular bundles and diffuse vascular bundles in stem nodes | Plasma membrane | [52] |
| OsLCD | LOC_Os01g72670 | Companion cells in phloem in the leaf, root vascular bundle | Cytoplasm, nucleus | [53] |
| OsMTP1 | LOC_Os05g03780 | Root and shoot tissues, leaf-specific sieve tube cells | Plasma membrane | [54] |
| OsMTP2 | LOC_Os01g62070 | Vascular bundles of stems and leaves, embryo sac wall of immature seeds | \ | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Du, R.; Wang, X. Genetic Regulation Mechanism of Cadmium Accumulation and Its Utilization in Rice Breeding. Int. J. Mol. Sci. 2023, 24, 1247. https://doi.org/10.3390/ijms24021247
Chen G, Du R, Wang X. Genetic Regulation Mechanism of Cadmium Accumulation and Its Utilization in Rice Breeding. International Journal of Molecular Sciences. 2023; 24(2):1247. https://doi.org/10.3390/ijms24021247
Chicago/Turabian StyleChen, Guang, Ruiying Du, and Xu Wang. 2023. "Genetic Regulation Mechanism of Cadmium Accumulation and Its Utilization in Rice Breeding" International Journal of Molecular Sciences 24, no. 2: 1247. https://doi.org/10.3390/ijms24021247
APA StyleChen, G., Du, R., & Wang, X. (2023). Genetic Regulation Mechanism of Cadmium Accumulation and Its Utilization in Rice Breeding. International Journal of Molecular Sciences, 24(2), 1247. https://doi.org/10.3390/ijms24021247







