The Barley Chloroplast Mutator (cpm) Mutant: All Roads Lead to the Msh1 Gene
Abstract
1. Introduction
2. Results
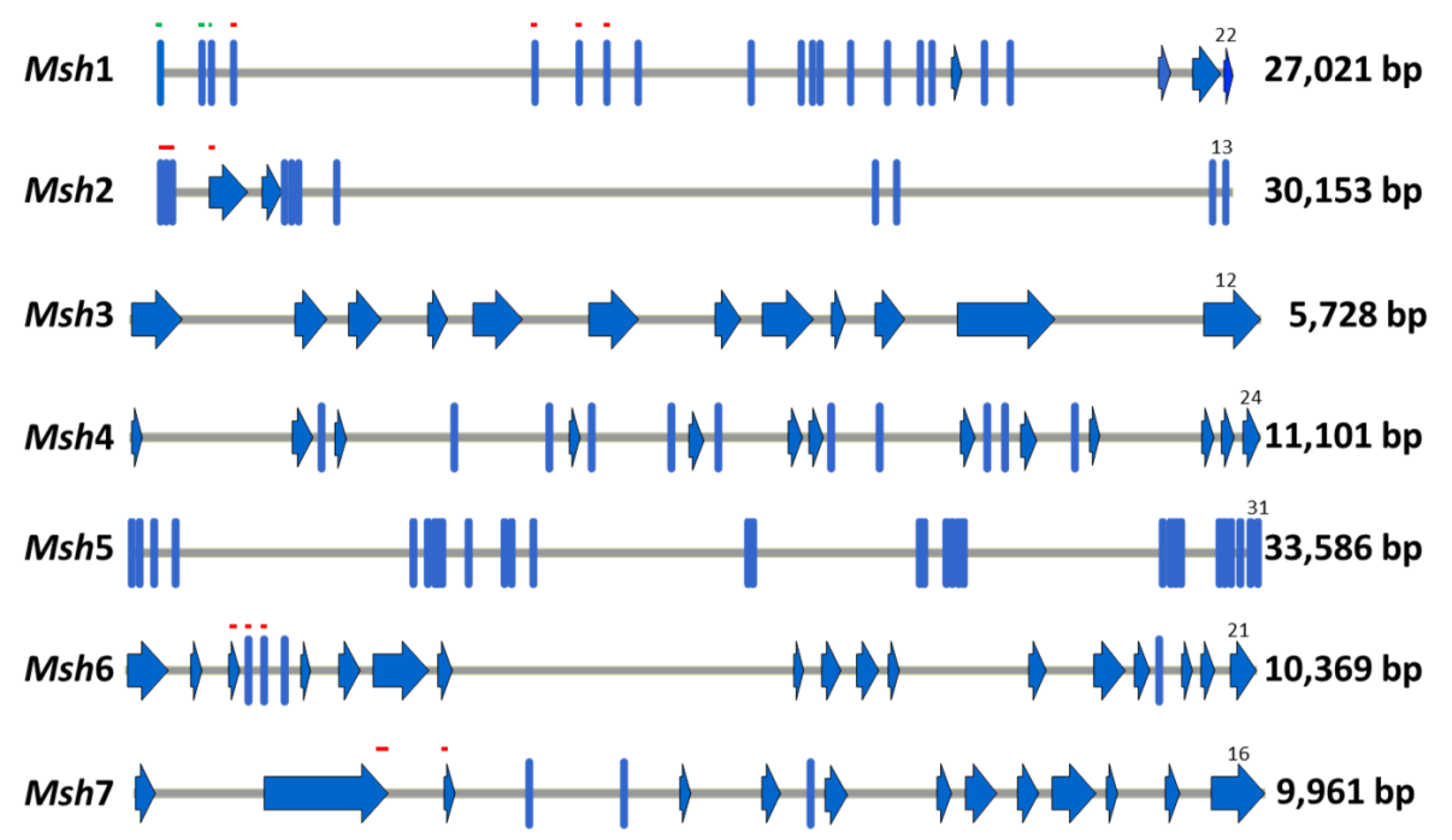
2.1. Identification and Amplification of the Coding Sequences of Barley Msh Genes
2.2. Sequencing and Detection of a Premature Stop-Codon Mutation in the Cpm Msh1 Gene
2.3. Analysis of the Msh1 DNA Region Carrying the Premature Stop Codon in Cpm and CLs Seedlings
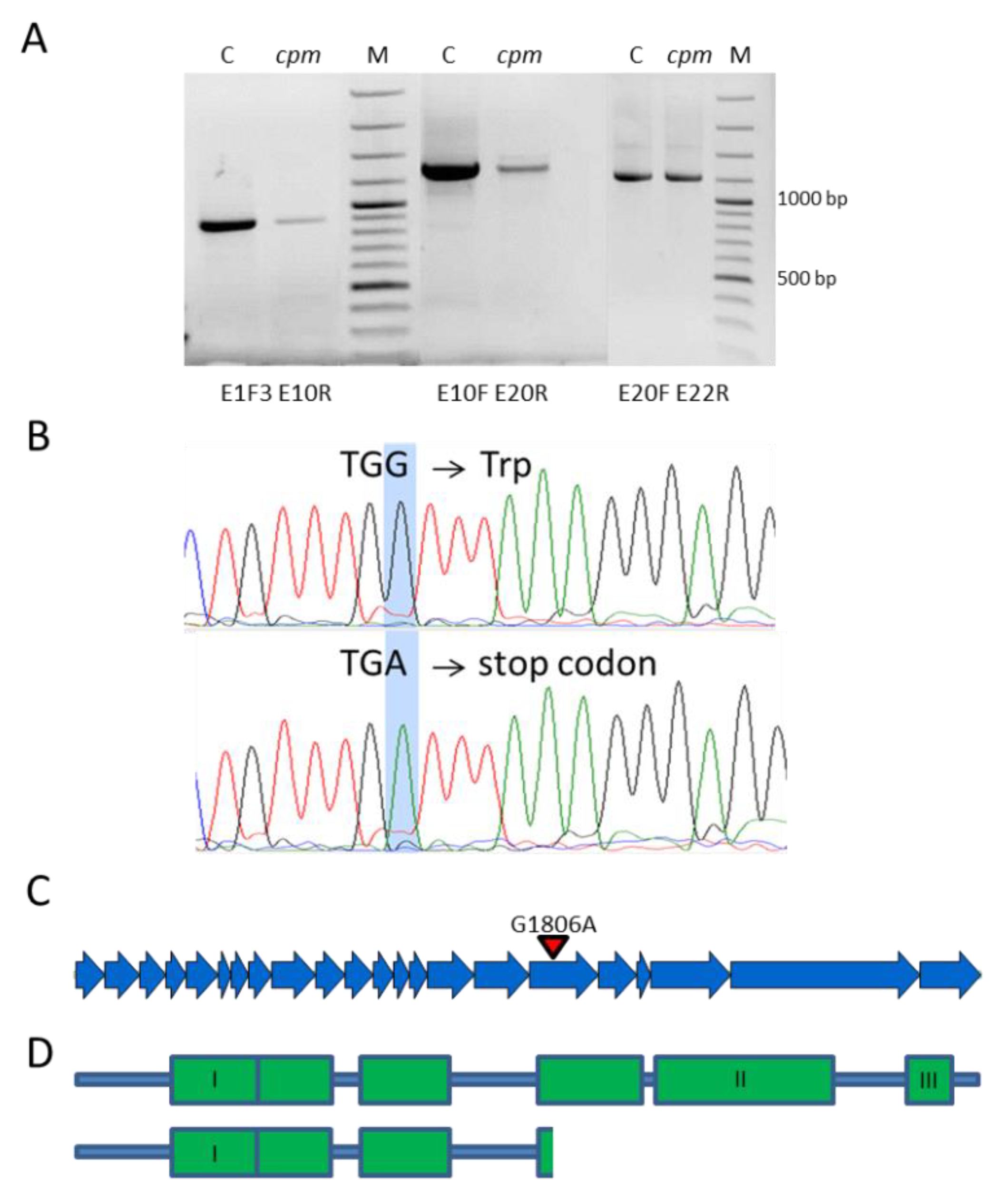
2.4. Prediction of Alternative Subcellular Locations of MSH1 Protein in Barley
3. Discussion
3.1. A Mutation in the Msh1 Gene as Candidate for the Barley cpm
3.2. Barley and Arabidopsis Mutants Exhibit Different Phenotypes
4. Materials and Methods
4.1. Plant Material
4.2. Identification of Msh Genes of Barley
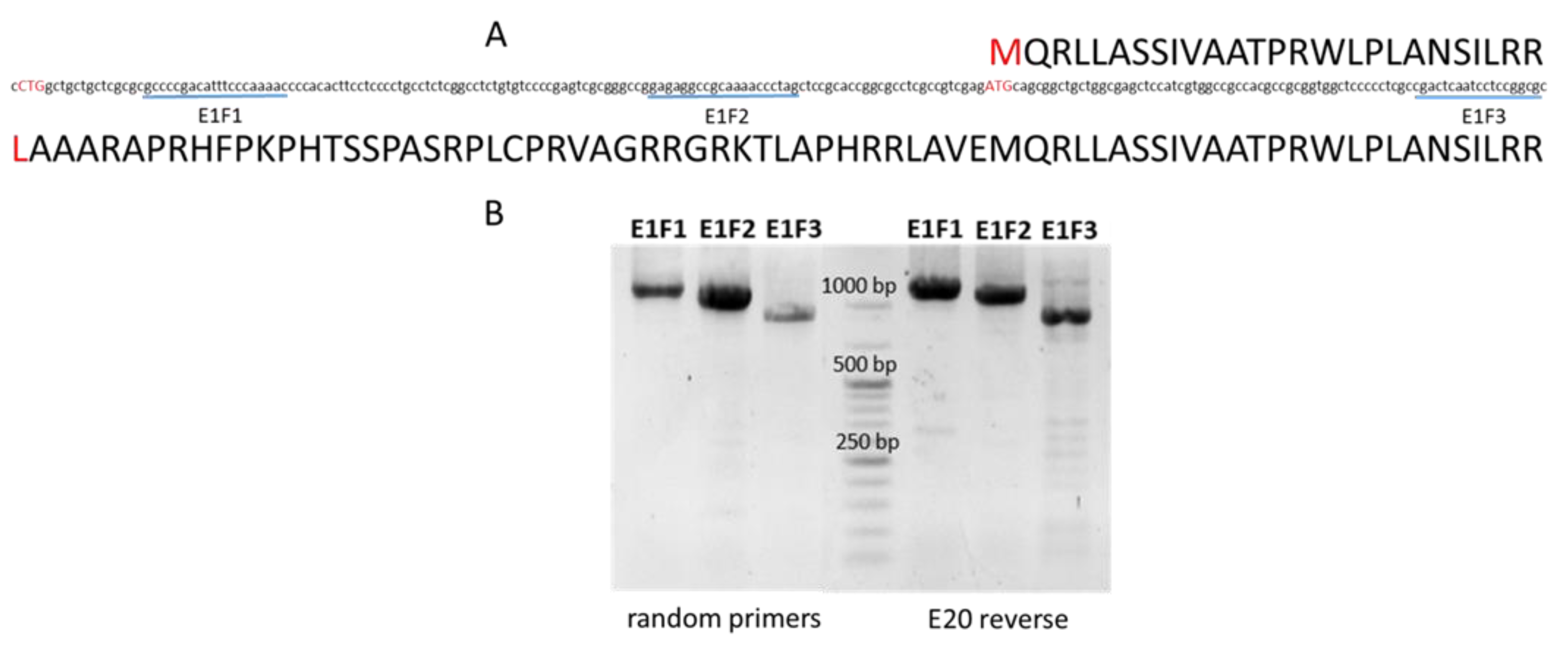
4.3. Amplification and Sequencing of the Coding Sequences of Msh Barley Genes
4.4. Analyses of DNA Sequences of cpm, Control and CLs Seedlings
4.5. Alignments and Polymorphism Identification
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morley, S.A.; Ahmad, N.; Nielsen, B.L. Plant Organelle Genome Replication. Plants 2019, 8, 358. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Waneka, G.; Broz, A.K.; King, C.R.; Sloan, D.B. MSH1 Is Required for Maintenance of the Low Mutation Rates in Plant Mitochondrial and Plastid Genomes. Proc. Natl. Acad. Sci. USA 2020, 117, 16448–16455. [Google Scholar] [CrossRef] [PubMed]
- Chevigny, N.; Schatz-Daas, D.; Lotfi, F.; Gualberto, J.M. DNA Repair and the Stability of the Plant Mitochondrial Genome. Int. J. Mol. Sci. 2020, 21, 328. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Nielsen, B.L. Plant Organelle DNA Maintenance. Plants 2020, 9, 683. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D. Comparative Organization of Chloroplast Genomes. Annu. Rev. Genet. 1985, 19, 325–354. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D. Contrasting Modes and Tempos of Genome Evolution in Land Plant Organelles. Trends Genet. 1990, 6, 115–120. [Google Scholar] [CrossRef]
- Wolfe, K.H.; Li, W.H.; Sharp, P.M. Rates of Nucleotide Substitution Vary Greatly among Plant Mitochondrial, Chloroplast, and Nuclear DNAs. Proc. Natl. Acad. Sci. USA 1987, 84, 9054–9058. [Google Scholar] [CrossRef]
