Biocompatible Conductive Hydrogels: Applications in the Field of Biomedicine
Abstract
:1. Introduction
2. Materials for Preparing CHs
2.1. Matrix Materials for Preparing CHs
| Types of CHs | Characteristics | Conductive Components | Materials | Conductive Hydrogel Polymer/System | Applications | Year | Refs |
|---|---|---|---|---|---|---|---|
| Electron-CHs (E-CHs) | Highly stretchable Better conductivity Better biocompatibility | Metallic nanoparticles | Ag | Polyacrylic acid (PAA) | Nanoelectronic devices Artificial muscles | 2014 | [16] |
| Polyethylene glycol diacrylate (PEGDA) | Tissue engineering Chemistry reactions ware | 2016 | [17] | ||||
| Au | Poly(acrylamide) [poly (AAm)] and poly(N-isopropyl-acrylamide) [poly (NIPAAm)] | Chemoresistors Biosensors | 2010 | [18] | |||
| Gelatin methacrylate (GelMA) | Cardiac tissue engineering | 2016 | [19] | ||||
| Cu | Polyacrylamide grafted poly(vinyl alcohol) (PAM-g-PVA) | Biosensors Drug delivery system | 2008 | [20] | |||
| Carbon-based materials | CNTs (carbon nanotubes) | Gelatin-grafted-dopamine (GT-DA) Chitosan (CS) | Multifunctional bioactive dressings | 2019 | [21] | ||
| N-isopropyl acrylamide (NIPAM) | Wearable electronics | 2019 | [22] | ||||
| Grphene /GO/rGO | Methacryloyl-substituted tropoelastin (MeTro) | Promising different biomedical applications | 2016 | [23] | |||
| Poly(N-isopropylacrylamide) (PNIPAM) | Wearable electronics | 2014 | [24] | ||||
| Conducting polymers | Polyaniline (PANI) | Chitosan-graft-aniline tetramer (CS-AT) Dibenzaldehyde-terminated poly (ethylene glycol) (PEG-DA) | Drug delivery system | 2016 | [25] | ||
| Poly(N-isopropylacrylamide) (PNIPAM) | Strain sensors Wearable electronics | 2018 | [26] | ||||
| Polypyrrole (PPy) | Polydopamine (PDA) | Wound dressing Biosensors | 2018 | [27] | |||
| PEDOT: PSS | Iota-carrageenan (CRG) Polyvinyl alcohol (PVA) | Biomedical engineering Biomedical devices | 2019 | [28] | |||
| Hybrid | Pt+ polyaniline | Pt nanoparticle (PtNP) Polyaniline (PANI) | Biosensors Biomedical devices | 2015 | [29] | ||
| SWCNTs+ polyaniline | Single-walled CNTs (SWCNTs) Polyaniline (PANI) | Supercapacitor Wearable electronics | 2018 | [30] | |||
| Ion-CHs (I-CHs) | Highly stretchable Conductive Biocompatible Transparency Generate ionic gradients | Acids | H2SO4 | Polyvinyl alcohol (PVA)-H2SO4 hydrogel Polyaniline (PANI) | Supercapacitor | 2018 | [31] |
| H3PO4 | Vinyl hybrid silica nanoparticles (VSNPs) Polyacrylamide (PAM) | Supercapacitor | 2017 | [32] | |||
| Metallic salts | LiCl | Lithium chloride (LiCl) Acrylamide (AAm) | Soft actuators Electronic fish | 2017 | [33] | ||
| Na+ | Polyacrylamide (PAAm) Sodium chloride (NaCl) | Epidermal strain sensor Biosensors | 2018 | [34] | |||
| Ca2+ | Polyacrylamide-alginate (PAAm–alginate) Calcium chloride (CaCl2) | Stretchable ionic touch sensor | 2018 | [35] | |||
| Al3+ | Cellular-structured nanofibrous hydrogels (NFHs) Alginate | Biomedical engineering Biomedical devices | 2017 | [36] | |||
| Fe3+ | Polyethylene glycol/poly(acrylic acid) (PEG/PAA) double network hydrogel | Electronic skin Wearable electronics | 2018 | [37] | |||
| K+ | Polyacrylamide (PAAm)/carrageenan double network hydrogel | Thermistor | 2018 | [38] | |||
| Ionic liquids | 1-Ethyl-3-methylimidazolium chloride | Supercapacitor | 2014 | [39] | |||
| E-CHs and I-CHs | Highly stretchable Conductive Biocompatible Transparent/ semitransparent | Electron conductive components and ions | H2SO4+ PEDOT | PEDOT: PSS H2SO4 | Wearable energy-storage devices | 2017 | [40] |
| Na+ + Ca2++ SWCNTs | Single-wall carbon nanotubes (SWCNTs) Sodium alginate Calcium chloride (CaCl2) | Wearable pressure sensor | 2015 | [41] | |||
| Fe3+ + rGO | Poly(acrylic acid) (PAA) Polydopamine (PDA) Iron chloride (FeCl3) | Electronic skins Biosensors Tissue engineering | 2018 | [42] | |||
| CuPcTs+ Polypyrrole | Crystal molecular copper-phthalocyanine-3,4′,4″,4‴-tetrasulfonic acid tetrasodium salt (CuPcTs)Polypyrrole(PPy) | Biomedical devices Biosensors | 2015 | [43] | |||
2.2. Conductive Materials for Preparing CHs
2.2.1. E-CHs
2.2.2. I-CHs
2.2.3. E-CHs and I-CHs
3. General Methods for Synthesizing CHs
3.1. Polymerization Techniques
3.1.1. Chain Growth Polymerization Method
3.1.2. Step-Growth Polymerization Method
3.2. Cross-Linking Techniques
3.2.1. Physical Cross-Linking Method
3.2.2. Chemical Cross-Linking Method
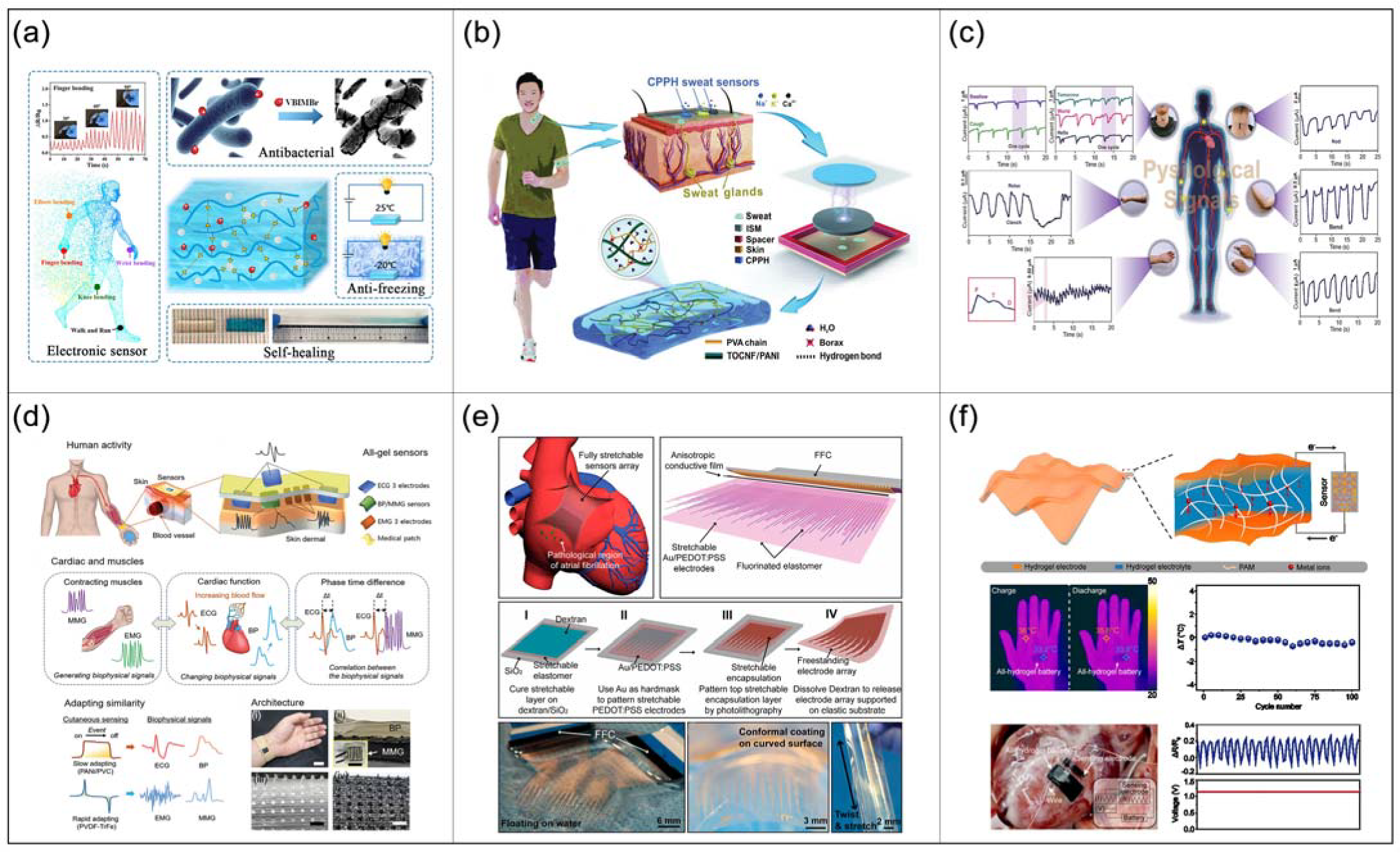
4. Development and Applications of CHs in the Field of Biomedicine
4.1. Regenerative Medicine
4.2. Artificial Organs
4.3. Biosensors
4.3.1. Wearable Biosensors
4.3.2. Implantable Biosensors
4.3.3. Energy Supply for Biosensors
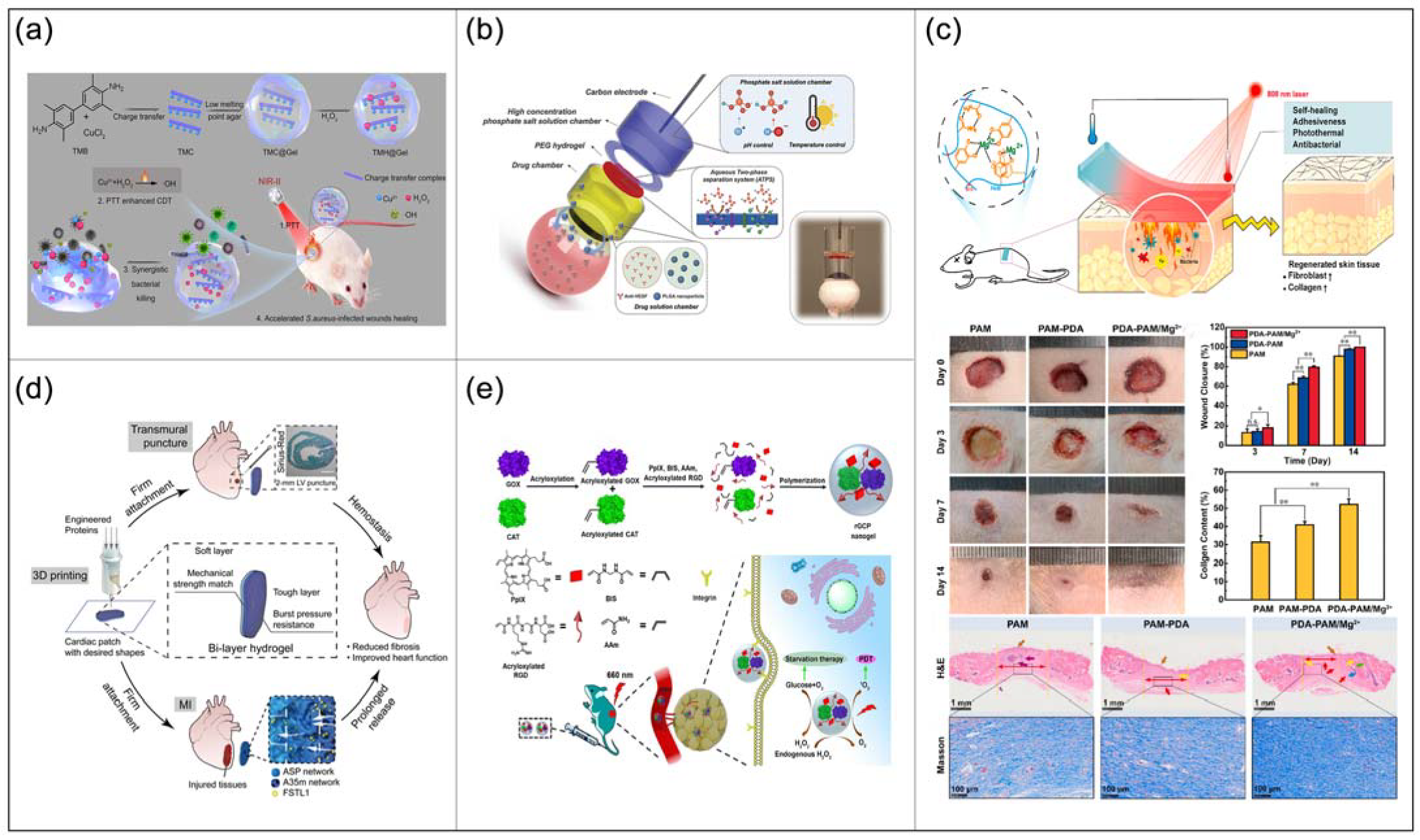
4.4. Drug Delivery Systems
4.5. Other Biomedical Applications
4.5.1. Wound Dressings
4.5.2. Cancer Treatment
5. Summary and Prospects
5.1. 3D Bioprinting of Microtissue Structures
5.2. Practical Biomedical Application Progress of CHs
5.3. Programmable CHs
5.4. Cross-Applications of CHs in Frontier Fields
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, B.; Zhu, H.; Liu, J.; Solovev, A.; Mei, Y. Requirement and Development of Hydrogel Micromotors towards Biomedical Applications. Research 2020, 2020, 7659749. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.-M.; Chen, H.-X.; Li, H.-D. Recent advances in tough and self-healing nanocomposite hydrogels for shape morphing and soft actuators. Eur. Polym. J. 2020, 124, 109448. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, Y.Z.; Wang, S.; Chen, Y.L.; Gao, S.Y.; Wang, F.; Lai, W.Y.; Huang, W. Conductive Hydrogel-Based Electrodes and Electrolytes for Stretchable and Self-Healable Supercapacitors. Adv. Functional Mater. 2021, 31, 2101303. [Google Scholar] [CrossRef]
