Zileuton, a 5-Lipoxygenase Inhibitor, Attenuates Haemolysate-Induced BV-2 Cell Activation by Suppressing the MyD88/NF-κB Pathway
Abstract
:1. Introduction
2. Results
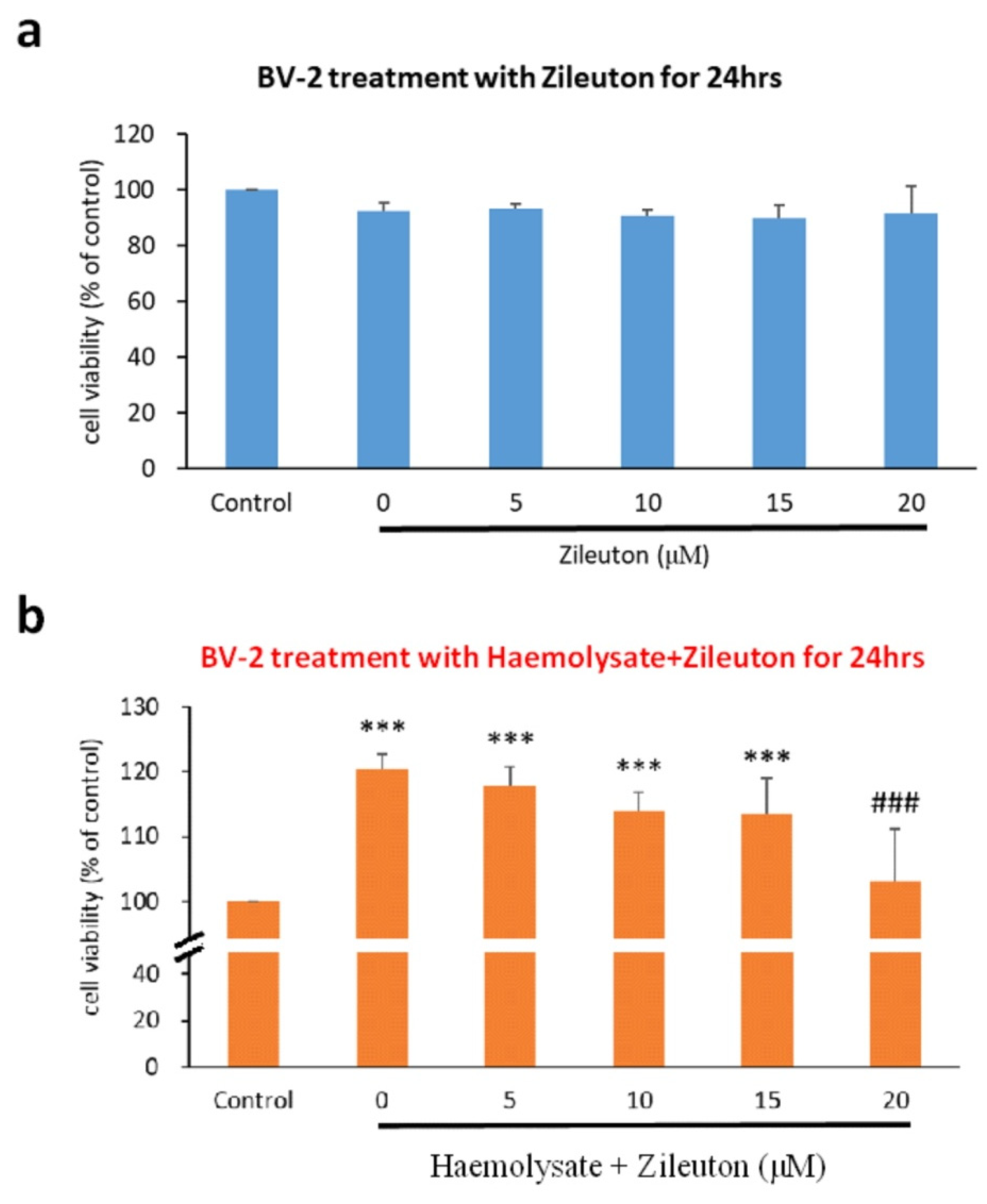
2.1. Viability of BV2 Cells under Different Concentrations of Zileuton
2.2. Viability of Haemolysate-Exposed BV-2 Cells Treated with Different Concentrations of ZILEUTON