- Clegg, M.T. Chloroplast Gene Sequences and the Study of Plant Evolution. Proc. Natl. Acad. Sci. USA 1993, 90, 363–367. [Google Scholar] [CrossRef]
- Wicke, S.; Schneeweiss, G.M.; de Pamphilis, C.W.; Müller, K.F.; Quandt, D. The Evolution of the Plastid Chromosome in Land Plants: Gene Content, Gene Order, Gene Function. Plant Mol. Biol. 2011, 76, 273–297. [Google Scholar] [CrossRef]
- Zhang, J.; Ruhlman, T.A.; Sabir, J.S.M.; Blazier, J.C.; Weng, M.-L.; Park, S.; Jansen, R.K. Coevolution between Nuclear-Encoded DNA Replication, Recombination, and Repair Genes and Plastid Genome Complexity. Genome Biol. Evol. 2016, 8, 622–634. [Google Scholar] [CrossRef]
- Kirk, J.T.; Tilney-Bassett, R.A. The Plastids: Their Chemistry, Structure, Growth and Inheritance; Elsevier/North: Amsterdam, The Netherland, 1978. [Google Scholar]
- Börner, T.; Sears, B. Plastome mutants. Plant Mol. Biol. Rep. 1986, 4, 69–72. [Google Scholar] [CrossRef]
- Gressel, J.; Levy, A.A. Stress, Mutators, Mutations and Stress Resistance. In Abiotic Stress Adaptation in Plants: Physiological, Molecular and Genomic Foundation; Pareek, A., Sopory, S.K., Bohnert, H.J., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 471–483. ISBN 978-90-481-3112-9. [Google Scholar]
- Greiner, S. Plastome mutants of higher plants. In Genomics of Chloroplasts and Mitochondria; Advances in Photosynthesis and Respiration 35 Including Bioenergy and Related Processes; Bock, R., Knoop, V., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 237–266. ISBN 978-94-007-2919-3. [Google Scholar]
- Prina, A.R.; Pacheco, M.G.; Landau, A.M. Mutation Induction in Cytoplasmic Genomes. In Plant Mutation Breeding and Biotechnology; Shu, Q.S., Forster, B.P., Nakagawa, H., Eds.; FAO-IAEA: Rome, Italy, 2012; pp. 201–206. [Google Scholar]
- Prina, A.R.; Landau, A.; Colombo, N.; Jaureguialzo, M.; Arias, M.C.; Rios, R.D.; Pacheco, M.G. Genetically Unstable Mutants as Novel Sources of Genetic Variability: The Chloroplast Mutator Genotype in Barley as a Tool for Exploring the Plastid Genome. In Induced Plant Mutations in the Genomics Era; Shu, Q.Y., Ed.; FAO-IAEA: Rome, Italy, 2009; pp. 255–256. [Google Scholar]
