MFG-E8 Reduces Aortic Intimal Proliferation in a Murine Model of Transplant Vasculopathy
Abstract
:1. Introduction
2. Materials and Methods
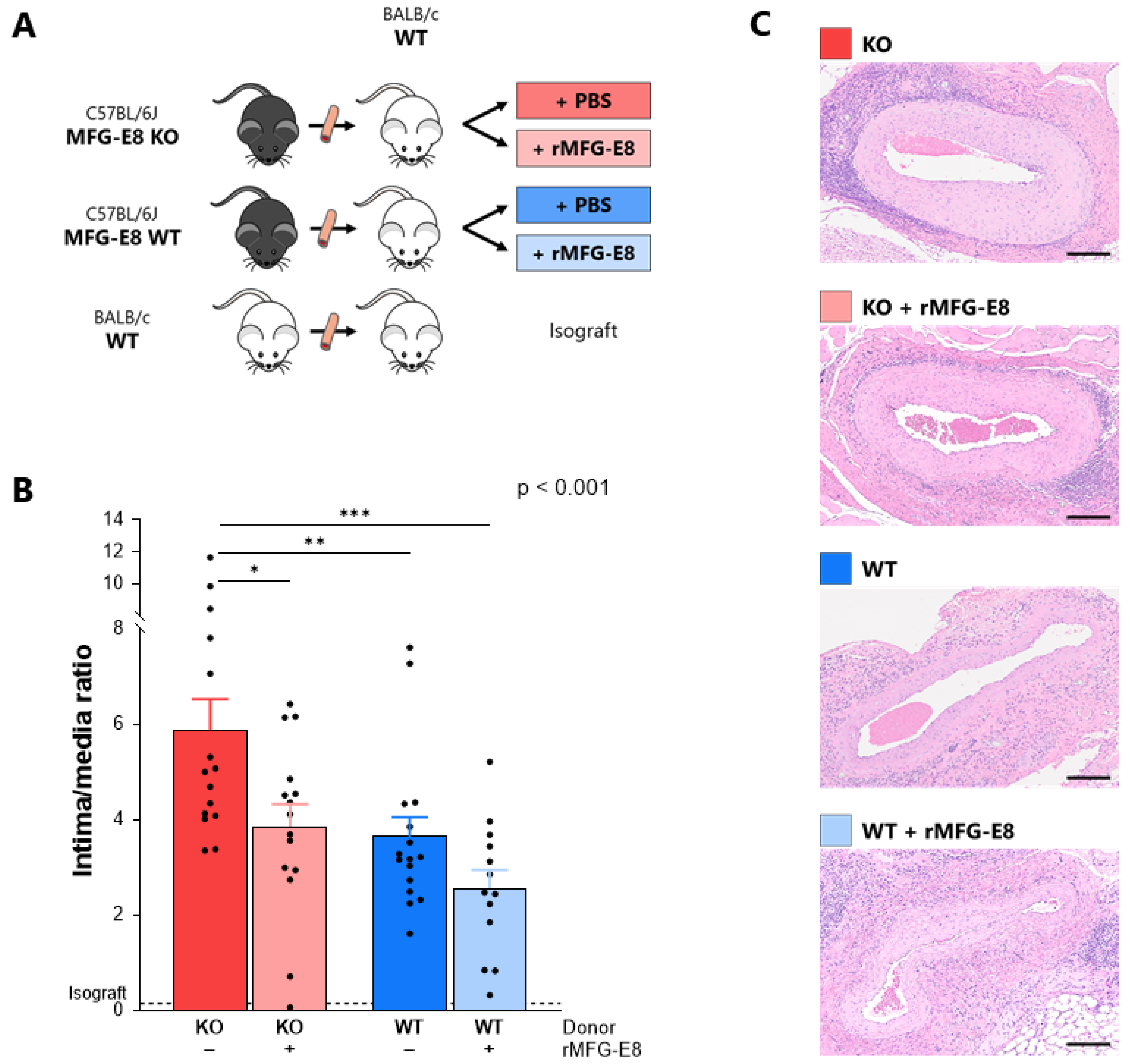
2.1. Transplant Vasculopathy Model and Injection of Murine Recombinant MFG-E8
2.2. Morphometric and Immunofluorescence Analysis of Aortic Grafts
2.3. Flow Cytometric Analyses of Lymphocytes
2.4. Assessment of Humoral Responses and Anti-Donor Antibodies
2.5. Statistical Analyses
3. Results
3.1. Absence of MFG-E8 Promoted Intimal Proliferation in the Transplanted Aorta
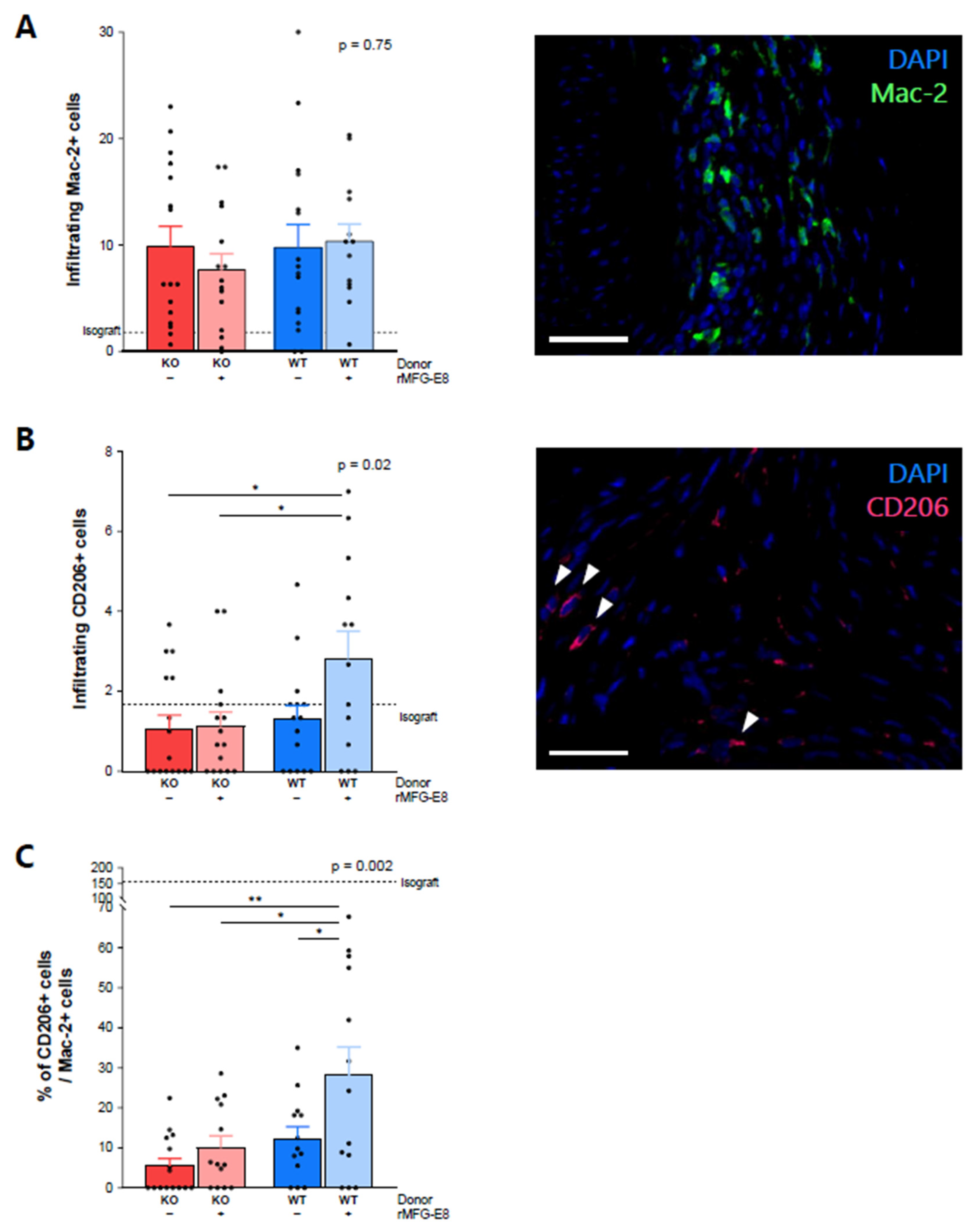
3.2. MFG-E8 Increased the Proportion of M2 Anti-Inflammatory Macrophages among Infiltrative Macrophages
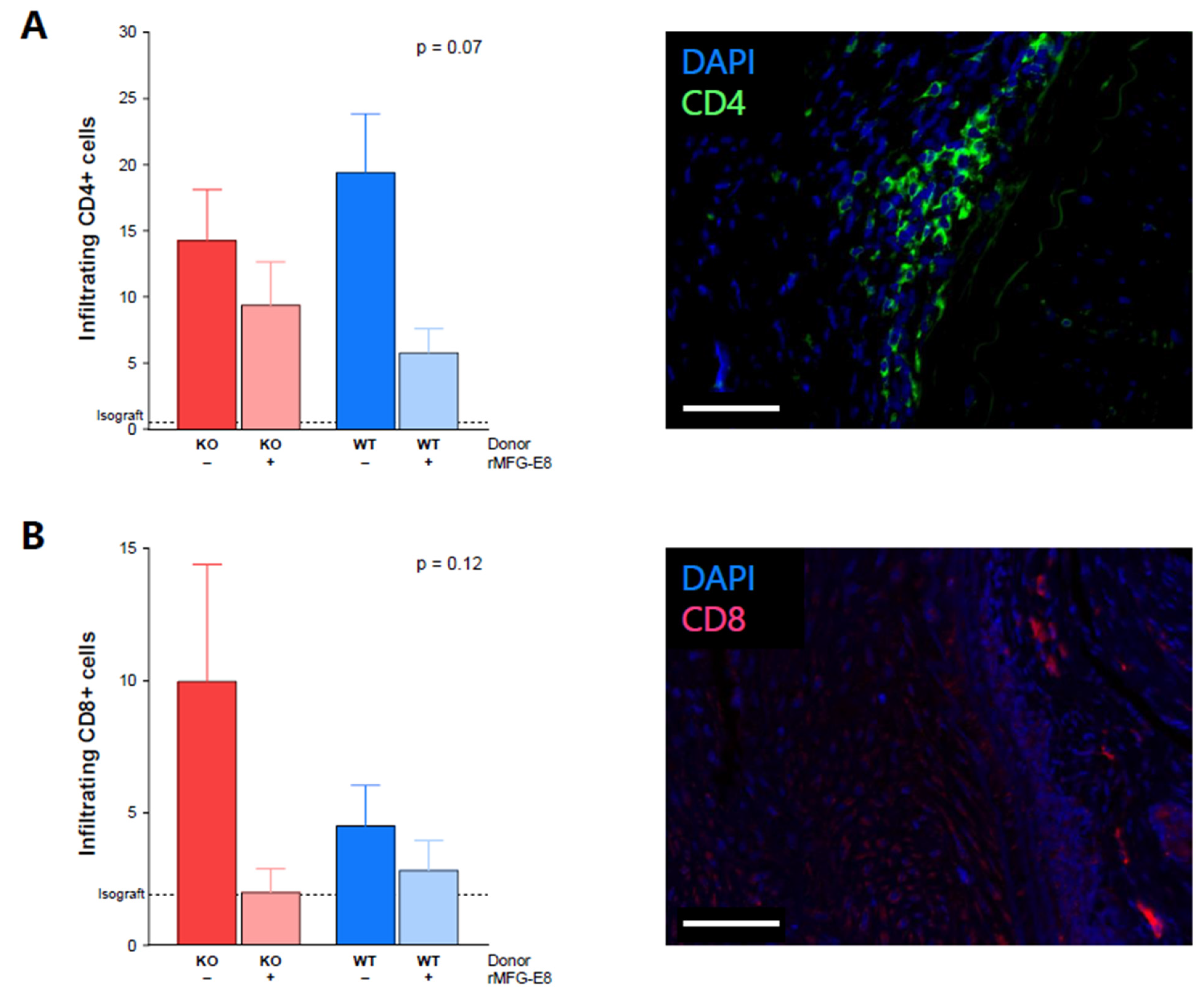
3.3. MFG-E8 Administration and T-Cell Infiltration into Allografts
3.4. MFG-E8 Dampened Systemic T-Cell Activation
3.5. MFG-E8 Did Not Significantly Impact Humoral Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | Anti-Donor Antibody |
| DGF | Delayed Graft Function |
| DSA | Donor-Specific Antibody |
| GFR | Glomerular Filtration Rate |
| IQR | Interquartile Range |
| KO | Knockout |
| MFG-E8 | Milk Fat Globule Epidermal Growth Factor 8 |
| PBS | Phosphate-Buffered Saline |
| PE | Phycoerythrin |
| rMFG-E8 | Recombinant MFG-E8 |
| Tregs | Regulatory T cells |
| TV | Transplant Vasculopathy |
| VSMCs | Vascular Smooth Muscle Cells |
| WT | Wild Type |
References
- Nankivell, B.J.; Borrows, R.J.; Fung, C.L.-S.; O’Connell, P.J.; Allen, R.D.M.; Chapman, J.R. The Natural History of Chronic Allograft Nephropathy. New Engl. J. Med. 2003, 349, 2326–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cailhier, J.-F.; Laplante, P.; Hébert, M.-J. Endothelial Apoptosis and Chronic Transplant Vasculopathy: Recent Results, Novel Mechanisms. Am. J. Transplant. 