Lessons on Drug Development: A Literature Review of Challenges Faced in Nonalcoholic Fatty Liver Disease (NAFLD) Clinical Trials
Abstract
1. Introduction
1.1. Prevalence of NAFLD
1.2. Pathogenesis of NAFLD
1.3. Risk Factors for NAFLD
2. Diagnosis and Prognosis of NAFLD
2.1. Noninvasive Methods for NAFLD
2.2. Noninvasive Methods for NAFLD with Fibrosis
2.3. Invasive Methods for NASH
3. Current Management of NAFLD
4. Methods
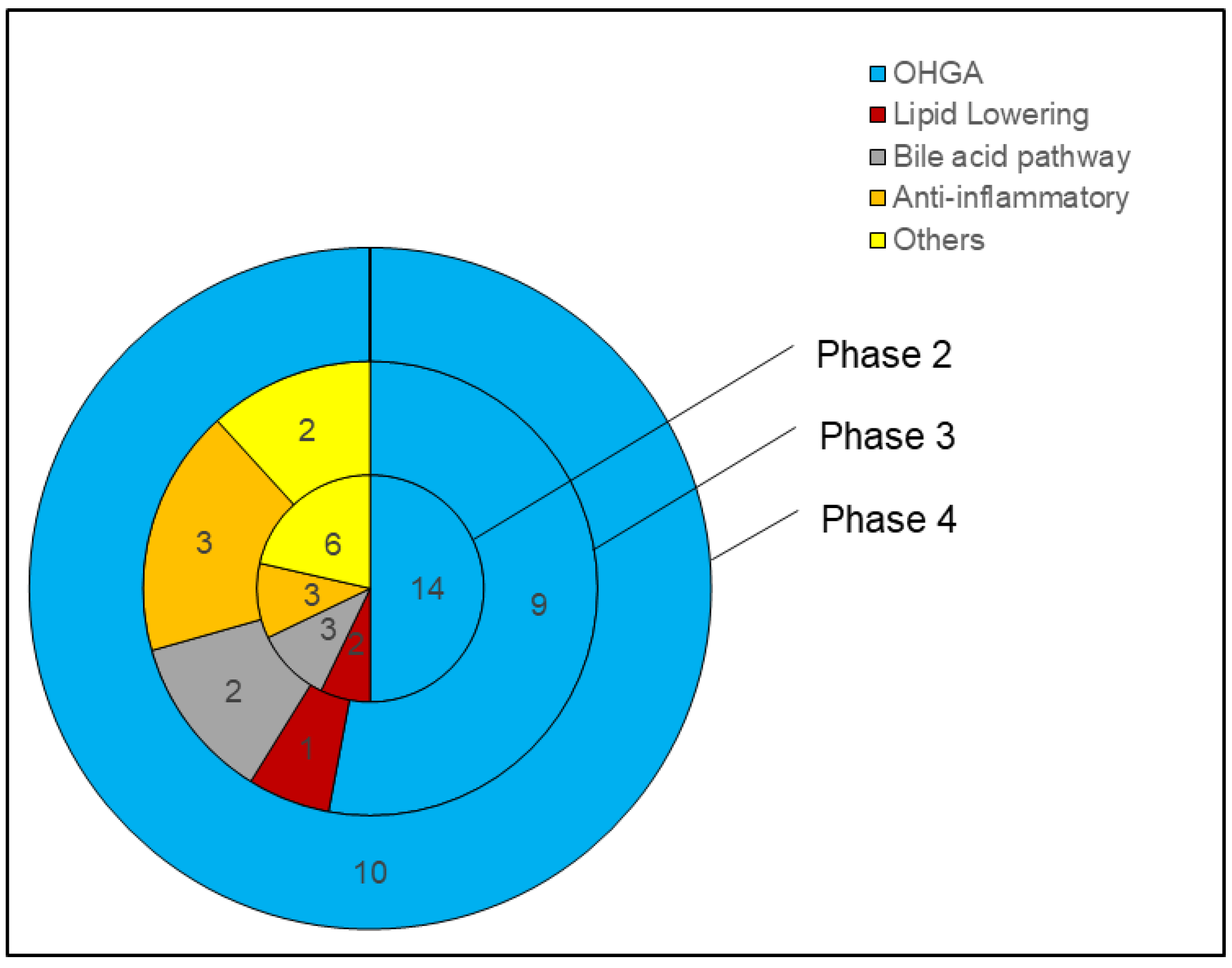
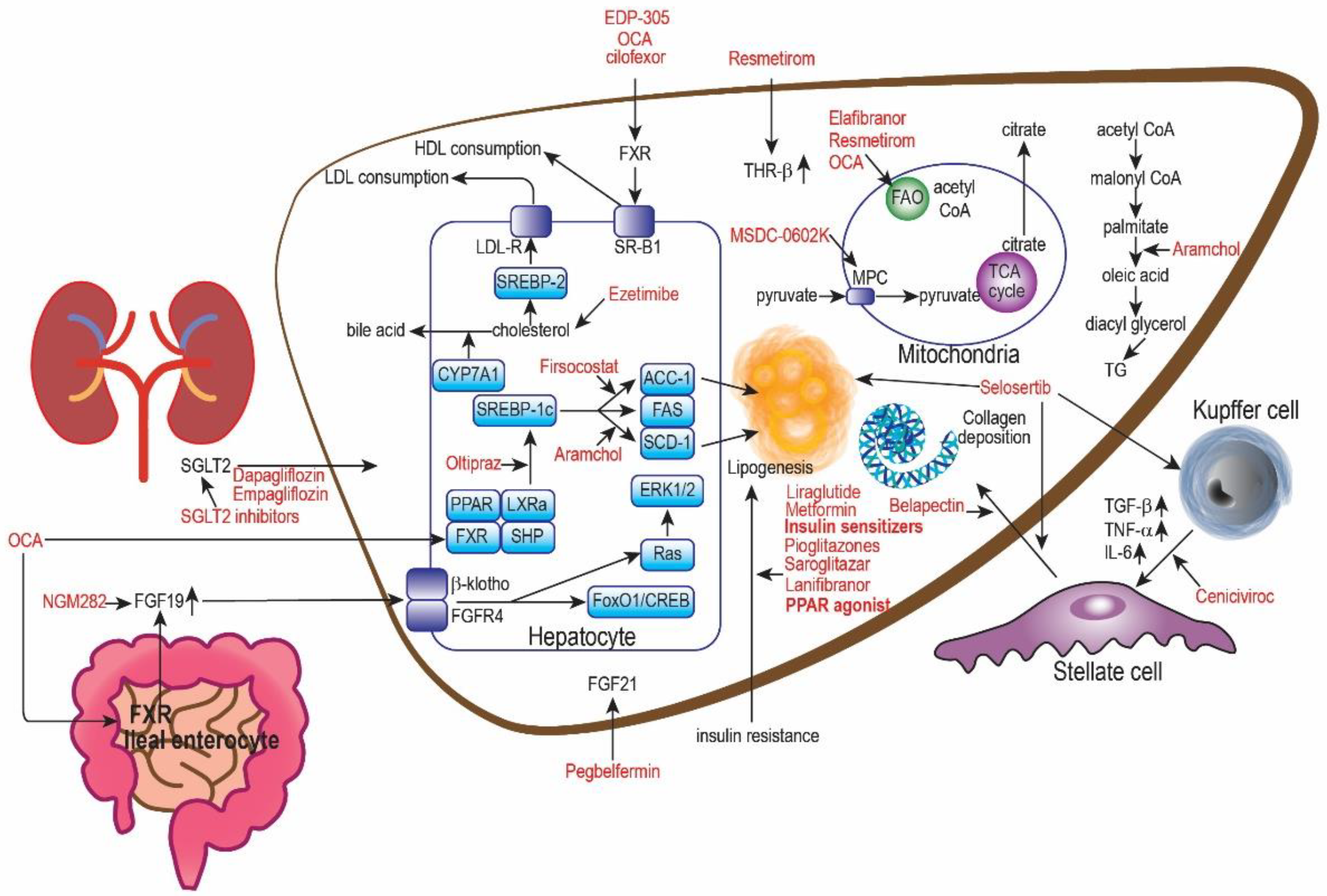
5. Oral Hypoglycemic Drugs (OHGA) for NAFLD
5.1. PPAR Agonist
5.2. GLP-1 Analog
5.3. AMPK Inhibitor
5.4. Comparing PPAR vs. SGLT-2 vs. GLP-1 vs. AMPK Inhibitor
6. Lipid-Lowering Drugs for NAFLD
6.1. NPC1L1 Inhibitor
6.2. HMG-CoA Inhibitor
6.3. SCD Inhibitor
6.4. LXRα Inhibitor
7. Bile Pathway Drugs for NAFLD
FXR Agonists
8. Anti-Inflammatory Drugs for NAFLD
8.1. Phosphodiesterase Inhibitors
| Anti-Inflammatory Drugs | |||||||
|---|---|---|---|---|---|---|---|
| Drug | Mechanism of Action | Trial (Type and Identifier) | Patient (Type and Number) | Dosage | Duration | Results (Histology, Imaging, Biomarker) ✓ X | Side Effects |
| Pentoxifylline (PTX) | Phosphodiesterase inhibitor | Phase 2 NCT00267670 [130] | Adult biopsy-proven NASH 26 | 1200 mg/day OR Placebo | 12 months | Histology: X Imaging: N Biomarker: X | Well tolerated |
| Phase 2 NCT00590161 [131] | Adult biopsy-proven noncirrhotic NASH with T2DM 55 | 1200 mg/day OR Placebo | 12 months | Histology: X Imaging: N Biomarker: ✓ (ALT) | |||
| Phase 2 [132] | Adult biopsy or US-proven NAFLD 90 | 1200 mg/day with 300 mg/day fenofibrate OR 300 mg/day fenofibrate | 24 weeks | Histology: N Imaging: ✓ (via Fibrosacan) Biomarker: ✓ (TNF-α, insulin, FBG, HOMA-IR, AST, ALT) | |||
| Selosertib | ASK-1 inhibitor | Phase 3 NCT03053050 (STELLAR-3) [134] TERMINATED | Adult NASH with F3 808 | 18 mg/day OR 6 mg/day OR Placebo | 48, 240 weeks | Histology: X (18 mg/day and 6 mg/day did worse than placebo; Did not meet primary endpoint during interim analysis at 48 weeks, hence terminated) Imaging: N Biomarker: N | Well tolerated |
| Phase 3 NCT03053063 (STELLAR-4) [134] TERMINATED | Adult NASH with F4 883 | 18 mg/day OR 6 mg/day OR Placebo | 48, 240 weeks | Histology: X (Did not meet primary outcome during interim analysis at 48 weeks, hence terminated) Imaging: N Biomarker: N | |||
| Ceniciviroc (CVC) | CCR2/5 antagonists | Phase 2b NCT02217475 (CENTAUR) [135] | Adult biopsy-proven NASH with F1-F3 289 | 150 mg/day for 2 years OR Placebo for 1 year followed by 150 mg/day for 2 years OR Placebo for 2 years | 2 years | Histology: X (for the 1st year of CVC) Imaging: N Biomarker: X (ALT, AST, FIB-4, NAFLD fibrosis score, ELF) | Well tolerated |
| Phase 3 NCT03028740 (141) TERMINATED | Adult biopsy-proven NASH with F2–F3 Part 1: 1200 Part 2: 800 | 150 mg/day OR Placebo | 40 months | Histology: X (based on Part 1) Imaging: N Biomarker: X | |||
8.2. ASK-1 Inhibitor
8.3. CCR2/5 Antagonists
9. Other Drugs for NAFLD
9.1. THR Agonist
9.2. FGF Analog
9.3. Nutraceuticals
10. Targeting the Gut Microbiome
11. Perspectives
11.1. Challenges in NAFLD Drug Development
11.2. What Can We Learn from NAFLD Clinical Trials?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Marjot, T.; Moolla, A.; Cobbold, J.F.; Hodson, L.; Tomlinson, J.W. Nonalcoholic Fatty Liver Disease in Adults: Current Concepts in Etiology, Outcomes, and Management. Endocr. Rev. 2020, 41, 66–117. [Google Scholar] [CrossRef]
- Finucane, M.M.; Stevens, G.A.; Cowan, M.J.; Danaei, G.; Lin, J.K.; Paciorek, C.J.; Singh, G.M.; Gutierrez, H.R.; Lu, Y.; Bahalim, A.N.; et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 2011, 377, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Definition & Facts of NAFLD & NASH. Available online: https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash/definition-facts (accessed on 8 September 2022).
