Intestinal Flora Changes Induced by a High-Fat Diet Promote Activation of Primordial Follicles through Macrophage Infiltration and Inflammatory Factor Secretion in Mouse Ovaries
Abstract
1. Introduction
2. Results
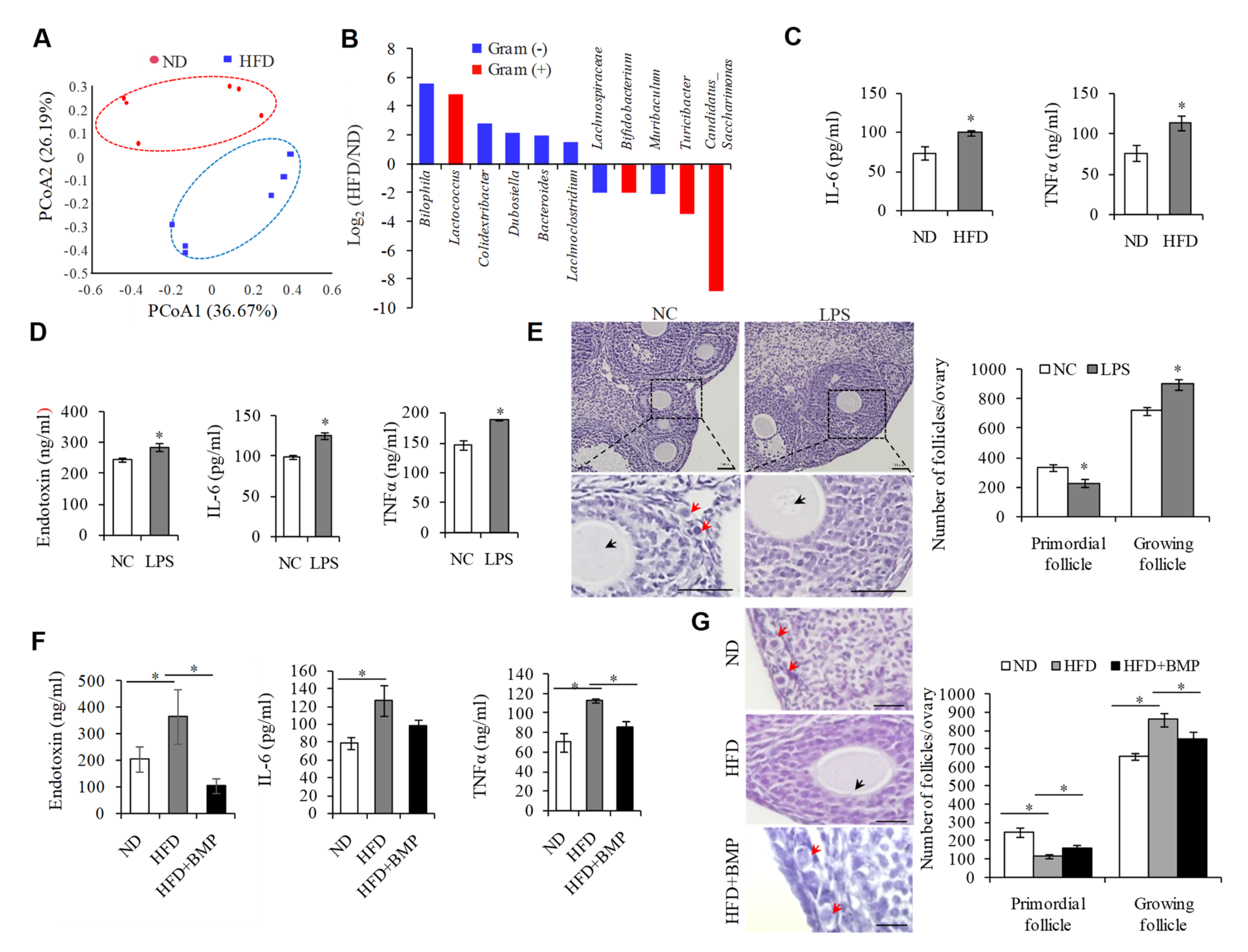
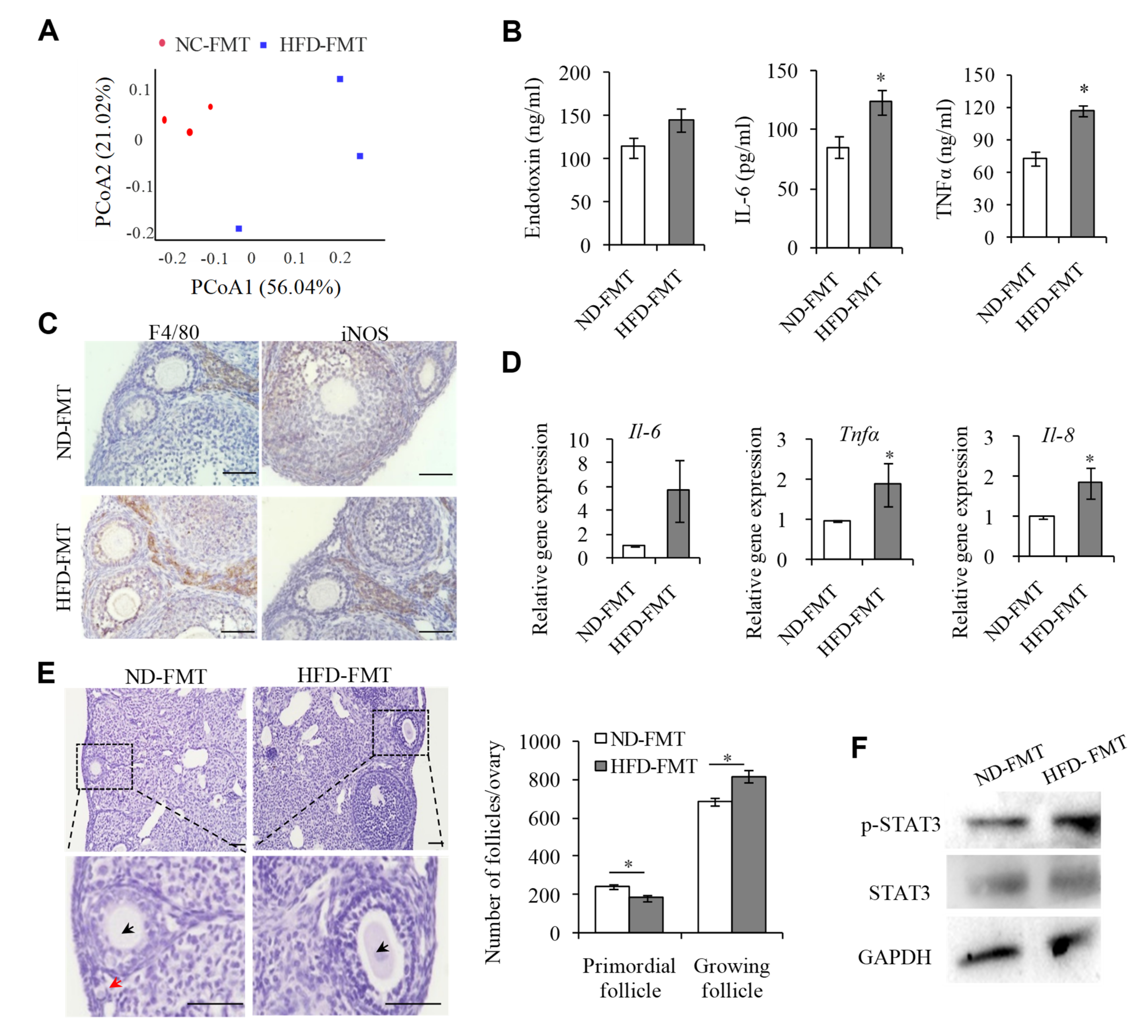
2.1. Endotoxemia Due to an Imbalance of Intestinal Microflora in HFD Mice Contributes to the Overactivation of PFs
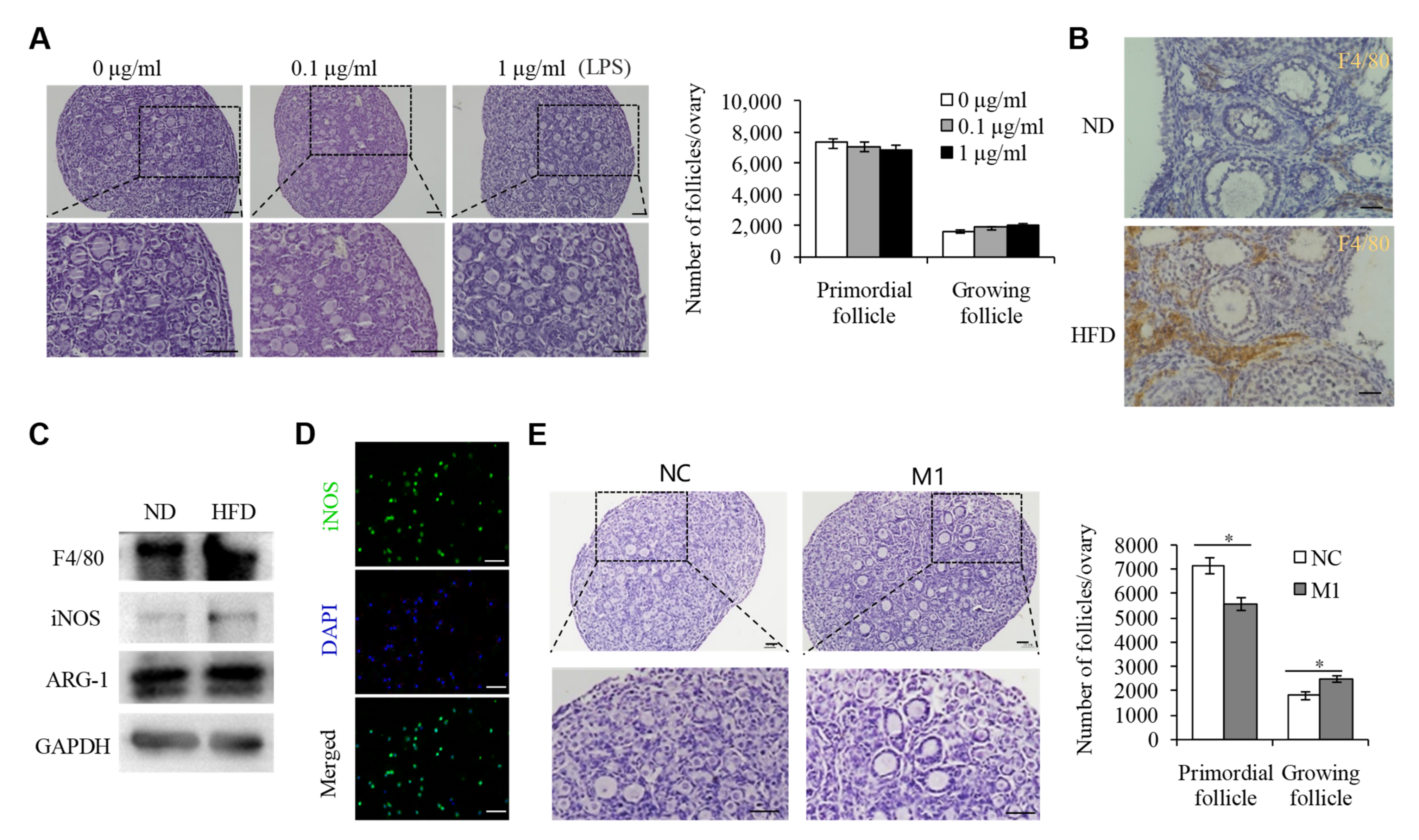
2.2. M1 Macrophages Promoted the Overactivation of PFs in HFD Mice
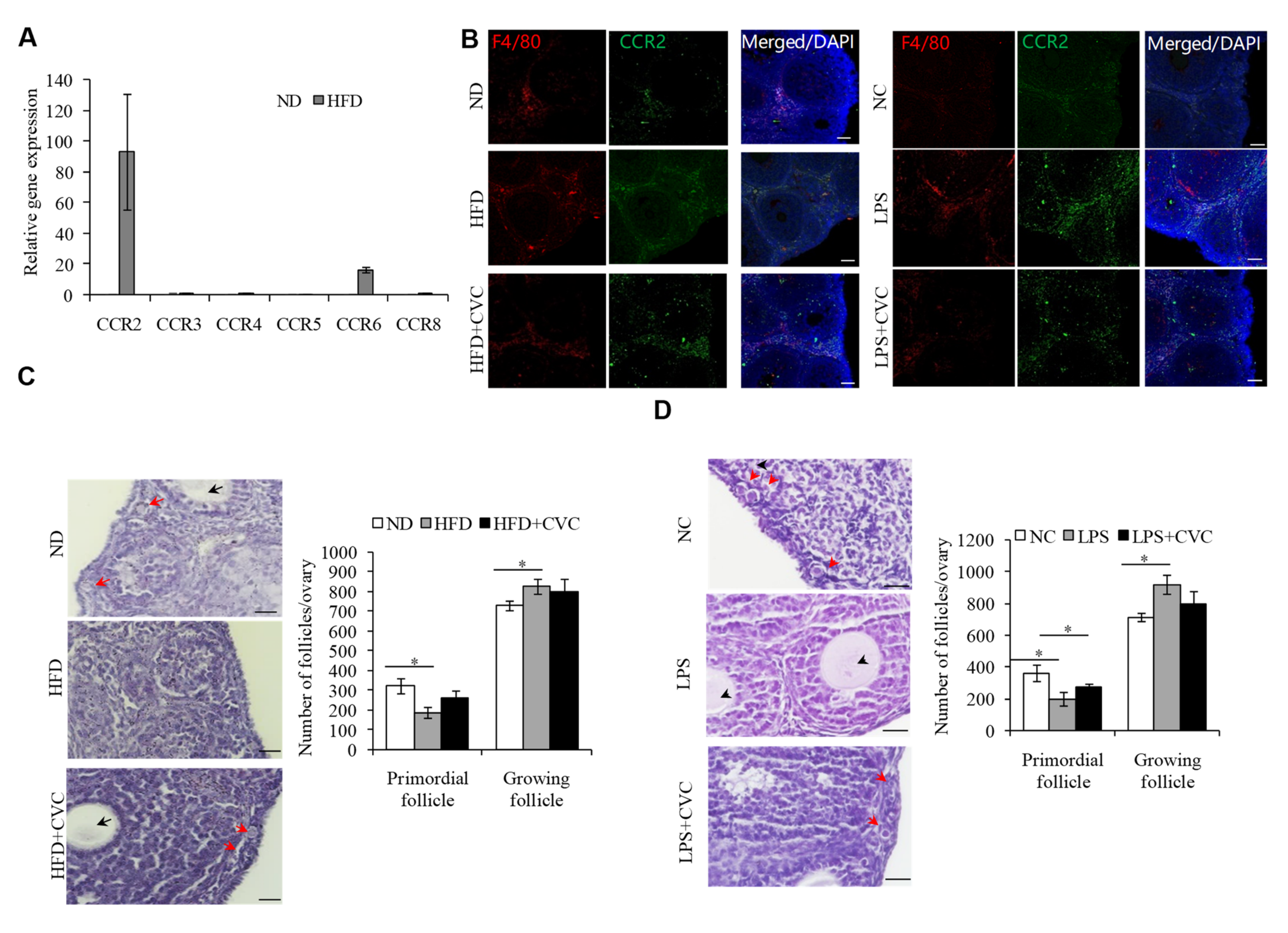
2.3. M1 Macrophage Infiltration intothe OvariesIs the Main Reason for the Overactivation of PFs in HFD Mice
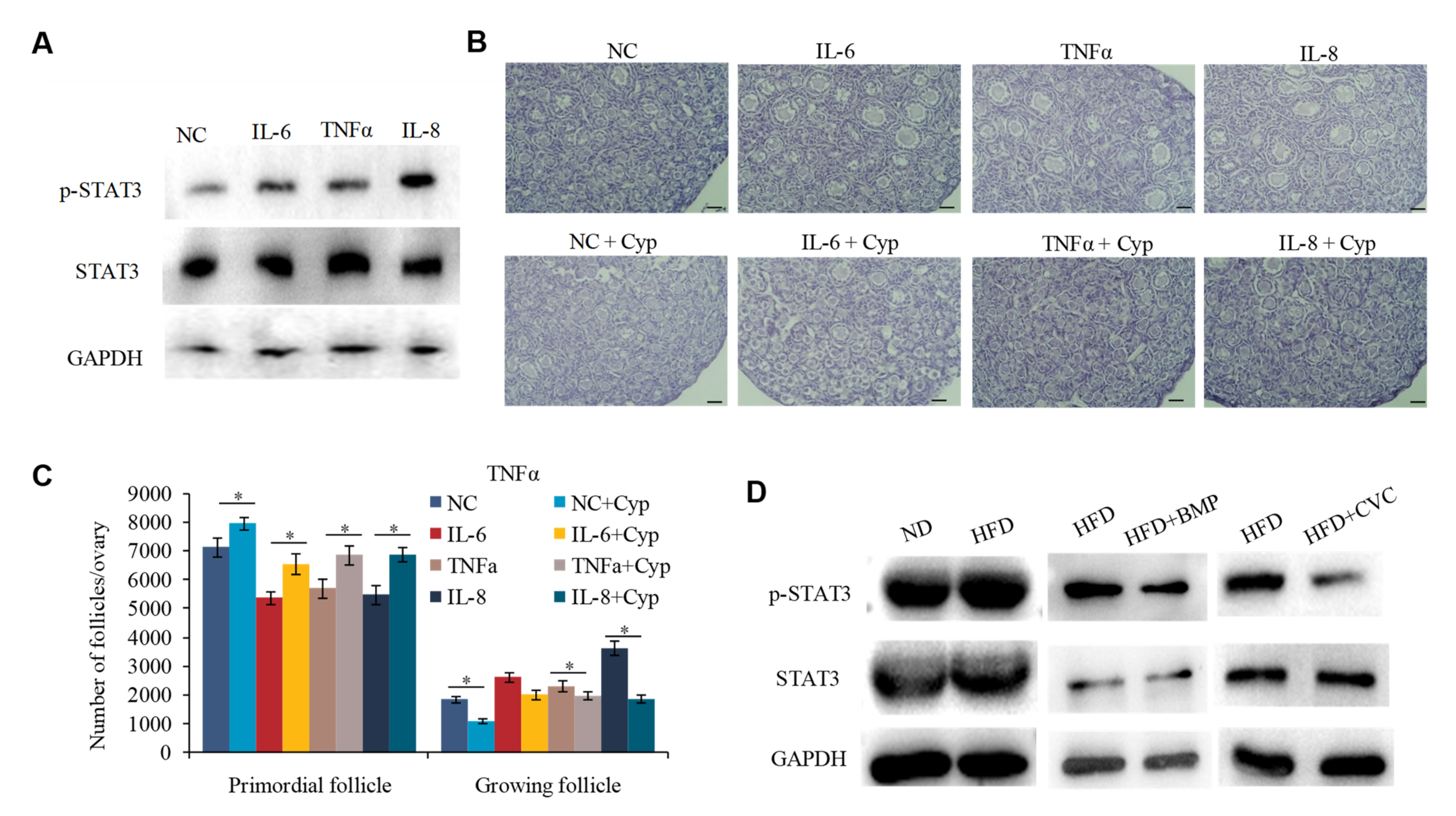
2.4. M1 Macrophages Exhibit Proinflammatory Actions by Secreting Cytokine IL-6, IL-8, and TNFα to Promote the Overactivation of PFs
2.5. IL-6, IL-8, and TNFα Promote the Overactivation of PFs in HFD Mice by Activating STAT3 Expression
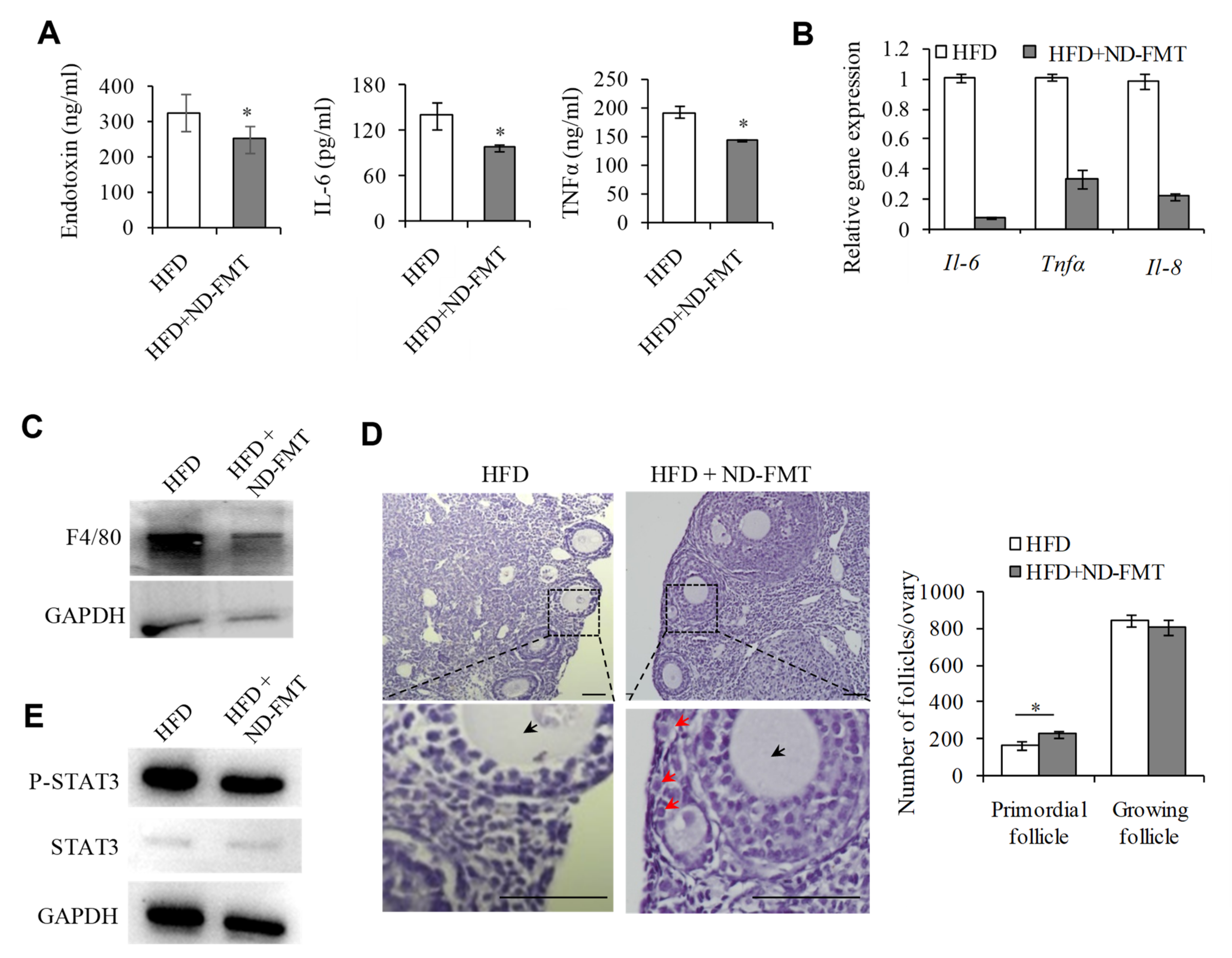
2.6. Changing IntestinalMicroflora of HFD Mice May Correct the Ovarian Inflammation Status and the Overactivation of PFs
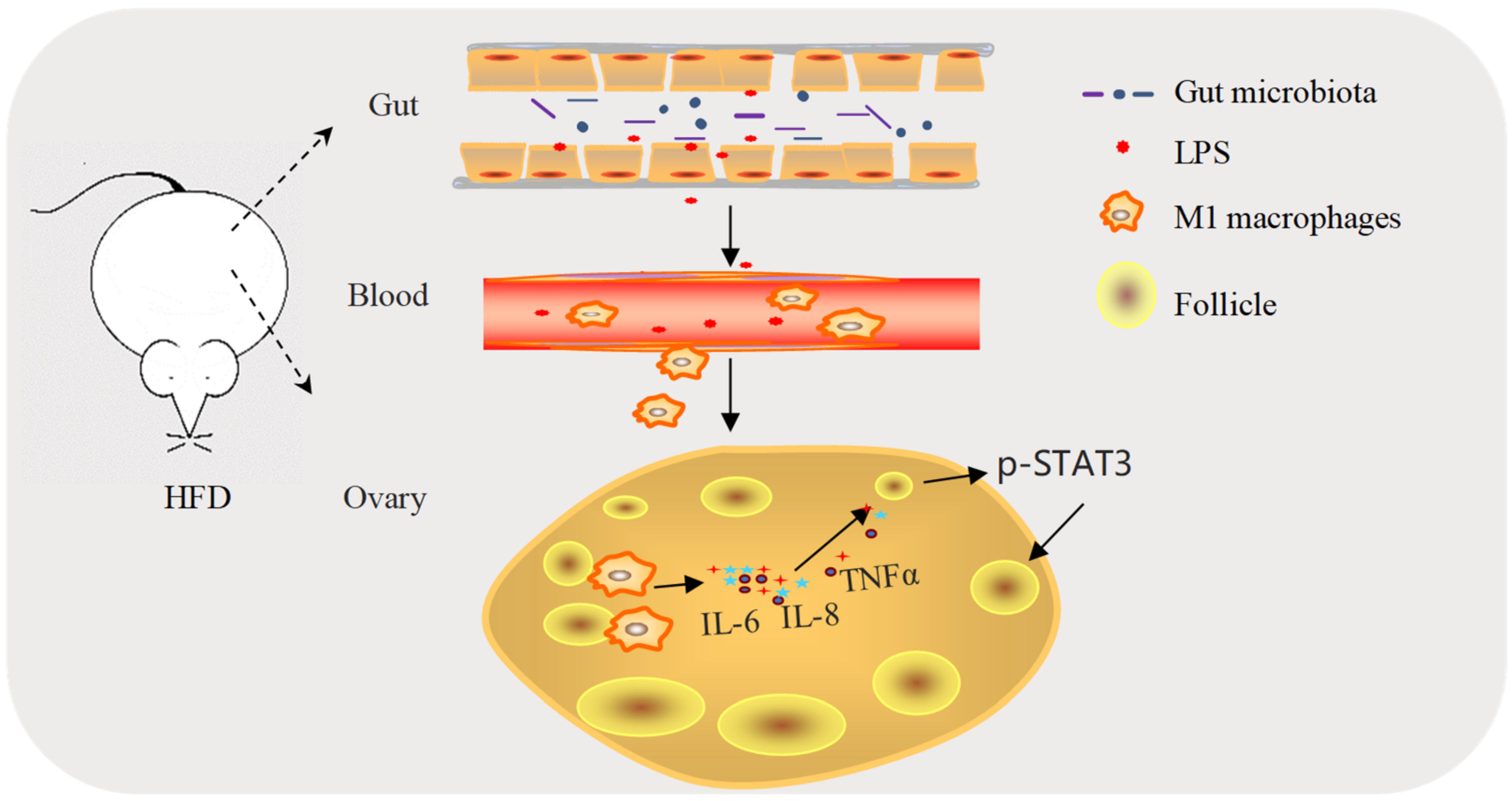
3. Discussion
4. Materials and Methods
4.1. Animal Study
4.2. Ovary Culture
4.3. Quantitative Real-Time PCR
4.4. Western Blotting
4.5. Immunofluorescence
4.6. Follicle Counting
4.7. Isolation of Macrophages
4.8. Immunohistochemistry
4.9. Enzyme-Linked Immunosorbant Assay (ELISA)
4.10. 16.S rDNA High-Throughput Sequencing and Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, C.; Chen, Y.; Lin, Q.; Yao, J.; Wu, W.; Xie, J. The significance of polymorphism and expression of oestrogen metabolism-related genes in Chinese women with premature ovarian insufficiency. Reprod. Biomed. Online 2017, 35, 609–615. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Skaznik-Wikiel, M.E.; Swindle, D.C.; Allshouse, A.A.; Polotsky, A.J.; McManaman, J.L. High-Fat Diet Causes Subfertility and Compromised Ovarian Function Independent of Obesity in Mice. Biol. Reprod. 2016, 94, 108. [Google Scholar] [CrossRef]
- Wang, N.; Luo, L.L.; Xu, J.J.; Xu, M.Y.; Zhang, X.M.; Zhou, X.L.; Liu, W.J.; Fu, Y.C. Obesity accelerates ovarian follicle development and follicle loss in rats. Metab. Clin. Exp. 2014, 63, 94–103. [Google Scholar] [CrossRef]
- Tsoulis, M.W.; Chang, P.E.; Moore, C.J.; Chan, K.A.; Gohir, W.; Petrik, J.J.; Vickers, M.H.; Connor, K.L.; Sloboda, D.M. Maternal High-Fat Diet-Induced Loss of Fetal Oocytes Is Associated with Compromised Follicle Growth in Adult Rat Offspring. Biol. Reprod. 2016, 94, 94. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Ostan, R.; Borelli, V.; Castellani, G.; Franceschi, C. Inflammaging and human longevity in the omics era. Mech. Ageing Dev. 2017, 165, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Said, R.S.; El-Demerdash, E.; Nada, A.S.; Kamal, M.M. Resveratrol inhibits inflammatory signaling implicated in ionizing radiation-induced premature ovarian failure through antagonistic crosstalk between silencing information regulator 1 (SIRT1) and poly(ADP-ribose) polymerase 1 (PARP-1). Biochem. Pharmacol. 2016, 103, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Zhang, X.; Zhang, X.D.; Jing, J.; Liu, S.S.; Mu, Y.P.; Peng, L.L.; Yan, Y.J.; Xiao, G.M.; Bi, X.Y.; et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 2020, 69, 1608–1619. [Google Scholar] [CrossRef]