- Jalili, N.A.; Muscarello, M.; Gaharwar, A.K. Nanoengineered thermoresponsive magnetic hydrogels for biomedical applications. Bioeng. Transl. Med. 2016, 1, 297–305. [Google Scholar] [CrossRef]
- Yang, D. Recent Advances in Hydrogels. Chem. Mater. 2022, 34, 1987–1989. [Google Scholar] [CrossRef]
- Vazquez-Gonzalez, M.; Willner, I. Stimuli-Responsive Biomolecule-Based Hydrogels and Their Applications. Angew. Chem. Int. Ed. Engl. 2020, 59, 15342–15377. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels: A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, L.A.; Daily, A.M.; Horava, S.D.; Peppas, N.A. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin. Drug Deliv. 2014, 11, 901–915. [Google Scholar] [CrossRef] [Green Version]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-Responsive Nanocomposite Hydrogels for Biomedical Applications. Adv. Functional Mater. 2021, 31, 2005941. [Google Scholar] [CrossRef]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar] [CrossRef] [Green Version]
- Ying, B.; Liu, X. Skin-like hydrogel devices for wearable sensing, soft robotics and beyond. iScience 2021, 24, 103174. [Google Scholar] [CrossRef] [PubMed]
- Ghadban, A.; Ahmed, A.S.; Ping, Y.; Ramos, R.; Arfin, N.; Cantaert, B.; Ramanujan, R.V.; Miserez, A. Bioinspired pH and magnetic responsive catechol-functionalized chitosan hydrogels with tunable elastic properties. Chem. Commun. 2016, 52, 697–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT:PSS hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Bai, R.; Suo, Z. Topological Adhesion of Wet Materials. Adv. Mater. 2018, 30, e1800671. [Google Scholar] [CrossRef] [PubMed]
- Devaki, S.J.; Narayanan, R.K.; Sarojam, S. Electrically conducting silver nanoparticle–polyacrylic acid hydrogel by in situ reduction and polymerization approach. Mater. Lett. 2014, 116, 135–138. [Google Scholar] [CrossRef]
- Fantino, E.; Chiappone, A.; Roppolo, I.; Manfredi, D.; Bongiovanni, R.; Pirri, C.F.; Calignano, F. 3D Printing of Conductive Complex Structures with In Situ Generation of Silver Nanoparticles. Adv. Mater. 2016, 28, 3712–3717. [Google Scholar] [CrossRef]
- Janovák, L.; Dékány, I. Optical properties and electric conductivity of gold nanoparticle-containing, hydrogel-based thin layer composite films obtained by photopolymerization. Appl. Surface Sci. 2010, 256, 2809–2817. [Google Scholar] [CrossRef]
- Navaei, A.; Saini, H.; Christenson, W.; Sullivan, R.T.; Ros, R.; Nikkhah, M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016, 41, 133–146. [Google Scholar] [CrossRef]
- Wei, Q.; Luo, Y.; Zhang, C.; Fan, L.; Chen, Y. Assembly of Cu nanoparticles in a polyacrylamide grafted poly(vinyl alcohol) copolymer matrix and vapor-induced response. Sens. Actuators B Chem. 2008, 134, 49–56. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Han, Y.; Guo, B. Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J. Colloid Interface Sci. 2019, 556, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Hu, T.; Lei, Q.; He, J.; Ma, P.X.; Guo, B. Stimuli-Responsive Conductive Nanocomposite Hydrogels with High Stretchability, Self-Healing, Adhesiveness, and 3D Printability for Human Motion Sensing. ACS Appl. Mater. Interfaces 2019, 11, 6796–6808. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Shin, S.R.; Tamayol, A.; Miscuglio, M.; Bakooshli, M.A.; Assmann, A.; Mostafalu, P.; Sun, J.Y.; Mithieux, S.; Cheung, L.; et al. Highly Elastic and Conductive Human-Based Protein Hybrid Hydrogels. Adv. Mater. 2016, 28, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Liu, D.; Wang, Y.; Cheng, C.; Zhou, K.; Ding, J.; Truong, V.T.; Li, D. Mechanically robust, electrically conductive and stimuli-responsive binary network hydrogels enabled by superelastic graphene aerogels. Adv. Mater. 2014, 26, 3333–3337. [Google Scholar] [CrossRef]
- Dong, R.; Zhao, X.; Guo, B. Self-healing Conductive Injectable Hydrogels with Anti-bacterial Activity as Cell Delivery Carrier for Cardiac Cell Therapy. ACS Appl. Mater. Interfaces 2016, 8, 17138–17150. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Ma, P.X.; Guo, B. Rapid thermal responsive conductive hybrid cryogels with shape memory properties, photothermal properties and pressure dependent conductivity. J. Colloid Interface Sci. 2018, 526, 281–294. [Google Scholar] [CrossRef]
- Han, L.; Yan, L.; Wang, M.; Wang, K.; Fang, L.; Zhou, J.; Fang, J.; Ren, F.; Lu, X. Transparent, Adhesive, and Conductive Hydrogel for Soft Bioelectronics Based on Light-Transmitting Polydopamine-Doped Polypyrrole Nanofibrils. Chem. Mater. 2018, 30, 5561–5572. [Google Scholar] [CrossRef]