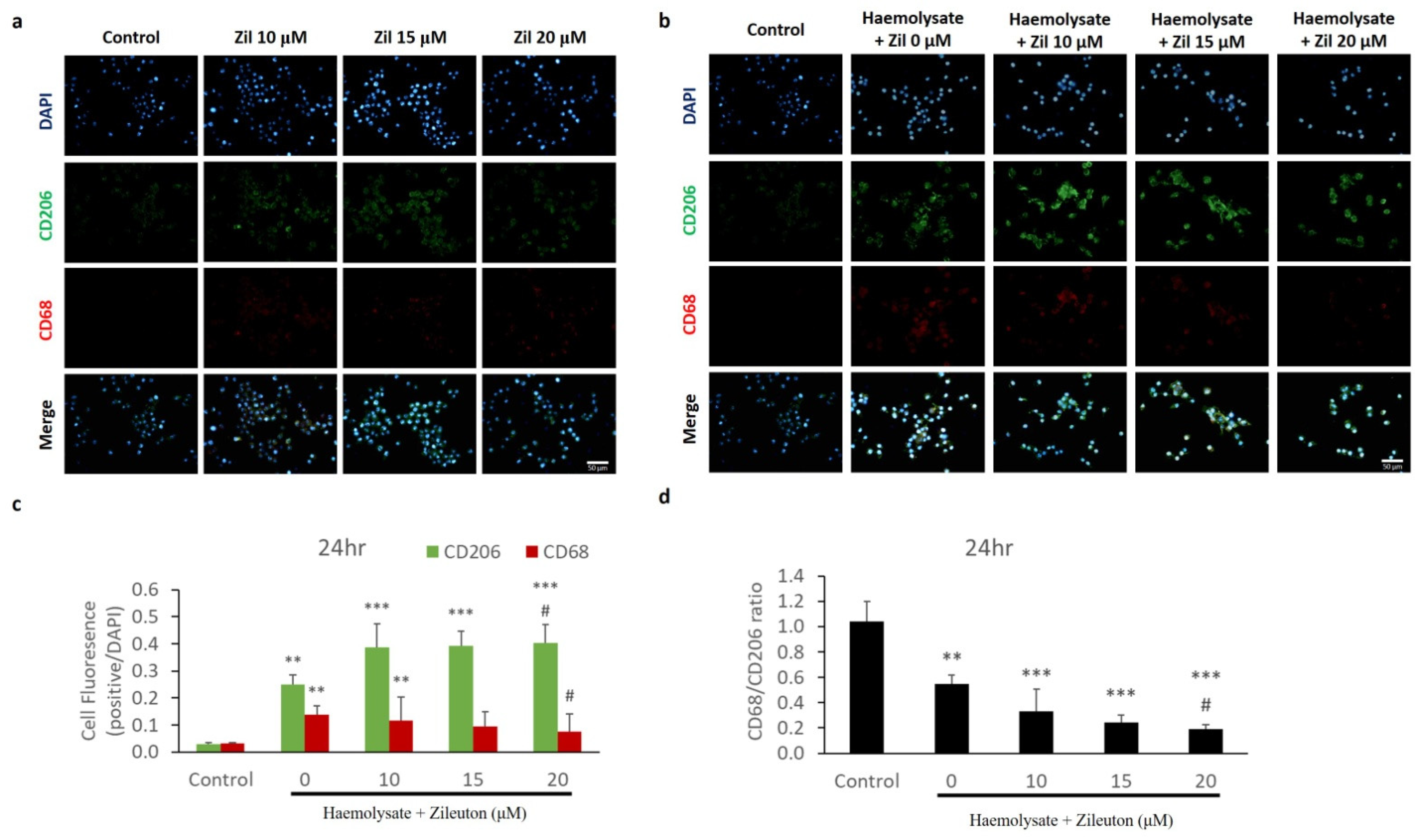
2.3. Immunofluorescence Staining of CD68 and CD206 Expressed on BV-2 Cells
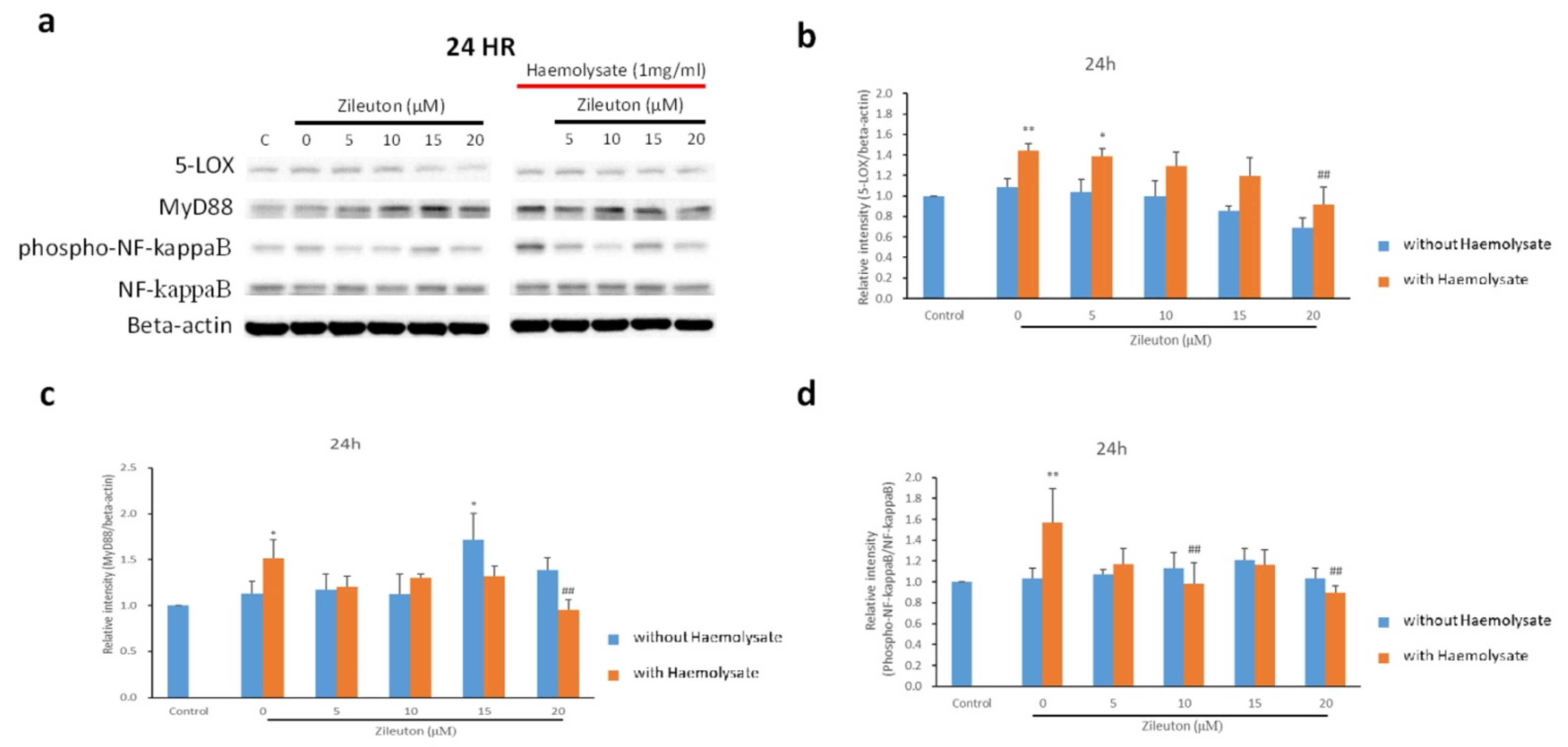
2.4. Western Blot Results for IL-10, TNF-α, 5-LOX, MyD88, and NF-κB
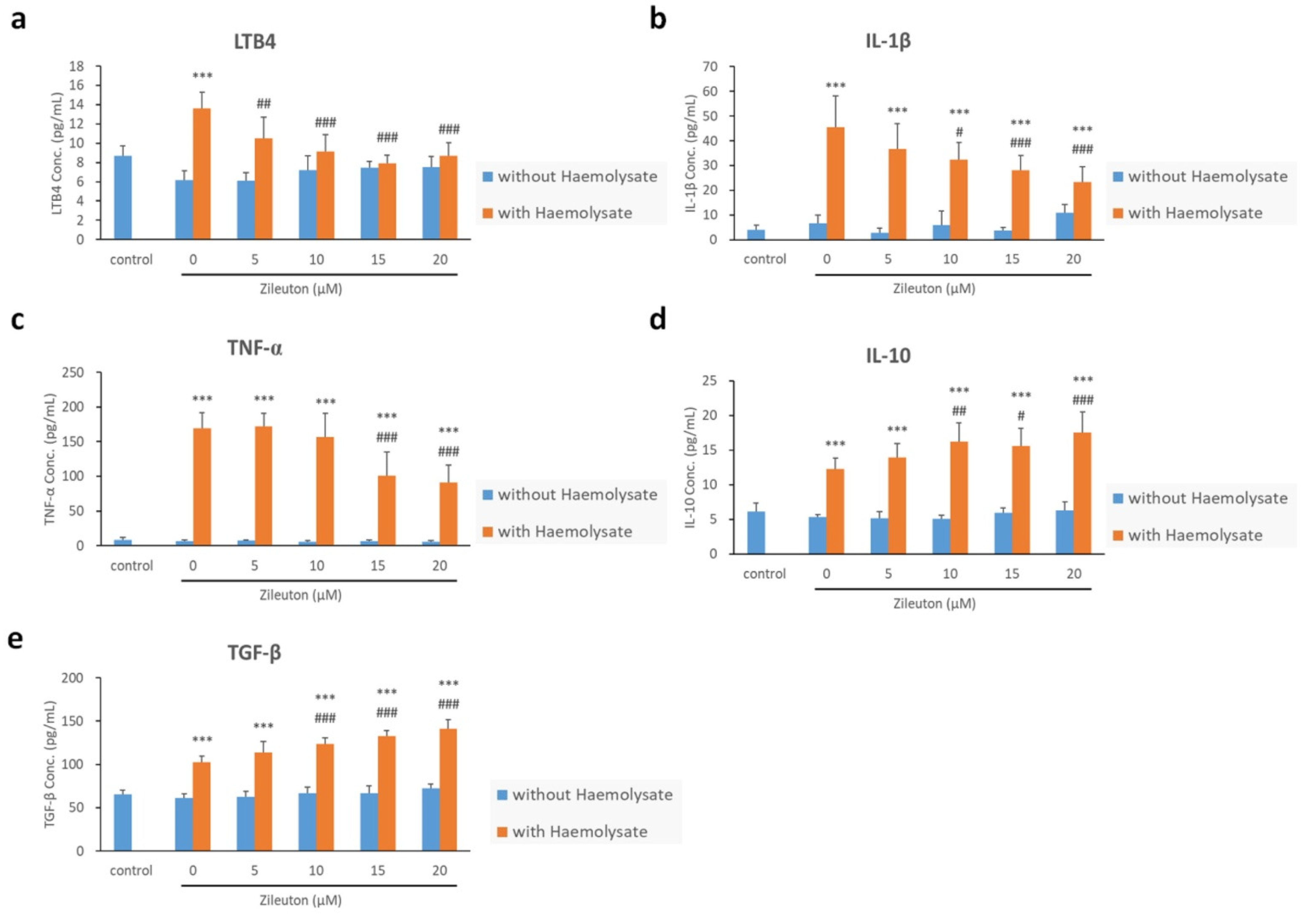
2.5. Enzyme-Linked Immunosorbent Assay (ELISA) Results for LTB4, IL-1β, TNF-α, IL-10, and TGF-β
3. Discussion
4. Materials and Methods
4.1. Cellular Model
4.2. Preparation of Haemolysate
4.3. Treatment with Zileuton
4.4. Cell Viability Assay
4.5. Immunostaining of Cells
4.6. Western Blotting
- Microglial markers: M1 marker, CD68 (1:500, MCA341R, Serotec); M2 marker, CD206 (1:1000, 18704-1-AP, proteintech).
- 5-LOX (1:1000, ab169755, Abcam), TLR4 (1:1000, PA5-23124, Thermo Fisher Scientific), MyD88 (1:1000, 23230-1-AP, Proteintech), NF-κB (1:1000, 10754-1-AP, Proteintech), pNF-κB (1:1000, 3033, Cell Signalling Technology), IL-10, TNF-α, and β-actin (1:10,000, A5441, Sigma-Aldrich).