- Bai, Y.F.; Wang, S.W.; Wang, X.X.; Weng, Y.Y.; Fan, X.Y.; Sheng, H.; Zhu, X.T.; Lou, L.J.; Zhang, F. The flavonoid-rich Quzhou Fructus Aurantii extract modulates gut microbiota and prevents obesity in high-fat diet-fed mice. Nutr. Diabetes 2019, 9, 30. [Google Scholar] [CrossRef]
- Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J.; Shen, M. Flavonoids from Whole-Grain Oat Alleviated High-Fat Diet-Induced Hyperlipidemia via Regulating Bile Acid Metabolism and Gut Microbiota in Mice. J. Agric. Food Chem. 2021, 69, 7629–7640. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.K.; Seo, S.H.; Park, S.E.; Kim, H.W.; Kim, E.J.; Kim, J.S.; Pyo, J.Y.; Cho, K.M.; Kwon, S.J.; Park, D.H.; et al. Gut Microbiome and Metabolome Profiles Associated with High-Fat Diet in Mice. Metabolites 2021, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Nteeba, J.; Ortinau, L.C.; Perfield, J.W., 2nd; Keating, A.F. Diet-induced obesity alters immune cell infiltration and expression of inflammatory cytokine genes in mouse ovarian and peri-ovarian adipose depot tissues. Mol. Reprod. Dev. 2013, 80, 948–958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, M.; Pang, X.; Zhao, Y.; Wang, L.; Zhao, L. Structural resilience of the gut microbiota in adult mice under high-fat dietary perturbations. ISME J. 2012, 6, 1848–1857. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Van der Hoek, K.H.; Ryan, N.K.; Norman, R.J.; Robker, R.L. Macrophage contributions to ovarian function. Hum. Reprod. Update 2004, 10, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Bromfield, J.J.; Sheldon, I.M. Lipopolysaccharide reduces the primordial follicle pool in the bovine ovarian cortex ex vivo and in the murine ovary in vivo. Biol. Reprod. 2013, 88, 98. [Google Scholar] [CrossRef] [PubMed]