2006, 6, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Nankivell, B.J.; Fenton-Lee, C.A.; Kuypers, D.R.; Cheung, E.; Allen, R.D.; O’Connell, P.J.; Chapman, J.R. Effect of Histological Damage on Long-Term Kidney Transplant Outcome. Transplantation 2001, 71, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.N.; Libby, P. Vascular Remodeling in Transplant Vasculopathy. Circ. Res. 2007, 100, 967–978. [Google Scholar] [CrossRef]
- Raymond, M.-A. Apoptosis of Endothelial Cells Triggers a Caspase-Dependent Anti-Apoptotic Paracrine Loop Active on Vascular Smooth Muscle Cells. FASEB J. 2004, 18, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Cornell, L.D.; Smith, R.N.; Colvin, R.B. Kidney Transplantation: Mechanisms of Rejection and Acceptance. Annu. Rev. Pathol. 2008, 3, 189–220. [Google Scholar] [CrossRef] [PubMed]
- Lauber, K.; Bohn, E.; Kröber, S.M.; Xiao, Y.; Blumenthal, S.G.; Lindemann, R.K.; Marini, P.; Wiedig, C.; Zobywalski, A.; Baksh, S.; et al. Apoptotic Cells Induce Migration of Phagocytes via Caspase-3-Mediated Release of a Lipid Attraction Signal. Cell 2003, 113, 717–730. [Google Scholar] [CrossRef] [Green Version]
- Laplante, P.; Raymond, M.-A.; Gagnon, G.; Vigneault, N.; Sasseville, A.M.-J.; Langelier, Y.; Bernard, M.; Raymond, Y.; Hébert, M.-J. Novel Fibrogenic Pathways Are Activated in Response to Endothelial Apoptosis: Implications in the Pathophysiology of Systemic Sclerosis. J. Immunol. 2005, 174, 5740–5749. [Google Scholar] [CrossRef] [Green Version]
- Pallet, N.; Dieudé, M.; Cailhier, J.; Hébert, M. The Molecular Legacy of Apoptosis in Transplantation: The Apoptotic Legacy in Transplantation. Am. J. Transplant. 2012, 12, 1378–1384. [Google Scholar] [CrossRef] [Green Version]