- Prina, A.R. A Mutator Nuclear Gene Inducing a Wide Spectrum of Cytoplasmically Inherited Chlorophyll Deficiencies in Barley. Theoret. Appl. Genet. 1992, 85, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Prina, A.R. Mutator-Induced Cytoplasmic Mutants in Barley: Genetic Evidence of Activation of a Putative Chloroplast Transposon. J. Hered. 1996, 87, 385–389. [Google Scholar] [CrossRef][Green Version]
- Landau, A.M.; Pacheco, M.G.; Prina, A.R. A Second InfA Plastid Gene Point Mutation Shows a Compensatory Effect on the Expression of the Cytoplasmic Line 2 (CL2) Syndrome in Barley. J. Hered. 2011, 102, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Landau, A.; Díaz Paleo, A.; Civitillo, R.; Jaureguialzo, M.; Prina, A.R. Two InfA Gene Mutations Independently Originated from a Mutator Genotype in Barley. J. Hered. 2007, 98, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Landau, A.M.; Lokstein, H.; Scheller, H.V.; Lainez, V.; Maldonado, S.; Prina, A.R. A Cytoplasmically Inherited Barley Mutant Is Defective in Photosystem I Assembly Due to a Temperature-Sensitive Defect in Ycf3 Splicing. Plant Physiol. 2009, 151, 1802–1811. [Google Scholar] [CrossRef]
- Rios, R.D.; Saione, H.; Robredo, C.; Acevedo, A.; Colombo, N.; Prina, A.R. Isolation and Molecular Characterization of Atrazine Tolerant Barley Mutants. Theor. Appl. Genet. 2003, 106, 696–702. [Google Scholar] [CrossRef]
- Landau, A.; Lencina, F.; Pacheco, M.G.; Prina, A.R. Plastome Mutations and Recombination Events in Barley Chloroplast Mutator Seedlings. J. Hered. 2016, 107, 266–273. [Google Scholar] [CrossRef][Green Version]
- Lencina, F.; Landau, A.M.; Petterson, M.E.; Pacheco, M.G.; Kobayashi, K.; Prina, A.R. The Rpl23 Gene and Pseudogene Are Hotspots of Illegitimate Recombination in Barley Chloroplast Mutator Seedlings. Sci. Rep. 2019, 9, 9960. [Google Scholar] [CrossRef]
- Lencina, F.; Landau, A.M.; Pacheco, M.G.; Kobayashi, K.; Prina, A.R. Four Large Indels Detected by CpTILLING in Barley Chloroplast Mutator Seedlings|BioRxiv. Available online: https://www.biorxiv.org/content/10.1101/2021.02.19.432034v1 (accessed on 17 August 2021).