- Fuller, E.A.; Sominsky, L.; Sutherland, J.M.; Redgrove, K.A.; Harms, L.; McLaughlin, E.A.; Hodgson, D.M. Neonatal immune activation depletes the ovarian follicle reserve and alters ovarian acute inflammatory mediators in neonatal rats. Biol. Reprod. 2017, 97, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Hunter, D.; Huber, R.; Lemieux, J.; Slaymaker, S.; Vaddi, K.; Charo, I.; Leibel, R.L.; Ferrante, A.W., Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Investig. 2006, 116, 115–124. [Google Scholar] [CrossRef]
- Giunti, S.; Barutta, F.; Perin, P.C.; Gruden, G. Targeting the MCP-1/CCR2 System in diabetic kidney disease. Curr. Vasc. Pharmacol. 2010, 8, 849–860. [Google Scholar] [CrossRef]
- Serbina, N.V.; Pamer, E.G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 2006, 7, 311–317. [Google Scholar] [CrossRef]
- Mills, C.D.; Lenz, L.L.; Harris, R.A. A Breakthrough: Macrophage-Directed Cancer Immunotherapy. Cancer Res. 2016, 76, 513–516. [Google Scholar] [CrossRef]
- Wu, J.; Gao, F.X.; Wang, C.; Qin, M.; Han, F.; Xu, T.; Hu, Z.; Long, Y.; He, X.-M.; Deng, X.; et al. IL-6 and IL-8 secreted by tumour cells impair the function of NK cells via the STAT3 pathway in oesophageal squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2019, 38, 321. [Google Scholar] [CrossRef]
- Yan, S.; Zhou, C.; Zhang, W.; Zhang, G.; Zhao, X.; Yang, S.; Hu, Z.; Long, Y.; He, X.M.; Deng, X.; et al. beta-Catenin/TCF pathway upregulates STAT3 expression in human esophageal squamous cell carcinoma. Cancer Lett. 2008, 271, 85–97. [Google Scholar] [CrossRef]
- Sutherland, J.M.; Frost, E.R.; Ford, E.A.; Peters, A.E.; Reed, N.L.; Seldon, A.N.; Mihalas, B.P.; Russel, D.L.; Dunning, K.R.; McLaughlin, E.A. Janus kinase JAK1 maintains the ovarian reserve of primordial follicles in the mouse ovary. Mol. Hum. Reprod. 2018, 24, 533–542. [Google Scholar] [CrossRef]