- Gotovtsev, P.M.; Badranova, G.U.; Zubavichus, Y.V.; Chumakov, N.K.; Antipova, C.G.; Kamyshinsky, R.A.; Presniakov, M.Y.; Tokaev, K.V.; Grigoriev, T.E. Electroconductive PEDOT:PSS-based hydrogel prepared by freezing-thawing method. Heliyon 2019, 5, e02498. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A Nanostructured Conductive Hydrogels-Based Biosensor Platform for Human Metabolite Detection. Nano Lett. 2015, 15, 1146–1151. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, K.Q.; Wan, P.B. A Flexible Stretchable Hydrogel Electrolyte for Healable All-in-One Configured Supercapacitors. Small 2018, 14, 1704497. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Li, C.; Sun, X.; Meng, Q.; Ma, Y.; Wei, Z. Chemically Crosslinked Hydrogel Film Leads to Integrated Flexible Supercapacitors with Superior Performance. Adv. Mater. 2015, 27, 7451–7457. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhong, M.; Shi, F.K.; Liu, X.Y.; Tang, Z.J.; Wang, Y.K.; Huang, Y.; Hou, H.Q.; Xie, X.M.; Zhi, C.Y. An Intrinsically Stretchable and Compressible Supercapacitor Containing a Polyacrylamide Hydrogel Electrolyte. Angew. Chem. Int. Edit. 2017, 56, 9141–9145. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, G.; Liang, Y.; Cheng, T.; Dai, J.; Yang, X.; Liu, B.; Zeng, Z.; Huang, Z.; Luo, Y.; et al. Fast-moving soft electronic fish. Sci. Adv. 2017, 3, e1602045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.Q.; Zheng, R.M.; Chen, S.; Wu, Y.H.; Liu, H.Z.; Wang, P.P.; Deng, Z.F.; Liu, L. A compliant, self-adhesive and self-healing wearable hydrogel as epidermal strain sensor. J. Mater. Chem. C 2018, 6, 4183–4190. [Google Scholar] [CrossRef]
- Morelle, X.P.; Illeperuma, W.R.; Tian, K. Highly Stretchable and Tough Hydrogels below Water Freezing Temperature. Adv. Mater. 2018, 30, 1801541. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Wang, L.H.; Wang, X.Q.; Tang, N.; Yu, J.Y.; Ding, B. Ultrahigh-Water-Content, Superelastic, and Shape-Memory Nanofiber-Assembled Hydrogels Exhibiting Pressure-Responsive Conductivity. Adv. Mater. 2017, 29, 1700339. [Google Scholar] [CrossRef]
- Liu, S.L.; Li, K.W.; Hussain, I.; Oderinde, O.; Yao, F.; Zhang, J.Y.; Fu, G.D. A Conductive Self-Healing Double Network Hydrogel with Toughness and Force Sensitivity. Chem. A Eur. J. 2018, 24, 6632–6638. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Lin, Y.-J.; Enriquez, E.; Peng, X.-F.; Turng, L.-S. Highly Stretchable and Biocompatible Strain Sensors Based on Mussel-Inspired Super-Adhesive Self-Healing Hydrogels for Human Motion Monitoring. ACS Appl. Mater. Interfaces 2018, 10, 20897–20909. [Google Scholar] [CrossRef]
- Liu, X.H.; Wu, D.B.; Wang, H.L.; Wang, Q.G. Self-Recovering Tough Gel Electrolyte with Adjustable Supercapacitor Performance. Adv. Mater. 2014, 26, 4370–4375. [Google Scholar] [CrossRef]
- Yao, B.; Wang, H.; Zhou, Q.; Wu, M.; Zhang, M.; Li, C.; Shi, G. Ultrahigh-Conductivity Polymer Hydrogels with Arbitrary Structures. Adv. Mater. 2017, 29, 1700974. [Google Scholar] [CrossRef]
- Tai, Y.; Mulle, M.; Aguilar Ventura, I.; Lubineau, G. A highly sensitive, low-cost, wearable pressure sensor based on conductive hydrogel spheres. Nanoscale 2015, 7, 14766–14773. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Mi, H.-Y.; Peng, X.-F.; Turng, L.-S. Biocompatible, self-healing, highly stretchable polyacrylic acid/reduced graphene oxide nanocomposite hydrogel sensors via mussel-inspired chemistry. Carbon 2018, 136, 63–72. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Pan, L.; Ding, Y.; Zhao, Y.; Li, Y.; Shi, Y.; Yu, G. Dopant-Enabled Supramolecular Approach for Controlled Synthesis of Nanostructured Conductive Polymer Hydrogels. Nano Lett. 2015, 15, 7736–7741. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically conductive hydrogels for flexible energy storage systems. Progress Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Gibot, L.; Fourquaux, I.; Golzio, M.; Rols, M.-P. Electric field-responsive nanoparticles and electric fields: Physical, chemical, biological mechanisms and therapeutic prospects. Adv. Drug Deliv. Rev. 2019, 138, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Ma, P.X.; Guo, B. Conductive biomaterials for muscle tissue engineering. Biomaterials 2020, 229, 119584. [Google Scholar] [CrossRef]
- Peng, Q.; Chen, J.; Wang, T.; Peng, X.; Liu, J.; Wang, X.; Wang, J.; Zeng, H. Recent advances in designing conductive hydrogels for flexible electronics. InfoMat 2020, 2, 843–865. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Narain, R.; Zeng, H. Rational Design of Self-Healing Tough Hydrogels: A Mini Review. Front. Chem. 2018, 6, 497. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Chen, J.; Wang, J.; Zeng, H.; Yu, J. Recent progress in synthesis and application of mussel-inspired adhesives. Nanoscale 2020, 12, 1307–1324. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Yang, J. Novel Mussel-Inspired Injectable Self-Healing Hydrogel with Anti-Biofouling Property. Adv. Mater. 2015, 27, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wei, W.; Danner, E.; Ashley, R.K.; Israelachvili, J.N.; Waite, J.H. Mussel protein adhesion depends on interprotein thiol-mediated redox modulation. Nat. Chem. Biol. 2011, 7, 588–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.L.; Ding, X.B.; Deng, Z.H.; Zheng, Z.H.; Peng, Y.X.; Long, X.P. Thermo switchable electronic properties of a gold nanoparticle/hydrogel composite. Macromol. Rapid Commun. 2005, 26, 1784–1787. [Google Scholar] [CrossRef]
- Walker, B.W.; Lara, R.P.; Mogadam, E.; Yu, C.H.; Kimball, W.; Annabi, N. Rational Design of Microfabricated Electroconductive Hydrogels for Biomedical Applications. Prog. Polym. Sci. 2019, 92, 135–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.X.; Lin, Z.Y.; Huang, X.Q.; Wang, Y.; Huang, Y.; Duan, X.F. Functionalized Graphene Hydrogel-Based High-Performance Supercapacitors. Adv. Mater. 2013, 25, 5779–5784. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.L.; Wu, G.Z.; Zhou, H.T.; Qian, K.; Hu, J.L. Preparation and Property Evaluation of Conductive Hydrogel Using Poly (Vinyl Alcohol)/Polyethylene Glycol/Graphene Oxide for Human Electrocardiogram Acquisition. Polymers 2017, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Mano, N.; Yoo, J.E.; Tarver, J.; Loo, Y.-L.; Heller, A. An Electron-Conducting Cross-Linked Polyaniline-Based Redox Hydrogel, Formed in One Step at pH 7.2, Wires Glucose Oxidase. J. Am. Chem. Soc. 2007, 129, 7006–7007. [Google Scholar] [CrossRef]