4.7. ELISA
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loch Macdonald, R.; Schweizer, T.A. Spontaneous subarachnoid haemorrhage. Lancet 2017, 389, 655–666. [Google Scholar] [CrossRef]
- Sehba, F.A.; Hou, J.; Pluta, R.M.; Zhang, J.H. The importance of early brain injury after subarachnoid hemorrhage. Prog. Neurobiol. 2012, 97, 14–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Feng, H.; Sherchan, P.; Klebe, D.; Zhao, G.; Sun, X.; Zhang, J.; Tang, J.; Zhang, J.H. Controversies and evolving new mechanisms in sub-arachnoid hemorrhage. Prog. Neurobiol. 2014, 115, 64–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaberel, T.; Gakuba, C.; Zheng, Y.; Lépine, M.; Lo, E.H.; Leyen, K.V. Impact of 12/15-lipoxygenase on brain injury after sub-arachnoid hemorrhage. Stroke 2019, 50, 520–523. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.V.; Wong, K.C.G. Microglial activation and polarisation after subarachnoid hemorrhage. Neuroimmunol. Neuroinflamm. 2019, 6, 1. [Google Scholar]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef]
- Walker, D.G.; Lue, L.-F. Immune phenotypes of microglia in human neurodegenerative disease: Challenges to detecting microglial polarization in human brains. Alzheimer’s Res. Ther. 2015, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Subramaniam, S.R.; Federoff, H.J. Targeting Microglial Activation States as a Therapeutic Avenue in Parkinson’s Disease. Front. Aging Neurosci. 2017, 9, 176. [Google Scholar] [CrossRef]
- Song, G.J.; Suk, K. Pharmacological Modulation of Functional Phenotypes of Microglia in Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 139. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhang, P.; Zhang, Z.; Liang, Y.; Chen, H.; He, Z.; Sun, X.; Guo, Z.; Deng, Y. 5-Lipoxygenase inhibition reduces inflammation and neuronal apoptosis via AKT signaling after subarachnoid hemorrhage in rats. Aging 2021, 13, 11752–11761. [Google Scholar] [CrossRef]
- Ye, Z.-N.; Zhuang, Z.; Wu, L.-y.; Liu, J.-p.; Chen, Q.; Zhang, X.-s.; Zhou, M.-l.; Zhang, Z.-h.; Li, W.; Wang, X.-l.; et al. Expression and cell distribution of leukotriene B4 receptor 1 in the rat brain cortex after experimental subarachnoid hemorrhage. Brain Res. 2016, 1652, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Wang, B.; Li, M.; Liu, J.; Yang, H.; Shi, Y. Baicalin and Geniposide Inhibit Polarization and Inflammatory Injury of OGD/R-Treated Microglia by Suppressing the 5-LOX/LTB4 Pathway. Neurochem. Res. 2021, 46, 1844–1858. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; Madhoun, A.; Arefanian, H.; Wilson, A.; Kochumon, S.; Thomas, R.; Shenouda, S.; Al-Mulla, F.; Ahmad, R.; Sindhu, S. Oxidative Stress Induces Expression of the Toll-Like Receptors (TLRs) 2 and 4 in the Human Peripheral Blood Mononuclear Cells: Implications for Metabolic Inflammation. Cell. Physiol. Biochem. 2019, 53, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, T.; Wang, X.; Wei, X.; Chen, Y.; Guo, L.; Zhang, J.; Wang, C. Curcumin modulates macrophage polarisation through the inhibition of the toll-Like receptor 4 expression and its signaling pathways. Cell. Physiol. Biochem. 2015, 36, 631–641. [Google Scholar] [CrossRef]
- Lan, X.; Han, X.; Li, Q.; Gao, Y.; Cheng, T.; Wan, J.; Zhu, W.; Wang, J. Pinocembrin protects hemorrhagic brain primarily by inhibiting toll-like receptor 4 and reducing M1 phenotype microglia. Brain Behav. Immun. 2017, 61, 326–339. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.; Khan, M.; Kahlert, U.; Niemelä, M.; Hänggi, D.; Chaudhry, S.; Muhammad, S. Role of Adaptor Protein Myeloid Differentiation 88 (MyD88) in Post-Subarachnoid Hemorrhage Inflammation: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 4185. [Google Scholar] [CrossRef]