- Sutherland, J.M.; Keightley, R.A.; Nixon, B.; Roman, S.D.; Robker, R.L.; Russell, D.L.; McLaughlin, E.A. Suppressor of cytokine signaling 4 (SOCS4): Moderator of ovarian primordial follicle activation. J. Cell. Physiol. 2012, 227, 1188–1198. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, C.; Ye, H.; Luo, R.; Fu, X.; Li, X.; Huang, J.; Chen, W.Y.; Zheng, Y.H. Inflamm-Aging: A New Mechanism Affecting Premature Ovarian Insufficiency. J. Immunol. Res. 2019, 2019, 8069898. [Google Scholar] [CrossRef]
- Lindheim, L.; Bashir, M.; Münzker, J.; Trummer, C.; Zachhuber, V.; Leber, B.; Horvath, A.; Pieber, T.R.; Gorkiewicz, G.; Stadlbauer, V.; et al. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS ONE 2017, 12, e0168390. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, C.; Shi, Y.; Zhang, F.; Li, L.; Wang, X.; Ling, Y.; Fu, H.; Dong, W.; Shen, J.; et al. Dysbiosis of Gut Microbiota Associated with Clinical Parameters in Polycystic Ovary Syndrome. Front. Microbiol. 2017, 8, 324. [Google Scholar] [CrossRef]
- He, F.; Li, Y. The gut microbial composition in polycystic ovary syndrome with insulin resistance: Findings from a normal-weight population. J. Ovarian Res. 2021, 14, 50. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Cani, P.D. Crosstalk between the gut microbiota and the endocannabinoid system: Impact on the gut barrier function and the adipose tissue. Clnical Microbiol. Infect. 2012, 18 (Suppl. 4), 50–53. [Google Scholar] [CrossRef]