- Yang, C.; Suo, Z. Hydrogel ionotronics. Nat. Rev. Mater. 2018, 3, 125–142. [Google Scholar] [CrossRef]
- Chen, B.H.; Bai, Y.Y.; Xiang, F.; Sun, J.Y.; Chen, Y.M.; Wang, H.; Zhou, J.X.; Suo, Z.G. Stretchable and Transparent Hydrogels as Soft Conductors for Dielectric Elastomer Actuators. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1055–1060. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, B.; Jiang, W.; Yang, Y.; Leow, W.R.; Wang, H.; Chen, X. A mechanically and electrically self-healing supercapacitor. Adv. Mater. 2014, 26, 3638–3643. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Huang, Y.; Zhi, C. Nanostructured Polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Billiet, T.; Vandenhaute, M.; Schelfhout, J.; Van Vlierberghe, S.; Dubruel, P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials 2012, 33, 6020–6041. [Google Scholar] [CrossRef] [PubMed]
- Rong, Q.; Lei, W.; Liu, M. Conductive Hydrogels as Smart Materials for Flexible Electronic Devices. Chemistry 2018, 24, 16930–16943. [Google Scholar] [CrossRef]
- Tran, V.T.; Mredha, M.T.I.; Pathak, S.K.; Yoon, H.; Cui, J.X.; Jeon, I. Conductive Tough Hydrogels with a Staggered Ion-Coordinating Structure for High Self-Recovery Rate. ACS Appl. Mater. Interfaces 2019, 11, 24598–24608. [Google Scholar] [CrossRef] [PubMed]
- Hajikarimi, P.; Rahi, M.; Nejad, F.M.; Ashourabadi, E.B.; Maniei, S.; MohammadGhasemi, P.; Fini, E.H. Introduction of Polymer Nanocomposites to Bitumen to Enhance its Thermomechanical Properties. J. Transp. Eng. Part B Pavements 2021, 147, 4021020. [Google Scholar] [CrossRef]
- Juliana Matos Seidel, S.M.M. Synthesis of PolyHEMA Hydrogels for Using as Biomaterials. Bulk and Solution Radical-Initiated Polymerization Techniques. Mater. Res. 2000, 3, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.L.; Liu, D.M.; Tian, D.; Zhang, X.Y.; Wu, W.; Wan, W.M. The introduction of the Barbier reaction into polymer chemistry. Nat. Commun. 2017, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ding, P.; Chang, B.S.; Qing, G.Y.; Sun, T.L. New approach for chiral separation: From polysaccharide-based materials to chirality-responsive polymers. Sci. China-Chem. 2014, 57, 1492–1506. [Google Scholar] [CrossRef]
- Liu, J.-L.; Eisenberg, B. Poisson–Fermi model of single ion activities in aqueous solutions. Chem. Physics Lett. 2015, 637, 1–6. [Google Scholar] [CrossRef]
- Jacobsen, E.K. JCE Resources for Chemistry and the Home. J. Chem. Ed. 2006, 83, 1444. [Google Scholar] [CrossRef]
- Zhai, M.; Xu, Y.; Zhou, B.; Jing, W. Keratin-chitosan/n-ZnO nanocomposite hydrogel for antimicrobial treatment of burn wound healing: Characterization and biomedical application. J. Photochem. Photobiol. B 2018, 180, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Huang, M.; Sun, D.; Hou, Y.; Li, Y.; Dong, T.; Wang, X.; Zhang, L.; Yang, W. Dual Physically Cross-Linked kappa-Carrageenan-Based Double Network Hydrogels with Superior Self-Healing Performance for Biomedical Application. ACS Appl. Mater. Interfaces 2018, 10, 37544–37554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauri, K.; Nandi, M.; De, P. Amino acid-derived stimuli-responsive polymers and their applications. Polymer Chemistry 2018, 9, 1257–1287. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Zhang, X.N.; Song, Y.; Zhao, Y.; Chen, L.; Su, F.; Li, L.; Wu, Z.L.; Zheng, Q. Ultrastiff and Tough Supramolecular Hydrogels with a Dense and Robust Hydrogen Bond Network. Chem. Mater. 2019, 31, 1430–1440. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, M.; Zhang, L.; He, B.; Chen, X.; Sun, J. Facile synthesis of self-healing and layered sodium alginate/polyacrylamide hydrogel promoted by dynamic hydrogen bond. Carbohydr. Polym. 2021, 256, 117580. [Google Scholar] [CrossRef]
- Palmese, L.L.; Thapa, R.K.; Sullivan, M.O.; Kiick, K.L. Hybrid hydrogels for biomedical applications. Curr. Opin. Chem. Eng. 2019, 24, 143–157. [Google Scholar] [CrossRef]
- Chang, B.; Ahuja, N.; Ma, C.; Liu, X. Injectable scaffolds: Preparation and application in dental and craniofacial regeneration. Mater. Sci. Eng. R Rep. 2017, 111, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Nian, G.; Yang, C.; Qu, S.; Suo, Z. Bonding dissimilar polymer networks in various manufacturing processes. Nat. Commun. 2018, 9, 846. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Yu, X.; Wang, C.; Wang, Z. Synthesis and characterization of a novel double cross-linked hydrogel based on Diels-Alder click reaction and coordination bonding. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Chang, H.; Wang, M.; Xu, F.; Yang, J. High-Strength, Tough, and Self-Healing Nanocomposite Physical Hydrogels Based on the Synergistic Effects of Dynamic Hydrogen Bond and Dual Coordination Bonds. ACS Appl. Mater Interfaces 2017, 9, 28305–28318. [Google Scholar] [CrossRef] [PubMed]
- Mredha, M.T.I.; Pathak, S.K.; Tran, V.T.; Cui, J.; Jeon, I. Hydrogels with superior mechanical properties from the synergistic effect in hydrophobic–hydrophilic copolymers. Chem. Eng. J. 2019, 362, 325–338. [Google Scholar] [CrossRef]