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Soares, M.J.; Mohan, V.; Anoop, S.; Abhishek, V.; Vaidya, R.; Pradeepa, R. Body fat, metabolic syndrome and hyperglycemia in South Asians. J. Diabetes Complicat. 2018, 32, 1068–1075. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Santos-Baez, L.S.; Ginsberg, H.N. Hypertriglyceridemia-Causes, Significance, and Approaches to Therapy. Front. Endocrinol. 2020, 11, 616. [Google Scholar] [CrossRef]
- Sanders, F.W.; Griffin, J.L. De novo lipogenesis in the liver in health and disease: More than just a shunting yard for glucose. Biol. Rev. Camb. Philos. Soc. 2016, 91, 452–468. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Yang, L.; McCall, S.; Huang, J.; Yu, X.X.; Pandey, S.K.; Bhanot, S.; Monia, B.P.; Li, Y.X.; Diehl, A.M. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology 2007, 45, 1366–1374. [Google Scholar] [CrossRef]
- Caussy, C.; Soni, M.; Cui, J.; Bettencourt, R.; Schork, N.; Chen, C.H.; Ikhwan, M.A.; Bassirian, S.; Cepin, S.; Gonzalez, M.P.; et al. Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. J. Clin. Investig. 2017, 127, 2697–2704. [Google Scholar] [CrossRef]
- Mahjoubin-Tehran, M.; De Vincentis, A.; Mikhailidis, D.P.; Atkin, S.L.; Mantzoros, C.S.; Jamialahmadi, T.; Sahebkar, A. Non-alcoholic fatty liver disease and steatohepatitis: State of the art on effective therapeutics based on the gold standard method for diagnosis. Mol. Metab. 2021, 50, 101049. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Ibdah, J.A. Role of mitochondria in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 8713–8742. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.; Carvalho, D.; Freitas, P. Gut Microbiota: Association with NAFLD and Metabolic Disturbances. BioMed Res. Int. 2015, 2015, 979515. [Google Scholar] [CrossRef] [PubMed]
- Kerdsuknirun, J.; Vilaichone, V.; Vilaichone, R.K. Clinical Outcome and Predictive Factors of Variceal Bleeding in Patients with Hepatocellular Carcinoma in Thailand. Asian Pac. J. Cancer Prev. 2018, 19, 3301–3305. [Google Scholar] [CrossRef]
- Kasper, P.; Martin, A.; Lang, S.; Kutting, F.; Goeser, T.; Demir, M.; Steffen, H.M. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol. 2021, 110, 921–937. [Google Scholar] [CrossRef]
- Korinkova, L.; Prazienkova, V.; Cerna, L.; Karnosova, A.; Zelezna, B.; Kunes, J.; Maletinska, L. Pathophysiology of NAFLD and NASH in Experimental Models: The Role of Food Intake Regulating Peptides. Front. Endocrinol. 2020, 11, 597583. [Google Scholar] [CrossRef]
- Sanyal, A.; Poklepovic, A.; Moyneur, E.; Barghout, V. Population-based risk factors and resource utilization for HCC: US perspective. Curr. Med. Res. Opin. 2010, 26, 2183–2191. [Google Scholar] [CrossRef]
- VanWagner, L.B.; Armstrong, M.J. Lean NAFLD: A not so benign condition? Hepatol. Commun. 2018, 2, 5–8. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Fowler, K.J.; Hamilton, G.; Cui, J.Y.; Sy, E.Z.; Balanay, M.; Hooker, J.C.; Szeverenyi, N.; Sirlin, C.B. Liver fat imaging-a clinical overview of ultrasound, CT, and MR imaging. Br. J. Radiol. 2018, 91, 20170959. [Google Scholar] [CrossRef]
- Tamaki, N.; Ajmera, V.; Loomba, R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD. Nat. Rev. Endocrinol. 2022, 18, 55–66. [Google Scholar] [CrossRef]
- Han, A.; Zhang, Y.N.; Boehringer, A.S.; Montes, V.; Andre, M.P.; Erdman, J.W., Jr.; Loomba, R.; Valasek, M.A.; Sirlin, C.B.; O’Brien, W.D., Jr. Assessment of Hepatic Steatosis in Nonalcoholic Fatty Liver Disease by Using Quantitative US. Radiology 2020, 295, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, B.G.; Lee, J.S.; Lee, C.K.; Yeon, J.E.; Chang, M.S.; Kim, J.H.; Kim, H.; Yi, S.; Lee, J.; et al. Randomised clinical trial: The efficacy and safety of oltipraz, a liver X receptor alpha-inhibitory dithiolethione in patients with non-alcoholic fatty liver disease. Aliment. Pharm. 2017, 45, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Neuschwander-Tetri, B.A.; Sanyal, A.; Chalasani, N.; Diehl, A.M.; Terrault, N.; Kowdley, K.; Dasarathy, S.; Kleiner, D.; Behling, C.; et al. Multicenter Validation of Association Between Decline in MRI-PDFF and Histologic Response in NASH. Hepatology 2020, 72, 1219–1229. [Google Scholar] [CrossRef]
- Bril, F.; Barb, D.; Lomonaco, R.; Lai, J.; Cusi, K. Change in hepatic fat content measured by MRI does not predict treatment-induced histological improvement of steatohepatitis. J. Hepatol. 2020, 72, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Pasanta, D.; Htun, K.T.; Pan, J.; Tungjai, M.; Kaewjaeng, S.; Kim, H.; Kaewkhao, J.; Kothan, S. Magnetic Resonance Spectroscopy of Hepatic Fat from Fundamental to Clinical Applications. Diagnostics 2021, 11, 842. [Google Scholar] [CrossRef]
- Gutierrez-Buey, G.; Nunez-Cordoba, J.M.; Llavero-Valero, M.; Gargallo, J.; Salvador, J.; Escalada, J. Is HOMA-IR a potential screening test for non-alcoholic fatty liver disease in adults with type 2 diabetes? Eur. J. Intern. Med. 2017, 41, 74–78. [Google Scholar] [CrossRef]
- Bonora, E.; Targher, G.; Alberiche, M.; Bonadonna, R.C.; Saggiani, F.; Zenere, M.B.; Monauni, T.; Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 2000, 23, 57–63. [Google Scholar] [CrossRef]
- Isokuortti, E.; Zhou, Y.; Peltonen, M.; Bugianesi, E.; Clement, K.; Bonnefont-Rousselot, D.; Lacorte, J.M.; Gastaldelli, A.; Schuppan, D.; Schattenberg, J.M.; et al. Use of HOMA-IR to diagnose non-alcoholic fatty liver disease: A population-based and inter-laboratory study. Diabetologia 2017, 60, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Pathik, P.; Ravindra, S.; Ajay, C.; Prasad, B.; Jatin, P.; Prabha, S. Fibroscan versus simple noninvasive screening tools in predicting fibrosis in high-risk nonalcoholic fatty liver disease patients from Western India. Ann. Gastroenterol. 2015, 28, 281–286. [Google Scholar] [PubMed]
- Lee, J.; Vali, Y.; Boursier, J.; Spijker, R.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int. 2021, 41, 261–270. [Google Scholar] [CrossRef]
- Chang, P.E.; Goh, G.B.; Ngu, J.H.; Tan, H.K.; Tan, C.K. Clinical applications, limitations and future role of transient elastography in the management of liver disease. World J. Gastrointest Pharm. 2016, 7, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, E.; Dhawan, A. Noninvasive biomarkers in non-alcoholic fatty liver disease: Current status and a glimpse of the future. World J. Gastroenterol. 2014, 20, 10851–10863. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Lucas, G.; Lucas, N.; Krzowska-Firych, J.; Tomasiewicz, K. A systematic review of the present and future of non-alcoholic fatty liver disease. Clin. Exp. Hepatol. 2018, 4, 165–174. [Google Scholar] [CrossRef]
- Petroni, M.L.; Brodosi, L.; Bugianesi, E.; Marchesini, G. Management of non-alcoholic fatty liver disease. BMJ 2021, 372, m4747. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gomez, M.; Zelber-Sagi, S.; Trenell, M. Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 2017, 67, 829–846. [Google Scholar] [CrossRef]
- Stine, J.G.; Soriano, C.; Schreibman, I.; Rivas, G.; Hummer, B.; Yoo, E.; Schmitz, K.; Sciamanna, C. Breaking Down Barriers to Physical Activity in Patients with Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2021, 66, 3604–3611. [Google Scholar] [CrossRef]
- Laursen, T.L.; Hagemann, C.A.; Wei, C.; Kazankov, K.; Thomsen, K.L.; Knop, F.K.; Gronbaek, H. Bariatric surgery in patients with non-alcoholic fatty liver disease—From pathophysiology to clinical effects. World J. Hepatol. 2019, 11, 138–149. [Google Scholar] [CrossRef]
- Schmitz, S.M.; Kroh, A.; Koch, A.; Brozat, J.F.; Stier, C.; Neumann, U.P.; Ulmer, T.F.; Alizai, P.H. Comparison of Liver Recovery After Sleeve Gastrectomy and Roux-en-Y-Gastric Bypass. Obes. Surg. 2021, 31, 3218–3226. [Google Scholar] [CrossRef]
- Taneja, S.; Roy, A. Nonalcoholic steatohepatitis recurrence after liver transplant. Transl. Gastroenterol. Hepatol. 2020, 5, 24. [Google Scholar] [CrossRef]
- Blazina, I.; Selph, S. Diabetes drugs for nonalcoholic fatty liver disease: A systematic review. Syst. Rev. 2019, 8, 295. [Google Scholar] [CrossRef]
- Bril, F.; Kalavalapalli, S.; Clark, V.C.; Lomonaco, R.; Soldevila-Pico, C.; Liu, I.C.; Orsak, B.; Tio, F.; Cusi, K. Response to Pioglitazone in Patients with Nonalcoholic Steatohepatitis with vs. without Type 2 Diabetes. Clin. Gastroenterol. Hepatol. 2018, 16, 558–566.e52. [Google Scholar] [CrossRef]