- Spampinato, C.P.; Gomez, R.L.; Galles, C.; Lario, L.D. From Bacteria to Plants: A Compendium of Mismatch Repair Assays. Mutat. Res. 2009, 682, 110–128. [Google Scholar] [CrossRef]
- Manova, V.; Gruszka, D. DNA Damage and Repair in Plants–from Models to Crops. Front. Plant Sci. 2015, 6, 885. [Google Scholar] [CrossRef] [PubMed]
- Harfe, B.D.; Jinks-Robertson, S. DNA Mismatch Repair and Genetic Instability. Annu. Rev. Genet. 2000, 34, 359–399. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.-H.; Kim, T.G.; Ban, C. DNA Mismatch Repair System. Classical and Fresh Roles. FEBS J. 2006, 273, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Nei, M.; Ma, H. The Origins and Early Evolution of DNA Mismatch Repair Genes—Multiple Horizontal Gene Transfers and Co-Evolution. Nucleic Acids Res. 2007, 35, 7591–7603. [Google Scholar] [CrossRef]
- Fukui, K.; Harada, A.; Wakamatsu, T.; Minobe, A.; Ohshita, K.; Ashiuchi, M.; Yano, T. The GIY-YIG Endonuclease Domain of Arabidopsis MutS Homolog 1 Specifically Binds to Branched DNA Structures. FEBS Lett. 2018, 592, 4066–4077. [Google Scholar] [CrossRef]
- Bray, C.M.; West, C.E. DNA Repair Mechanisms in Plants: Crucial Sensors and Effectors for the Maintenance of Genome Integrity. New Phytol. 2005, 168, 511–528. [Google Scholar] [CrossRef]
- Culligan, K.M.; Hays, J.B. Arabidopsis MutS Homologs-AtMSH2, AtMSH3, AtMSH6, and a Novel AtMSH7-Form Three Distinct Protein Heterodimers with Different Specificities for Mismatched DNA. Plant Cell 2000, 12, 991–1002. [Google Scholar] [CrossRef]
- Eisen, J. A Phylogenomic Study of the MutS Family of Proteins. Nucleic Acids Res. 1998, 26, 4291–4300. [Google Scholar] [CrossRef]
- Snowden, T.; Acharya, S.; Butz, C.; Berardini, M.; Fishel, R. HMSH4-HMSH5 Recognizes Holliday Junctions and Forms a Meiosis-Specific Sliding Clamp That Embraces Homologous Chromosomes. Mol. Cell 2004, 15, 437–451. [Google Scholar] [CrossRef]
- Abdelnoor, R.V.; Yule, R.; Elo, A.; Christensen, A.C.; Meyer-Gauen, G.; Mackenzie, S.A. Substoichiometric Shifting in the Plant Mitochondrial Genome Is Influenced by a Gene Homologous to MutS. Proc. Natl. Acad. Sci. USA 2003, 100, 5968–5973. [Google Scholar] [CrossRef]