- Walsh, C.J.; Healy, S.; O’Toole, P.W.; Murphy, E.F.; Cotter, P.D. The probiotic L. casei LC-XCAL™ improves metabolic health in a diet-induced obesity mouse model without altering the microbiome. Gut Microbes 2020, 12, 1704141. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Z.; Wang, B.; Xia, X.; Yao, W.; Huang, L.; Wang, Y.; Ding, W. Early-life high-fat diet-induced obesity programs hippocampal development and cognitive functions via regulation of gut commensal Akkermansiamuciniphila. Neuropsychopharmacology 2019, 44, 2054–2064. [Google Scholar] [CrossRef]
- Sominsky, L.; Meehan, C.L.; Walker, A.K.; Bobrovskaya, L.; McLaughlin, E.A.; Hodgson, D.M. Neonatal immune challenge alters reproductive development in the female rat. Horm. Behav. 2012, 62, 345–355. [Google Scholar] [CrossRef]
- Sominsky, L.; Sobinoff, A.P.; Jobling, M.S.; Pye, V.; McLaughlin, E.A.; Hodgson, D.M. Immune regulation of ovarian development: Programming by neonatal immune challenge. Front. Neurosci. 2013, 7, 100. [Google Scholar] [CrossRef]
- Guicciardi, M.E.; Trussoni, C.E.; Krishnan, A.; Bronk, S.F.; Lorenzo Pisarello, M.J.; Gao, Y.D.; Vig, P.; Revzin, A.; LaRusso, N.F.; Gores, G.J. Macrophages contribute to the pathogenesis of sclerosing cholangitis in mice. J. Hepatol. 2018, 69, 676–686. [Google Scholar] [CrossRef]
- Behmoaras, J.; Gil, J. Similarities and interplay between senescent cells and macrophages. J. Cell Biol. 2021, 220, e202010162. [Google Scholar] [CrossRef]
- Robker, R.L.; Wu, L.L.; Yang, X. Inflammatory pathways linking obesity and ovarian dysfunction. J. Reprod. Immunol. 2011, 88, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Feng, H.L. Extra- and intra-ovarian factors in polycystic ovary syndrome: Impact on oocyte maturation and embryo developmental competence. Hum. Reprod. Update 2011, 17, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, M.; Nejaty, J.; Fröysa, B.; Guan, Y.; Söder, O.; Bergqvist, A. Elevated expression of tumour necrosis factor alpha in cultured granulosa cells from women with endometriosis. Hum. Reprod. 