- Bril, F.; Biernacki, D.M.; Kalavalapalli, S.; Lomonaco, R.; Subbarayan, S.K.; Lai, J.; Tio, F.; Suman, A.; Orsak, B.K.; Hecht, J.; et al. Role of Vitamin E for Nonalcoholic Steatohepatitis in Patients with Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2019, 42, 1481–1488. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Bansal, G.; Thanikachalam, P.V.; Maurya, R.K.; Chawla, P.; Ramamurthy, S. An overview on medicinal perspective of thiazolidine-2,4-dione: A remarkable scaffold in the treatment of type 2 diabetes. J. Adv. Res. 2020, 23, 163–205. [Google Scholar] [CrossRef] [PubMed]
- Lange, N.F.; Graf, V.; Caussy, C.; Dufour, J.F. PPAR-Targeted Therapies in the Treatment of Non-Alcoholic Fatty Liver Disease in Diabetic Patients. Int. J. Mol. Sci. 2022, 23, 4305. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Alkhouri, N.; Davison, B.A.; Sanyal, A.; Edwards, C.; Colca, J.R.; Lee, B.H.; Loomba, R.; Cusi, K.; Kolterman, O.; et al. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase IIb study. J. Hepatol. 2020, 72, 613–626. [Google Scholar] [CrossRef]
- Dufour, J.F.; Caussy, C.; Loomba, R. Combination therapy for non-alcoholic steatohepatitis: Rationale, opportunities and challenges. Gut 2020, 69, 1877–1884. [Google Scholar] [CrossRef]
- Sven, M.F.; Pierre, B.; Manal, F.A.; Quentin, M.A.; Elisabetta, B.; Vlad, R.; Philippe, H.M.; Bruno, S.; Jean-Louis, J.; Pierre, B.; et al. A randomised, double-blind, placebo-controlled, multi-centre, dose-range, proof-of-concept, 24-week treatment study of lanifibranor in adult subjects with non-alcoholic steatohepatitis: Design of the NATIVE study. Contemp. Clin. Trials 2020, 98, 106170. [Google Scholar] [CrossRef]
- Prikhodko, V.A.; Bezborodkina, N.N.; Okovityi, S.V. Pharmacotherapy for Non-Alcoholic Fatty Liver Disease: Emerging Targets and Drug Candidates. Biomedicines 2022, 10, 274. [Google Scholar] [CrossRef]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, K.V.; Lai, M.; Schiff, E.; Parmar, D.; et al. Saroglitazar, a PPAR-alpha/gamma Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology 2021, 74, 1809–1824. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Idowu, M.O.; Parmar, D.; Borg, B.B.; Denham, D.; Loo, N.M.; Lazas, D.; Younes, Z.; Sanyal, A.J. A Phase 2 Double Blinded, Randomized Controlled Trial of Saroglitazar in Patients with Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2021, 19, 2670–2672. [Google Scholar] [CrossRef]
- Satiya, J.; Snyder, H.S.; Singh, S.P.; Satapathy, S.K. Narrative review of current and emerging pharmacological therapies for nonalcoholic steatohepatitis. Transl. Gastroenterol. Hepatol. 2021, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Harrison, S.A.; Francque, S.; Bedossa, P.; Lehert, P.; Serfaty, L.; Romero-Gomez, M.; Boursier, J.; Abdelmalek, M.; Caldwell, S.; et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-alpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis without Fibrosis Worsening. Gastroenterology 2016, 150, 1147–1159.e45. [Google Scholar] [CrossRef]
- Rupcic Rubin, V.; Bojanic, K.; Smolic, M.; Rubin, J.; Tabll, A.; Smolic, R. An Update on Efficacy and Safety of Emerging Hepatic Antifibrotic Agents. J. Clin. Transl. Hepatol. 2021, 9, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Phrueksotsai, S.; Pinyopornpanish, K.; Euathrongchit, J.; Leerapun, A.; Phrommintikul, A.; Buranapin, S.; Chattipakorn, N.; Thongsawat, S. The effects of dapagliflozin on hepatic and visceral fat in type 2 diabetes patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2021, 36, 2952–2959. [Google Scholar] [CrossRef] [PubMed]
- Aso, Y.; Kato, K.; Sakurai, S.; Kishi, H.; Shimizu, M.; Jojima, T.; Iijima, T.; Maejima, Y.; Shimomura, K.; Usui, I. Impact of dapagliflozin, an SGLT2 inhibitor, on serum levels of soluble dipeptidyl peptidase-4 in patients with type 2 diabetes and non-alcoholic fatty liver disease. Int. J. Clin. Pract. 2019, 73, e13335. [Google Scholar] [CrossRef] [PubMed]
- Yabiku, K. Efficacy of Sodium-Glucose Cotransporter 2 Inhibitors in Patients with Concurrent Type 2 Diabetes Mellitus and Non-Alcoholic Steatohepatitis: A Review of the Evidence. Front. Endocrinol. 2021, 12, 768850. [Google Scholar] [CrossRef] [PubMed]
- Taheri, H.; Malek, M.; Ismail-Beigi, F.; Zamani, F.; Sohrabi, M.; Reza Babaei, M.; Khamseh, M.E. Effect of Empagliflozin on Liver Steatosis and Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease without Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Adv. Ther. 2020, 37, 4697–4708. [Google Scholar] [CrossRef] [PubMed]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Farooqui, K.J.; Singh, M.K.; Wasir, J.S.; Bansal, B.; Kaur, P.; Jevalikar, G.; Gill, H.K.; et al. Effect of Empagliflozin on Liver Fat in Patients with Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care 2018, 41, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A.; Investigators, N.N. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; Team, L.T.; Abouda, G.; et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Bugianesi, E.; Gentilcore, E.; Manini, R.; Natale, S.; Vanni, E.; Villanova, N.; David, E.; Rizzetto, M.; Marchesini, G. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am. J. Gastroenterol. 2005, 100, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Haukeland, J.W.; Konopski, Z.; Eggesbo, H.B.; von Volkmann, H.L.; Raschpichler, G.; Bjoro, K.; Haaland, T.; Loberg, E.M.; Birkeland, K. Metformin in patients with non-alcoholic fatty liver disease: A randomized, controlled trial. Scand J. Gastroenterol. 2009, 44, 853–860. [Google Scholar] [CrossRef]
- Shields, W.W.; Thompson, K.E.; Grice, G.A.; Harrison, S.A.; Coyle, W.J. The Effect of Metformin and Standard Therapy versus Standard Therapy alone in Nondiabetic Patients with Insulin Resistance and Nonalcoholic Steatohepatitis (NASH): A Pilot Trial. Ther. Adv. Gastroenterol. 2009, 2, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARalpha action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Francque, S.; Verrijken, A.; Caron, S.; Prawitt, J.; Paumelle, R.; Derudas, B.; Lefebvre, P.; Taskinen, M.R.; Van Hul, W.; Mertens, I.; et al. PPARalpha gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J. Hepatol. 2015, 63, 164–173. [Google Scholar] [CrossRef]
- Bojic, L.A.; Huff, M.W. Peroxisome proliferator-activated receptor delta: A multifaceted metabolic player. Curr. Opin. Lipidol. 2013, 24, 171–177. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Arnold, S.V.; Inzucchi, S.E.; Echouffo-Tcheugui, J.B.; Tang, F.; Lam, C.S.P.; Sperling, L.S.; Kosiborod, M. Understanding Contemporary Use of Thiazolidinediones. Circ. Heart Fail. 2019, 12, e005855. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Paschetta, E.; Gambino, R. Thiazolidinediones and Advanced Liver Fibrosis in Nonalcoholic Steatohepatitis: A Meta-analysis. JAMA Intern. Med. 2017, 177, 633–640. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Wiley, S.E.; Rogers, G.W.; Andreyev, A.Y.; Petrosyan, S.; Loviscach, M.; Wall, E.A.; Yadava, N.; Heuck, A.P.; Ferrick, D.A.; et al. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc. Natl. Acad. Sci. USA 2013, 110, 5422–5427. [Google Scholar] [CrossRef] [PubMed]
- Nesto, R.W.; Bell, D.; Bonow, R.O.; Fonseca, V.; Grundy, S.M.; Horton, E.S.; Le Winter, M.; Porte, D.; Semenkovich, C.F.; Smith, S.; et al. Thiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the American Heart Association and American Diabetes Association. Diabetes Care 2004, 27, 256–263. [Google Scholar] [CrossRef] [PubMed]
- McCommis, K.S.; Chen, Z.; Fu, X.; McDonald, W.G.; Colca, J.R.; Kletzien, R.F.; Burgess, S.C.; Finck, B.N. Loss of Mitochondrial Pyruvate Carrier 2 in the Liver Leads to Defects in Gluconeogenesis and Compensation via Pyruvate-Alanine Cycling. Cell Metab. 2015, 22, 682–694. [Google Scholar] [CrossRef] [PubMed]
- Kamm, D.R.; Pyles, K.D.; Sharpe, M.C.; Healy, L.N.; Colca, J.R.; McCommis, K.S. Novel insulin sensitizer MSDC-0602K improves insulinemia and fatty liver disease in mice, alone and in combination with liraglutide. J. Biol. Chem. 2021, 296, 100807. [Google Scholar] [CrossRef]
- Lefere, S.; Puengel, T.; Hundertmark, J.; Penners, C.; Frank, A.K.; Guillot, A.; de Muynck, K.; Heymann, F.; Adarbes, V.; Defrene, E.; et al. Differential effects of selective- and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages. J. Hepatol. 2020, 73, 757–770. [Google Scholar] [CrossRef]
- Inventiva Receives FDA Breakthrough Therapy Designation for Lead Drug Candidate Lanifibranor in NASH. Available online: https://inventivapharma.com/inventiva-receives-fda-breakthrough-therapy-designation-for-lead-drug-candidate-lanifibranor-in-nash/ (accessed on 9 September 2022).
- Highleyman, L. Lanifibranor Both Resolves NASH and Reduces Liver Fibrosis. Available online: https://www.hepmag.com/article/lanifibranor-resolves-nash-reduces-liver-fibrosis (accessed on 8 September 2022).