- Zhang, X.N.; Wang, Y.J.; Sun, S.; Hou, L.; Wu, P.; Wu, Z.L.; Zheng, Q. A Tough and Stiff Hydrogel with Tunable Water Content and Mechanical Properties Based on the Synergistic Effect of Hydrogen Bonding and Hydrophobic Interaction. Macromolecules 2018, 51, 8136–8146. [Google Scholar] [CrossRef]
- Liang, Y.; Xue, J.; Du, B.; Nie, J. Ultrastiff, Tough, and Healable Ionic-Hydrogen Bond Cross-Linked Hydrogels and Their Uses as Building Blocks To Construct Complex Hydrogel Structures. ACS Appl. Mater. Interfaces 2019, 11, 5441–5454. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wang, Z.; Xiao, Y. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, S.; Zhang, C.; Liang, K.; Li, J.; Yang, H.; Gu, S.; Bai, Z.; Ye, D.; Xu, W. Photopolymerized maleilated chitosan/thiol-terminated poly (vinyl alcohol) hydrogels as potential tissue engineering scaffolds. Carbohydr. Polym. 2018, 184, 383–389. [Google Scholar] [CrossRef]
- Brown, T.E.; Carberry, B.J.; Worrell, B.T.; Dudaryeva, O.Y.; McBride, M.K.; Bowman, C.N.; Anseth, K.S. Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials 2018, 178, 496–503. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, K.; Zhao, S.; Zhang, C.; Li, J.; Yang, H.; Liu, X.; Yin, X.; Chen, D.; Xu, W.; et al. Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2018, 108, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Recent advances in photo-crosslinkable hydrogels for biomedical applications. Biotechniques 2019, 66, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Ranga, A.; Lutolf, M.P.; Hilborn, J.; Ossipov, D.A. Hyaluronic Acid Hydrogels Formed in Situ by Transglutaminase-Catalyzed Reaction. Biomacromolecules 2016, 17, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Miller, A.L., 2nd; Xu, H.; Waletzki, B.E.; Lu, L. Injectable Catalyst-Free Poly(Propylene Fumarate) System Cross-Linked by Strain Promoted Alkyne-Azide Cycloaddition Click Chemistry for Spine Defect Filling. Biomacromolecules 2019, 20, 3352–3365. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, N.; Ma, M. Electroconductive hydrogels for biomedical applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1568. [Google Scholar] [CrossRef] [PubMed]
- Sadat Ebrahimi, M.M.; Schönherr, H. Enzyme-Sensing Chitosan Hydrogels. Langmuir 2014, 30, 7842–7850. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; An, Y.-H.; Kim, H.D.; Kim, K.; Lee, S.-H.; Yim, H.-G.; Kim, B.-G.; Hwang, N.S. Enzyme-mediated tissue adhesive hydrogels for meniscus repair. Int. J. Biol. Macromol. 2018, 110, 479–487. [Google Scholar] [CrossRef]
- Chou, P.-Y.; Chen, S.-H.; Chen, C.-H.; Chen, S.-H.; Fong, Y.T.; Chen, J.-P. Thermo-responsive in-situ forming hydrogels as barriers to prevent post-operative peritendinous adhesion. Acta Biomater. 2017, 63, 85–95. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Zhao, X. Multifunctional Stimuli-Responsive Hydrogels with Self-Healing, High Conductivity, and Rapid Recovery through Host–Guest Interactions. Chem. Mater. 2018, 30, 1729–1742. [Google Scholar] [CrossRef]
- Xiang, Y.; Chen, D. Preparation of a novel pH-responsive silver nanoparticle/poly(HEMA–PEGMA–MAA) composite hydrogel. Eur. Polym. J. 2007, 43, 4178–4187. [Google Scholar] [CrossRef]
- Han, L.; Liu, K.; Wang, M.; Wang, K.; Fang, L.; Chen, H.; Zhou, J.; Lu, X. Mussel-Inspired Adhesive and Conductive Hydrogel with Long-Lasting Moisture and Extreme Temperature Tolerance. Adv. Funct. Mater. 2018, 28, 1704195. [Google Scholar] [CrossRef]
- Lijia, P.; Guihua, Y.; Dongyuan, Z.; Hye Ryoung, L.; Wenting, Z.; Nian, L.; Huiliang, W.; Tee, B.C.K.; Yi, S.; Yi, C.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef] [Green Version]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.L.; Qu, J.; Zhao, X.; Zhang, M.Y. Degradable conductive self-healing hydrogels based on dextran-graft-tetraaniline and N-carboxyethyl chitosan as injectable carriers for myoblast cell therapy and muscle regeneration. Acta Biomater. 2019, 84, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Bilalis, P.; Skoulas, D.; Karatzas, A.; Marakis, J.; Stamogiannos, A.; Tsimblouli, C.; Sereti, E.; Stratikos, E.; Dimas, K.; Vlassopoulos, D.; et al. Self-Healing pH- and Enzyme Stimuli-Responsive Hydrogels for Targeted Delivery of Gemcitabine To Treat Pancreatic Cancer. Biomacromolecules 2018, 19, 3840–3852. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pei, Y.; He, C.; Chen, L. Synthesis of novel thermo- and redox-sensitive polypeptide hydrogels. Polym. Int. 2017, 66, 712–718. [Google Scholar] [CrossRef]
- Hebeish, A.; Farag, S.; Sharaf, S.; Shaheen, T.I. Radically new cellulose nanocomposite hydrogels: Temperature and pH responsive characters. Int. J. Biol. Macromol. 2015, 81, 356–361. [Google Scholar] [CrossRef]
- Augurio, A.; Cortelletti, P.; Tognato, R.; Rios, A.; Levato, R.; Malda, J.; Alini, M.; Eglin, D.; Giancane, G.; Speghini, A.; et al. A Multifunctional Nanocomposite Hydrogel for Endoscopic Tracking and Manipulation. Adv. Intell. Syst. 2020, 2, 1900105. [Google Scholar] [CrossRef] [Green Version]
- Kabb, C.P.; O’Bryan, C.S.; Deng, C.C.; Angelini, T.E.; Sumerlin, B.S. Photoreversible Covalent Hydrogels for Soft-Matter Additive Manufacturing. ACS Appl. Mater. Interfaces 2018, 10, 16793–16801. [Google Scholar] [CrossRef]
- Feng, G.; Zha, Z.; Huang, Y.; Li, J.; Wang, Y.; Ke, W.; Chen, H.; Liu, L.; Song, Y.; Ge, Z. Sustained and Bioresponsive Two-Stage Delivery of Therapeutic miRNA via Polyplex Micelle-Loaded Injectable Hydrogels for Inhibition of Intervertebral Disc Fibrosis. Adv. Healthc. Mater. 2018, 7, 1800623. [Google Scholar] [CrossRef]
