Multiple Roles of SMC5/6 Complex during Plant Sexual Reproduction
Abstract
:1. Introduction
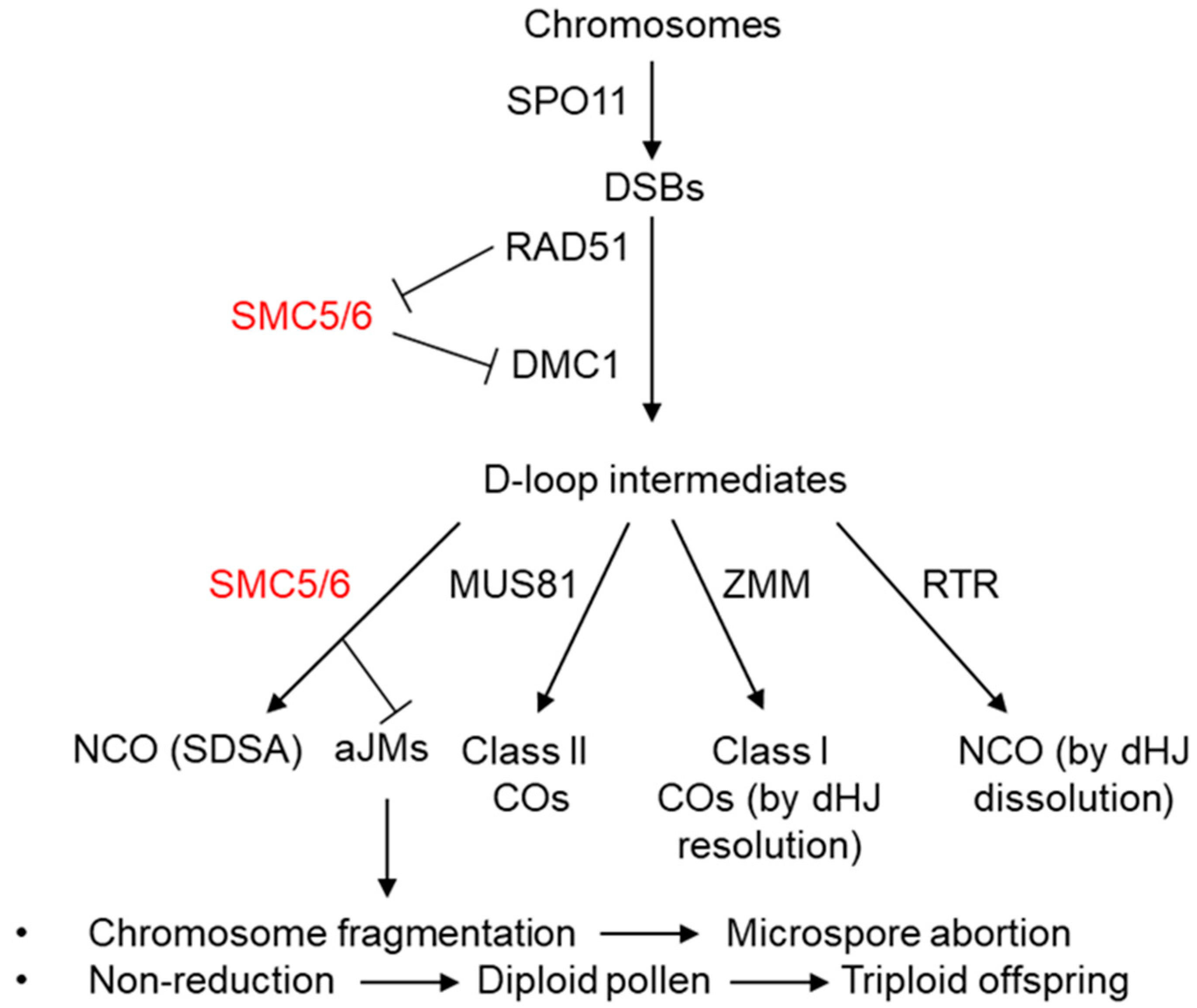
2. Essential Functions in Meiosis
| Gene Name | Gene ID | Mutant Allele | Stock ID/Mutation | Somatic Phenotype * | Meiotic Phenotype | Seed Phenotype | Reference |
|---|---|---|---|---|---|---|---|
| AtSMC5 | At5g15920 | Atsmc5-1 | SALK_107583 | Not viable | n.a. | Early embryo lethal (Type 1) | [31,45] |
| Atsmc5-2 | SALK_092081 | Not viable | n.a. | ||||
| AtSMC6A | At5g07660 | Atsmc6a-2 | SALK_091553 | WT-like | n.a. | WT-like | [51] |
| AtSMC6B | At5g61460 | Atsmc6b-4 | SALK_124719 | WT-like | n.a. | WT-like | |
| smc6b-2 | SALK_135638 | n.a. | Increased COs | n.a. | [39] | ||
| smc6a-2 smc6b-4 | SALK_091553 SALK_124719 | Not viable | n.a. | Early embryo lethal (Type 1) | [51] | ||
| smc6a-1 smc6b-2 | SALK_009818 SALK_135638 | n.a. | Increased COs | n.a. | [39] | ||
| AtNSE1 | At5g21140 | Atnse1-1 | CS16151 | Not viable | n.a. | Early embryo lethal (Type 1) | [52,53] |
| Atnse1-2 | SALK_136483 | Not viable | n.a. | Early embryo lethal (Type 1) | |||
| AtNSE2/AtHPY2/AtMMS21 | At3g15150 | Atnse2-1/Athpy2-1/Atmms21-1 | Q115STOP | Short roots and stems, deformed leaves, stem fasciations, irregular branching, triploid individuals | Increased COs, fragmented and lagging chromosomes, anaphase bridges, monads to tetrads | Large seeds, cellularization defects, increased seed abortion (Type 2) | [21,22,40,54,55] |
| Atnse2-2/Athpy2-2/Atmms21-2 | SAIL_77_G06 | ||||||
| AtNSE3 | At1g34770 | Atnse3-1 | GK-459F08 | Not viable | n.a. | Early embryo lethal (Type 1) | [52,53] |
| Atnse3-2 | GK-534A03 | Not viable | n.a. | Early embryo lethal (Type 1) | |||
| AtNSE4A | At1g51130 | Atnse4a-1 | SALK_057130 | Not viable | n.a. | Early embryo lethal (Type 1) | [56] |
| Atnse4a-2 | GK-768H08 | Weakly delayed, triploid individuals | Increased COs, fragmented and lagging chromosomes, anaphase bridges and micronuclei | Large seeds, cellularization defects, increased seed abortion (Type 2) | |||
| AtNSE4B | At3g20760 | Atnse4b-1 | SAIL_296_F02 | WT-like | n.a. | WT-like | |
| Atnse4b-2 | GK-175D10 | WT-like | n.a. | WT-like | |||
| AtASAP1 | At2g28130 | Atasap1 | GK-218F01 | Strongly reduced growth, short roots | Fragmentated chromosomes | Almost sterile | [31,37] |
| AtSNI1 | At4g18470 | Atsni1-1 | 11 bp del., premature stop | Reduced growth, short roots | Fragmented chromosomes, increased Class II COs, dyads | Reduced fertility | [30,31,37,39] |
| Atsni1-2 | SAIL_298_H07 | n.a. | Fragmented chromosomes | Almost sterile | [37] | ||