- Brissette, M.-J.; Lepage, S.; Lamonde, A.-S.; Sirois, I.; Groleau, J.; Laurin, L.-P.; Cailhier, J.-F. MFG-E8 Released by Apoptotic Endothelial Cells Triggers Anti-Inflammatory Macrophage Reprogramming. PLoS ONE 2012, 7, e36368. [Google Scholar] [CrossRef] [Green Version]
- Laplante, P.; Brillant-Marquis, F.; Brissette, M.-J.; Joannette-Pilon, B.; Cayrol, R.; Kokta, V.; Cailhier, J.-F. MFG-E8 Reprogramming of Macrophages Promotes Wound Healing by Increased BFGF Production and Fibroblast Functions. J. Investig. Dermatol. 2017, 137, 2005–2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aziz, M.; Jacob, A.; Matsuda, A.; Wu, R.; Zhou, M.; Dong, W.; Yang, W.-L.; Wang, P. Pre-Treatment of Recombinant Mouse MFG-E8 Downregulates LPS-Induced TNF-α Production in Macrophages via STAT3-Mediated SOCS3 Activation. PLoS ONE 2011, 6, e27685. [Google Scholar] [CrossRef] [PubMed]
- Brissette, M.-J.; Laplante, P.; Qi, S.; Latour, M.; Cailhier, J.-F. Milk Fat Globule Epidermal Growth Factor-8 Limits Tissue Damage through Inflammasome Modulation during Renal Injury. J. Leukoc. Biol. 2016, 100, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Wu, R.; Jacob, A.; Komura, H.; Zhou, M.; Wang, Z.; Aziz, M.M.; Wang, P. Protective Effect of Milk Fat Globule-Epidermal Growth Factor-Factor VIII after Renal Ischemia-Reperfusion Injury in Mice. Crit. Care Med. 2011, 39, 2039–2047. [Google Scholar] [CrossRef] [Green Version]
- Aziz, M.M.; Ishihara, S.; Mishima, Y.; Oshima, N.; Moriyama, I.; Yuki, T.; Kadowaki, Y.; Rumi, M.A.K.; Amano, Y.; Kinoshita, Y. MFG-E8 Attenuates Intestinal Inflammation in Murine Experimental Colitis by Modulating Osteopontin-Dependent v 3 Integrin Signaling. J. Immunol. 2009, 182, 7222–7232. [Google Scholar] [CrossRef] [Green Version]
