Anti-Inflammatory and Anti-Fibrotic Effect of Immortalized Mesenchymal-Stem-Cell-Derived Conditioned Medium on Human Lung Myofibroblasts and Epithelial Cells
Abstract
1. Introduction
2. Results
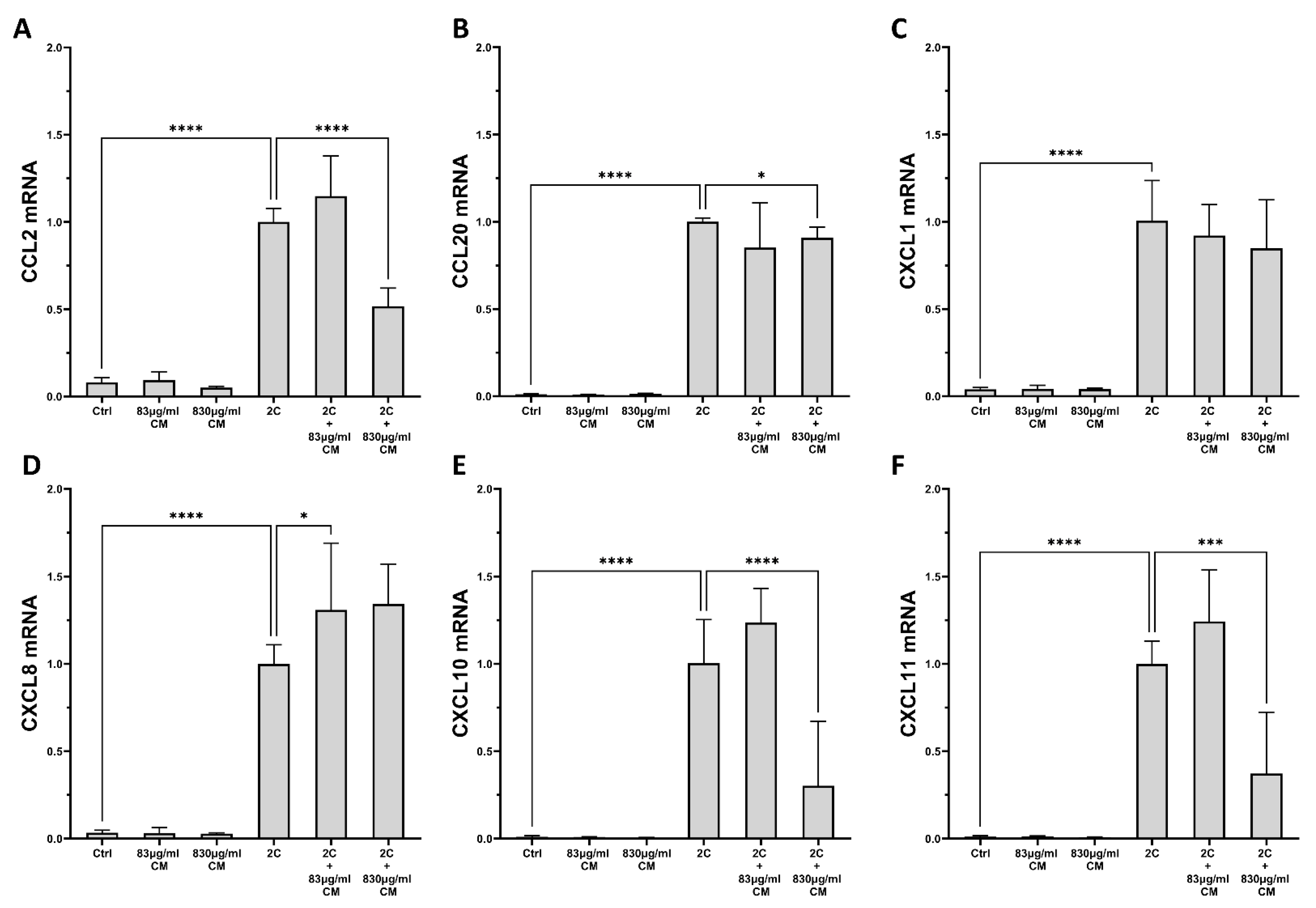
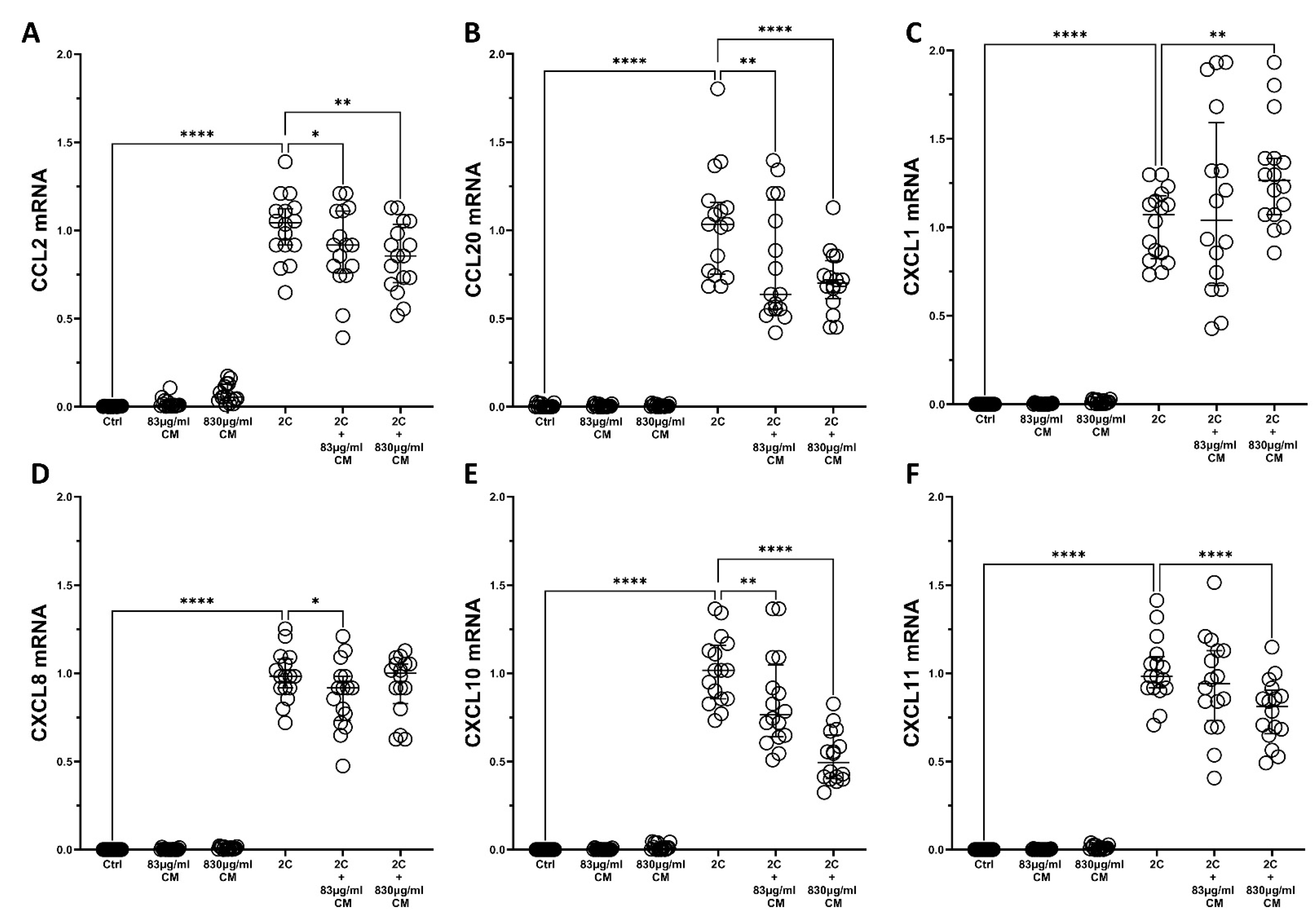
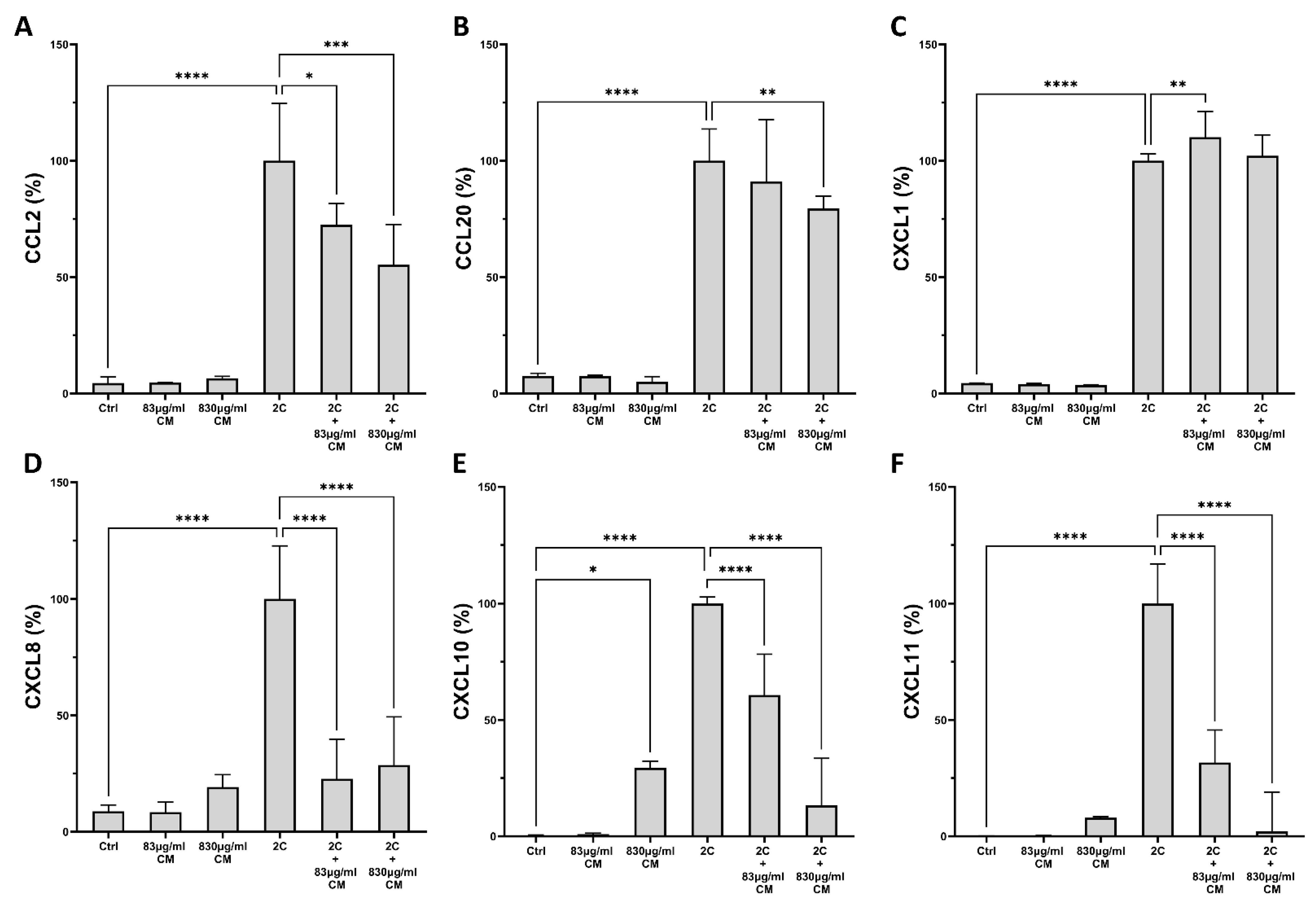
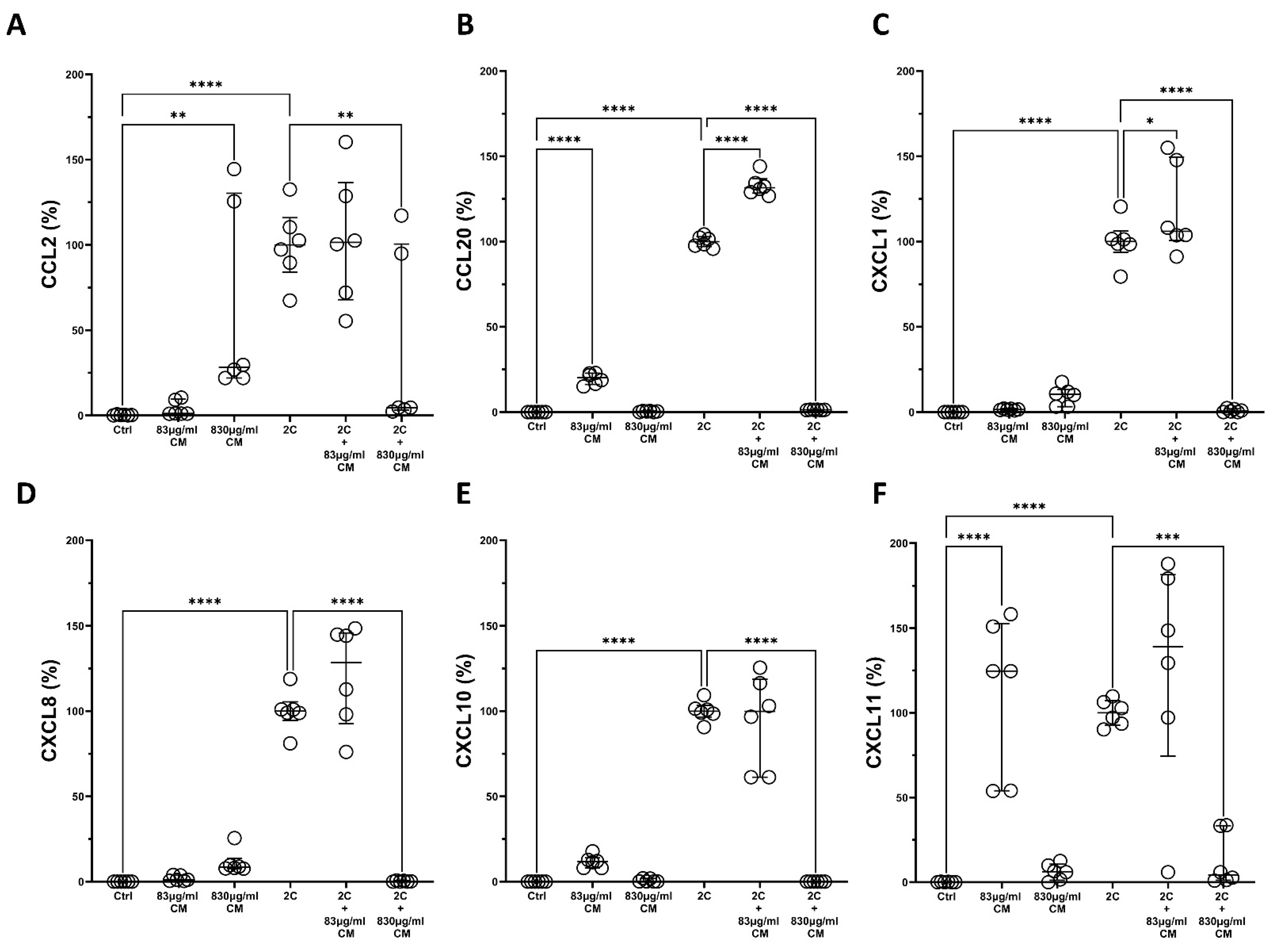
2.1. The Conditioned Medium Inhibits the IL-1α- and TNF-α-Induced mRNA and Protein Chemokine Production in the Pulmonary Epithelial Cell Line A549 and in hPSMs
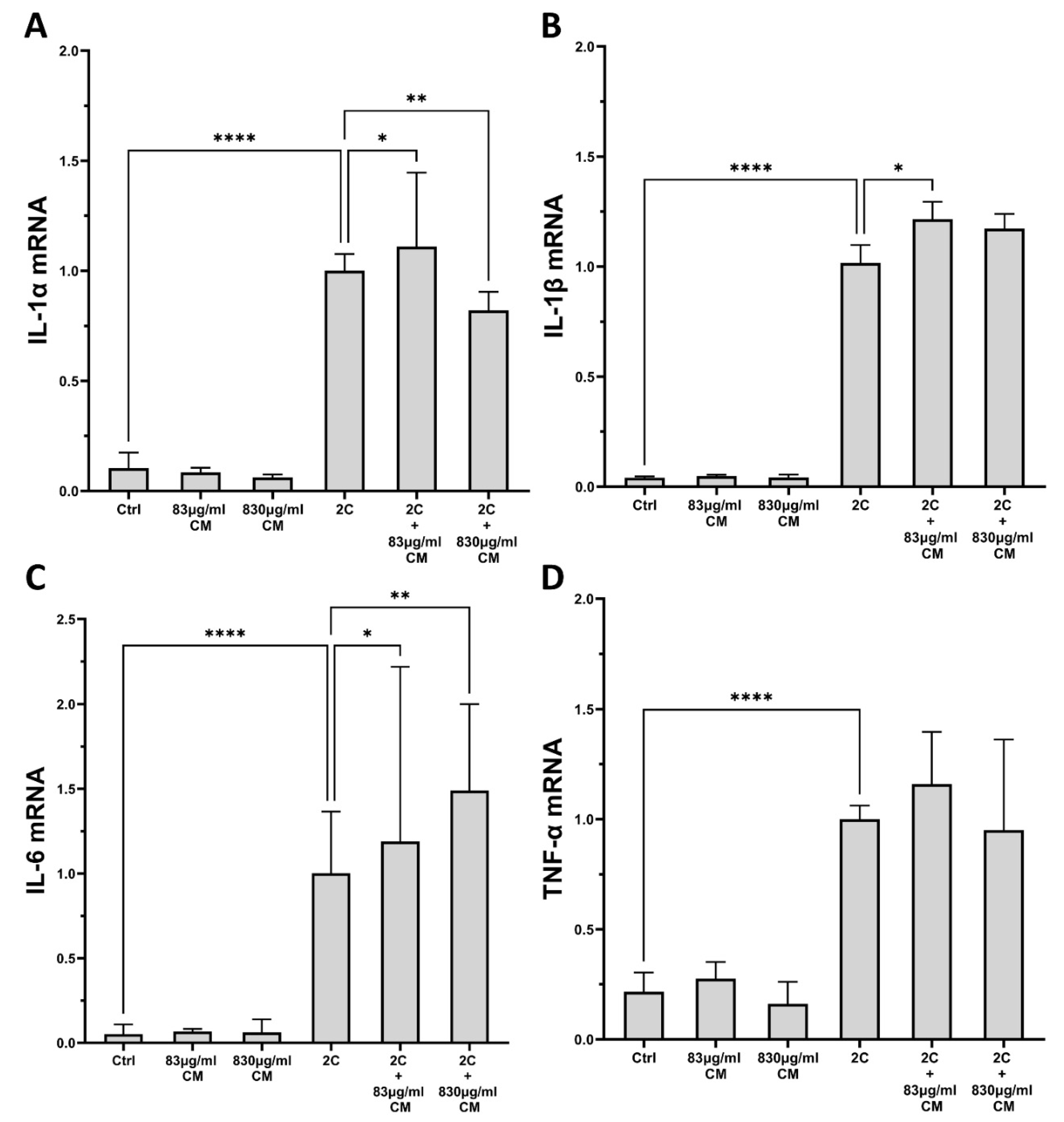
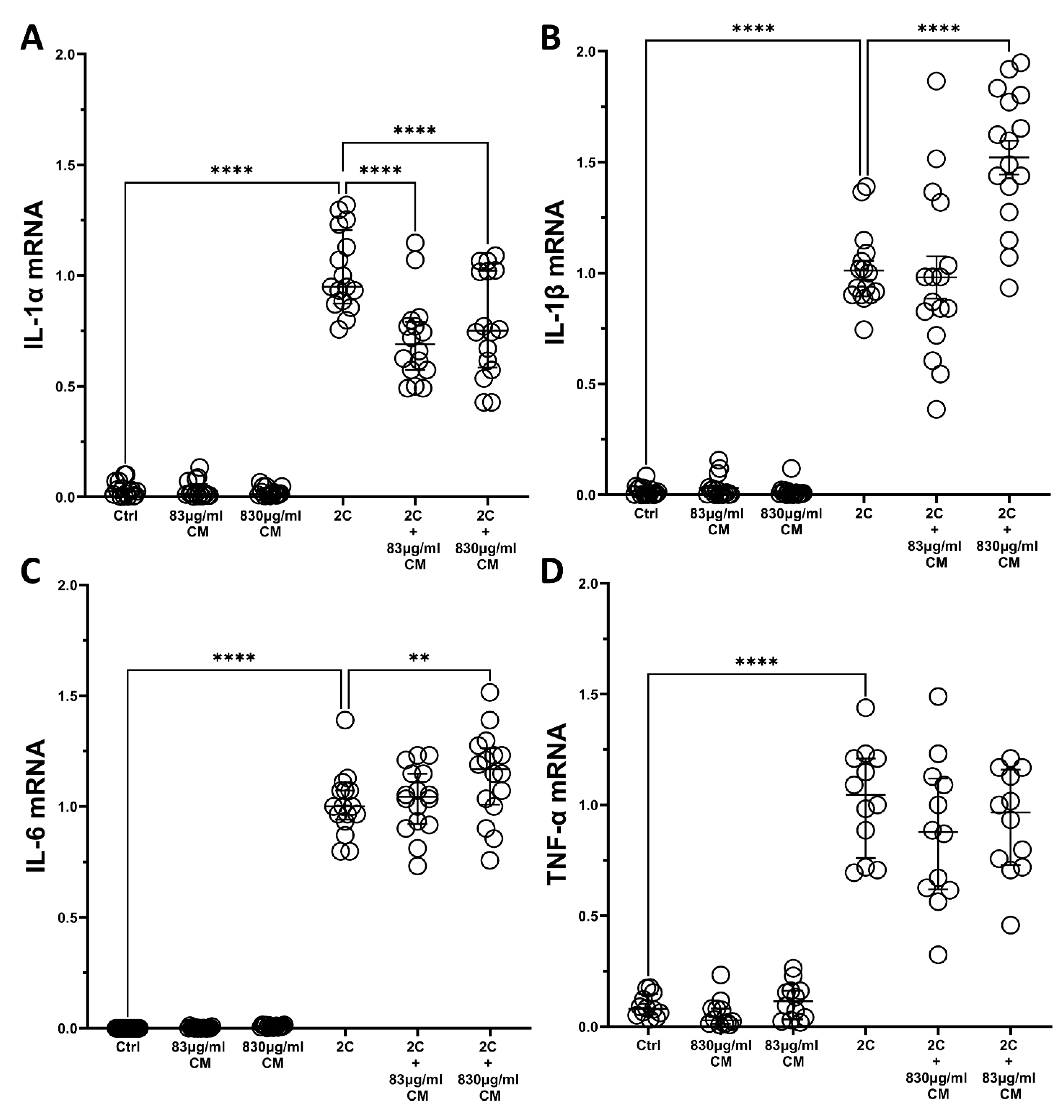
2.2. The Conditioned Medium Regulates the IL-1α- and TNF-α-Inducible mRNA Expression of Several Pro-Inflammatory Interleukins in the Pulmonary Epithelial Cell Line A549 and in hPSMs
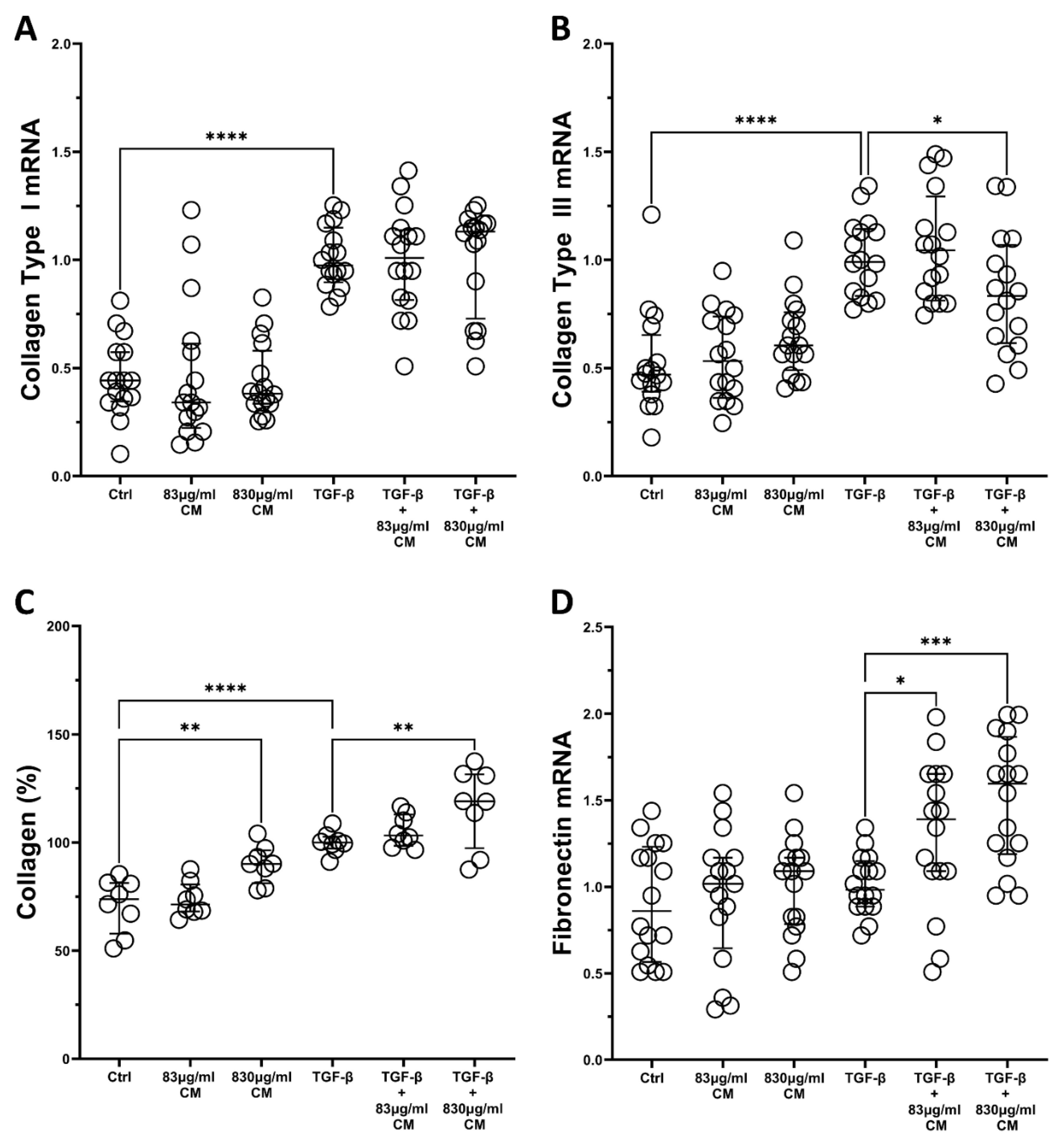
2.3. The Effect of the Conditioned Medium on the TGF-β-Induced Fibrotic Responses of hPSMs
2.4. The Conditioned Medium Inhibits the TGF-β-Induced Migration of hPSMs
3. Discussion
4. Materials and Methods
4.1. Adipose-Derived Mesenchymal Stem Cell Isolation and Immortalization
4.2. Characterization of hPSMs and ADSCs
4.3. Conditioned Medium Preparation
4.4. Patients
4.5. Isolation of Human Pulmonary Subepithelial Myofibroblast
4.6. A549 Pulmonary Epithelial Cell Line
4.7. Stimulation of A549 and hPSMs with Recombinant Cytokines and Conditioned Medium
4.8. Total RNA Extraction and DNase Treatment
4.9. cDNA Synthesis and Real-Time PCR
4.10. Enzyme Linked Immunosorbent Assay (ELISA)
4.11. Measurement of Total Protein Collagen Production
4.12. Wound Healing Assay
4.13. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Primers 2017, 3, 17074. [Google Scholar] [CrossRef]
- Galioto, F.; Palmucci, S.; Astuti, G.M.; Vancheri, A.; Distefano, G.; Tiralongo, F.; Libra, A.; Cusumano, G.; Basile, A.; Vancheri, C. Complications in Idiopathic Pulmonary Fibrosis: Focus on Their Clinical and Radiological Features. Diagnostics 2020, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Kishaba, T. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Medicina 2019, 55, 70. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R. Overview of idiopathic pulmonary fibrosis (IPF) and evidence-based guidelines. Am. J. Manag. Care 2017, 23, S176–S182. [Google Scholar] [PubMed]
- Huang, Y.; Oldham, J.M.; Ma, S.F.; Unterman, A.; Liao, S.Y.; Barros, A.J.; Bonham, C.A.; Kim, J.S.; Vij, R.; Adegunsoye, A.; et al. Blood Transcriptomics Predicts Progression of Pulmonary Fibrosis and Associated Natural Killer Cells. Am. J. Respir. Crit. Care Med. 2021, 204, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, R.; Varone, F.; Sgalla, G.; Richeldi, L. Existing and emerging biomarkers for disease progression in idiopathic pulmonary fibrosis. Expert Rev. Respir. Med. 2019, 13, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Jung, S.M.; Park, K.S.; Kim, K.J. Integrative analysis of lung molecular signatures reveals key drivers of idiopathic pulmonary fibrosis. BMC Pulm. Med. 2021, 21, 404. [Google Scholar] [CrossRef]
- Wang, Y.; Yella, J.; Chen, J.; McCormack, F.X.; Madala, S.K.; Jegga, A.G. Unsupervised gene expression analyses identify IPF-severity correlated signatures, associated genes and biomarkers. BMC Pulm. Med. 2017, 17, 133. [Google Scholar] [CrossRef]
- Canestaro, W.J.; Forrester, S.H.; Raghu, G.; Ho, L.; Devine, B.E. Drug Treatment of Idiopathic Pulmonary Fibrosis: Systematic Review and Network Meta-Analysis. Chest 2016, 149, 756–766. [Google Scholar] [CrossRef]
- Biondini, D.; Balestro, E.; Sverzellati, N.; Cocconcelli, E.; Bernardinello, N.; Ryerson, C.J.; Spagnolo, P. Acute exacerbations of idiopathic pulmonary fibrosis (AE-IPF): An overview of current and future therapeutic strategies. Expert. Rev. Respir. Med. 2020, 14, 405–414. [Google Scholar] [CrossRef]