| Atsni1-3 | SAIL_34_D11 | Short roots and stems, deformed leaves and triploid individuals | n.a. | Large seeds, cellularization defects, increased seed abortion (Type 2) | [40] | ||
| AtSNI1Ler | I235V | WT-like | Recombination QTL, increased COs | n.a. | [39] | ||
| Atsni1-4 (as Atsni1-2) | 14 bp del., premature stop | n.a. | n.a. | n.a. | |||
| ZmMMS21 | Zm00001d039007 | Zmmms21-1 | Mu ins., ex4 | Slow growth, severely stunted plants, short roots, fewer leaves at maturity | n.a. | Small kernels, pitted surface, reduced embryo size and an underfilled endosperm, poor germination | [57] |
| Zmmms21-2 | Mu ins., ex6 | n.a. | |||||
| Zmmms21-CR7 | 33 bp del., 11 aa del. ex1 | n.a. | |||||
| Zmmms21-CR1 | 1 bp del., ex2, premature stop | Not viable to early somatic lethal | n.a. | ||||
| Zmmms21-CR3 | 1 bp ins. ex2, new TSS producing a truncated protein | Not viable to early somatic lethal | n.a. | ||||
| Zmmms21-CR4 | 1bp ins., ex1; 2 bp del., ex2, premature stop | Not viable to early somatic lethal | n.a. | ||||
| Zmmms21-CR6 | 3 bp del., 1 aa del. ex1 | Not viable to early somatic lethal | n.a. | ||||
| Zmmms21-CR2 | 14 bp del., ex2, premature stop | Not viable | n.a. | Early embryo lethal | |||
| Zmmms21-CR5 | 6 bp del., 2 aa del. ex1; 8 bp del. ex2, premature stop | Not viable | n.a. | Early embryo lethal | |||
| OsMMS21 | LOC_ Os05g48880 | Osmms21 | 05Z11BH79 | Short roots, dwarf plants | n.a. | n.a. | [58] |
3. Emerging Roles in Plant Gametophytic Development
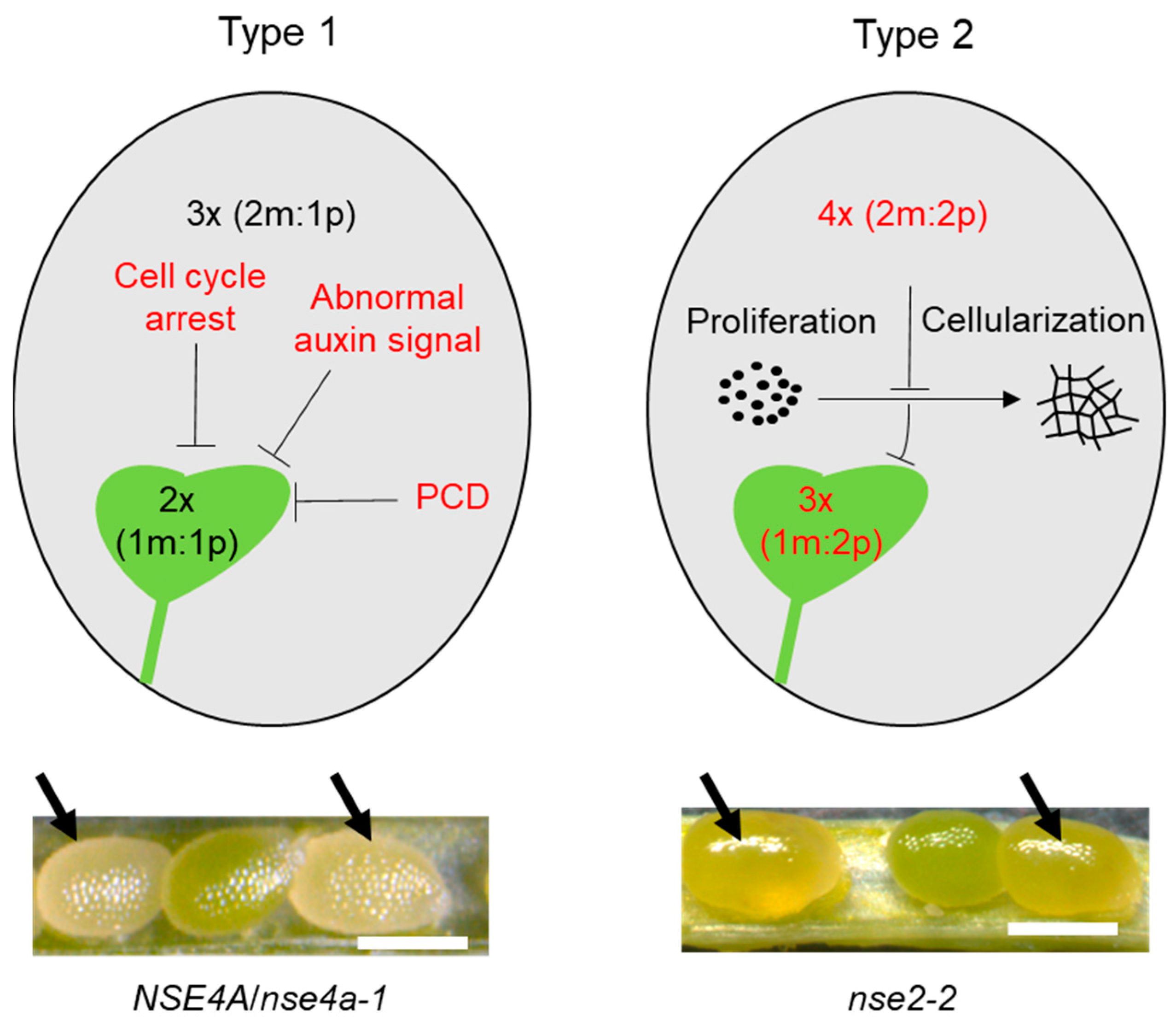
4. Direct and Indirect Effects on Seed Development
5. Importance of Crop Fertility
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosa, S.; Shaw, P. Insights into chromatin structure and dynamics in plants. Biology 2013, 2, 1378–1410. [Google Scholar] [CrossRef]
- Doğan, E.S.; Liu, C. Three-dimensional chromatin packing and positioning of plant genomes. Nat. Plants 2018, 4, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Pecinka, A.; Chevalier, C.; Colas, I.; Kalantidis, K.; Varotto, S.; Krugman, T.; Michailidis, C.; Vallés, M.-P.; Muñoz, A.; Pradillo, M. Chromatin dynamics during interphase and cell division: Similarities and differences between model and crop plants. J. Exp. Bot. 2020, 71, 5205–5222. [Google Scholar] [CrossRef]
- Kawashima, T.; Berger, F. Epigenetic reprogramming in plant sexual reproduction. Nat. Rev. Genet. 2014, 15, 613–624. [Google Scholar] [CrossRef] [PubMed]