- Cariou, B.; Staels, B. GFT505 for the treatment of nonalcoholic steatohepatitis and type 2 diabetes. Expert. Opin. Investig. Drugs 2014, 23, 1441–1448. [Google Scholar] [CrossRef]
- Tolbol, K.S.; Kristiansen, M.N.; Hansen, H.H.; Veidal, S.S.; Rigbolt, K.T.; Gillum, M.P.; Jelsing, J.; Vrang, N.; Feigh, M. Metabolic and hepatic effects of liraglutide, obeticholic acid and elafibranor in diet-induced obese mouse models of biopsy-confirmed nonalcoholic steatohepatitis. World J. Gastroenterol. 2018, 24, 179–194. [Google Scholar] [CrossRef]
- Ciardullo, S.; Perseghin, G. Prevalence of NAFLD, MAFLD and associated advanced fibrosis in the contemporary United States population. Liver Int. 2021, 41, 1290–1293. [Google Scholar] [CrossRef]
- GENFIT. GENFIT: Announces Results from Interim Analysis of RESOLVE-IT Phase 3 Trial of Elafibranor in Adults with NASH and Fibrosis; GENFIT: Cambridge, MA, USA, 2020. [Google Scholar]
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Freitas, H.S.; Anhe, G.F.; Melo, K.F.; Okamoto, M.M.; Oliveira-Souza, M.; Bordin, S.; Machado, U.F. Na(+) -glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: Involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinology 2008, 149, 717–724. [Google Scholar] [CrossRef]
- Kuhre, R.E.; Ghiasi, S.M.; Adriaenssens, A.E.; Wewer Albrechtsen, N.J.; Andersen, D.B.; Aivazidis, A.; Chen, L.; Mandrup-Poulsen, T.; Orskov, C.; Gribble, F.M.; et al. No direct effect of SGLT2 activity on glucagon secretion. Diabetologia 2019, 62, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Bonner, C.; Kerr-Conte, J.; Gmyr, V.; Queniat, G.; Moerman, E.; Thevenet, J.; Beaucamps, C.; Delalleau, N.; Popescu, I.; Malaisse, W.J.; et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat. Med. 2015, 21, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Baldi, S.; Frascerra, S.; Astiarraga, B.; Heise, T.; Bizzotto, R.; Mari, A.; Pieber, T.R.; Muscelli, E. Shift to Fatty Substrate Utilization in Response to Sodium-Glucose Cotransporter 2 Inhibition in Subjects without Diabetes and Patients with Type 2 Diabetes. Diabetes 2016, 65, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Lundkvist, P.; Pereira, M.J.; Kamble, P.G.; Katsogiannos, P.; Langkilde, A.M.; Esterline, R.; Johnsson, E.; Eriksson, J.W. Glucagon Levels During Short-Term SGLT2 Inhibition Are Largely Regulated by Glucose Changes in Patients with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 193–201. [Google Scholar] [CrossRef]
- Hodson, D.J.; Rorsman, P. A Variation on the Theme: SGLT2 Inhibition and Glucagon Secretion in Human Islets. Diabetes 2020, 69, 864–866. [Google Scholar] [CrossRef]
- Jeong, S.W. Nonalcoholic Fatty Liver Disease: A Drug Revolution Is Coming. Diabetes Metab. J. 2020, 44, 640–657. [Google Scholar] [CrossRef]
- Ku, E.J.; Lee, D.H.; Jeon, H.J.; Oh, T.K. Empagliflozin versus dapagliflozin in patients with type 2 diabetes inadequately controlled with metformin, glimepiride and dipeptidyl peptide 4 inhibitors: A 52-week prospective observational study. Diabetes Res. Clin. Pract. 2019, 151, 65–73. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, X.; Li, T.; Fang, T.; Cheng, Y.; Han, L.; Sun, B.; Chen, L. The SGLT2 inhibitor empagliflozin negatively regulates IL-17/IL-23 axis-mediated inflammatory responses in T2DM with NAFLD via the AMPK/mTOR/autophagy pathway. Int. Immunopharmacol. 2021, 94, 107492. [Google Scholar] [CrossRef]
- Blundell, J.; Finlayson, G.; Axelsen, M.; Flint, A.; Gibbons, C.; Kvist, T.; Hjerpsted, J.B. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes. Metab. 2017, 19, 1242–1251. [Google Scholar] [CrossRef]
- Sorli, C.; Harashima, S.I.; Tsoukas, G.M.; Unger, J.; Karsbol, J.D.; Hansen, T.; Bain, S.C. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): A double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017, 5, 251–260. [Google Scholar] [CrossRef]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Thethi, T.K.; Pratley, R.; Meier, J.J. Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: The PIONEER programme. Diabetes Obes. Metab. 2020, 22, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Liava, C.; Sinakos, E. Semaglutide for nonalcoholic steatohepatitis: Closer to a solution? Hepatobiliary Surg. Nutr. 2021, 10, 541–544. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Approves First Oral GLP-1 Treatment for Type 2 Diabetes; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019.
- Ohki, T.; Isogawa, A.; Iwamoto, M.; Ohsugi, M.; Yoshida, H.; Toda, N.; Tagawa, K.; Omata, M.; Koike, K. The effectiveness of liraglutide in nonalcoholic fatty liver disease patients with type 2 diabetes mellitus compared to sitagliptin and pioglitazone. Sci. World J. 2012, 2012, 496453. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gu, J.; Shao, W.; Pang, J.; Qian, X.; Jin, T. Comparison of Beneficial Metabolic Effects of Liraglutide and Semaglutide in Male C57BL/6J Mice. Can. J. Diabetes 2022, 46, 216–224.e12. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.J.T.; Campbell, D.B.; Ogundeji, Y.; Au, F.; Beall, R.; Ronksley, P.E.; Quinn, A.E.; Manns, B.J.; Hemmelgarn, B.R.; Tonelli, M.; et al. First-line pharmacotherapy for incident type 2 diabetes: Prescription patterns, adherence and associated costs. Diabet. Med. 2021, 38, e14622. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Wang, B.; Wang, J.; Chen, D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed. Rep. 2013, 1, 57–64. [Google Scholar] [CrossRef]
- Takeshita, Y.; Takamura, T.; Honda, M.; Kita, Y.; Zen, Y.; Kato, K.; Misu, H.; Ota, T.; Nakamura, M.; Yamada, K.; et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: A randomised controlled trial. Diabetologia 2014, 57, 878–890. [Google Scholar] [CrossRef]
- Montgomery, M.K.; Osborne, B.; Brown, S.H.; Small, L.; Mitchell, T.W.; Cooney, G.J.; Turner, N. Contrasting metabolic effects of medium- versus long-chain fatty acids in skeletal muscle. J. Lipid Res. 2013, 54, 3322–3333. [Google Scholar] [CrossRef]
- Spiekerkoetter, U.; Lindner, M.; Santer, R.; Grotzke, M.; Baumgartner, M.R.; Boehles, H.; Das, A.; Haase, C.; Hennermann, J.B.; Karall, D.; et al. Treatment recommendations in long-chain fatty acid oxidation defects: Consensus from a workshop. J. Inherit. Metab. Dis. 2009, 32, 498–505. [Google Scholar] [CrossRef]
- Nelson, A.; Torres, D.M.; Morgan, A.E.; Fincke, C.; Harrison, S.A. A pilot study using simvastatin in the treatment of nonalcoholic steatohepatitis: A randomized placebo-controlled trial. J. Clin. Gastroenterol. 2009, 43, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; de Guevara, L.; Safadi, R.; Poordad, F.; Fuster, F.; Flores-Figueroa, J.; Arrese, M.; Fracanzani, A.L.; Ben Bashat, D.; Lackner, K.; et al. Aramchol in patients with nonalcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase 2b trial. Nat. Med. 2021, 27, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Ajmera, V.H.; Cachay, E.; Ramers, C.; Vodkin, I.; Bassirian, S.; Singh, S.; Mangla, N.; Bettencourt, R.; Aldous, J.L.; Park, D.; et al. MRI Assessment of Treatment Response in HIV-associated NAFLD: A Randomized Trial of a Stearoyl-Coenzyme-A-Desaturase-1 Inhibitor (ARRIVE Trial). Hepatology 2019, 70, 1531–1545. [Google Scholar] [CrossRef] [PubMed]
- Min, H.K.; Kapoor, A.; Fuchs, M.; Mirshahi, F.; Zhou, H.; Maher, J.; Kellum, J.; Warnick, R.; Contos, M.J.; Sanyal, A.J. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 2012, 15, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Hyogo, H.; Yamagishi, S.; Takeuchi, M.; Ishitobi, T.; Nabeshima, Y.; Arihiro, K.; Chayama, K. Atorvastatin decreases serum levels of advanced glycation endproducts (AGEs) in nonalcoholic steatohepatitis (NASH) patients with dyslipidemia: Clinical usefulness of AGEs as a biomarker for the attenuation of NASH. J. Gastroenterol. 2010, 45, 750–757. [Google Scholar] [CrossRef]
- Abel, T.; Feher, J.; Dinya, E.; Gamal Eldin, M.; Kovacs, A. Efficacy and safety of ezetimibe/simvastatin combination therapy in patients with type 2 diabetes and nonalcoholic fatty liver disease. Orv. Hetil. 2009, 150, 989–993. [Google Scholar] [CrossRef]
- Jose, J.; Al-Tamimi, F.A.; Helal, M.M.; Jimmy, B.; Al Riyami, Q. Statin associated hepatic adverse effects: A retrospective review from a regional hospital in sultanate of oman. Oman Med. J. 2014, 29, 351–357. [Google Scholar] [CrossRef]
- Bril, F.; Portillo Sanchez, P.; Lomonaco, R.; Orsak, B.; Hecht, J.; Tio, F.; Cusi, K. Liver Safety of Statins in Prediabetes or T2DM and Nonalcoholic Steatohepatitis: Post Hoc Analysis of a Randomized Trial. J. Clin. Endocrinol. Metab. 2017, 102, 2950–2961. [Google Scholar] [CrossRef]
- Fernandez-Ramos, D.; Lopitz-Otsoa, F.; Delacruz-Villar, L.; Bilbao, J.; Pagano, M.; Mosca, L.; Bizkarguenaga, M.; Serrano-Macia, M.; Azkargorta, M.; Iruarrizaga-Lejarreta, M.; et al. Arachidyl amido cholanoic acid improves liver glucose and lipid homeostasis in nonalcoholic steatohepatitis via AMPK and mTOR regulation. World J. Gastroenterol. 2020, 26, 5101–5117. [Google Scholar] [CrossRef]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wai-Sun Wong, V.; Dufour, J.F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Ali, A.H.; Carey, E.J.; Lindor, K.D. Recent advances in the development of farnesoid X receptor agonists. Ann. Transl. Med. 2015, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Cariello, M.; Piccinin, E.; Moschetta, A. Transcriptional Regulation of Metabolic Pathways via Lipid-Sensing Nuclear Receptors PPARs, FXR, and LXR in NASH. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 1519–1539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jackson, J.P.; St Claire, R.L., 3rd; Freeman, K.; Brouwer, K.R.; Edwards, J.E. Obeticholic acid, a selective farnesoid X receptor agonist, regulates bile acid homeostasis in sandwich-cultured human hepatocytes. Pharm. Res. Perspect. 2017, 5, e00329. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef]