- Jang, H.J.; Yong, S.H.; Leem, A.Y.; Lee, S.H.; Kim, S.Y.; Lee, S.H.; Kim, E.Y.; Chung, K.S.; Jung, J.Y.; Kang, Y.A.; et al. Corticosteroid responsiveness in patients with acute exacerbation of interstitial lung disease admitted to the emergency department. Sci. Rep. 2021, 11, 5762. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.F.; Raghu, G. Antifibrotic therapy for fibrotic lung disease beyond idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2019, 28, 190022. [Google Scholar] [CrossRef] [PubMed]
- Fleetwood, K.; McCool, R.; Glanville, J.; Edwards, S.C.; Gsteiger, S.; Daigl, M.; Fisher, M. Systematic Review and Network Meta-analysis of Idiopathic Pulmonary Fibrosis Treatments. J. Manag. Care Spec. Pharm. 2017, 23, S5–S16. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Shah, B.A.; Chowdhury, B.A. Forced vital capacity in idiopathic pulmonary fibrosis—FDA review of pirfenidone and nintedanib. N. Engl. J. Med. 2015, 372, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, N.; Shea, B.S.; Tager, A.M. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am. J. Respir. Crit. Care Med. 2014, 190, 867–878. [Google Scholar] [CrossRef]
- Somogyi, V.; Chaudhuri, N.; Torrisi, S.E.; Kahn, N.; Müller, V.; Kreuter, M. The therapy of idiopathic pulmonary fibrosis: What is next? Eur. Respir. Rev. 2019, 28, 190021. [Google Scholar] [CrossRef]
- Glass, D.S.; Grossfeld, D.; Renna, H.A.; Agarwala, P.; Spiegler, P.; DeLeon, J.; Reiss, A.B. Idiopathic pulmonary fibrosis: Current and future treatment. Clin. Respir. J. 2022, 16, 84–96. [Google Scholar] [CrossRef]
- Glassberg, M.K.; Minkiewicz, J.; Toonkel, R.L.; Simonet, E.S.; Rubio, G.A.; DiFede, D.; Shafazand, S.; Khan, A.; Pujol, M.V.; LaRussa, V.F.; et al. Allogeneic Human Mesenchymal Stem Cells in Patients with Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest 2017, 151, 971–981. [Google Scholar] [CrossRef]
- Serrano-Mollar, A.; Gay-Jordi, G.; Guillamat-Prats, R.; Closa, D.; Hernandez-Gonzalez, F.; Marin, P.; Burgos, F.; Martorell, J.; Sánchez, M.; Arguis, P.; et al. Safety and Tolerability of Alveolar Type II Cell Transplantation in Idiopathic Pulmonary Fibrosis. Chest 2016, 150, 533–543. [Google Scholar] [CrossRef]
- Ghadiri, M.; Young, P.M.; Traini, D. Cell-based therapies for the treatment of idiopathic pulmonary fibrosis (IPF) disease. Expert Opin. Biol. Ther. 2016, 16, 375–387. [Google Scholar] [CrossRef]
- Tzouvelekis, A.; Toonkel, R.; Karampitsakos, T.; Medapalli, K.; Ninou, I.; Aidinis, V.; Bouros, D.; Glassberg, M.K. Mesenchymal Stem Cells for the Treatment of Idiopathic Pulmonary Fibrosis. Front. Med. (Lausanne) 2018, 5, 142. [Google Scholar] [CrossRef] [PubMed]
- Purdon, S.; Patete, C.L.; Glassberg, M.K. Multipotent Mesenchymal Stromal Cells for Pulmonary Fibrosis? Am. J. Med. Sci. 2019, 357, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Abreu, S.C.; Rolandsson Enes, S.; Dearborn, J.; Goodwin, M.; Coffey, A.; Borg, Z.D.; Dos Santos, C.C.; Wargo, M.J.; Cruz, F.F.; Loi, R.; et al. Lung inflammatory environments differentially alter mesenchymal stromal cell behavior. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L823–L831. [Google Scholar] [CrossRef] [PubMed]
- Rolandsson Enes, S.; Hampton, T.H.; Barua, J.; McKenna, D.H.; Dos Santos, C.C.; Amiel, E.; Ashare, A.; Liu, K.D.; Krasnodembskaya, A.D.; English, K.; et al. Healthy versus inflamed lung environments differentially affect mesenchymal stromal cells. Eur. Respir. J. 2021, 58, 2004149. [Google Scholar] [CrossRef]
- Bogatcheva, N.V.; Coleman, M.E. Conditioned Medium of Mesenchymal Stromal Cells: A New Class of Therapeutics. Biochemistry 2019, 84, 1375–1389. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, X.; He, H.; Zhou, L.; Naito, Y.; Sugita, S.; Lee, J.W. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin. Biol. Ther. 2020, 20, 125–140. [Google Scholar] [CrossRef]