- She, W.; Baroux, C. Chromatin dynamics during plant sexual reproduction. Front. Plant Sci. 2014, 5, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroux, C.; Autran, D. Chromatin dynamics during cellular differentiation in the female reproductive lineage of flowering plants. Plant J. 2015, 83, 160–176. [Google Scholar] [CrossRef] [Green Version]
- Jeppsson, K.; Kanno, T.; Shirahige, K.; Sjögren, C. The maintenance of chromosome structure: Positioning and functioning of SMC complexes. Nat. Rev. Mol. Cell Biol. 2014, 15, 601–614. [Google Scholar] [CrossRef]
- Uhlmann, F. SMC complexes: From DNA to chromosomes. Nat. Rev. Mol. Cell Biol. 2016, 17, 399–412. [Google Scholar] [CrossRef]
- Liu, C.M.; McElver, J.; Tzafrir, I.; Joosen, R.; Wittich, P.; Patton, D.; Van Lammeren, A.A.M.; Meinke, D. Condensin and cohesin knockouts in Arabidopsis exhibit a titan seed phenotype. Plant J. 2002, 29, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Díaz, M.; Pecinka, A. Scaffolding for repair: Understanding molecular functions of the SMC5/6 complex. Genes 2018, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Kegel, A.; Sjögren, C. The Smc5/6 complex: More than repair? Cold Spring Harb. Symp. Quant. Biol. 2010, 75, 179–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, N.; Yu, H. The Smc complexes in DNA damage response. Cell Biosci. 2012, 2, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragón, L. The Smc5/6 complex: New and old functions of the enigmatic long-distance relative. Annu. Rev. Genet. 2018, 52, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Schubert, V. SMC proteins and their multiple functions in higher plants. Cytogenet. Genome Res. 2009, 124, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Palecek, J.J.; Gruber, S. Kite proteins: A superfamily of SMC/kleisin partners conserved across bacteria, archaea, and eukaryotes. Structure 2015, 23, 2183–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haering, C.H.; Gruber, S. SnapShot: SMC protein complexes part i. Cell 2016, 164, 326–326.e1. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.M.; Gao, J.; Wang, J.; Yang, M.; Potts, P.R. MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol. Cell 2010, 39, 963–974. [Google Scholar] [CrossRef] [Green Version]
- Kolesar, P.; Stejskal, K.; Potesil, D.; Murray, J.M.; Palecek, J.J. Role of Nse1 subunit of SMC5/6 complex as a ubiquitin ligase. Cells 2022, 11, 165. [Google Scholar] [CrossRef]