- Shedge, V.; Arrieta-Montiel, M.; Christensen, A.C.; Mackenzie, S.A. Plant Mitochondrial Recombination Surveillance Requires Unusual RecA and MutS Homologs. Plant Cell 2007, 19, 1251–1264. [Google Scholar] [CrossRef]
- Arrieta-Montiel, M.P.; Shedge, V.; Davila, J.; Christensen, A.C.; Mackenzie, S.A. Diversity of the Arabidopsis Mitochondrial Genome Occurs via Nuclear-Controlled Recombination Activity. Genetics 2009, 183, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Boesch, P.; Weber-Lotfi, F.; Ibrahim, N.; Tarasenko, V.; Cosset, A.; Paulus, F.; Lightowlers, R.N.; Dietrich, A. DNA Repair in Organelles: Pathways, Organization, Regulation, Relevance in Disease and Aging. Biochim. Biophys. Acta 2011, 1813, 186–200. [Google Scholar] [CrossRef]
- Odahara, M.; Kishita, Y.; Sekine, Y. MSH1 Maintains Organelle Genome Stability and Genetically Interacts with RECA and RECG in the Moss Physcomitrella Patens. Plant J. 2017, 91, 455–465. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Culligan, K.; Lamers, M.; Hays, J. Dissimilar Mispair-Recognition Spectra of Arabidopsis DNA-Mismatch-Repair Proteins MSH2·MSH6 (MutSα) and MSH2·MSH7 (MutSγ). Nucleic Acids Res. 2003, 31, 6027–6034. [Google Scholar] [CrossRef]
- Hoffman, P.D.; Leonard, J.M.; Lindberg, G.E.; Bollmann, S.R.; Hays, J.B. Rapid Accumulation of Mutations during Seed-to-Seed Propagation of Mismatch-Repair-Defective Arabidopsis. Genes Dev. 2004, 18, 2676–2685. [Google Scholar] [CrossRef]
- Emmanuel, E.; Yehuda, E.; Melamed-Bessudo, C.; Avivi-Ragolsky, N.; Levy, A.A. The Role of AtMSH2 in Homologous Recombination in Arabidopsis Thaliana. EMBO Rep. 2006, 7, 100–105. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, X.; Song, Y.; Shen, H.; Cui, H. Effects of OsMSH6 Mutations on Microsatellite Stability and Homeologous Recombination in Rice. Front. Plant Sci. 2020, 11, 220. [Google Scholar] [CrossRef]
- Rayssiguier, C.; Thaler, D.S.; Radman, M. The Barrier to Recombination between Escherichia Coli and Salmonella Typhimurium Is Disrupted in Mismatch-Repair Mutants. Nature 1989, 342, 396–401. [Google Scholar] [CrossRef]