- Kadota, T.; Fujita, Y.; Araya, J.; Watanabe, N.; Fujimoto, S.; Kawamoto, H.; Minagawa, S.; Hara, H.; Ohtsuka, T.; Yamamoto, Y.; et al. Human bronchial epithelial cell-derived extracellular vesicle therapy for pulmonary fibrosis via inhibition of TGF-β-WNT crosstalk. J. Extracell. Vesicles 2021, 10, e12124. [Google Scholar] [CrossRef]
- Kadota, T.; Yoshioka, Y.; Fujita, Y.; Araya, J.; Minagawa, S.; Hara, H.; Miyamoto, A.; Suzuki, S.; Fujimori, S.; Kohno, T.; et al. Extracellular Vesicles from Fibroblasts Induce Epithelial-Cell Senescence in Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 2020, 63, 623–636. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Y.; Kang, X.; Liu, Z.; Zhang, W.; Xu, F. microRNA-186 in extracellular vesicles from bone marrow mesenchymal stem cells alleviates idiopathic pulmonary fibrosis via interaction with SOX4 and DKK1. Stem Cell Res. Ther. 2021, 12, 96. [Google Scholar] [CrossRef]
- Ionescu, L.; Byrne, R.N.; van Haaften, T.; Vadivel, A.; Alphonse, R.S.; Rey-Parra, G.J.; Weissmann, G.; Hall, A.; Eaton, F.; Thébaud, B. Stem cell conditioned medium improves acute lung injury in mice: In vivo evidence for stem cell paracrine action. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L967–L977. [Google Scholar] [CrossRef]
- Yang, K.Y.; Shih, H.C.; How, C.K.; Chen, C.Y.; Hsu, H.S.; Yang, C.W.; Lee, Y.C.; Perng, R.P.; Peng, C.H.; Li, H.Y.; et al. IV delivery of induced pluripotent stem cells attenuates endotoxin-induced acute lung injury in mice. Chest 2011, 140, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Hsu, C.C.; Hsu, S.L.; Chou, W.Y.; Wu, Y.N.; Kuo, C.A.; Hsu, T.C.; Shiu, L.Y.; Jhan, S.W. Adipose-Derived Mesenchymal Stem Cells-Conditioned Medium Modulates the Expression of Inflammation Induced Bone Morphogenetic Protein-2, -5 and -6 as Well as Compared with Shockwave Therapy on Rat Knee Osteoarthritis. Biomedicines 2021, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ding, F.; Hu, D.; Zhou, Y.; Long, C.; Shen, L.; Zhang, Y.; Zhang, D.; Wei, G. Human umbilical cord mesenchymal stem cell conditioned medium attenuates renal fibrosis by reducing inflammation and epithelial-to-mesenchymal transition via the TLR4/NF-κB signaling pathway in vivo and in vitro. Stem Cell Res. Ther. 2018, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hu, D.; Zhou, Y.; Yu, Y.; Shen, L.; Long, C.; Butnaru, D.; Timashev, P.; He, D.; Lin, T.; et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against renal interstitial fibrosis through ROS-mediated P38MAPK/ERK signaling pathway. Am. J. Transl. Res. 2020, 12, 4998–5014. [Google Scholar] [PubMed]
- Tang, Y.; Ding, F.; Wu, C.; Liu, B. hucMSC Conditioned Medium Ameliorate Lipopolysaccharide-Induced Acute Lung Injury by Suppressing Oxidative Stress and Inflammation via Nrf2/NF-κB Signaling Pathway. Anal. Cell Pathol. 2021, 2021, 6653681. [Google Scholar] [CrossRef]
- Ali, K.M.; Fattah, F.H.R.; Omar, S.H.; Gubari, M.I.M.; Yousefifard, M.; Hosseini, M. Mesenchymal Stromal Cells Derived Conditioned Medium in Pulmonary Fibrosis: A Systematic Review and Meta-analysis. Arch. Iran Med. 2020, 23, 870–879. [Google Scholar] [CrossRef]
- Gad, E.S.; Salama, A.A.A.; El-Shafie, M.F.; Arafa, H.M.M.; Abdelsalam, R.M.; Khattab, M. The Anti-fibrotic and Anti-inflammatory Potential of Bone Marrow-Derived Mesenchymal Stem Cells and Nintedanib in Bleomycin-Induced Lung Fibrosis in Rats. Inflammation 2020, 43, 123–134. [Google Scholar] [CrossRef]
- Kheirollahi, V.; Wasnick, R.M.; Biasin, V.; Vazquez-Armendariz, A.I.; Chu, X.; Moiseenko, A.; Weiss, A.; Wilhelm, J.; Zhang, J.S.; Kwapiszewska, G.; et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat. Commun. 2019, 10, 2987. [Google Scholar] [CrossRef]