- Andrews, E.A.; Palecek, J.; Sergeant, J.; Taylor, E.; Lehmann, A.R.; Watts, F.Z. Nse2, a component of the Smc5-6 complex, is a SUMO ligase required for the response to DNA damage. Mol. Cell. Biol. 2005, 25, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Potts, P.R.; Yu, H. Human MMS21/NSE2 is a SUMO ligase required for DNA repair. Mol. Cell. Biol. 2005, 25, 7021–7032. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Fujiwara, S.; Miura, K.; Stacey, N.; Yoshimura, M.; Schneider, K.; Adachi, S.; Minamisawa, K.; Umeda, M.; Sugimoto, K. SUMO E3 ligase HIGH PLOIDY2 regulates endocycle onset and meristem maintenance in Arabidopsis. Plant Cell 2009, 21, 2284–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Yang, S.; Zhang, S.; Liu, M.; Lai, J.; Qi, Y.; Shi, S.; Wang, J.; Wang, Y.; Xie, Q.; et al. The Arabidopsis SUMO E3 ligase AtMMS21, a homologue of NSE2/MMS21, regulates cell proliferation in the root. Plant J. 2009, 60, 666–678. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, N.; Wong, R.P.; Ulrich, H.D. Functions of Ubiquitin and SUMO in DNA replication and replication stress. Front. Genet. 2016, 7, 87. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Wei, L.; Peng, X.P.; Zhao, X. Sumoylation of the DNA polymerase ε by the Smc5/6 complex contributes to DNA replication. PLoS Genet. 2019, 15, e1008426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rytz, T.C.; Miller, M.J.; McLoughlin, F.; Augustine, R.C.; Marshall, R.S.; Juan, Y.; Charng, Y.; Scalf, M.; Smith, L.M.; Vierstra, R.D. SUMOylome profiling reveals a diverse array of nuclear targets modified by the SUMO ligase SIZ1 during heat stress. Plant Cell 2018, 30, 1077–1099. [Google Scholar] [CrossRef] [PubMed]
- Pebernard, S.; Wohlschlegel, J.; McDonald, W.H.; Yates, J.R.; Boddy, M.N. The Nse5-Nse6 dimer mediates DNA repair roles of the Smc5-Smc6 complex. Mol. Cell. Biol. 2006, 26, 1617–1630. [Google Scholar] [CrossRef] [Green Version]
- Leung, G.P.; Lee, L.; Schmidt, T.I.; Shirahige, K.; Kobor, M.S. Rtt107 is required for recruitment of the SMC5/6 complex to DNA double strand breaks. J. Biol. Chem. 2011, 286, 26250–26257. [Google Scholar] [CrossRef] [Green Version]
- Oravcová, M.; Gadaleta, M.C.; Nie, M.; Reubens, M.C.; Limbo, O.; Russell, P.; Boddy, M.N. Brc1 promotes the focal accumulation and SUMO ligase activity of Smc5-Smc6 during replication stress. Mol. Cell. Biol. 2019, 39, e00271-18. [Google Scholar] [CrossRef] [Green Version]