- Abdelnoor, R.V.; Christensen, A.C.; Mohammed, S.; Munoz-Castillo, B.; Moriyama, H.; Mackenzie, S.A. Mitochondrial Genome Dynamics in Plants and Animals: Convergent Gene Fusions of a MutS Homologue. J. Mol. Evol. 2006, 63, 165–173. [Google Scholar] [CrossRef]
- Maréchal, A.; Brisson, N. Recombination and the Maintenance of Plant Organelle Genome Stability. New Phytol. 2010, 186, 299–317. [Google Scholar] [CrossRef]
- Redei, G.P. Extrachromosomal mutability determined by a nuclear gene locus in Arabidopsis. Mutat Res. 1973, 18, 149–162. [Google Scholar] [CrossRef]
- Martínez-Zapater, J.M.; Gil, P.; Capel, J.; Somerville, C.R. Mutations at the Arabidopsis CHM Locus Promote Rearrangements of the Mitochondrial Genome. Plant Cell 1992, 4, 889–899. [Google Scholar]
- Sakamoto, W.; Kondo, H.; Murata, M.; Motoyoshi, F. Altered Mitochondrial Gene Expression in a Maternal Distorted Leaf Mutant of Arabidopsis Induced by Chloroplast Mutator. Plant Cell 1996, 8, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Z.; Arrieta-Montiel, M.P.; Virdi, K.S.; de Paula, W.B.M.; Widhalm, J.R.; Basset, G.J.; Davila, J.I.; Elthon, T.E.; Elowsky, C.G.; Sato, S.J.; et al. Muts Homolog1 Is a Nucleoid Protein That Alters Mitochondrial and Plastid Properties and Plant Response to High Light. Plant Cell 2011, 23, 3428–3441. [Google Scholar] [CrossRef] [PubMed]
- Christensen, A.C.; Lyznik, A.; Mohammed, S.; Elowsky, C.G.; Elo, A.; Yule, R.; Mackenzie, S.A. Dual-Domain, Dual-Targeting Organellar Protein Presequences in Arabidopsis Can Use Non-AUG Start Codons. Plant Cell 2005, 17, 2805–2816. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-Z.; Santamaria, R.D.L.R.; Virdi, K.S.; Arrieta-Montiel, M.P.; Razvi, F.; Li, S.; Ren, G.; Yu, B.; Alexander, D.; Guo, L.; et al. The Chloroplast Triggers Developmental Reprogramming When MutS HOMOLOG1 Is Suppressed in Plants. Plant Physiol. 2012, 159, 710–720. [Google Scholar] [CrossRef]
- Virdi, K.S.; Laurie, J.D.; Xu, Y.Z.; Yu, J.; Shao, M.R.; Sanchez, R.; Kundariya, H.; Wang, D.; Riethoven, J.J.M.; Wamboldt, Y.; et al. Arabidopsis MSH1 Mutation Alters the Epigenome and Produces Heritable Changes in Plant Growth. Nat. Commun. 2015, 6, 6386. [Google Scholar] [CrossRef]