- Rathinasabapathy, A.; Bruce, E.; Espejo, A.; Horowitz, A.; Sudhan, D.R.; Nair, A.; Guzzo, D.; Francis, J.; Raizada, M.K.; Shenoy, V.; et al. Therapeutic potential of adipose stem cell-derived conditioned medium against pulmonary hypertension and lung fibrosis. Br. J. Pharmacol. 2016, 173, 2859–2879. [Google Scholar] [CrossRef]
- Shologu, N.; Scully, M.; Laffey, J.G.; O’Toole, D. Human Mesenchymal Stem Cell Secretome from Bone Marrow or Adipose-Derived Tissue Sources for Treatment of Hypoxia-Induced Pulmonary Epithelial Injury. Int. J. Mol. Sci. 2018, 19, 2996. [Google Scholar] [CrossRef]
- Su, V.Y.; Lin, C.S.; Hung, S.C.; Yang, K.Y. Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-κB Pathway in Endotoxin-Induced Acute Lung Injury. Int. J. Mol. Sci. 2019, 20, 2208. [Google Scholar] [CrossRef] [PubMed]
- Cargnoni, A.; Piccinelli, E.C.; Ressel, L.; Rossi, D.; Magatti, M.; Toschi, I.; Cesari, V.; Albertini, M.; Mazzola, S.; Parolini, O. Conditioned medium from amniotic membrane-derived cells prevents lung fibrosis and preserves blood gas exchanges in bleomycin-injured mice-specificity of the effects and insights into possible mechanisms. Cytotherapy 2014, 16, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Felix, R.G.; Bovolato, A.L.C.; Cotrim, O.S.; Leão, P.D.S.; Batah, S.S.; Golim, M.A.; Velosa, A.P.; Teodoro, W.; Martins, V.; Cruz, F.F.; et al. Adipose-derived stem cells and adipose-derived stem cell-conditioned medium modulate in situ imbalance between collagen I- and collagen V-mediated IL-17 immune response recovering bleomycin pulmonary fibrosis. Histol. Histopathol. 2020, 35, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Vasse, G.F.; Van Os, L.; De Jager, M.; Jonker, M.R.; Borghuis, T.; Van Den Toorn, L.T.; Jellema, P.; White, E.S.; Van Rijn, P.; Harmsen, M.C.; et al. Adipose Stromal Cell-Secretome Counteracts Profibrotic Signals From IPF Lung Matrices. Front. Pharmacol. 2021, 12, 669037. [Google Scholar] [CrossRef]
- Emukah, C.; Dittmar, E.; Naqvi, R.; Martinez, J.; Corral, A.; Moreira, A.; Moreira, A. Mesenchymal stromal cell conditioned media for lung disease: A systematic review and meta-analysis of preclinical studies. Respir. Res. 2019, 20, 239. [Google Scholar] [CrossRef]
- Liang, X.; Lin, F.; Ding, Y.; Zhang, Y.; Li, M.; Zhou, X.; Meng, Q.; Ma, X.; Wei, L.; Fan, H.; et al. Conditioned medium from induced pluripotent stem cell-derived mesenchymal stem cells accelerates cutaneous wound healing through enhanced angiogenesis. Stem Cell Res. Ther. 2021, 12, 295. [Google Scholar] [CrossRef]
- Moreira, A.; Naqvi, R.; Hall, K.; Emukah, C.; Martinez, J.; Moreira, A.; Dittmar, E.; Zoretic, S.; Evans, M.; Moses, D.; et al. Effects of mesenchymal stromal cell-conditioned media on measures of lung structure and function: A systematic review and meta-analysis of preclinical studies. Stem Cell Res. Ther. 2020, 11, 399. [Google Scholar] [CrossRef]
- Filidou, E.; Kandilogiannakis, L.; Tarapatzi, G.; Su, C.; Po, E.N.F.; Paspaliaris, V.; Kolios, G. Conditioned medium from a human adipose-derived stem cell line ameliorates inflammation and fibrosis in a lung experimental model of idiopathic pulmonary fibrosis. Life Sci. 2021, 287, 120123. [Google Scholar] [CrossRef]
- Mohammadipoor, A.; Antebi, B.; Batchinsky, A.I.; Cancio, L.C. Therapeutic potential of products derived from mesenchymal stem/stromal cells in pulmonary disease. Respir. Res. 2018, 19, 218. [Google Scholar] [CrossRef]