- Räschle, M.; Smeenk, G.; Hansen, R.K.; Temu, T.; Oka, Y.; Hein, M.Y.; Nagaraj, N.; Long, D.T.; Walter, J.C.; Hofmann, K.; et al. Proteomics reveals dynamic assembly of repair complexes during bypass of DNA cross-links. Science 2015, 348, 1253671. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhang, Y.; Clarke, J.D.; Li, Y.; Dong, X. Identification and cloning of a negative regulator of systemic acquired resistance, SNI1, through a screen for suppressors of npr1-1. Cell 1999, 98, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Wang, W.; Marqués, J.; Mohan, R.; Saleh, A.; Durrant, W.E.; Song, J.; Dong, X. Salicylic acid activates DNA damage responses to potentiate plant immunity. Mol. Cell 2013, 52, 602–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercier, R.; Mézard, C.; Jenczewski, E.; Macaisne, N.; Grelon, M. The molecular biology of meiosis in plants. Annu. Rev. Plant Biol. 2015, 66, 297–327. [Google Scholar] [CrossRef] [PubMed]
- Borde, V. The multiple roles of the Mre11 complex for meiotic recombination. Chromosom. Res. 2007, 15, 551–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercier, R.; Jolivet, S.; Vezon, D.; Huppe, E.; Chelysheva, L.; Giovanni, M.; Nogué, F.; Doutriaux, M.-P.; Horlow, C.; Grelon, M.; et al. Two Meiotic Crossover Classes Cohabit in Arabidopsis: One Is Dependent on MER3, whereas the Other One Is Not. Curr. Biol. 2005, 15, 692–701. [Google Scholar] [CrossRef] [Green Version]
- Knoll, A.; Schröpfer, S.; Puchta, H. The RTR complex as caretaker of genome stability and its unique meiotic function in plants. Front. Plant Sci. 2014, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Lynn, A.; Soucek, R.; Börner, G.V. ZMM proteins during meiosis: Crossover artists at work. Chromosom. Res. 2007, 15, 591–605. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; He, C.; Wang, C.; Wang, X.; Ruan, F.; Yan, J.; Yin, P.; Wang, Y.; Yan, S. RAD51 supports DMC1 by inhibiting the SMC5/6 complex during meiosis. Plant Cell 2021, 33, 2869–2882. [Google Scholar] [CrossRef]
- Xaver, M.; Huang, L.; Chen, D.; Klein, F. Smc5/6-Mms21 prevents and eliminates inappropriate recombination intermediates in meiosis. PLoS Genet. 2013, 9, e1004067. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Fernández-Jiménez, N.; Szymanska-Lejman, M.; Pelé, A.; Underwood, C.J.; Serra, H.; Lambing, C.; Dluzewska, J.; Bieluszewski, T.; Pradillo, M.; et al. Natural variation identifies SNI1, the SMC5/6 component, as a modifier of meiotic crossover in Arabidopsis. Proc. Natl. Acad. Sci. USA 2021, 118, e2021970118. [Google Scholar] [CrossRef]
- Yang, F.; Fernández-Jiménez, N.; Tučková, M.; Vrána, J.; Cápal, P.; Díaz, M.; Pradillo, M.; Pecinka, A. Defects in meiotic chromosome segregation lead to unreduced male gametes in Arabidopsis SMC5/6 complex mutants. Plant Cell 2021, 33, 3104–3119. [Google Scholar] [CrossRef]