- Deroide, N.; Li, X.; Lerouet, D.; Van Vré, E.; Baker, L.; Harrison, J.; Poittevin, M.; Masters, L.; Nih, L.; Margaill, I.; et al. MFGE8 Inhibits Inflammasome-Induced IL-1β Production and Limits Postischemic Cerebral Injury. J. Clin. Investig. 2013, 123, 1176–1181. [Google Scholar] [CrossRef] [Green Version]
- Hanayama, R.; Tanaka, M.; Miwa, K.; Shinohara, A.; Iwamatsu, A.; Nagata, S. Identification of a Factor That Links Apoptotic Cells to Phagocytes. Nature 2002, 417, 182–187. [Google Scholar] [CrossRef]
- Miyasaka, K.; Hanayama, R.; Tanaka, M.; Nagata, S. Expression of Milk Fat Globule Epidermal Growth Factor 8 in Immature Dendritic Cells for Engulfment of Apoptotic Cells. Eur. J. Immunol. 2004, 34, 1414–1422. [Google Scholar] [CrossRef]
- Aziz, M.; Matsuda, A.; Yang, W.-L.; Jacob, A.; Wang, P. Milk Fat Globule-Epidermal Growth Factor-Factor 8 Attenuates Neutrophil Infiltration in Acute Lung Injury via Modulation of CXCR2. J. Immunol. 2012, 189, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Hirano, Y.; Yang, W.-L.; Aziz, M.; Zhang, F.; Sherry, B.; Wang, P. MFG-E8-Derived Peptide Attenuates Adhesion and Migration of Immune Cells to Endothelial Cells. J. Leukoc. Biol. 2017, 101, 1201–1209. [Google Scholar] [CrossRef]
- Dieude, M.; Bell, C.; Turgeon, J.; Beillevaire, D.; Pomerleau, L.; Yang, B.; Hamelin, K.; Qi, S.; Pallet, N.; Beland, C.; et al. The 20S Proteasome Core, Active within Apoptotic Exosome-like Vesicles, Induces Autoantibody Production and Accelerates Rejection. Sci. Transl. Med. 2015, 7, ra200–ra318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koulack, J.; McAlister, V.C.; Giacomantonio, C.A.; Bitter-Suermann, H.; MacDonald, A.S.; Lee, T.D. Development of a Mouse Aortic Transplant Model of Chronic Rejection. Microsurgery 1995, 16, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; Akalin, E.; et al. The Banff 2015 Kidney Meeting Report: Current Challenges in Rejection Classification and Prospects for Adopting Molecular Pathology. Am. J. Transplant. 