2000, 15, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Knebel, B.; Janssen, O.E.; Hahn, S.; Jacob, S.; Gleich, J.; Kotzka, J.; Muller-Wieland, D. Increased low grade inflammatory serum markers in patients with Polycystic ovary syndrome (PCOS) and their relationship to PPARgamma gene variants. Exp. Clin. Endocrinol. Diabetes 2008, 116, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar Ram, M.; Sundararaman, P.G.; Mahadevan, S.; Malathi, R. Cytokines and leptin correlation in patients with polycystic ovary syndrome: Biochemical evaluation in south Indian population. Reprod. Med. Biol. 2005, 4, 247–254. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–2348. [Google Scholar] [CrossRef]
- De Simone, V.; Franzè, E.; Ronchetti, G.; Colantoni, A.; Fantini, M.C.; Di Fusco, D.; Sica, G.S.; Sileri, P.; MacDonald, T.T.; Pallone, F.; et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2015, 34, 3493–3503. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Karin, M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010, 21, 11–19. [Google Scholar] [CrossRef]
- Frost, E.R.; Ford, E.A.; Peters, A.E.; Reed, N.L.; McLaughlin, E.A.; Baker, M.A.; Lovell-Badge, R.; Sutherland, J.M. Signal transducer and activator of transcription (STAT) 1 and STAT3 are expressed in the human ovary and have Janus kinase 1-independent functions in the COV434 human granulosa cell line. Reprod. Fertil. Dev. 2020, 32, 1027–1039. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, Y.; Du, J.; Han, J.; Sun, W.; Gao, F.; Zhang, P.; Zhao, H.; Chen, M.; Wang, J.; et al. Polymyxin B Attenuates LPS-Induced Death but Aggravates Radiation-Induced Death via TLR4-Myd88-IL-6 Pathway. Cell Physiol. Biochem. 