- Jatana, M.; Giri, S.; Ansari, M.A.; Elango, C.; Singh, A.K.; Singh, I.; Khan, M. Inhibition of NF-kappaB activation by 5-lipoxygenase inhibitors protects brain against injury in a rat model of focal cerebral ischemia. J. Neuroinflamm. 2006, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Helmy, M.M.; Hashim, A.A.; Mouneir, S.M. Zileuton alleviates acute cisplatin nephrotoxicity: Inhibition of lipoxygenase pathway favorably modulates the renal oxidative/inflammatory/caspase-3 axis. Prostaglandins Other Lipid Mediat. 2018, 135, 1–10. [Google Scholar] [CrossRef]
- Tu, X.-K.; Yang, W.-Z.; Shi, S.-S.; Chen, C.-M.; Wang, C.-H. 5-lipoxygenase inhibitor zileuton at-tenuates ischemic brain damage: Involvement of matrix metalloproteinase 9. Neurol Res. 2009, 31, 848–852. [Google Scholar] [CrossRef]
- Rass, V.; Helbok, R. Early Brain Injury After Poor-Grade Subarachnoid Hemorrhage. Curr. Neurol. Neurosci. Rep. 2019, 19, 78. [Google Scholar] [CrossRef] [Green Version]
- Rinkel, G.J.; Algra, A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011, 10, 349–356. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Higashida, R.T.; Keller, E.; Mayer, S.A.; Molyneux, A.; Raabe, A.; Vajkoczy, P.; Wanke, I.; Bach, D.; Pharm, A.F.; et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: A randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011, 10, 618–625. [Google Scholar] [CrossRef]
- Treichl, S.A.; Ho, W.M.; Steiger, R.; Grams, A.E.; Rietzler, A.; Luger, M.; Gizewski, E.R.; Thomé, C.; Petr, O. Cerebral Energy Status and Altered Metabolism in Early Brain Injury After Aneurysmal Subarachnoid Hemorrhage: A Prospective 31P-MRS Pilot Study. Front. Neurol. 2022, 13, 319. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Kong, Y.; Liu, Z.; Zang, D.; Yang, X.; Wood, K.; Li, M.; Liu, Q. Selective NLRP3 (Pyrin Domain–Containing Protein 3) in-flammasome inhibitor reduces brain injury after intracerebral hemorrhage. Stroke 2018, 49, 184–192. [Google Scholar] [CrossRef]
- Xu, C.-R.; Li, J.-R.; Jiang, S.-W.; Wan, L.; Zhang, X.; Xia, L.; Hua, X.-M.; Li, S.-T.; Chen, H.-J.; Fu, X.-J.; et al. CD47 Blockade Accelerates Blood Clearance and Alleviates Early Brain Injury After Experimental Subarachnoid Hemorrhage. Front. Immunol. 2022, 13, 823999. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; You, W.; Yu, Z.; Li, X.; Shen, H.; Li, H.; Sun, Q.; Li, W.; Chen, G. Enhancing S-nitrosoglutathione reductase decreases S-nitrosylation of Drp1 and reduces neuronal apoptosis in experimental subarachnoid hemorrhage both in vivo and in vitro. Brain Res. Bull. 2022, 183, 184–200. [Google Scholar] [CrossRef]
- Lucke-Wold, B.P.; Logsdon, A.F.; Manoranjan, B.; Turner, R.C.; McConnell, E.; Vates, G.E.; Huber, J.D.; Rosen, C.L.; Simard, J.M. Aneurysmal Subarachnoid Hemorrhage and Neuroinflammation: A Comprehensive Review. Int. J. Mol. Sci. 2016, 17, 497. [Google Scholar] [CrossRef]
- Young, A.M.; Karri, S.K.; Helmy, A.; Budohoski, K.P.; Kirollos, R.W.; Bulters, D.; Kirkpatrick, P.J.; Ogilvy, C.S.; Trivedi, R.A. Pharmacologic Management of Subarachnoid Hemorrhage. World Neurosurg. 2015, 84, 28–35. [Google Scholar] [CrossRef]
- Savarraj, J.P.J.; Parsha, K.; Hergenroeder, G.W.; Zhu, L.; Bajgur, S.S.; Ahn, S.; Lee, K.; Chang, T.; Kim, D.H.; Liu, Y.; et al. Systematic model of peripheral inflammation after subarachnoid hemorrhage. Neurology 2017, 88, 1535–1545. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wu, Q.; Lu, Y.; Wan, J.; Dai, H.; Zhou, X.; Lv, S.; Chen, X.; Zhang, X.; Hang, C.; et al. Cerebroprotection by salvianolic acid B after experimental sub-arachnoid hemorrhage occurs via Nrf2- and SIRT1-dependent pathways. Free Radic. Biol. Med. 2018, 124, 504–516. [Google Scholar] [CrossRef]