- Xu, C.; Guan, S.; Wang, S.; Gong, W.; Liu, T.; Ma, X.; Sun, C. Biodegradable and electroconductive poly(3,4-ethylenedioxythiophene)/carboxymethyl chitosan hydrogels for neural tissue engineering. Mater. Sci. Eng. C 2018, 84, 32–43. [Google Scholar] [CrossRef]
- Dong, M.; Shi, B.; Liu, D.; Liu, J.-H.; Zhao, D.; Yu, Z.-H.; Shen, X.-Q.; Gan, J.-M.; Shi, B.-l.; Qiu, Y.; et al. Conductive Hydrogel for a Photothermal-Responsive Stretchable Artificial Nerve and Coalescing with a Damaged Peripheral Nerve. ACS Nano 2020, 14, 16565–16575. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-K.; Sharma, R.; Ma, H.; Chen, W.-S.; Yao, C.-L. In situ polymerizable hydrogel incorporated with specific pathogen-free porcine platelet-rich plasma for the reconstruction of the corneal endothelium. J. Taiwan Inst. Chem. Eng. 2017, 78, 65–74. [Google Scholar] [CrossRef]
- Bush, J.R.; Liang, H.; Dickinson, M.; Botchwey, E.A. Xylan hemicellulose improves chitosan hydrogel for bone tissue regeneration. Polym. Adv. Technol. 2016, 27, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, X.; Xu, Q.; Lu, Y.; Zhang, Y.; Xia, H.; Lu, A.; Zhang, L. Rubbery Chitosan/Carrageenan Hydrogels Constructed through an Electroneutrality System and Their Potential Application as Cartilage Scaffolds. Biomacromolecules 2018, 19, 340–352. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Q.; Song, S.; Duan, L.; Gao, G. Bioinspired Dynamic Cross-Linking Hydrogel Sensors with Skin-like Strain and Pressure Sensing Behaviors. Chem. Mater. 2019, 31, 9522–9531. [Google Scholar] [CrossRef]
- Lim, S.L.; Ooi, C.-W.; Low, L.E.; Tan, W.S.; Chan, E.-S.; Ho, K.L.; Tey, B.T. Synthesis of poly(acrylamide)-based hydrogel for bio-sensing of hepatitis B core antigen. Mater. Chem. Phys. 2020, 243, 122578. [Google Scholar] [CrossRef]
- Gan, D.; Han, L.; Wang, M.; Xing, W.; Xu, T.; Zhang, H.; Wang, K.; Fang, L.; Lu, X. Conductive and Tough Hydrogels Based on Biopolymer Molecular Templates for Controlling in Situ Formation of Polypyrrole Nanorods. ACS Appl. Mater. Interfaces 2018, 10, 36218–36228. [Google Scholar] [CrossRef]
- Souza, S.F.; Kogikoski, S.; Silva, E.R.; Alves, W.A. Nanostructured Antigen-Responsive Hydrogels Based on Peptides for Leishmaniasis Detection. J. Braz. Chem. Soc. 2017, 28, 1619–1629. [Google Scholar] [CrossRef]
- Huebsch, N.; Kearney, C.J.; Zhao, X.; Kim, J.; Cezar, C.A.; Suo, Z.; Mooney, D.J. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc. Natl. Acad. Sci. USA 2014, 111, 9762–9767. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jiang, R.; Fang, A.; Zhao, Y.; Wu, T.; Cao, X.; Liang, P.; Xia, D.; Zhang, G. A highly transparent, elastic, injectable sericin hydrogel induced by ultrasound. Polym. Test. 2019, 77, 105890. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, K.; Huang, S.; Yang, C.; Wang, M. Near-Infrared Light-Responsive Semiconductor Polymer Composite Hydrogels: Spatial/Temporal-Controlled Release via a Photothermal “Sponge” Effect. ACS Appl. Mater. Interfaces 2017, 9, 13602–13610. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.-Q.; Luo, L.-Z.; Xue, P.-P.; Han, Y.-H.; Wang, L.-F.; Zhuge, D.-L.; Yao, Q.; Chen, B.; Zhao, Y.-Z.; Xu, H.-L. Glucose-responsive hydrogel enhances the preventive effect of insulin and liraglutide on diabetic nephropathy of rats. Acta Biomater. 2021, 122, 111–132. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.M.; Kumar, A.; Han, S.S. Poly(acrylamidoglycolic acid) nanocomposite hydrogels reinforced with cellulose nanocrystals for pH-sensitive controlled release of diclofenac sodium. Polym. Test. 2017, 64, 175–182. [Google Scholar] [CrossRef]
- Zhang, K.; Lv, H.; Zheng, Y.; Yao, Y.; Li, X.; Yu, J.; Ding, B. Nanofibrous hydrogels embedded with phase-change materials: Temperature-responsive dressings for accelerating skin wound healing. Compos. Commun. 2021, 25, 100752. [Google Scholar] [CrossRef]
- Rasool, A.; Ata, S.; Islam, A. Stimuli responsive biopolymer (chitosan) based blend hydrogels for wound healing application. Carbohydr. Polym. 2019, 203, 423–429. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Napiwocki, B.N.; Peng, X.-F.; Turng, L.-S. Mussel-inspired electroactive chitosan/graphene oxide composite hydrogel with rapid self-healing and recovery behavior for tissue engineering. Carbon 2017, 125, 557–570. [Google Scholar] [CrossRef]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Le, P.; Lai, K.; Fernandes-Cunha, G.M.; Myung, D. Simultaneous Interpenetrating Polymer Network of Collagen and Hyaluronic Acid as an In Situ-Forming Corneal Defect Filler. Chem. Mater. 2020, 32, 5208–5216. [Google Scholar] [CrossRef]
- Yazdanpanah, G.; Shen, X.; Nguyen, T.; Anwar, K.N.; Jeon, O.; Jiang, Y.; Pachenari, M.; Pan, Y.; Shokuhfar, T.; Rosenblatt, M.I.; et al. A Light-Curable and Tunable Extracellular Matrix Hydrogel for In Situ Suture-Free Corneal Repair. Adv. Funct. Mater. 2022, 2113383. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Wang, Y.; Liu, Z.; Li, Z.; Li, J.; Chen, Q.; Meng, Q.; Shu, W.W.; Wu, J.; et al. Endothelialized microvessels fabricated by microfluidics facilitate osteogenic differentiation and promote bone repair. Acta Biomater. 2022, 142, 85–98. [Google Scholar] [CrossRef]
- Ishikawa, S.; Iijima, K.; Matsukuma, D.; Asawa, Y.; Hoshi, K.; Osawa, S.; Otsuka, H. Interpenetrating Polymer Network Hydrogels via a One-Pot and in Situ Gelation System Based on Peptide Self-Assembly and Orthogonal Cross-Linking for Tissue Regeneration. Chem. Mater. 2020, 32, 2353–2364. [Google Scholar] [CrossRef]