- Cui, H.; Xie, N.; Banerjee, S.; Ge, J.; Jiang, D.; Dey, T.; Matthews, Q.L.; Liu, R.M.; Liu, G. Lung Myofibroblasts Promote Macrophage Profibrotic Activity through Lactate-induced Histone Lactylation. Am. J. Respir. Cell Mol. Biol. 2021, 64, 115–125. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Li, Z.; Yao, K.; Zhang, J.; Si, W.; Liu, X.; Jiang, Y.; Zhu, M. Identification of Potential Pathogenic Super-Enhancers-Driven Genes in Pulmonary Fibrosis. Front. Genet. 2021, 12, 644143. [Google Scholar] [CrossRef] [PubMed]
- Pakshir, P.; Noskovicova, N.; Lodyga, M.; Son, D.O.; Schuster, R.; Goodwin, A.; Karvonen, H.; Hinz, B. The myofibroblast at a glance. J. Cell Sci. 2020, 133, jcs227900. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Dey, T.; Liu, G. Recent developments in the pathobiology of lung myofibroblasts. Expert Rev. Respir. Med. 2021, 15, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Andugulapati, S.B.; Gourishetti, K.; Tirunavalli, S.K.; Shaikh, T.B.; Sistla, R. Biochanin-A ameliorates pulmonary fibrosis by suppressing the TGF-β mediated EMT, myofibroblasts differentiation and collagen deposition in in vitro and in vivo systems. Phytomedicine 2020, 78, 153298. [Google Scholar] [CrossRef]
- Artaud-Macari, E.; Goven, D.; Brayer, S.; Hamimi, A.; Besnard, V.; Marchal-Somme, J.; Ali, Z.E.; Crestani, B.; Kerdine-Römer, S.; Boutten, A.; et al. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid. Redox. Signal. 2013, 18, 66–79. [Google Scholar] [CrossRef]
- Li, J.; Feng, M.; Sun, R.; Li, Z.; Hu, L.; Peng, G.; Xu, X.; Wang, W.; Cui, F.; Yue, W.; et al. Andrographolide ameliorates bleomycin-induced pulmonary fibrosis by suppressing cell proliferation and myofibroblast differentiation of fibroblasts via the TGF-β1-mediated Smad-dependent and -independent pathways. Toxicol. Lett. 2020, 321, 103–113. [Google Scholar] [CrossRef]
- Liu, S.S.; Liu, C.; Lv, X.X.; Cui, B.; Yan, J.; Li, Y.X.; Li, K.; Hua, F.; Zhang, X.W.; Yu, J.J.; et al. The chemokine CCL1 triggers an AMFR-SPRY1 pathway that promotes differentiation of lung fibroblasts into myofibroblasts and drives pulmonary fibrosis. Immunity 2021, 54, 2042–2056.e8. [Google Scholar] [CrossRef]
- Molina-Molina, M.; Machahua-Huamani, C.; Vicens-Zygmunt, V.; Llatjós, R.; Escobar, I.; Sala-Llinas, E.; Luburich-Hernaiz, P.; Dorca, J.; Montes-Worboys, A. Anti-fibrotic effects of pirfenidone and rapamycin in primary IPF fibroblasts and human alveolar epithelial cells. BMC Pulm. Med. 2018, 18, 63. [Google Scholar] [CrossRef]
- Ng, B.; Dong, J.; D’Agostino, G.; Viswanathan, S.; Widjaja, A.A.; Lim, W.W.; Ko, N.S.J.; Tan, J.; Chothani, S.P.; Huang, B.; et al. Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci. Transl. Med. 2019, 11, eaaw1237. [Google Scholar] [CrossRef]
- Ono, S.J.; Nakamura, T.; Miyazaki, D.; Ohbayashi, M.; Dawson, M.; Toda, M. Chemokines: Roles in leukocyte development, trafficking, and effector function. J. Allergy Clin. Immunol. 2003, 111, 1185–1199. [Google Scholar] [CrossRef]
- Kandilogiannakis, L.; Filidou, E.; Drygiannakis, I.; Tarapatzi, G.; Didaskalou, S.; Koffa, M.; Arvanitidis, K.; Bamias, G.; Valatas, V.; Paspaliaris, V.; et al. Development of a Human Intestinal Organoid Model for In Vitro Studies on Gut Inflammation and Fibrosis. Stem Cells Int. 2021, 2021, 9929461. [Google Scholar] [CrossRef] [PubMed]
- Kolios, G.; Wright, K.L.; Jordan, N.J.; Leithead, J.B.; Robertson, D.A.; Westwick, J. C-X-C and C-C chemokine expression and secretion by the human colonic epithelial cell line, HT-29: Differential effect of T lymphocyte-derived cytokines. Eur. J. Immunol. 1999, 29, 530–536. [Google Scholar] [CrossRef]
- Santarlasci, V.; Cosmi, L.; Maggi, L.; Liotta, F.; Annunziato, F. IL-1 and T Helper Immune Responses. Front. Immunol. 2013, 4, 182. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Van Damme, J.; Mortier, A.; Proost, P. Regulation of Chemokine Activity—A Focus on the Role of Dipeptidyl Peptidase IV/CD26. Front. Immunol. 2016, 7, 483. [Google Scholar] [CrossRef] [PubMed]