- Intercept. Intercept Announces Positive Data in Fibrosis due to Nash from a New Analysis of Its Phase 3 Regenerate Study of Obeticholic Acid (OCA); Intercept Pharmaceuticals, Inc.: Morristown, NJ, USA, 2022. [Google Scholar]
- Drescher, H.K.; Weiskirchen, R.; Fulop, A.; Hopf, C.; de San Roman, E.G.; Huesgen, P.F.; de Bruin, A.; Bongiovanni, L.; Christ, A.; Tolba, R.; et al. The Influence of Different Fat Sources on Steatohepatitis and Fibrosis Development in the Western Diet Mouse Model of Non-alcoholic Steatohepatitis (NASH). Front. Physiol. 2019, 10, 770. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Ratziu, V.; Loomba, R.; Rinella, M.; Anstee, Q.M.; Goodman, Z.; Bedossa, P.; Geier, A.; Beckebaum, S.; Newsome, P.N.; et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: Interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019, 394, 2184–2196. [Google Scholar] [CrossRef]
- Loomba, R.; Noureddin, M.; Kowdley, K.V.; Kohli, A.; Sheikh, A.; Neff, G.; Bhandari, B.R.; Gunn, N.; Caldwell, S.H.; Goodman, Z.; et al. Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH. Hepatol. 2021, 73, 625–643. [Google Scholar] [CrossRef]
- Ratziu, V.; Rinella, M.E.; Neuschwander-Tetri, B.A.; Lawitz, E.; Denham, D.; Kayali, Z.; Sheikh, A.; Kowdley, K.V.; Desta, T.; Elkhashab, M.; et al. EDP-305 in patients with NASH: A phase II double-blind placebo-controlled dose-ranging study. J. Hepatol. 2022, 76, 506–517. [Google Scholar] [CrossRef]
- Xin, Z.; Himmelbauer, M.K.; Jones, J.H.; Enyedy, I.; Gilfillan, R.; Hesson, T.; King, K.; Marcotte, D.J.; Murugan, P.; Santoro, J.C.; et al. Discovery of CNS-Penetrant Apoptosis Signal-Regulating Kinase 1 (ASK1) Inhibitors. ACS Med. Chem. Lett. 2020, 11, 485–490. [Google Scholar] [CrossRef]
- Alkhouri, N.; Lawitz, E.; Noureddin, M.; DeFronzo, R.; Shulman, G.I. GS-0976 (Firsocostat): An investigational liver-directed acetyl-CoA carboxylase (ACC) inhibitor for the treatment of non-alcoholic steatohepatitis (NASH). Expert. Opin. Investig. Drugs 2020, 29, 135–141. [Google Scholar] [CrossRef]
- Loomba, R.; Lawitz, E.; Mantry, P.S.; Jayakumar, S.; Caldwell, S.H.; Arnold, H.; Diehl, A.M.; Djedjos, C.S.; Han, L.; Myers, R.P.; et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 2018, 67, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Chianelli, D.; Rucker, P.V.; Roland, J.; Tully, D.C.; Nelson, J.; Liu, X.; Bursulaya, B.; Hernandez, E.D.; Wu, J.; Prashad, M.; et al. Nidufexor (LMB763), a Novel FXR Modulator for the Treatment of Nonalcoholic Steatohepatitis. J. Med. Chem. 2020, 63, 3868–3880. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. Pentoxifylline for vascular health: A brief review of the literature. Open Heart 2016, 3, e000365. [Google Scholar] [CrossRef] [PubMed]
- Van Wagner, L.B.; Koppe, S.W.; Brunt, E.M.; Gottstein, J.; Gardikiotes, K.; Green, R.M.; Rinella, M.E. Pentoxifylline for the treatment of non-alcoholic steatohepatitis: A randomized controlled trial. Ann. Hepatol. 2011, 10, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Ma, Y.Y.; Yu, C.H.; Li, Y.M. Effects of pentoxifylline on nonalcoholic fatty liver disease: A meta-analysis. World J. Gastroenterol. 2014, 20, 569–577. [Google Scholar] [CrossRef]
- El-Haggar, S.M.; Mostafa, T.M. Comparative clinical study between the effect of fenofibrate alone and its combination with pentoxifylline on biochemical parameters and liver stiffness in patients with non-alcoholic fatty liver disease. Hepatol. Int. 2015, 9, 471–479. [Google Scholar] [CrossRef]
- Shirakami, Y.; Shimizu, M.; Kubota, M.; Ohno, T.; Kochi, T.; Nakamura, N.; Sumi, T.; Tanaka, T.; Moriwaki, H.; Seishima, M. Pentoxifylline prevents nonalcoholic steatohepatitis-related liver pre-neoplasms by inhibiting hepatic inflammation and lipogenesis. Eur. J. Cancer Prev. 2016, 25, 206–215. [Google Scholar] [CrossRef]
- Harrison, S.A.; Wong, V.W.; Okanoue, T.; Bzowej, N.; Vuppalanchi, R.; Younes, Z.; Kohli, A.; Sarin, S.; Caldwell, S.H.; Alkhouri, N.; et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J. Hepatol. 2020, 73, 26–39. [Google Scholar] [CrossRef]
- Ratziu, V.; Sanyal, A.; Harrison, S.A.; Wong, V.W.; Francque, S.; Goodman, Z.; Aithal, G.P.; Kowdley, K.V.; Seyedkazemi, S.; Fischer, L.; et al. Cenicriviroc Treatment for Adults with Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology 2020, 72, 892–905. [Google Scholar] [CrossRef]
- Wang, P.X.; Ji, Y.X.; Zhang, X.J.; Zhao, L.P.; Yan, Z.Z.; Zhang, P.; Shen, L.J.; Yang, X.; Fang, J.; Tian, S.; et al. Targeting CASP8 and FADD-like apoptosis regulator ameliorates nonalcoholic steatohepatitis in mice and nonhuman primates. Nat. Med. 2017, 23, 439–449. [Google Scholar] [CrossRef]
- Rinella, M.E.; Noureddin, M. STELLAR 3 and STELLAR 4: Lessons from the fall of Icarus. J. Hepatol. 2020, 73, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Abdelmalek, M.F.; Caldwell, S.; Shiffman, M.L.; Diehl, A.M.; Ghalib, R.; Lawitz, E.J.; Rockey, D.C.; Schall, R.A.; Jia, C.; et al. Simtuzumab Is Ineffective for Patients with Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology 2018, 155, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, E.; Moyle, G.; Reshef, R.; Richman, L.P.; Thompson, M.; Hong, F.; Chou, H.L.; Hashiguchi, T.; Plato, C.; Poulin, D.; et al. Antifibrotic Effects of the Dual CCR2/CCR5 Antagonist Cenicriviroc in Animal Models of Liver and Kidney Fibrosis. PLoS ONE 2016, 11, e0158156. [Google Scholar] [CrossRef] [PubMed]
- Krenkel, O.; Puengel, T.; Govaere, O.; Abdallah, A.T.; Mossanen, J.C.; Kohlhepp, M.; Liepelt, A.; Lefebvre, E.; Luedde, T.; Hellerbrand, C.; et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology 2018, 67, 1270–1283. [Google Scholar] [CrossRef]
- Baeck, C.; Wehr, A.; Karlmark, K.R.; Heymann, F.; Vucur, M.; Gassler, N.; Huss, S.; Klussmann, S.; Eulberg, D.; Luedde, T.; et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 2012, 61, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.J.; Hokeness-Antonelli, K.L.; Salazar-Mather, T.P. Regulation of inflammatory monocyte/macrophage recruitment from the bone marrow during murine cytomegalovirus infection: Role for type I interferons in localized induction of CCR2 ligands. J. Immunol. 2009, 183, 2810–2817. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Neuschwander-Tetri, B.A.; Wong, V.W.; Abdelmalek, M.F.; Younossi, Z.M.; Yuan, J.; Pecoraro, M.L.; Seyedkazemi, S.; Fischer, L.; Bedossa, P.; et al. Cenicriviroc for the treatment of liver fibrosis in adults with nonalcoholic steatohepatitis: AURORA Phase 3 study design. Contemp. Clin. Trials 2020, 89, 105922. [Google Scholar] [CrossRef]
- Kelly, M.J.; Pietranico-Cole, S.; Larigan, J.D.; Haynes, N.E.; Reynolds, C.H.; Scott, N.; Vermeulen, J.; Dvorozniak, M.; Conde-Knape, K.; Huang, K.S.; et al. Discovery of 2-[3,5-dichloro-4-(5-isopropyl-6-oxo-1,6-dihydropyridazin-3-yloxy)phenyl]-3,5-dio xo-2,3,4,5-tetrahydro[1,2,4]triazine-6-carbonitrile (MGL-3196), a Highly Selective Thyroid Hormone Receptor beta agonist in clinical trials for the treatment of dyslipidemia. J. Med. Chem. 2014, 57, 3912–3923. [Google Scholar] [CrossRef]
- Kizivat, T.; Maric, I.; Mudri, D.; Curcic, I.B.; Primorac, D.; Smolic, M. Hypothyroidism and Nonalcoholic Fatty Liver Disease: Pathophysiological Associations and Therapeutic Implications. J. Clin. Transl. Hepatol. 2020, 8, 347–353. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bashir, M.; Moussa, S.E.; McCarty, K.; Pablo Frias, J.; Taub, R.; Alkhouri, N. Effects of Resmetirom on Noninvasive Endpoints in a 36-Week Phase 2 Active Treatment Extension Study in Patients with NASH. Hepatol. Commun. 2021, 5, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Rinella, M.E.; Abdelmalek, M.F.; Trotter, J.F.; Paredes, A.H.; Arnold, H.L.; Kugelmas, M.; Bashir, M.R.; Jaros, M.J.; Ling, L.; et al. NGM282 for treatment of non-alcoholic steatohepatitis: A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2018, 391, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Rossi, S.J.; Paredes, A.H.; Trotter, J.F.; Bashir, M.R.; Guy, C.D.; Banerjee, R.; Jaros, M.J.; Owers, S.; Baxter, B.A.; et al. NGM282 Improves Liver Fibrosis and Histology in 12 Weeks in Patients with Nonalcoholic Steatohepatitis. Hepatology 2020, 71, 1198–1212. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.; Charles, E.D.; Neuschwander-Tetri, B.A.; Loomba, R.; Harrison, S.A.; Abdelmalek, M.F.; Lawitz, E.J.; Halegoua-DeMarzio, D.; Kundu, S.; Noviello, S.; et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: A randomised, double-blind, placebo-controlled, phase 2a trial. Lancet 2019, 392, 2705–2717. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Charles, E.D.; Sanyal, A.J.; Harrison, S.A.; Neuschwander-Tetri, B.A.; Goodman, Z.; Ehman, R.A.; Karsdal, M.; Nakajima, A.; Du, S.; et al. The FALCON program: Two phase 2b randomized, double-blind, placebo-controlled studies to assess the efficacy and safety of pegbelfermin in the treatment of patients with nonalcoholic steatohepatitis and bridging fibrosis or compensated cirrhosis. Contemp. Clin. Trials 2021, 104, 106335. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Abdelaziz, A.E.; Hussien, M.; Ali, A.A.; Abdelkawy, K.S.; Elbarbry, F. A randomized controlled trial comparing the effects of Vitamin E, Ursodeoxycholic acid and Pentoxifylline on Egyptian non-alcoholic steatohepatitis patients. Eur. Rev. Med. Pharm. Sci. 2021, 25, 7449–7459. [Google Scholar] [CrossRef]