- Virdi, K.S.S.; Wamboldt, Y.; Kundariya, H.; Laurie, J.D.D.; Keren, I.; Kumar, K.R.S.; Block, A.; Basset, G.; Luebker, S.; Elowsky, C.; et al. MSH1 Is a Plant Organellar DNA Binding and Thylakoid Protein under Precise Spatial Regulation to Alter Development. Mol. Plant 2016, 9, 245–260. [Google Scholar] [CrossRef]
- Shao, M.R.; Kumar Kenchanmane Raju, S.; Laurie, J.D.; Sanchez, R.; Mackenzie, S.A. Stress-Responsive Pathways and Small RNA Changes Distinguish Variable Developmental Phenotypes Caused by MSH1 Loss. BMC Plant Biol. 2017, 17, 47. [Google Scholar] [CrossRef]
- Beltrán, J.; Wamboldt, Y.; Sanchez, R.; LaBrant, E.W.; Kundariya, H.; Virdi, K.S.; Elowsky, C.; Mackenzie, S.A. Specialized Plastids Trigger Tissue-Specific Signaling for Systemic Stress Response in Plants. Plant Physiol. 2018, 178, 672–683. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, S.A.; Kundariya, H. Organellar Protein Multi-Functionality and Phenotypic Plasticity in Plants. Philos. Trans. R. Soc. B Biol. Sci. 2019, 375. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sanchez, R.; Kundariya, H.; Maher, T.; Dopp, I.; Schwegel, R.; Virdi, K.; Axtell, M.J.; Mackenzie, S.A. Segregation of an MSH1 RNAi Transgene Produces Heritable Non-Genetic Memory in Association with Methylome Reprogramming. Nat. Commun. 2020, 11, 2214. [Google Scholar] [CrossRef] [PubMed]
- Prina, A.R.; Arias, M.C.; Lainez, V.; Landau, A.; Maldonado, S. A Cytoplasmically Inherited Mutant Controlling Early Chloroplast Development in Barley Seedlings. Theor. Appl. Genet. 2003, 107, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Dellaporta, S. Plant DNA Miniprep and Microprep: Versions 2.1–2.3. In The Maize Handbook; Springer Lab Manuals; Freeling, M., Walbot, V., Eds.; Springer: New York, NY, USA, 1994; pp. 522–525. ISBN 978-1-4612-2694-9. [Google Scholar]

| Gene | Nucleotide Change a | Exon Location | Amino-Acid Change | Genotype |
|---|---|---|---|---|
| Msh1 | A216G | 2 | - | cpm and control |
| T225C | 2 | - | cpm and control | |
| A263C | 3 | Q88P | cpm and control | |
| A300G | 3 | - | cpm and control | |
| T696C | 8 | - | cpm and control | |
| T831C | 9 | - | cpm and control | |