- Hua, L.; Zhao, C.; Guan, X.; Lu, J.; Zhang, J. Cold-induced shape memory hydrogels for strong and programmable artificial muscles. Sci. China Mater. 2022. [Google Scholar] [CrossRef]
- Lawlor, K.T.; Vanslambrouck, J.M.; Higgins, J.W.; Chambon, A.; Bishard, K.; Arndt, D.; Er, P.X.; Wilson, S.B.; Howden, S.E.; Tan, K.S.; et al. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat. Mater. 2021, 20, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Chrisnandy, A.; Blondel, D.; Rezakhani, S.; Broguiere, N.; Lutolf, M.P. Synthetic dynamic hydrogels promote degradation-independent in vitro organogenesis. Nat. Mater. 2021, 21, 479–487. [Google Scholar] [CrossRef]
- Barrs, R.W.; Jia, J.; Ward, M.; Richards, D.J.; Yao, H.; Yost, M.J.; Mei, Y. Engineering a Chemically Defined Hydrogel Bioink for Direct Bioprinting of Microvasculature. Biomacromolecules 2021, 22, 275–288. [Google Scholar] [CrossRef]
- Lee, S.; Sani, E.S.; Spencer, A.R.; Guan, Y.; Weiss, A.S.; Annabi, N. Human-Recombinant-Elastin-Based Bioinks for 3D Bioprinting of Vascularized Soft Tissues. Adv. Mater. 2020, 32, 2003915. [Google Scholar] [CrossRef]
- Zhou, Y.; Fei, X.; Tian, J.; Xu, L.; Li, Y. A ionic liquid enhanced conductive hydrogel for strain sensing applications. J. Colloid Interface Sci. 2022, 606, 192–203. [Google Scholar] [CrossRef]
- Lin, G.; Si, M.; Wang, L.; Wei, S.; Lu, W.; Liu, H.; Zhang, Y.; Li, D.; Chen, T. Dual-Channel Flexible Strain Sensors Based on Mechanofluorescent and Conductive Hydrogel Laminates. Adv. Opt. Mater. 2022, 10, 2102306. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Wu, Z.; Zhang, Y.; Lin, J.; Chen, T.; Liu, H.; Wang, F.; Sun, L. Wearable multichannel pulse condition monitoring system based on flexible pressure sensor arrays. Microsyst. Nanoeng. 2022, 8, 16. [Google Scholar] [CrossRef]
- Qin, Y.; Mo, J.; Liu, Y.; Zhang, S.; Wang, J.; Fu, Q.; Wang, S.; Nie, S. Stretchable Triboelectric Self-Powered Sweat Sensor Fabricated from Self-Healing Nanocellulose Hydrogels. Adv. Funct. Mater. 2022. [Google Scholar] [CrossRef]
- Lin, X.; Li, F.; Bing, Y.; Fei, T.; Liu, S.; Zhao, H.; Zhang, T. Biocompatible Multifunctional E-Skins with Excellent Self-Healing Ability Enabled by Clean and Scalable Fabrication. Nano-Micro Lett. 2021, 13, 200. [Google Scholar] [CrossRef]
- Su, G.; Zhang, Y.; Zhang, X.; Feng, J.; Cao, J.; Zhang, X.; Zhou, T. Soft yet Tough: A Mechanically and Functionally Tissue-like Organohydrogel for Sensitive Soft Electronics. Chem. Mater. 2022, 34, 1392–1402. [Google Scholar] [CrossRef]
- Chun, K.-Y.; Seo, S.; Han, C.-S. A Wearable All-Gel Multimodal Cutaneous Sensor Enabling Simultaneous Single-Site Monitoring of Cardiac-Related Biophysical Signals. Adv. Mater. 2022, e2110082. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, X.; Liu, Y.; Rodrigo, M.; Loftus, P.D.; Aparicio-Valenzuela, J.; Zheng, J.; Pong, T.; Cyr, K.J.; Babakhanian, M.; et al. Intrinsically stretchable electrode array enabled in vivo electrophysiological mapping of atrial fibrillation at cellular resolution. Proc. Natl. Acad. Sci. USA 2020, 117, 14769–14778. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, C.F.; Kim, N.; Zhang, J.X.; Wang, T.M.; Stowe, J.; Nasiri, R.; Li, J.F.; Zhang, D.B.; Yang, A.; et al. Three-dimensional transistor arrays for intra- and inter-cellular recording. Nat. Nanotechnol. 2022, 17, 20. [Google Scholar] [CrossRef]
- Ye, T.; Wang, J.; Jiao, Y.; Li, L.; He, E.; Wang, L.; Li, Y.; Yun, Y.; Li, D.; Lu, J.; et al. A Tissue-Like Soft All-Hydrogel Battery. Adv. Mater. 2022, 34, 2105120. [Google Scholar] [CrossRef]
- Xia, M.; Pan, N.; Zhang, C.; Zhang, C.; Fan, W.; Xia, Y.; Wang, Z.; Sui, K. Self-Powered Multifunction Ionic Skins Based on Gradient Polyelectrolyte Hydrogels. ACS Nano 2022. [Google Scholar] [CrossRef]
- Onaciu, A.; Munteanu, R.A.; Moldovan, A.I.; Moldovan, C.S.; Berindan-Neagoe, I. Hydrogels Based Drug Delivery Synthesis, Characterization and Administration. Pharmaceutics 2019, 11, 432. [Google Scholar] [CrossRef] [Green Version]
- Mauri, E.; Negri, A.; Rebellato, E.; Masi, M.; Perale, G.; Rossi, F. Hydrogel-Nanoparticles Composite System for Controlled Drug Delivery. Gels 2018, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Niu, N.; Yang, N.; Yu, C.; Wang, D.; Tang, B.Z. NIR-II Absorbing Charge Transfer Complexes for Synergistic Photothermal–Chemodynamic Antimicrobial Therapy and Wounds Healing. ACS Mater. Lett. 2022, 4, 692–700. [Google Scholar] [CrossRef]
- Zhao, F.; Fan, S.; Ghate, D.; Romanova, S.; Bronich, T.K.; Zhao, S. A Hydrogel Ionic Circuit Based High-Intensity Iontophoresis Device for Intraocular Macromolecule and Nanoparticle Delivery. Adv. Mater. 2022, 34, e2107315. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, W.; Yang, J.; Wang, J.; Wang, J.; Zhu, G.; Li, D.; Ding, J.; Sun, T. Tumor Microenvironments-Adapted Polypeptide Hydrogel/Nanogel Composite Boosts Antitumor Molecular Targeted Inhibition and Immunoactivation. Adv. Mater. 2022, 2200449. [Google Scholar] [CrossRef] [PubMed]
- Di, Z.; Shi, Z.J.; Ullah, M.W.; Li, S.X.; Yang, G. A transparent wound dressing based on bacterial cellulose whisker and poly(2-hydroxyethyl methacrylate). Int. J. Biol. Macromol. 2017, 105, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, Z.; Zhang, N.; Gao, W.; Li, J.; Pu, Y.; He, B.; Xie, J. A Mg2+/polydopamine composite hydrogel for the acceleration of infected wound healing. Bioactive Mater. 2021, 15, 203–213. [Google Scholar] [CrossRef]