- Kedarisetty, C.K.; Bhardwaj, A.; Kumar, G.; Rastogi, A.; Bihari, C.; Kumar, M.; Sarin, S.K. Efficacy of combining pentoxiphylline and vitamin E versus vitamin E alone in non-alcoholic steatohepatitis—A randomized pilot study. Indian J. Gastroenterol. 2021, 40, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Schaap, F.G. Role of fibroblast growth factor 19 in the control of glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 386–391. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Chazouilleres, O.; Drenth, J.P.; Thorburn, D.; Harrison, S.A.; Landis, C.S.; Mayo, M.J.; Muir, A.J.; Trotter, J.F.; Leeming, D.J.; et al. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: A multicenter, randomized, double-blind, placebo-controlled phase II trial. J. Hepatol. 2019, 70, 483–493. [Google Scholar] [CrossRef]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, T.; Zhou, Y.; Zheng, J.; Lin, Z. Advances in Biological Functions and Clinical Studies of FGF21. Diabetes Metab. Syndr. Obes. 2021, 14, 3281–3290. [Google Scholar] [CrossRef]
- Rana, J.C.; Singh, M.; Chauhan, R.S.; Chahota, R.K.; Sharma, T.R.; Yadav, R.; Archak, S. Chapter nine—Genetic Resources of Buckwheat in India. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.-H., Chrungoo, N., Wieslander, G., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 109–135. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- Salvoza, N.; Giraudi, P.J.; Tiribelli, C.; Rosso, N. Natural Compounds for Counteracting Nonalcoholic Fatty Liver Disease (NAFLD): Advantages and Limitations of the Suggested Candidates. Int. J. Mol. Sci. 2022, 23, 2764. [Google Scholar] [CrossRef]
- Cicero, A.F.; Colletti, A.; Bellentani, S. Nutraceutical approach to non-alcoholic fatty liver disease (NAFLD): The available clinical evidence. Nutrients 2018, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, S.; Ji, X.; Shen, X.; You, J.; Liang, X.; Yin, H.; Zhao, L. Current innovations in nutraceuticals and functional foods for intervention of non-alcoholic fatty liver disease. Pharmacol. Res. 2021, 166, 105517. [Google Scholar] [CrossRef] [PubMed]
- Presa, N.; Clugston, R.D.; Lingrell, S.; Kelly, S.E.; Merrill, A.H., Jr.; Jana, S.; Kassiri, Z.; Gomez-Munoz, A.; Vance, D.E.; Jacobs, R.L.; et al. Vitamin E alleviates non-alcoholic fatty liver disease in phosphatidylethanolamine N-methyltransferase deficient mice. Biochim Biophys Acta Mol. Basis Dis. 2019, 1865, 14–25. [Google Scholar] [CrossRef]
- Hariri, M.; Zohdi, S. Effect of Vitamin D on Non-Alcoholic Fatty Liver Disease: A Systematic Review of Randomized Controlled Clinical Trials. Int. J. Prev. Med. 2019, 10, 14. [Google Scholar] [CrossRef]
- Barchetta, I.; Cimini, F.A.; Cavallo, M.G. Vitamin D Supplementation and Non-Alcoholic Fatty Liver Disease: Present and Future. Nutrients 2017, 9, 1015. [Google Scholar] [CrossRef]
- Kalopitas, G.; Antza, C.; Doundoulakis, I.; Siargkas, A.; Kouroumalis, E.; Germanidis, G.; Samara, M.; Chourdakis, M. Impact of Silymarin in individuals with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutrition 2021, 83, 111092. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Shastri, Y.; Al Otaibi, M.; Buchel, E.; Saleh, H.; Ahmad, R.; Ahmed, H.; Al Idris, F.; Ahmed, S.; Guda, M. Expert opinion on the management of Non-alcoholic fatty liver disease (NAFLD) in the Middle east with a focus on the use of silymarin. Gastroenterol. Insights 2021, 12, 155–165. [Google Scholar] [CrossRef]
- Savic, D.; Hodson, L.; Neubauer, S.; Pavlides, M. The importance of the fatty acid transporter L-carnitine in non-alcoholic fatty liver disease (NAFLD). Nutrients 2020, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, C.; Zhang, Y.; Hu, S.; Li, D. Dietary supplementation of L-carnitine ameliorates metabolic syndrome independent of trimethylamine N-oxide produced by gut microbes in high fat diet induced obese mice. Food Funct. 2022, 13, 12039–12050. [Google Scholar] [PubMed]
- Oh, H.; Park, C.H.; Jun, D.W. Impact of l-Carnitine Supplementation on Liver Enzyme Normalization in Patients with Chronic Liver Disease: A Meta-Analysis of Randomized Trials. J. Pers. Med. 2022, 12, 1053. [Google Scholar] [CrossRef]
- Li, N.; Zhao, H. Role of carnitine in non-alcoholic fatty liver disease and other related diseases: An update. Front. Med. 2021, 8, 689042. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.S.; Lordan, R.; Horbańczuk, O.K.; Atanasov, A.G.; Chopra, I.; Horbańczuk, J.O.; Jóźwik, A.; Huang, L.; Pirgozliev, V.; Banach, M.; et al. The current use and evolving landscape of nutraceuticals. Pharmacol. Res. 2022, 175, 106001. [Google Scholar] [CrossRef]
- Colletti, A.; Fratter, A.; Pellizzato, M.; Cravotto, G. Nutraceutical Approaches to Dyslipidaemia: The Main Formulative Issues Preventing Efficacy. Nutrients 2022, 14, 4769. [Google Scholar] [CrossRef] [PubMed]
- Manocha, S.; Dhiman, S.; Grewal, A.S.; Guarve, K. Nanotechnology: An approach to overcome bioavailability challenges of nutraceuticals. J. Drug Deliv. Sci. Technol. 2022, 72, 103418. [Google Scholar] [CrossRef]
- Poulos, J.E.; Kalogerinis, P.T.; Milanov, V.; Kalogerinis, C.T.; Poulos, E.J. The Effects of Vitamin E, Silymarin and Carnitine on the Metabolic Abnormalities Associated with Nonalcoholic Liver Disease. J. Diet. Suppl. 2022, 19, 287–302. [Google Scholar] [CrossRef]
- Ferro, Y.; Pujia, R.; Mazza, E.; Lascala, L.; Lodari, O.; Maurotti, S.; Pujia, A.; Montalcini, T. A new nutraceutical (Livogen Plus®) improves liver steatosis in adults with non-alcoholic fatty liver disease. J. Transl. Med. 2022, 20, 377. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.H.S.; Wong, S.H. Microbiota, Obesity and NAFLD. Adv. Exp. Med. Biol. 2018, 1061, 111–125. [Google Scholar] [CrossRef]
- Bajzer, M.; Seeley, R.J. Physiology: Obesity and gut flora. Nature 2006, 444, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- Le Roy, T.; Llopis, M.; Lepage, P.; Bruneau, A.; Rabot, S.; Bevilacqua, C.; Martin, P.; Philippe, C.; Walker, F.; Bado, A.; et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2013, 62, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clement, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.; Christodoulatos, G.S.; Karampela, I.; Tsilingiris, D.; Magkos, F.; Stratigou, T.; Kounatidis, D.; Dalamaga, M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules 2021, 12, 56. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Hoffmann, D.E.; Fraser, C.M.; Palumbo, F.; Ravel, J.; Rowthorn, V.; Schwartz, J. Probiotics: Achieving a better regulatory fit. Food Drug Law J. 2014, 69, 237. [Google Scholar]
- Guo, W.; Wang, P.; Liu, Z.H.; Ye, P. Analysis of differential expression of tight junction proteins in cultured oral epithelial cells altered by Porphyromonas gingivalis, Porphyromonas gingivalis lipopolysaccharide, and extracellular adenosine triphosphate. Int. J. Oral Sci. 2018, 10, e8. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, P.; Liu, Y.; Zhang, Y. Efficacy of Probiotics and Synbiotics in Patients with Nonalcoholic Fatty Liver Disease: A Meta-Analysis. Dig. Dis. Sci. 2019, 64, 3402–3412. [Google Scholar] [CrossRef]
- Carpi, R.Z.; Barbalho, S.M.; Sloan, K.P.; Laurindo, L.F.; Gonzaga, H.F.; Grippa, P.C.; Zutin, T.L.M.; Girio, R.J.S.; Repetti, C.S.F.; Detregiachi, C.R.P.; et al. The Effects of Probiotics, Prebiotics and Synbiotics in Non-Alcoholic Fat Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8805. [Google Scholar] [CrossRef]
- Kim, K.O.; Gluck, M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin. Endosc. 2019, 52, 137–143. [Google Scholar] [CrossRef]
- Sadowsky, M.J.; Khoruts, A. Faecal microbiota transplantation is promising but not a panacea. Nat. Microbiol. 2016, 1, 16015. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.J.; Smits, L.P.; Pekmez, C.T.; Prodan, A.; Meijnikman, A.S.; Troelstra, M.A.; Bouter, K.E.C.; Herrema, H.; Levin, E.; Holleboom, A.G.; et al. Donor Fecal Microbiota Transplantation Alters Gut Microbiota and Metabolites in Obese Individuals with Steatohepatitis. Hepatol. Commun. 2020, 4, 1578–1590. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Deng, Z.; Luo, W.; He, X.; Chen, Y. Effect of Fecal Microbiota Transplantation on Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Front. Cell Infect. Microbiol. 2022, 12, 759306. [Google Scholar] [CrossRef] [PubMed]
- Gangarapu, V.; Ince, A.T.; Baysal, B.; Kayar, Y.; Kilic, U.; Gok, O.; Uysal, O.; Senturk, H. Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Friedman, S.L.; McCullough, A.J.; Dimick-Santos, L.; American Association for the Study of Liver, D.; United States, F.; Drug, A. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: Findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology 2015, 61, 1392–1405. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Yu, M.; Ma, J.; Zhu, Y. Metabolic classification of bladder cancer based on multi-omics integrated analysis to predict patient prognosis and treatment response. J. Transl. Med. 2021, 19, 205. [Google Scholar] [CrossRef]
- Marcucci, G.; Kumar, A.; Castro, M.; Grover, H.; Patil, V.; Alam, A.; Azam, H.; Mohapatra, S.; Tyagi, A.; Kumari, P.; et al. Biosimulation Using the Cellworks Computational Omics Biology Model (CBM) Identifies Novel Biomarkers to Inform Mitoxantrone, Etoposide, and Cytarabine (MEC)-Based Combination Therapy in Refractory & Relapsed Acute Myeloid Leukemia (AML) Patients. Blood 2021, 138, 4453. [Google Scholar] [CrossRef]
- Doumas, M.; Imprialos, K.; Dimakopoulou, A.; Stavropoulos, K.; Binas, A.; Athyros, V.G. The Role of Statins in the Management of Nonalcoholic Fatty Liver Disease. Curr. Pharm. Des. 2018, 24, 4587–4592. [Google Scholar] [CrossRef]
- Risk Evaluation and Mitigation Strategies|REMS. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems (accessed on 10 September 2022).