2017, 17, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bu, H.-F.; Zhong, W.; Asai, A.; Zhou, Z.; Tan, X. MFG-E8 and HMGB1 Are Involved in the Mechanism Underlying Alcohol-Induced Impairment of Macrophage Efferocytosis. Mol. Med. 2013, 19, 170–182. [Google Scholar] [CrossRef]
- Fricker, M.; Neher, J.J.; Zhao, J.-W.; Théry, C.; Tolkovsky, A.M.; Brown, G.C. MFG-E8 Mediates Primary Phagocytosis of Viable Neurons during Neuroinflammation. J. Neurosci. 2012, 32, 2657–2666. [Google Scholar] [CrossRef] [Green Version]
- Braza, M.S.; Conde, P.; Garcia, M.; Cortegano, I.; Brahmachary, M.; Pothula, V.; Fay, F.; Boros, P.; Werner, S.A.; Ginhoux, F.; et al. Neutrophil Derived CSF1 Induces Macrophage Polarization and Promotes Transplantation Tolerance. Am. J. Transpl. 2018, 18, 1247–1255. [Google Scholar] [CrossRef] [Green Version]
- Conde, P.; Rodriguez, M.; van der Touw, W.; Jimenez, A.; Burns, M.; Miller, J.; Brahmachary, M.; Chen, H.; Boros, P.; Rausell-Palamos, F.; et al. DC-SIGN(+) Macrophages Control the Induction of Transplantation Tolerance. Immunity 2015, 42, 1143–1158. [Google Scholar] [CrossRef] [Green Version]
- Braza, M.S.; van Leent, M.M.T.; Lameijer, M.; Sanchez-Gaytan, B.L.; Arts, R.J.W.; Pérez-Medina, C.; Conde, P.; Garcia, M.R.; Gonzalez-Perez, M.; Brahmachary, M.; et al. Inhibiting Inflammation with Myeloid Cell-Specific Nanobiologics Promotes Organ Transplant Acceptance. Immunity 2018, 49, 819–828.e6. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.; AlKhamees, B.; Jia, D.; Li, L.; Couture, J.-F.; Figeys, D.; Jinushi, M.; Wang, L. MFG-E8 Is Critical for Embryonic Stem Cell-Mediated T Cell Immunomodulation. Stem Cell Rep. 2015, 5, 741–752. [Google Scholar] [CrossRef] [Green Version]