- Jiang, X.; Feng, T.; An, B.; Ren, S.; Meng, J.; Li, K.; Liu, S.; Wu, H.; Zhang, H.; Zhong, C. A Bi-layer Hydrogel Cardiac Patch Made of Recombinant Functional Proteins. Adv. Mater. 2022, 2201411. [Google Scholar] [CrossRef]
- Fan, X.; Luo, Z.; Chen, Y.; Yeo, J.C.C.; Li, Z.; Wu, Y.-L.; He, C. Oxygen self-supplied enzyme nanogels for tumor targeting with amplified synergistic starvation and photodynamic therapy. Acta Biomater. 2022, 142, 274–283. [Google Scholar] [CrossRef]
- Prince, E.; Cruickshank, J.; Ba-Alawi, W.; Hodgson, K.; Haight, J.; Tobin, C.; Wakeman, A.; Avoulov, A.; Topolskaia, V.; Elliott, M.J.; et al. Biomimetic hydrogel supports initiation and growth of patient-derived breast tumor organoids. Nat. Commun. 2022, 13, 1466. [Google Scholar] [CrossRef]
- Guan, L.; Liu, H.; Ren, X.; Wang, T.; Zhu, W.; Zhao, Y.; Feng, Y.; Shen, C.; Zvyagin, A.V.; Fang, L.; et al. Balloon Inspired Conductive Hydrogel Strain Sensor for Reducing Radiation Damage in Peritumoral Organs During Brachytherapy. Adv. Funct. Mater. 2022, 2112281. [Google Scholar] [CrossRef]


| Types of Cross-Linking | Advantages | Disadvantages | Refs |
|---|---|---|---|
| Physical cross-linking (Non-permanent) |
|
| [1,76,77,78] |
| Chemical cross-linking (Permanent) |
|
| [1,78,79,80,81] |
| Major Biomedical Applications | Conductive Hydrogel Polymer | Conductive Hydrogel System | Specific Biomedical Applications | Year | Refs |
|---|---|---|---|---|---|
| Regenerative medicine | Polyethylene glycol (PEG) | miRNA/PGPC polyplex encapsulated in PEG hydrogels (miRNA/PGPC@PEG HG) | Intervertebral disc tissue engineering | 2018 | [109] |
| Carboxymethyl chitosan (CMCH) Poly(3,4-ethylenedioxythiophene) (PEDOT) | Conductive hydrogels (PEDOT/CMCH) | Neural tissue engineering | 2018 | [110] | |
| Polyacrylamide (PAM) | Conducting polymer hydrogel (CPH) based on copolymerized PANI and PAM (PAM/PANI CPH) | Neural tissue engineering | 2020 | [111] | |
| Hyaluronic acid (HA) | HA and Pluronic F-127 (HA-F) | Corneal tissue engineering | 2017 | [112] | |
| Chitosan (CH) | Hemicellulose xylan/CH composite | Osseous tissue engineering | 2016 | [113] | |
| Chitosan (CH) | CH/CG composites | Cartilaginous tissue engineering | 2018 | [114] | |
| Artificial organs | Polypyrrole (PPY) | Multiwalled carbon nanotubes (CNT) Beta-cyclodextrin (beta-CD) N-isopropylacrylamide (NIPAM) | Artificial heart | 2018 | [98] |
| Polyacrylic acid (PAA) | Electrically conducting hydrogel nanocomposite based on silver nanoparticles–polyacrylic acid (PAA) | Artificial muscles | 2014 | [16] | |
| Biosensors | Polyaniline (PANI) | Acid-templated polyaniline (PANI) Poly (ethylene glycol diglycidyl ether) | Glucose biosensor | 2007 | [57] |
| Poly(acrylamide-co-lauryl methacrylate) (P(AAM-co-LMA)) | Hybrid latex nanoparticles (HLPs) crosslinked P(AAM-co-LMA) | Motion/respiration biosensor | 2019 | [115] | |
| Polyacrylamide (PAAM) | Bio-conjugated polyacrylamide-based hydrogel (HBPAAM hydrogel) | Hepatitis B core antigen biosensor | 2020 | [116] | |
| Chitosan (CH) | PAAM–CH–PPy | Wearable biosensor | 2018 | [117] | |
| N-(9-fluorenylmethoxycarbonyl)-L, L-diphenylalanine (Fmoc-FF) | Peptide hydrogels encapsulating leishmania antigen(N-(9-fluorenylmethoxycarbonyl)-L, L-diphenylalanine (Fmoc-FF) encapsulating leishmania antigen) | Antigen biosensor | 2017 | [118] | |
| Drug delivery systems | Alginate (Alg.) | Ionic crosslinked alginate hydrogels (calcium alginate hydrogels) | Drug delivery systems for the treatment of tumors | 2014 | [119] |
| Polyethylene Glycol (PEG) | Sericin/hydrogel scaffold | Controlled drug delivery system | 2019 | [120] | |
| Poly(N-isopropylacrylamide) (PNIPAAM) | Poly(diketopyrrolopyrrole-alt-3,4-ethylenedioxythiophene)-PNIPAAM | Near-infrared light-controlled drug delivery system | 2017 | [121] | |
| Phenylboronic acid-grafted γ-Polyglutamic acid (PBA-PGA) | KGM/PBA-PGA | Insulin (Ins) with liraglutide (Lir) delivery system | 2021 | [122] | |
| Poly(acrylamidoglycolic acid) (PAGA) | Poly(acrylamidoglycolic acid) based nanocomposite (PAGA-NC) | Diclofenac sodium (DCF) delivery system | 2017 | [123] | |
| Wound dressings | Methylacrylate gelatin (GelMA) | GelMA-PDA-ASP nanocomposite hydrogels | Dermal tissue engineering | 2021 | [124] |
| Chitosan (CH) | Silver sulfadiazine loaded CH/PVP | Dermal tissue engineering | 2019 | [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Lin, Z.; Yang, Y.; Jiang, T.; Shang, J.; Luo, Z. Biocompatible Conductive Hydrogels: Applications in the Field of Biomedicine. Int. J. Mol. Sci. 2022, 23, 4578. https://doi.org/10.3390/ijms23094578
Hong Y, Lin Z, Yang Y, Jiang T, Shang J, Luo Z. Biocompatible Conductive Hydrogels: Applications in the Field of Biomedicine. International Journal of Molecular Sciences. 2022; 23(9):4578. https://doi.org/10.3390/ijms23094578
Chicago/Turabian StyleHong, Yang, Zening Lin, Yun Yang, Tao Jiang, Jianzhong Shang, and Zirong Luo. 2022. "Biocompatible Conductive Hydrogels: Applications in the Field of Biomedicine" International Journal of Molecular Sciences 23, no. 9: 4578. https://doi.org/10.3390/ijms23094578
APA StyleHong, Y., Lin, Z., Yang, Y., Jiang, T., Shang, J., & Luo, Z. (2022). Biocompatible Conductive Hydrogels: Applications in the Field of Biomedicine. International Journal of Molecular Sciences, 23(9), 4578. https://doi.org/10.3390/ijms23094578