- Capelli, A.; Di Stefano, A.; Gnemmi, I.; Donner, C.F. CCR5 expression and CC chemokine levels in idiopathic pulmonary fibrosis. Eur. Respir. J. 2005, 25, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.M.; Worrell, J.C.; Fabre, A.; Hinz, B.; Kane, R.; Keane, M.P. Novel differences in gene expression and functional capabilities of myofibroblast populations in idiopathic pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L697–L710. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Qiu, X.; Tian, Y.; Xie, M.; Li, H.; Gao, Y.; Zhuang, Y.; Cao, M.; Ding, H.; Ding, J.; et al. Prognostic value of IFN-γ, sCD163, CCL2 and CXCL10 involved in acute exacerbation of idiopathic pulmonary fibrosis. Int. Immunopharmacol. 2019, 70, 208–215. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, J.H.; Cho, S.S.; Bae, C.S.; Kim, G.Y.; Park, D.H. Establishment of a chronic obstructive pulmonary disease mouse model based on the elapsed time after LPS intranasal instillation. Lab. Anim. Res. 2018, 34, 1–10. [Google Scholar] [CrossRef]
- Cargnoni, A.; Romele, P.; Bonassi Signoroni, P.; Farigu, S.; Magatti, M.; Vertua, E.; Toschi, I.; Cesari, V.; Silini, A.R.; Stefani, F.R.; et al. Amniotic MSCs reduce pulmonary fibrosis by hampering lung B-cell recruitment, retention, and maturation. Stem Cells Transl. Med. 2020, 9, 1023–1035. [Google Scholar] [CrossRef]
- Liu, D.; Kong, F.; Yuan, Y.; Seth, P.; Xu, W.; Wang, H.; Xiao, F.; Wang, L.; Zhang, Q.; Yang, Y.; et al. Decorin-Modified Umbilical Cord Mesenchymal Stem Cells (MSCs) Attenuate Radiation-Induced Lung Injuries via Regulating Inflammation, Fibrotic Factors, and Immune Responses. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 945–956. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, T.; Bozkanat, M.; Ibe, J.C.; Christman, J.W.; Raj, J.U.; Zhou, G. Intratracheal instillation of high dose adenoviral vectors is sufficient to induce lung injury and fibrosis in mice. PLoS ONE 2014, 9, e116142. [Google Scholar] [CrossRef] [PubMed]
- Ogushi, F.; Tani, K.; Endo, T.; Tada, H.; Kawano, T.; Asano, T.; Huang, L.; Ohmoto, Y.; Muraguchi, M.; Moriguchi, H.; et al. Autoantibodies to IL-1 alpha in sera from rapidly progressive idiopathic pulmonary fibrosis. J. Med. Investig. 2001, 48, 181–189. [Google Scholar]
- Li, F.; Han, F.; Li, H.; Zhang, J.; Qiao, X.; Shi, J.; Yang, L.; Dong, J.; Luo, M.; Wei, J.; et al. Human placental mesenchymal stem cells of fetal origins-alleviated inflammation and fibrosis by attenuating MyD88 signaling in bleomycin-induced pulmonary fibrosis mice. Mol. Immunol. 2017, 90, 11–21. [Google Scholar] [CrossRef]
- Takao, S.; Nakashima, T.; Masuda, T.; Namba, M.; Sakamoto, S.; Yamaguchi, K.; Horimasu, Y.; Miyamoto, S.; Iwamoto, H.; Fujitaka, K.; et al. Human bone marrow-derived mesenchymal stromal cells cultured in serum-free media demonstrate enhanced antifibrotic abilities via prolonged survival and robust regulatory T cell induction in murine bleomycin-induced pulmonary fibrosis. Stem Cell Res. Ther. 2021, 12, 506. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhao, Q.; Yang, G.; Huang, R.; Li, C.; Qi, Y.; Hao, C.; Yao, W. Mesenchymal stem cells ameliorate silica-induced pulmonary fibrosis by inhibition of inflammation and epithelial-mesenchymal transition. J. Cell Mol. Med. 2021, 25, 6417–6428. [Google Scholar] [CrossRef]
- He, F.; Wang, Y.; Li, Y.; Yu, L. Human amniotic mesenchymal stem cells alleviate paraquat-induced pulmonary fibrosis in rats by inhibiting the inflammatory response. Life Sci. 2020, 243, 117290. [Google Scholar] [CrossRef]
- Li, X.; An, G.; Wang, Y.; Liang, D.; Zhu, Z.; Tian, L. Targeted migration of bone marrow mesenchymal stem cells inhibits silica-induced pulmonary fibrosis in rats. Stem Cell Res. Ther. 2018, 9, 335. [Google Scholar] [CrossRef]