- Ait-Oufella, H.; Kinugawa, K.; Zoll, J.; Simon, T.; Boddaert, J.; Heeneman, S.; Blanc-Brude, O.; Barateau, V.; Potteaux, S.; Merval, R.; et al. Lactadherin Deficiency Leads to Apoptotic Cell Accumulation and Accelerated Atherosclerosis in Mice. Circulation 2007, 115, 2168–2177. [Google Scholar] [CrossRef] [Green Version]
- Jinushi, M.; Nakazaki, Y.; Dougan, M.; Carrasco, D.R.; Mihm, M.; Dranoff, G. MFG-E8–Mediated Uptake of Apoptotic Cells by APCs Links the pro- and Antiinflammatory Activities of GM-CSF. J. Clin. Investig. 2007, 117, 1902–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jinushi, M.; Sato, M.; Kanamoto, A.; Itoh, A.; Nagai, S.; Koyasu, S.; Dranoff, G.; Tahara, H. Milk Fat Globule Epidermal Growth Factor–8 Blockade Triggers Tumor Destruction through Coordinated Cell-Autonomous and Immune-Mediated Mechanisms. J. Exp. Med. 2009, 206, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Donor-Specific Antibodies in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2018, 13, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Lefaucheur, C.; Loupy, A.; Vernerey, D.; Duong-Van-Huyen, J.-P.; Suberbielle, C.; Anglicheau, D.; Vérine, J.; Beuscart, T.; Nochy, D.; Bruneval, P.; et al. Antibody-Mediated Vascular Rejection of Kidney Allografts: A Population-Based Study. Lancet 2013, 381, 313–319. [Google Scholar] [CrossRef]
- Yacoub-Youssef, H.; Marcheix, B.; Calise, D.; Thiers, J.C.; Benoist, H.; Blaes, N.; Ségui, B.; Dambrin, C.; Thomsen, M. Chronic Vascular Rejection: Histologic Comparison Between Two Murine Experimental Models. Transplant. Proc. 2005, 37, 2886–2887. [Google Scholar] [CrossRef]
- Dun, H.; Ye, L.; Zhu, Y.; Wong, B.W. Combined Abdominal Heterotopic Heart and Aorta Transplant Model in Mice. PLoS ONE 2020, 15, e0230649. [Google Scholar] [CrossRef]