| G1806A | 17 | W602 * | cpm | |
| T2060C | 18 | M687T | cpm and control | |
| G2799A | 21 | - | cpm and control | |
| G2861A | 21 | R954Q | cpm and control | |
| C2988T | 21 | - | cpm and control | |
| G3000A | 21 | - | cpm and control | |
| G3046A | 21 | V1016I | cpm and control | |
| G3135T | 21 | - | cpm and control | |
| C3226T | 22 | - | cpm and control | |
| A3235G | 22 | M1079V | cpm and control | |
| Msh6 | G498A | 3 | - | cpm and control |
| T1144C | 9 | - | cpm and control | |
| T1577C | 10 | L526S | cpm and control | |
| A2340G | 15 | - | cpm and control | |
| G2464A | 16 | V822I | cpm and control | |
| Msh7 | C337T | 2 | P113S | cpm and control |
| T440C | 2 | V147A | cpm and control | |
| T1739C | 7 | F580S | cpm and control | |
| G1860T | 9 | M620I | cpm and control | |
| A3030G | 14 | - | cpm and control | |
| C3171T | 15 | - | cpm and control | |
| G3317A | 16 | R1106K | cpm and control |
| Protein | Mitochondrial Transfer Peptide | Chloroplast Transfer Peptide | Signal Peptide to Secretory Pathway | Any Other Location | Location |
|---|---|---|---|---|---|
| MSH1 typical start codon | 0.791 | 0.620 | 0.004 | 0.006 | Mitochondria Chloroplast |
| MSH1 alternative start codon | 0.279 | 0.754 | 0 | 0.098 | Chloroplast |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lencina, F.; Landau, A.; Prina, A.R. The Barley Chloroplast Mutator (cpm) Mutant: All Roads Lead to the Msh1 Gene. Int. J. Mol. Sci. 2022, 23, 1814. https://doi.org/10.3390/ijms23031814
Lencina F, Landau A, Prina AR. The Barley Chloroplast Mutator (cpm) Mutant: All Roads Lead to the Msh1 Gene. International Journal of Molecular Sciences. 2022; 23(3):1814. https://doi.org/10.3390/ijms23031814
Chicago/Turabian StyleLencina, Franco, Alejandra Landau, and Alberto R. Prina. 2022. "The Barley Chloroplast Mutator (cpm) Mutant: All Roads Lead to the Msh1 Gene" International Journal of Molecular Sciences 23, no. 3: 1814. https://doi.org/10.3390/ijms23031814
APA StyleLencina, F., Landau, A., & Prina, A. R. (2022). The Barley Chloroplast Mutator (cpm) Mutant: All Roads Lead to the Msh1 Gene. International Journal of Molecular Sciences, 23(3), 1814. https://doi.org/10.3390/ijms23031814