- Copsey, A.; Tang, S.; Jordan, P.W.; Blitzblau, H.G.; Newcombe, S.; Chan, A.C.; Newnham, L.; Li, Z.; Gray, S.; Herbert, A.D.; et al. Smc5/6 coordinates formation and resolution of joint molecules with chromosome morphology to ensure meiotic divisions. PLoS Genet. 2013, 9, e1004071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lilienthal, I.; Kanno, T.; Sjögren, C. Inhibition of the Smc5/6 complex during meiosis perturbs joint molecule formation and resolution without significantly changing crossover or non-crossover levels. PLoS Genet. 2013, 9, e1003898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verver, D.E.; Langedijk, N.S.M.; Jordan, P.W.; Repping, S.; Hamer, G. The SMC5/6 complex is involved in crucial processes during human spermatogenesis. Biol. Reprod. 2014, 91, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potts, P.R.; Porteus, M.H.; Yu, H. Human SMC5/6 complex promotes sister chromatid homologous recombination by recruiting the SMC1/3 cohesin complex to double-strand breaks. EMBO J. 2006, 25, 3377–3388. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Pacher, M.; Dukowic, S.; Schubert, V.; Puchta, H.; Schubert, I. The STRUCTURAL MAINTENANCE of CHROMOSOMES 5/6 complex promotes sister chromatid alignment and homologous recombination after DNA damage in Arabidopsis thaliana. Plant Cell 2009, 21, 2688–2699. [Google Scholar] [CrossRef] [Green Version]
- Masson, J.-Y.; West, S.C. The Rad51 and Dmc1 recombinases: A non-identical twin relationship. Trends Biochem. Sci. 2001, 26, 131–136. [Google Scholar] [CrossRef]
- Roy, M.-A.; D’Amours, D. DNA-binding properties of Smc6, a core component of the Smc5–6 DNA repair complex. Biochem. Biophys. Res. Commun. 2011, 416, 80–85. [Google Scholar] [CrossRef]
- Roy, M.-A.; Siddiqui, N.; D’Amours, D. Dynamic and selective DNA-binding activity of Smc5, a core component of the Smc5-Smc6 complex. Cell Cycle 2011, 10, 690–700. [Google Scholar] [CrossRef] [Green Version]
- Cromer, L.; Heyman, J.; Touati, S.; Harashima, H.; Araou, E.; Girard, C.; Horlow, C.; Wassmann, K.; Schnittger, A.; De Veylder, L.; et al. OSD1 promotes meiotic progression via APC/C inhibition and forms a regulatory network with TDM and CYCA1;2/TAM. PLoS Genet. 2012, 8, e1002865. [Google Scholar] [CrossRef] [Green Version]
- De Jaeger-Braet, J.; Krause, L.; Buchholz, A.; Schnittger, A. Heat stress reveals a specialized variant of the pachytene checkpoint in meiosis of Arabidopsis thaliana. Plant Cell 2022, 34, 433–454. [Google Scholar] [CrossRef]