- Zhao, Q.; Hao, C.; Wei, J.; Huang, R.; Li, C.; Yao, W. Bone marrow-derived mesenchymal stem cells attenuate silica-induced pulmonary fibrosis by inhibiting apoptosis and pyroptosis but not autophagy in rats. Ecotoxicol. Environ. Saf. 2021, 216, 112181. [Google Scholar] [CrossRef]
- Wang, X.; Gao, J.L.; Zhao, M.M.; Zhu, H.X.; Tian, Y.X.; Li, R.; Jiang, X.H.; Yu, L.; Tian, J.R.; Cui, J.Z. Therapeutic effects of conditioned medium from bone marrow-derived mesenchymal stem cells on epithelial-mesenchymal transition in A549 cells. Int. J. Mol. Med. 2018, 41, 659–668. [Google Scholar] [CrossRef]
- Filidou, E.; Valatas, V.; Drygiannakis, I.; Arvanitidis, K.; Vradelis, S.; Kouklakis, G.; Kolios, G.; Bamias, G. Cytokine receptor profiling in human colonic subepithelial myofibroblasts: A differential effect of Th polarization associated cytokines in intestinal fibrosis. Inflamm. Bowel. Dis. 2018, 24, 2224–2241. [Google Scholar] [CrossRef]
- Bouros, E.; Filidou, E.; Arvanitidis, K.; Mikroulis, D.; Steiropoulos, P.; Bamias, G.; Bouros, D.; Kolios, G. Lung fibrosis-associated soluble mediators and bronchoalveolar lavage from idiopathic pulmonary fibrosis patients promote the expression of fibrogenic factors in subepithelial lung myofibroblasts. Pulm. Pharmacol. Ther. 2017, 46, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In Vitro Cultivation of Human Tumors: Establishment of Cell Lines Derived From a Series of Solid Tumors2. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Jordan, N.J.; Kolios, G.; Abbot, S.E.; Sinai, M.A.; Thompson, D.A.; Petraki, K.; Westwick, J. Expression of functional CXCR4 chemokine receptors on human colonic epithelial cells. J. Clin. Investig. 1999, 104, 1061–1069. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sperling, T.; Ołdak, M.; Walch-Rückheim, B.; Wickenhauser, C.; Doorbar, J.; Pfister, H.; Malejczyk, M.; Majewski, S.; Keates, A.C.; Smola, S. Human papillomavirus type 8 interferes with a novel C/EBPβ-mediated mechanism of keratinocyte CCL20 chemokine expression and Langerhans cell migration. PLoS Pathog. 2012, 8, e1002833. [Google Scholar] [CrossRef] [PubMed]
- He, J.T.; Huang, H.Y.; Qu, D.; Xue, Y.; Zhang, K.K.; Xie, X.L.; Wang, Q. CXCL1 and CXCR2 as potential markers for vital reactions in skin contusions. Forensic Sci. Med. Pathol. 2018, 14, 174–179. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, S.; Chen, X.; Cheng, S.; Zhang, Z.; Li, F.; Huang, L.; Yang, Y.; Zhou, B.; Yue, D.; et al. Cancer-cell-secreted CXCL11 promoted CD8(+) T cells infiltration through docetaxel-induced-release of HMGB1 in NSCLC. J. Immunother. Cancer 2019, 7, 42. [Google Scholar] [CrossRef]
- Li, P.B.; Tang, W.J.; Wang, K.; Zou, K.; Che, B. Expressions of IL-1α and MMP-9 in degenerated lumbar disc tissues and their clinical significance. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4007–4013. [Google Scholar]
- Stordeur, P.; Poulin, L.F.; Craciun, L.; Zhou, L.; Schandené, L.; de Lavareille, A.; Goriely, S.; Goldman, M. Cytokine mRNA quantification by real-time PCR. J. Immunol. Methods 2002, 259, 55–64. [Google Scholar] [CrossRef]
- Ocmant, A.; Michils, A.; Schandené, L.; Peignois, Y.; Goldman, M.; Stordeur, P. IL-4 and IL-13 mRNA real-time PCR quantification on whole blood to assess allergic response. Cytokine 2005, 31, 375–381. [Google Scholar] [CrossRef]
- Matsui, S.; Ogata, Y. Effects of miR-223 on expression of IL-1β and IL-6 in human gingival fibroblasts. J. Oral Sci. 2016, 58, 101–108. [Google Scholar] [CrossRef]