- Karuppagounder, S.S.; Alin, L.; Chen, Y.; Brand, D.; Bourassa, M.W.; Dietrich, K.; Wilkinson, C.M.; Nadeau, C.A.; Kumar, A.; Perry, S.; et al. N-acetylcysteine targets 5 lipoxygen-ase-derived, toxic lipids and can synergize with prostaglandin E2 to inhibit ferroptosis and improve outcomes following hemorrhagic stroke in mice. Ann. Neurol. 2018, 84, 854–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Liu, B.; Li, Y.; Zhang, Y.; Shao, J.; Wu, P.; Xu, C.; Chen, G.; Shi, H. Activation of RARα Receptor Attenuates Neuroinflammation After SAH via Promoting M1-to-M2 Phenotypic Polarization of Microglia and Regulating Mafb/Msr1/PI3K-Akt/NF-κB Pathway. Front. Immunol. 2022, 13, 839796. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Cheng, Y.; Peng, W.; Wu, Y.; Rui, T.; Luo, C.; Zhang, J. Targeting iNOS Alleviates Early Brain Injury After Experimental Subarachnoid Hemorrhage via Promoting Ferroptosis of M1 Microglia and Reducing Neuroinflammation. Mol. Neurobiol. 2022, 59, 3124–3139. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.-K.; Zhang, H.-B.; Shi, S.-S.; Liang, R.-S.; Wang, C.-H.; Chen, C.-M.; Yang, W.-Z. 5-LOX Inhibitor Zileuton Reduces Inflammatory Reaction and Ischemic Brain Damage Through the Activation of PI3K/Akt Signaling Pathway. Neurochem. Res. 2016, 41, 2779–2787. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Gaire, B.P.; Kang, M.G.; Choi, J.W. S1P2 contributes to microglial activation and M1 polarisation following cerebral ischemia through ERK1/2 and JNK. Sci. Rep. 2019, 9, 12106. [Google Scholar] [CrossRef]
- Lin, F.L.; Yen, J.L.; Kuo, Y.C.; Kang, J.J.; Cheng, Y.W.; Huang, W.J.; Hsiao, G. HADC8 inhibitor WK2-16 therapeu-tically targets lipopolysaccharide-induced mouse model of neuroinflammation and microglial activation. Int. J. Mol. Sci. 2019, 20, 410. [Google Scholar] [CrossRef] [Green Version]
- Monif, M.; Burnstock, G.; Williams, D.A. Microglia: Proliferation and activation driven by the P2X7 receptor. Int. J. Biochem. Cell Biol. 2010, 42, 1753–1756. [Google Scholar] [CrossRef]
- Wu, M.; Xu, L.; Wang, Y.; Zhou, N.; Zhen, F.; Zhang, Y.; Qu, X.; Fan, H.; Liu, S.; Chen, Y.; et al. S100A8/A9 induces microglia activation and promotes the apoptosis of oligodendrocyte precursor cells by activating the NF-κB signaling pathway. Brain Res. Bull. 2018, 143, 234–245. [Google Scholar] [CrossRef]
- Möller, T.; Hanisch, U.-K.; Ransom, B.R. Thrombin-Induced Activation of Cultured Rodent Microglia. J. Neurochem. 2002, 75, 1539–1547. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.V.; Lyu, H.; Lam, S.Y.E.; Lam, P.K.; Poon, W.S.; Wong, G.K.C. The dynamics of microglial polarisation reveal the resident neuroinflammatory responses after subarachnoid hemorrhage. Transl. Stroke Res. 2020, 11, 433–449. [Google Scholar] [CrossRef]
- Huang, M.; Li, Y.; Wu, K.; Yan, W.; Tian, T.; Wang, Y.; Yang, H. Paraquat modulates microglia M1/M2 polarisation via activation of TLR4-mediated NF-κB signaling pathway. Chem. Biol. Interact. 2019, 310, 108743. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Ying, M.; Gu, S.; Yin, W.; Li, Y.; Yuan, H.; Fang, S.; Li, M. Cysteinyl leukotriene receptor 2 is involved in inflammation and neuronal damage by mediating microglia M1/M2 polarisation through NF-κB pathway. Neuroscience 2019, 422, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.H.; Yan, W.F.; Zhang, Z.; Ma, K.L.; Peng, S.Y.; Cao, Y.L.; Yuan, Y.-H.; Chen, N.-H. Nurr1: A vital participant in the TLR4-NF-κB signal pathway stimulated by α-synuclein in BV-2 cells. Neuropharmacology 2019, 144, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.G.; Reichmann, G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J. Immunol. 2001, 166, 2717–2726. [Google Scholar] [CrossRef] [Green Version]
- Thivierge, M.; Stankova, J.; Rola-Pleszczynski, M. Toll-like receptor agonists differentially regulate cysteinyl-leukotriene re-ceptor 1 expression and function in human dendritic cells. J. Allergy Clin. Immunol. 2006, 117, 1155–1162. [Google Scholar] [CrossRef]