- Eggleton, J.S.; Jialal, I. Thiazolidinediones; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- McLaughlin, T.; Lamendola, C.; Liu, A.; Abbasi, F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J. Clin. Endocrinol. Metab. 2011, 96, E1756–E1760. [Google Scholar] [CrossRef]
- Lecka-Czernik, B. Bone loss in diabetes: Use of antidiabetic thiazolidinediones and secondary osteoporosis. Curr. Osteoporos Rep. 2010, 8, 178–184. [Google Scholar] [CrossRef]
- Vuppalanchi, R.; Jain, A.K.; Deppe, R.; Yates, K.; Comerford, M.; Masuoka, H.C.; Neuschwander-Tetri, B.A.; Loomba, R.; Brunt, E.M.; Kleiner, D.E.; et al. Relationship between changes in serum levels of keratin 18 and changes in liver histology in children and adults with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2014, 12, 2121–2130.e2. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, S.; Scamporrino, A.; Filippello, A.; Di Pino, A.; Scicali, R.; Malaguarnera, R.; Purrello, F.; Piro, S. Clinical and Molecular Biomarkers for Diagnosis and Staging of NAFLD. Int. J. Mol. Sci. 2021, 22, 11905. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Akita, M.; Takeda, Y.; Yamada, S.; Fujii, H.; Sawai, Y.; Doi, Y.; Asazawa, H.; Nakayama, K.; Mizutani, K.; et al. Serum Fucosylated Haptoglobin as a Novel Diagnostic Biomarker for Predicting Hepatocyte Ballooning and Nonalcoholic Steatohepatitis. PLoS ONE 2013, 8, e66328. [Google Scholar] [CrossRef]
- Adams, L.A.; Lymp, J.F.; St Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Lonardo, A.; Nascimbeni, F.; Ballestri, S.; Fairweather, D.; Win, S.; Than, T.A.; Abdelmalek, M.F.; Suzuki, A. Sex Differences in Nonalcoholic Fatty Liver Disease: State of the Art and Identification of Research Gaps. Hepatology 2019, 70, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Esmaili, S.; Rogers, G.B.; Bugianesi, E.; Petta, S.; Marchesini, G.; Bayoumi, A.; Metwally, M.; Azardaryany, M.K.; Coulter, S.; et al. Lean NAFLD: A Distinct Entity Shaped by Differential Metabolic Adaptation. Hepatology 2020, 71, 1213–1227. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef]
- Pan, Z.; Fan, J.-G.; Eslam, M. An update on drug development for the treatment of metabolic (dysfunction) associated fatty liver disease: Progress and opportunities. Curr. Opin. Pharmacol. 2021, 60, 170–176. [Google Scholar] [CrossRef]
- Ampuero, J.; Romero-Gomez, M. Stratification of patients in NASH clinical trials: A pitfall for trial success. JHEP Rep. 2020, 2, 100148. [Google Scholar] [CrossRef]
- De, A.; Ahmad, N.; Mehta, M.; Singh, P.; Duseja, A. NAFLD vs. MAFLD–It is not the name but the disease that decides the outcome in fatty liver. J. Hepatol. 2022, 76, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Siden, E.; Zoratti, M.J.; Dron, L.; Harari, O.; Singer, J.; Lester, R.T.; Thorlund, K.; Mills, E.J. Systematic review of basket trials, umbrella trials, and platform trials: A landscape analysis of master protocols. Trials 2019, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pericàs, J.M.; Tacke, F.; Anstee, Q.M.; Di Prospero, N.A.; Kjær, M.S.; Mesenbrink, P.; Koenig, F.; Genescà, J.; Ratziu, V.; Mesenbrinck, P. Platform trials to overcome major shortcomings of traditional clinical trials in non-alcoholic steatohepatitis? Pros and Cons. J. Hepatol. 2022, 72, 103418. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Vallejo, L.; Guatibonza-García, V.; Mantzoros, C.S. Recent Guidelines for Non-Alcoholic Fatty Liver Disease (NAFLD): Are They Already Outdated and in Need of Supplementation? Elsevier: Amsterdam, The Netherlands, 2022; p. 155248. [Google Scholar]
- Brouwers, M.C.; Simons, N.; Stehouwer, C.D.; Isaacs, A. Non-alcoholic fatty liver disease and cardiovascular disease: Assessing the evidence for causality. Diabetologia 2020, 63, 253–260. [Google Scholar] [CrossRef]
- Mu, W.; Cheng, X.-F.; Liu, Y.; Lv, Q.-Z.; Liu, G.-L.; Zhang, J.-G.; Li, X.-Y. Potential nexus of non-alcoholic fatty liver disease and type 2 diabetes mellitus: Insulin resistance between hepatic and peripheral tissues. Front. Pharmacol. 2019, 9, 1566. [Google Scholar] [CrossRef]
- Caussy, C.; Aubin, A.; Loomba, R. The relationship between type 2 diabetes, NAFLD, and cardiovascular risk. Curr. Diabetes Rep. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Oh, J.H.; Jun, D.W.; Kim, H.Y.; Lee, S.M.; Yoon, E.L.; Hwang, J.; Park, J.H.; Lee, H.; Kim, W.; Kim, H. Discovery of dipeptidyl peptidase-4 inhibitor specific biomarker in non-alcoholic fatty liver disease mouse models using modified basket trial. Clin. Mol. Hepatol. 2022, 28, 497. [Google Scholar] [CrossRef]


| NAFLD Activity Score | |||
|---|---|---|---|
| Score | Steatosis | Lobular Inflammation | Ballooning Degeneration |
| 0 | <5% | None | None |
| 1 | 5–33% | <2 foci/20× field | Few |
| 2 | >33–66% | 2–4 foci/20× field | Many |
| 3 | >60% | >4 foci/20× field | |
| Fibrosis Score | |||
| Stage | Histological Findings | ||
| 1a | mild pericellular fibrosis (only seen on connective tissue stain) | ||
| 1b | moderate pericellular fibrosis (readily seen on H&E) | ||
| 1c | portal/periportal fibrosis without pericellular fibrosis | ||
| 2 | pericelluar and portal/periportal fibrosis | ||
| 3 | bridging fibrosis | ||
| 4 | cirrhosis | ||
| SAF (Steatosis, Activity, Fibrosis) Score | |||
|---|---|---|---|
| Score | Steatosis | ||
| 0 | <5% | ||
| 1 | 5–33% | ||
| 2 | >33–66% | ||
| 3 | >60% | ||
| Activity | Score | Lobular Inflammation (LI) | Ballooning Degeneration (BD) |
| A0–4 | 0 | none | none |
| (LI + BD) | 1 | <2 foci/20× field | hepatocytes with rounded shape and pale cytoplasm usually reticulated. size is quite similar to normal hepatocytes. |
| 2 | >2 foci/20× field | hepatocytes with rounded shape and pale cytoplasm usually reticulated. some cells are twice the size of normal hepatocytes. | |
| Fibrosis | Histological Findings | ||
| 1a | mild pericellular fibrosis (only seen on connective tissue stain) | ||
| 1b | moderate pericellular fibrosis (readily seen on H&E) | ||
| 1c | portal/periportal fibrosis without pericellular fibrosis | ||
| 2 | pericelluar and portal/periportal fibrosis | ||
| 3 | bridging fibrosis | ||
| 4 | cirrhosis | ||
| Intervention | NAFL | NASH |
|---|---|---|
| Lifestyle Modification | Yes | Yes |
| Pharmacotherapy | No | Yes, although pediatric differs among guidelines |
| Bariatric surgery | Yes, if obese | Yes, if obese |
| Liver Transplantation | No | Yes, only with end-stage liver disease such as liver failure |
| Guidelines | Pharmacotherapy | Considerations | Therapy Duration | Care Settings |
|---|---|---|---|---|
| National Institute for Health Care and Excellence | Pioglitazone or Vitamin E | Adults, advanced fibrosis, with or without diabetes | 24 months | Secondary to Tertiary |
| National Institute for Health Care and Excellence | Vitamin E | Pediatric, advanced fibrosis, with or without diabetes | 24 months | Tertiary |
| European Association for the Study of the Liver | Pioglitazone or Vitamin E or combination | Advanced fibrosis (F2 or higher), NASH | 6 months | Unspecified |
| American Association for the Study of Liver Diseases | Pioglitazone | Biopsy-proven NASH, with or without diabetes | Unspecified | Unspecified |
| Oral Hypoglycemic Agents | |||||||
|---|---|---|---|---|---|---|---|
| Drug | Mechanism of Action | Trial (Type and Identifier) | Patient (Type and Number) | Dosage | Duration | Results (Histology, Imaging, Biomarker) ✓ X | Side Effects |
| Pioglitazones | PPARγ agonist | Phase 4 NCT00994682 (UTHSCSA) [42] | Adult NASH 176 | 45 mg/day with 500 kcal deficit OR Placebo with 500 kcal deficit | 18 months, 36 months | Histology: ✓ Imaging: X Biomarker: ✓ (adipose insulin sensitivity) | Long-term osteoporosis, weight gain, fluid retention |
| Phase 4 NCT01002547 [43] | Adult NASH with T2DM 105 | 45 mg/day with 800 mg/day Vitamin E and 500 kcal deficit OR 800IU/day Vitamin E with 500 kcal deficit OR Placebo with 500 kcal deficit | 18 months | Histology: ✓ Imaging: X Biomarker: X | Mild hypoglycemia, dyspnea | ||
| Phase 3 NCT00063622 (PIVENS) [44] | Adult NASH 247 | 30 mg/day OR 800IU/day Vitamin E OR Placebo | 24 months | Histology: ✓ Imaging: N Biomarker: N | |||
| Phase 4 NCT02365233 [45] | Pioglitazone or Lantus Insulin, DPP4 inhibitor Saxagliptin | Pending | |||||
| Phase 4 NCT03910361 [46] | Pioglitazone or Evogliptin | Pending | |||||
| Phase 4 NCT03796975 [46] | Pioglitazone + Metformin or Metformin | Pending | |||||
| Phase 4 NCT03646292 [46] | Pioglitazone or Empagliflozin or both | Pending | |||||
| Phase 3 NCT02265276 [46] | Pioglitazone or Saroglitazar | Pending | |||||
| MSDC-0602K | MPC inhibition | Phase 2 NCT02784444 (EMMINENCE) [47] | Adult NASH with F1-F3 392 | 250 mg/day OR 125 mg/day OR 62.5 mg/day OR Placebo | 12 months | Histology: X Imaging: N Biomarker: ✓ | Mild gastrointestinal disorders, peripheral edema, weight gain |
| Phase 3 NCT03970031 [48] | Adult NAFLD with pre-T2DM or T2DM and macrovascular cardiovascular disease 1800 | 62.5 mg/day OR Placebo | 26 weeks, 15 months | Pending (September 2024) | |||
| Lanifibranor | Pan-PPAR agonist | Phase 2 NCT03008070 (NATIVE) [49] | Adult NASH 247 | 1200 mg/day OR 800 mg/day OR Placebo | 24 weeks | Histology: ✓ Imaging: ✓ Biomarker: ✓ (HbA1c, fasting plasma glucose, HOMA-IR and adiponectin) | Mild gastrointestinal disorders, peripheral edema, weight gain |
| Phase 3 NCT04849728 [50] | Adult noncirrhotic NASH with F2–F3 2000 | 1200 mg/day OR 800 mg/day OR Placebo | 72 weeks, 7 years | Pending (September 2028) | |||