- Woodward, E.A.; Kolesnik, T.B.; Nicholson, S.E.; Prêle, C.M.; Hart, P.H. The anti-inflammatory actions of IL-4 in human monocytes are not mediated by IL-10, RP105 or the kinase activity of RIPK2. Cytokine 2012, 58, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Raouf-Rahmati, A.; Ansar, A.R.; Rezaee, S.A.; Hosseini, S.M.; Garweg, J.G.; Ghezeldasht, S.A.; Vaghei, S.; Zarean, M.; Shamsian, S.A.; Moghaddas, E. Local and systemic gene expression levels of IL-10, IL-17 and TGF-β in active ocular toxoplasmosis in humans. Cytokine 2021, 146, 155643. [Google Scholar] [CrossRef] [PubMed]
- Guedes, P.M.; de Andrade, C.M.; Nunes, D.F.; de Sena Pereira, N.; Queiroga, T.B.; Machado-Coelho, G.L.; Nascimento, M.S.; Do-Valle-Matta, M.A.; da Câmara, A.C.; Chiari, E.; et al. Inflammation Enhances the Risks of Stroke and Death in Chronic Chagas Disease Patients. PLoS Negl. Trop. Dis. 2016, 10, e0004669. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward | Reverse | Reference |
|---|---|---|---|
| GapdH | GACATCAAGAAGGTGGTGAA | TGTCATACCAGGAAATGAGC | [80] |
| COL1 | CCCTGGAAAGAATGGAGATGAT | ACTGAAACCTCTGTGTCCCTTCA | |
| COL3 | GCTCTGCTTCATCCCACTATTA | TGCGAGTCCTCCTACTGCTAC | |
| FD-A | CCAGTCCACAGCTATTCCTG | ACAACCACGGATGAGCTG | |
| CCL2 | AGGAAGATCTCAGTGCAGAGG | AGTCTTCGGAGTTTGGGTTTG | [83] |
| CCL20 | GCTGCTTTGATGTCAGTGC | GCAGTCAAAGTTGCTTGCTTC | [84] |
| CXCL1 | GCCCAAACCGAAGTCATAGCC | ATCCGCCAGCCTCTATCACA | [85] |
| CXCL8 | TGGGTGCAGAGGGTTGTG | CAGACTAGGGTTGCCAGATTTA | [83] |
| CXCL10 | CCTGCTTCAAATATTTCCCT | CCTTCCTGTATGTGTTTGGA | |
| CXCL11 | GACGCTGTCTTTGCATAGGC | GGATTTAGGCATCGTTGTCCTTT | [86] |
| IL-1α | AGATGCCTGAGATACCCAAAACC | CCAAGCACACCCAGTAGTCT | [87] |
| IL-1β | ACAGATGAAGTGCTCCTTCCA | GTCGGAGATTCGTAGCTGGAT | [88] |
| IL-4 | ACTTTGAACAGCCTCACAGAG | TTGGAGGCAGCAAAGATGTC | [89] |
| IL-6 | AAGCCAGAGCTGTGCAGATGAGTA | TGTCCTGCAGCCACTGGTTC | [90] |
| IL-10 | CATCAAGGCGCATGTGAACT | GATGTCAAACTCACTCATGGCTTT | [91] |
| IL-13 | TGAGGAGCTGGTCAACATCA | CAGGTTGATGCTCCATACCAT | [89] |
| IL-17 | GTCAACCTGAACATCCATAACCG | ACTTTGCCTCCCAGATCACAG | [92] |
| IL-22 | TTCCAGCAGCCCTATATCACC | GCTCACTCATACTGACTCCGTG | [93] |
| TNF-α | CCCAGGGACCTCTCTCTAATC | ATGGGCTACAGGCTTGTCACT | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filidou, E.; Kandilogiannakis, L.; Tarapatzi, G.; Spathakis, M.; Steiropoulos, P.; Mikroulis, D.; Arvanitidis, K.; Paspaliaris, V.; Kolios, G. Anti-Inflammatory and Anti-Fibrotic Effect of Immortalized Mesenchymal-Stem-Cell-Derived Conditioned Medium on Human Lung Myofibroblasts and Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 4570. https://doi.org/10.3390/ijms23094570
Filidou E, Kandilogiannakis L, Tarapatzi G, Spathakis M, Steiropoulos P, Mikroulis D, Arvanitidis K, Paspaliaris V, Kolios G. Anti-Inflammatory and Anti-Fibrotic Effect of Immortalized Mesenchymal-Stem-Cell-Derived Conditioned Medium on Human Lung Myofibroblasts and Epithelial Cells. International Journal of Molecular Sciences. 2022; 23(9):4570. https://doi.org/10.3390/ijms23094570
Chicago/Turabian StyleFilidou, Eirini, Leonidas Kandilogiannakis, Gesthimani Tarapatzi, Michail Spathakis, Paschalis Steiropoulos, Dimitrios Mikroulis, Konstantinos Arvanitidis, Vasilis Paspaliaris, and George Kolios. 2022. "Anti-Inflammatory and Anti-Fibrotic Effect of Immortalized Mesenchymal-Stem-Cell-Derived Conditioned Medium on Human Lung Myofibroblasts and Epithelial Cells" International Journal of Molecular Sciences 23, no. 9: 4570. https://doi.org/10.3390/ijms23094570
APA StyleFilidou, E., Kandilogiannakis, L., Tarapatzi, G., Spathakis, M., Steiropoulos, P., Mikroulis, D., Arvanitidis, K., Paspaliaris, V., & Kolios, G. (2022). Anti-Inflammatory and Anti-Fibrotic Effect of Immortalized Mesenchymal-Stem-Cell-Derived Conditioned Medium on Human Lung Myofibroblasts and Epithelial Cells. International Journal of Molecular Sciences, 23(9), 4570. https://doi.org/10.3390/ijms23094570