- Zou, W.; Li, G.; Jian, L.; Qian, J.; Liu, Y.; Zhao, J. Arabidopsis SMC6A and SMC6B have redundant function in seed and gametophyte development. J. Exp. Bot. 2021, 72, 4871–4887. [Google Scholar] [CrossRef]
- Li, G.; Zou, W.; Jian, L.; Qian, J.; Deng, Y.; Zhao, J. Non-SMC elements 1 and 3 are required for early embryo and seedling development in Arabidopsis. J. Exp. Bot. 2017, 68, 1039–1054. [Google Scholar] [CrossRef]
- Li, G.; Zou, W.; Jian, L.; Qian, J.; Zhao, J. AtNSE1 and AtNSE3 are required for embryo pattern formation and maintenance of cell viability during Arabidopsis embryogenesis. J. Exp. Bot. 2019, 70, 6229–6244. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Fernández Jiménez, N.; Majka, J.; Pradillo, M.; Pecinka, A. Structural maintenance of chromosomes 5/6 complex is necessary for tetraploid genome stability in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 2139. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Shi, S.; Zhang, S.; Xu, P.; Lai, J.; Liu, Y.; Yuan, D.; Wang, Y.; Du, J.; Yang, C. SUMO E3 ligase AtMMS21 is required for normal meiosis and gametophyte development in Arabidopsis. BMC Plant Biol. 2014, 14, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz, M.; Pečinková, P.; Nowicka, A.; Baroux, C.; Sakamoto, T.; Yuliani Gandha, P.; Jeřábková, H.; Matsunaga, S.; Grossniklaus, U.; Pecinka, A. The SMC5/6 complex subunit NSE4A is involved in DNA damage repair and seed development in Arabidopsis. Plant Cell 2019, 31, 1579–1597. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Augustine, R.C.; Suzuki, M.; Feng, J.; Char, S.N.; Yang, B.; McCarty, D.R.; Vierstra, R.D. The SUMO ligase MMS21 profoundly influences maize development through its impact on genome activity and stability. PLoS Genet. 2021, 17, e1009830. [Google Scholar] [CrossRef]
- Jiang, J.; Xie, Y.; Du, J.; Yang, C.; Lai, J. A SUMO ligase OsMMS21 regulates rice development and auxin response. J. Plant Physiol. 2021, 263, 153447. [Google Scholar] [CrossRef]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Grandont, L.; Jenczewski, E.; Lloyd, A. Meiosis and Its Deviations in Polyploid Plants. Cytogenet. Genome Res. 2013, 140, 171–184. [Google Scholar] [CrossRef]
- Zelkowski, M.; Zelkowska, K.; Conrad, U.; Hesse, S.; Lermontova, I.; Marzec, M.; Meister, A.; Houben, A.; Schubert, V. Arabidopsis NSE4 proteins act in somatic nuclei and meiosis to ensure plant viability and fertility. Front. Plant Sci. 2019, 10, 774. [Google Scholar] [CrossRef] [Green Version]