| Saroglitazar | Dual PPARα/γ agonist | Phase 2 NCT03061721 [51] | US or biopsy-proven NAFLD 106 | 4 mg/day OR 2 mg/day OR 1 mg/day OR Placebo | 16 weeks | Histology: X Imaging: ✓ (MRI-PDFF, only 4 mg/day) Biomarker: ✓ (ALT, AST, ELF, APRI, triglycerides, VLDL, HOMA-IR) | Well-tolerated |
| Phase 2 [52] | Biopsy-proven NASH 16 | 4 mg/day OR 2 mg/day OR Placebo | 24 weeks | Histology: X Imaging: N Biomarker: ✓ (LDL-C) | |||
| Phase 3 NCT04193982 [53] | Pending | ||||||
| Phase 2 NCT05011305 [46] | Pending | ||||||
| Elafibranor | Dual-PPAR α/β agonist | Phase 2 NCT01694849 [54] | Adult NASH 270 | 120 mg/day OR 80 mg/day OR Placebo | 56 weeks | Histology: ✓ Imaging: N Biomarker: ✓ (ALT, AST, HbA1c, triglycerides, LDL cholesterol, HOMA-IR) | Well tolerated |
| Phase 3 NCT02704403 (RESOLVE-IT) [55] TERMINATED | Adult NASH with F1–3 2157 | 120 mg/day OR Placebo | 72 weeks, 54 months | Histology: X (at 72 weeks, resulting in termination) Imaging: N Biomarker: X | |||
| Dapagliflozin | SGLT2 inhibitor | Phase 2 [56] | NAFLD with T2DM 38 | 10 mg/day OR Placebo | 12 weeks | Histology: N Imaging: ✓ (non-contrast CT) Biomarker: ✓ (ALT, HbA1c) | Well tolerated |
| Phase 2 [57] | NAFLD with T2DM 57 | 5 mg/day OR Placebo | 24 weeks | Histology: N Imaging: N Biomarker: ✓ (AST, ALT, HOMA-IR) | |||
| Phase 4 NCT02637973 | Adult NAFLD with T2DM | 25 mg/day OR Placebo | Pending | ||||
| Phase 4 NCT02964715 | Adult NASH with T2DM | 25 mg/day OR Placebo | Pending | ||||
| Phase 4 NCT03646292 | Adult NAFLD with T2DM | 10 mg/day OR 15 mg/day pioglitazone OR Both | Pending | ||||
| Phase 4 NCT04642261 | Adult NAFLD | 10 mg/day OR Placebo | Pending | ||||
| Phase 3 NCT03723252 (DEAN) [58] | Adult NASH with T2DM | 10 mg/day OR Placebo | Pending | ||||
| Empagliflozin | SGLT2 inhibitor | Phase 3 IRCT20190122042450N1 [59] | Adult NAFLD with T2DM 91 | 10 mg/day OR Placebo | 24 weeks | Histology: N Imaging: ✓ (FibroScan) Biomarker: ✓ (AST, ALT, fasting insulin) | Mild fungal genitourinary infections, increased truncal fat mass |
| Phase 2 NCT02686476 (E-LIFT) [60] | Adult NAFLD with T2DM 50 | 10 mg/day OR Placebo | 20 weeks | Histology: N Imaging: ✓ (MRI-PDFF) Biomarker: ✓ (ALT) | |||
| Semaglutide | GLP-1 analog | Phase 2 NCT02970942 [61] | Adult NASH | 0.4 mg/day OR 0.2 mg/day OR 0.1 mg/day OR Placebo | 72 weeks | Histology: ✓ Imaging N Biomarker: X | Mild gastrointestinal disorders |
| Phase 3 NCT04822181 (ESSENCE) | Adult NASH with F2–F3 1200 | Per week (dosage unknown) OR Placebo | 72, 240 weeks | Pending (May 2028) | |||
| Liraglutide | GLP-1 analog | Phase 2 NCT01237119 (LEAN) [62] | Adult biopsy-proven NASH | 1.8 mg/day OR Placebo | 48 weeks | Histology: ✓ Imaging: N Biomarker: ✓ (GGT) | Well-tolerated |
| Metformin | AMPK inhibitor | Phase 2 [63] | Adult biopsy-proven NAFLD without diabetes 55 | 2 g/day OR 800 IU/day Vitamin E OR Placebo with diet | 12 months | Histology: ✓ Imaging: N Biomarker: ✓ (ALT, AST, HOMA-IR) | Well-tolerated |
| Phase 2 [64] | Adult biopsy-proven NAFLD 48 | 5 g/day OR Placebo | 6 months | Histology: X Imaging: N Biomarker: ✓ (glucose, HbA1c, cholesterol, LDL-C, body weight) | |||
| Phase 2 [65] | Adult biopsy-proven NAFLD without diabetes 19 | 1 g/day OR Placebo with weight loss program | 12 months | Histology: X Imaging: N Biomarker: X | |||
| Lipid-Lowering Drugs | |||||||
|---|---|---|---|---|---|---|---|
| Drug | Mechanism of Action | Trial (Type and Identifier) | Patient (Type and Number) | Dosage | Duration | Results (Histology, Imaging, Biomarker) ✓ X | Side Effects |
| Ezetimibe | NPC1L1 inhibitor | Phase 2 (UMIN000005250) [103] | Adult biopsy-proven NASH 32 | 10 mg/day OR Placebo | 6 months | Histology: ✓ Imaging: N Biomarker: X | Worsened insulin resistance with increased risk of T2DM |
| Statins | HMG-CoA inhibitor | Phase 2 [106] | Adult biopsy-proven NASH 16 | Unspecified dosage OR Placebo | 12 months | Histology: X Imaging: N Biomarker: X | Well tolerated |
| Aramchol | SCD-1 inhibitor | Phase 2b (NCT02279524) [107] | Adult biopsy-proven NASH with prediabetes or T2DM 247 | 600 mg/day OR 400 mg/day OR Placebo | 52 weeks | Histology: X Imaging: ✓ Biomarker: ✓ (HbA1c) | Well tolerated |
| Phase 3 NCT04104321 (ARMOUR) [108] | Adult NASH with F1-F3 150 | 300 mg/day OR Placebo | 24, 48, 72, 96, 120 weeks | Pending (June 2027) | |||
| Oltipraz | LXRα inhibitor | Phase 2 (NCT01373554) [23]. | Adult US-proven NAFLD Asian 68 | 120 mg/day OR 60 mg/day OR Placebo | 24 weeks | Histology: N Imaging: ✓ Biomarker: ✓ (BMI, HDL-C) | Well tolerated |
| Bile Pathway Drugs | |||||||
|---|---|---|---|---|---|---|---|
| Drug | Mechanism of Action | Trial (Type and Identifier) | Patient (Type and Number) | Dosage | Duration | Results (Histology, Imaging, Biomarker) ✓ X | Side Effects |
| Obeticholic acid | FXR agonist | Phase 3 NCT02548351 (REGENERATE) [122] | Adult non-cirrhotic biopsy-proven NASH, with F2–F3 or F1 with at least 1 comorbidity 2480 (931 F2–F3 patients in interim analysis) | 25 mg/day OR 10 mg/day OR Placebo | 18 months (interim analysis), 7 years (end of study) | Histology: ✓ (based on interim analysis, NASH resolution outcome not met yet) Imaging: N Biomarker: X | Significant pruritus (lead to rejection of accelerated approval pathway) |
| Phase 2 (FLINT) [119] | Adult non-cirrhotic biopsy-proven NAFLD 283 | 25 mg/day OR Placebo | 18 months | Histology: ✓ Imaging: N Biomarker: X | |||
| Phase 3 NCT03439254 [55] | Adult NASH 919 | 10 to 25 mg/day OR 10 mg/day OR Placebo | 18 months | Pending | |||
| Cilofexor | Selective non-bile acid intestinal FXR agonist | Phase 2b NCT03449446 (ALTAS) [123] | Adult biopsy-proven NASH, F3-F4 392 | 30 mg/day OR 18 mg/day selonsertib OR 20 mg/day firsocostat OR 30 mg/day with 18 mg/day selonsertib OR 30 mg/day with 20 mg/day firsocostat OR Placebo | 48 weeks | Histology: X Imaging: X (measured by FibroScan) Biomarker: X (ALT, AST, bilirubin, cytokeratin-18, insulin) (All results were significant for cilofexor-firsocostat) | Cilofexor has the highest percentage of adverse events |
| Phase 2 (NCT02854605) [62] | Adult non-cirrhotic biopsy-proven NASH 140 | 100 mg/day OR 30 mg/day OR Placebo | 24 weeks | Histology: ✓ Imaging: ✓ (via MRI-PDFF) Biomarker: ✓ (GGT, bile acids, C4) | Pruritus (especially in 100 mg/day group) | ||
| EDP-305 | FXR agonist | Phase 2 [124] | Adult fibrotic NASH 134 | 2.5 mg/day OR 1.5 mg/day OR Placebo | 12 weeks | Histology: N Imaging: ✓ (via MRI-PDFF) Biomarker: ✓ (ALT) | Pruritus |
| Other Drugs | |||||||
|---|---|---|---|---|---|---|---|
| Drug | Mechanism of Action | Trial (Type and Identifier) | Patient (Type and Number) | Dosage | Duration | Results (Histology, Imaging, Biomarker) ✓ X | Side Effects |
| Resemtirom | THR-β agonist | Phase 2 NCT02912260 [146] | Adult biopsy-proven non-cirrhotic NASH 348 | 80 mg/day OR Placebo | 36 weeks | Histology: ✓ Imaging: ✓ (via MRI-PDFF) Biomarker: ✓ (ALT, AST, GGT, ELF, CK-18, adiponectin, LDL, triglycerides) | Well tolerated |
| Phase 3 NCT03900429 (MAESTRO-NASH) [147] | Adult biopsy-proven NASH with F2–3 2000 | 100 mg/day OR 60 mg/day Or Placebo | 52 weeks | Pending | |||
| NGM282 | FGF19 analog | Phase 2 NCT02443116 [148] | Adult biopsy-proven NASH | 6 mg/day OR 3 mg/day OR Placebo | Histology: ✓ Imaging: ✓ (via MRI-PDFF) Biomarker: ✓ (ALT, AST, triglycerides, pro-C3, ELF) | Well tolerated | |
| Open label [149] | Adult biopsy-proven NASH | 3 mg/day OR 1 mg/day OR Placebo | 12 weeks | Histology: ✓ Imaging: X Biomarker: X | |||
| Pegbelfermin | FGF21 analog | Phase 2a NCT02413372 [150] | Adult biopsy-confirmed NASH 75 | 20 mg/week OR 10 mg/week OR Placebo | 16 weeks | Histology: N Imaging: ✓ (via MRI-PDFF) Biomarker: X | Well tolerated |
| Phase 2 (FALCON) [151] | Pending | ||||||
| Phase 2 (FALCON 2) [151] | Pending | ||||||
| Vitamin E | Antioxidant | Phase 3 Random double blind NCT00063622 (PIVENS) [44] | Adult NASH 247 | 800 mg/day OR 30 mg/day pioglitazone OR Placebo | 24 months | Histology: ✓ Imaging: N Biomarker: ✓ (ALT, AST) | Well tolerated |
| Phase 2 [152] | Adult NASH 102 | 800 mg/day OR 500 mg/day UDCA OR 800 mg/day PTX | 3 months | Histology: N Imaging: N Biomarker: ✓ (IL6, CCL2/MCP-1 AST, ALT) | |||
| Phase 2 [153] | Adult NASH 69 | 800 mg/day with 1200 mg/day PTX OR 800 mg/day | 12 months | Histology: ✓ Imaging: X (via FibroScan) Biomarker: X | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.Y.S.; Chua, D.; Lim, C.O.; Ho, W.X.; Tan, N.S. Lessons on Drug Development: A Literature Review of Challenges Faced in Nonalcoholic Fatty Liver Disease (NAFLD) Clinical Trials. Int. J. Mol. Sci. 2023, 24, 158. https://doi.org/10.3390/ijms24010158
Chen JYS, Chua D, Lim CO, Ho WX, Tan NS. Lessons on Drug Development: A Literature Review of Challenges Faced in Nonalcoholic Fatty Liver Disease (NAFLD) Clinical Trials. International Journal of Molecular Sciences. 2023; 24(1):158. https://doi.org/10.3390/ijms24010158
Chicago/Turabian StyleChen, Joel Yeh Siang, Damien Chua, Carissa Odelia Lim, Wan Xi Ho, and Nguan Soon Tan. 2023. "Lessons on Drug Development: A Literature Review of Challenges Faced in Nonalcoholic Fatty Liver Disease (NAFLD) Clinical Trials" International Journal of Molecular Sciences 24, no. 1: 158. https://doi.org/10.3390/ijms24010158
APA StyleChen, J. Y. S., Chua, D., Lim, C. O., Ho, W. X., & Tan, N. S. (2023). Lessons on Drug Development: A Literature Review of Challenges Faced in Nonalcoholic Fatty Liver Disease (NAFLD) Clinical Trials. International Journal of Molecular Sciences, 24(1), 158. https://doi.org/10.3390/ijms24010158