2017, 42, 1120–1126. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Zhang, X.; Shang, Y.; Zou, M.; Zhou, M.; E, Q.; Fei, S.; Chen, W.; Li, J.; Zhang, X.; et al. Intestinal Flora Changes Induced by a High-Fat Diet Promote Activation of Primordial Follicles through Macrophage Infiltration and Inflammatory Factor Secretion in Mouse Ovaries. Int. J. Mol. Sci. 2022, 23, 4797. https://doi.org/10.3390/ijms23094797
Fan Z, Zhang X, Shang Y, Zou M, Zhou M, E Q, Fei S, Chen W, Li J, Zhang X, et al. Intestinal Flora Changes Induced by a High-Fat Diet Promote Activation of Primordial Follicles through Macrophage Infiltration and Inflammatory Factor Secretion in Mouse Ovaries. International Journal of Molecular Sciences. 2022; 23(9):4797. https://doi.org/10.3390/ijms23094797
Chicago/Turabian StyleFan, Zhihao, Xiaoqian Zhang, Yanxing Shang, Maosheng Zou, Meng Zhou, Qiukai E, Shujia Fei, Wei Chen, Jing Li, Xuesen Zhang, and et al. 2022. "Intestinal Flora Changes Induced by a High-Fat Diet Promote Activation of Primordial Follicles through Macrophage Infiltration and Inflammatory Factor Secretion in Mouse Ovaries" International Journal of Molecular Sciences 23, no. 9: 4797. https://doi.org/10.3390/ijms23094797
APA StyleFan, Z., Zhang, X., Shang, Y., Zou, M., Zhou, M., E, Q., Fei, S., Chen, W., Li, J., Zhang, X., & Liu, X. (2022). Intestinal Flora Changes Induced by a High-Fat Diet Promote Activation of Primordial Follicles through Macrophage Infiltration and Inflammatory Factor Secretion in Mouse Ovaries. International Journal of Molecular Sciences, 23(9), 4797. https://doi.org/10.3390/ijms23094797