- Chaudhury, A.M.; Koltunow, A.; Payne, T.; Luo, M.; Tucker, M.R.; Dennis, E.S.; Peacock, W.J. Control of early seed development. Annu. Rev. Cell Dev. Biol. 2001, 17, 677–699. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Vinkenoog, R.; Spielman, M.; Dickinson, H.G.; Scott, R.J. Parent-of-origin effects on seed development in Arabidopsis thaliana require DNA methylation. Development 2000, 127, 2493–2502. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Meinke, D.W. The titan mutants of Arabidopsis are disrupted in mitosis and cell cycle control during seed development. Plant J. 1998, 16, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Tzafrir, I.; Mcelver, J.A.; Liu, C.; Yang, L.J.; Wu, J.Q.; Martinez, A.; Patton, D.A.; Meinke, D.W.; Oklahoma, I.T. Diversity of TITAN functions in Arabidopsis seed development. Plant Physiol. 2002, 74078, 38–51. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.; Wang, C.; Hu, Z.; Yan, S. Negative regulator of E2F transcription factors links cell cycle checkpoint and DNA damage repair. Proc. Natl. Acad. Sci. USA 2018, 115, E3837–E3845. [Google Scholar] [CrossRef] [Green Version]
- The Arabidopsis Genome Initiative. Arabidopsis Genome Initiative Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 2000, 408, 796–815. [Google Scholar] [CrossRef] [Green Version]
- Hoencamp, C.; Dudchenko, O.; Elbatsh, A.M.O.; Brahmachari, S.; Raaijmakers, J.A.; van Schaik, T.; Cacciatore, Á.S.; Contessoto, V.G.; van Heesbeen, R.G.; van den Broek, B.; et al. 3D genomics across the tree of life reveals condensin II as a determinant of architecture type. Science 2021, 372, 984–989. [Google Scholar] [CrossRef]
- Schnable, P.S.; Ware, D.H.; Fulton, R.S.; Stein, J.C.; Wei, F.; Pasternak, S.; Liang, C.; Zhang, J.; Fulton, L.L.; Graves, T.; et al. The B73 maize genome: Complexity, diversity, and dynamics. Science 2009, 326, 1112–1115. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Pecinka, A. Multiple Roles of SMC5/6 Complex during Plant Sexual Reproduction. Int. J. Mol. Sci. 2022, 23, 4503. https://doi.org/10.3390/ijms23094503
Yang F, Pecinka A. Multiple Roles of SMC5/6 Complex during Plant Sexual Reproduction. International Journal of Molecular Sciences. 2022; 23(9):4503. https://doi.org/10.3390/ijms23094503
Chicago/Turabian StyleYang, Fen, and Ales Pecinka. 2022. "Multiple Roles of SMC5/6 Complex during Plant Sexual Reproduction" International Journal of Molecular Sciences 23, no. 9: 4503. https://doi.org/10.3390/ijms23094503
APA StyleYang, F., & Pecinka, A. (2022). Multiple Roles of SMC5/6 Complex during Plant Sexual Reproduction. International Journal of Molecular Sciences, 23(9), 4503. https://doi.org/10.3390/ijms23094503