- Zhao, Y.; Gan, Y.; Xu, G.; Yin, G.; Liu, D. MSCs-derived exosomes attenuate acute brain injury and inhibit microglial inflammation by reversing CysLT2R-ERK1/2 mediated microglia M1 polarisation. Neurochem. Res. 2020, 45, 1180–1190. [Google Scholar] [CrossRef]
- Okada, T.; Suzuki, H. Toll-like receptor 4 as a possible therapeutic target for delayed brain injuries after aneurysmal sub-arachnoid hemorrhage. Neural Regen. Res. 2017, 12, 193–196. [Google Scholar]
- Serezani, C.H.; Lewis, C.; Jancar, S.; Peters-Golden, M. Leukotriene B4 amplifies NF-κB activation in mouse macrophages by reducing SOCS1 inhibition of MyD88 expression. J. Clin. Investig. 2011, 121, 671–682. [Google Scholar] [CrossRef] [Green Version]
- Hijioka, M.; Futokoro, R.; Ohto-Nakanishi, T.; Nakanishi, H.; Katsuki, H.; Kitamura, Y. Microglia-released leukotriene B4 promotes neutrophil infiltration and microglial activation following intracerebral hemorrhage. Int. Immunopharmacol. 2020, 85, 106678. [Google Scholar] [CrossRef]
- Hijioka, M.; Anan, J.; Ishibashi, H.; Kurauchi, Y.; Hisatsune, A.; Seki, T.; Koga, T.; Yokomizo, T.; Shimizu, T.; Katsuki, H. Inhibition of Leu-kotriene B4 Action Mitigates Intracerebral Hemorrhage-Associated Pathological Events in Mice. J. Pharm. Exp. Ther. 2017, 360, 399–408. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.; Li, J.-G.; Praticò, D. Zileuton Improves Memory Deficits, Amyloid and Tau Pathology in a Mouse Model of Alzheimer’s Disease with Plaques and Tangles. PLoS ONE 2013, 8, e70991. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-D.; Xie, H.; Du, Y.-F.; Long, Y.; Reed, M.N.; Hu, M.; Suppiramaniam, V.; Hong, H.; Tang, S.-S. Antidepressant-like effect of zileuton is ac-companied by hippocampal neuroinflammation reduction and CREB/BDNF upregulation in lipopolysaccharide-challenged mice. J. Affect Disord. 2018, 227, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Kubavat, A.H.; Khippal, N.; Tak, S.; Rijhwani, P.; Bhargava, S.; Patel, T.; Shah, N.; Kshatriya, R.R.; Mittal, R. A Randomized, Comparative, Multicentric Clinical Trial to Assess the Efficacy and Safety of Zileuton Extended-Release Tablets With Montelukast Sodium Tablets in Patients Suffering From Chronic Persistent Asthma. Am. J. Ther. 2013, 20, 154–162. [Google Scholar] [CrossRef]
- Blasi, E.; Barluzzi, R.; Bocchini, V.; Mazzolla, R.; Bistoni, F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J. Neuroimmunol. 1990, 27, 229–237. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Chen, L.; Yang, Y.; Xu, D.-M.; Zhang, S.-R.; Li, C.-T.; Zheng, W.; Yu, S.-Y.; Wei, E.-Q.; Zhang, L.-H. Regulation of rotenone-induced microglial activation by 5-lipoxygenase and cysteinyl leukotriene receptor 1. Brain Res. 2014, 1572, 59–71. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.-Y.; Tsai, Y.-C.; Tsai, H.-P.; Lin, C.-L. Zileuton, a 5-Lipoxygenase Inhibitor, Attenuates Haemolysate-Induced BV-2 Cell Activation by Suppressing the MyD88/NF-κB Pathway. Int. J. Mol. Sci. 2022, 23, 4910. https://doi.org/10.3390/ijms23094910
Su H-Y, Tsai Y-C, Tsai H-P, Lin C-L. Zileuton, a 5-Lipoxygenase Inhibitor, Attenuates Haemolysate-Induced BV-2 Cell Activation by Suppressing the MyD88/NF-κB Pathway. International Journal of Molecular Sciences. 2022; 23(9):4910. https://doi.org/10.3390/ijms23094910
Chicago/Turabian StyleSu, Hui-Yuan, Yi-Cheng Tsai, Hung-Pei Tsai, and Chih-Lung Lin. 2022. "Zileuton, a 5-Lipoxygenase Inhibitor, Attenuates Haemolysate-Induced BV-2 Cell Activation by Suppressing the MyD88/NF-κB Pathway" International Journal of Molecular Sciences 23, no. 9: 4910. https://doi.org/10.3390/ijms23094910
APA StyleSu, H.-Y., Tsai, Y.-C., Tsai, H.-P., & Lin, C.-L. (2022). Zileuton, a 5-Lipoxygenase Inhibitor, Attenuates Haemolysate-Induced BV-2 Cell Activation by Suppressing the MyD88/NF-κB Pathway. International Journal of Molecular Sciences, 23(9), 4910. https://doi.org/10.3390/ijms23094910