- Siemeni, T.; Knöfel, A.-K.; Madrahimov, N.; Sommer, W.; Avsar, M.; Salman, J.; Ius, F.; Frank, N.; Büchler, G.; Jonigk, D.; et al. In Vivo Development of Transplant Arteriosclerosis in Humanized Mice Reflects Alloantigen Recognition and Peripheral Treg Phenotype of Lung Transplant Recipients. Am. J. Transpl. 2016, 16, 3150–3162. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brilland, B.; Laplante, P.; Thebault, P.; Geoffroy, K.; Brissette, M.-J.; Latour, M.; Chassé, M.; Qi, S.; Hébert, M.-J.; Cardinal, H.; et al. MFG-E8 Reduces Aortic Intimal Proliferation in a Murine Model of Transplant Vasculopathy. Int. J. Mol. Sci. 2022, 23, 4094. https://doi.org/10.3390/ijms23084094
Brilland B, Laplante P, Thebault P, Geoffroy K, Brissette M-J, Latour M, Chassé M, Qi S, Hébert M-J, Cardinal H, et al. MFG-E8 Reduces Aortic Intimal Proliferation in a Murine Model of Transplant Vasculopathy. International Journal of Molecular Sciences. 2022; 23(8):4094. https://doi.org/10.3390/ijms23084094
Chicago/Turabian StyleBrilland, Benoit, Patrick Laplante, Pamela Thebault, Karen Geoffroy, Marie-Joëlle Brissette, Mathieu Latour, Michaël Chassé, Shijie Qi, Marie-Josée Hébert, Héloïse Cardinal, and et al. 2022. "MFG-E8 Reduces Aortic Intimal Proliferation in a Murine Model of Transplant Vasculopathy" International Journal of Molecular Sciences 23, no. 8: 4094. https://doi.org/10.3390/ijms23084094
APA StyleBrilland, B., Laplante, P., Thebault, P., Geoffroy, K., Brissette, M.-J., Latour, M., Chassé, M., Qi, S., Hébert, M.-J., Cardinal, H., & Cailhier, J.-F. (2022). MFG-E8 Reduces Aortic Intimal Proliferation in a Murine Model of Transplant Vasculopathy. International Journal of Molecular Sciences, 23(8), 4094. https://doi.org/10.3390/ijms23084094





