Reduced Expression of PD-1 in Circulating CD4+ and CD8+ Tregs Is an Early Feature of RRMS
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Population
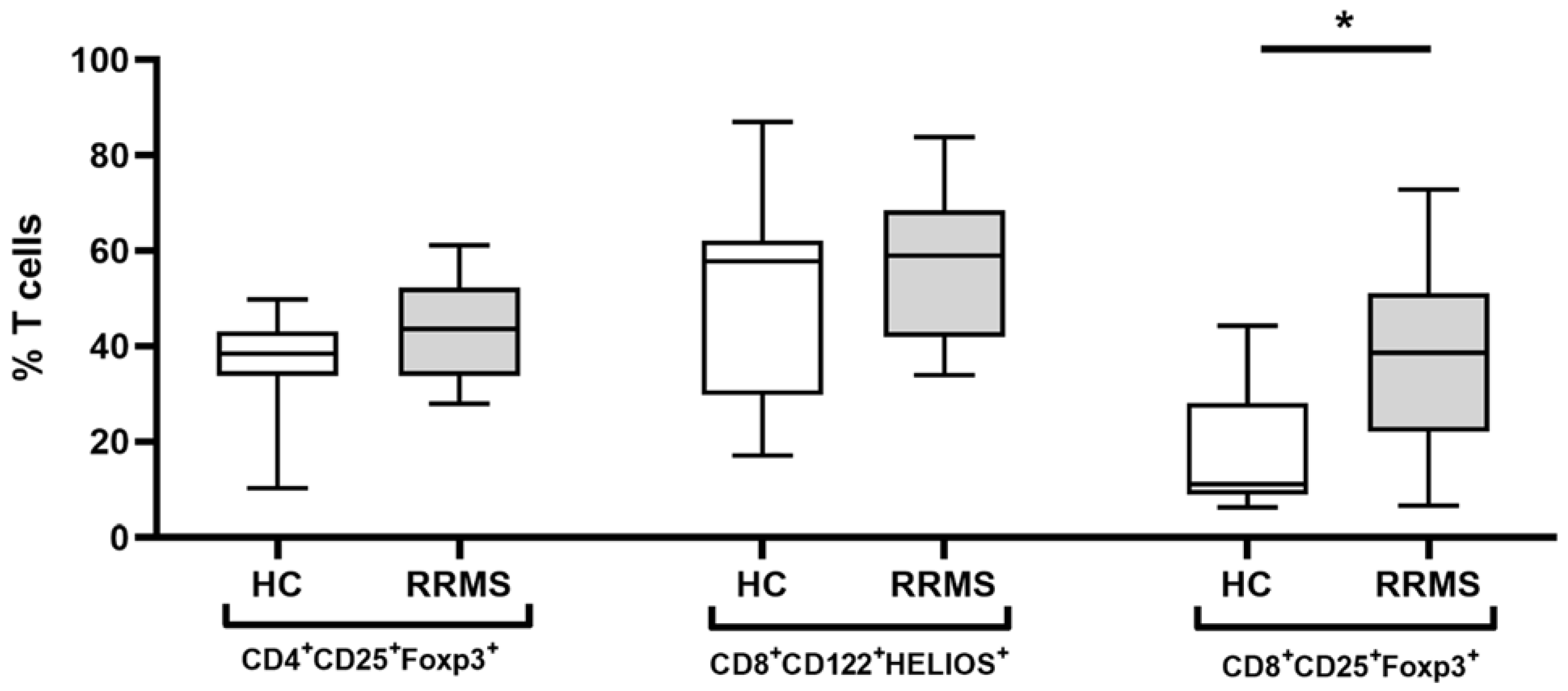
2.2. Demographic Factors Affect the Treg Populations
2.3. Expression of Immunomodulatory Cell-Surface Receptors and Transcriptional Factors on CD4+ and CD8+ Tregs
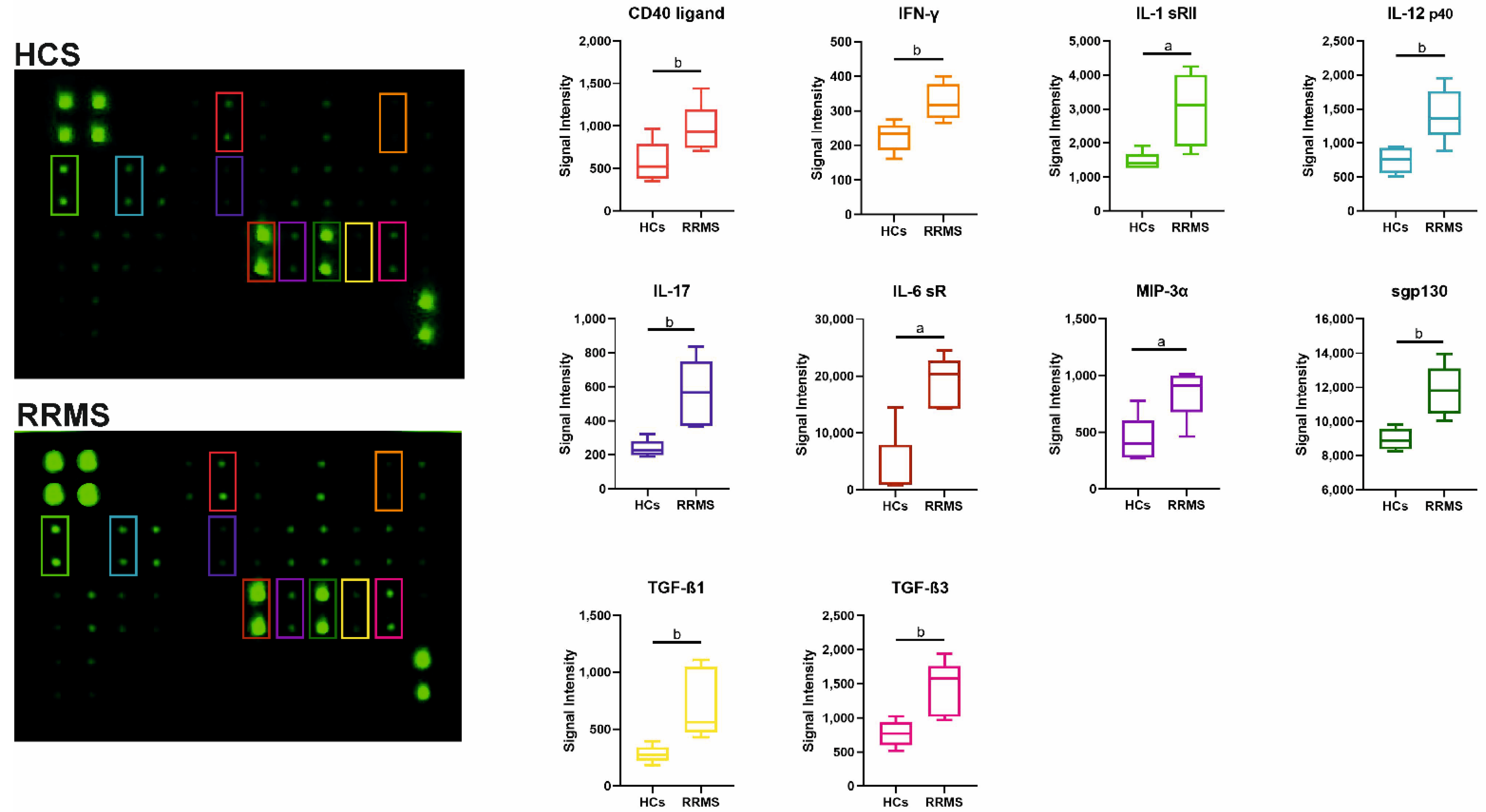
2.4. Profiles of Cytokines in Serum from RRMS Patients
3. Discussion
4. Materials and Methods
4.1. Study Design and Human Subjects
4.2. Collection of Serum Samples
4.3. Peripheral Blood Mononuclear Cell (PBMC) Isolation
4.4. Flow Cytometry Analysis
4.5. Cytokine Profiles
4.6. ELISA
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef]
- Pilli, D.; Zou, A.; Tea, F.; Dale, R.C.; Brilot, F. Expanding Role of T Cells in Human Autoimmune Diseases of the Central Nervous System. Front. Immunol. 2017, 8, 652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friese, M.A.; Fugger, L. Pathogenic CD8+ T cells in multiple sclerosis. Ann. Neurol. 2009, 66, 132–141. [Google Scholar] [CrossRef]
- Gravano, D.M.; Hoyer, K.K. Promotion and prevention of autoimmune disease by CD8+ T cells. J. Autoimmun. 2013, 45, 68–79. [Google Scholar] [CrossRef]
- Sinha, S.; Itani, F.R.; Karandikar, N.J. Immune regulation of multiple sclerosis by CD8+ T cells. Immunol. Res. 2014, 59, 254–265. [Google Scholar] [CrossRef]
- Donskow-Łysoniewska, K.; Krawczak, K.; Bocian, K.; Doligalska, M. The Effects of Intestinal Nematode L4 Stage on Mouse Experimental Autoimmune Encephalomyelitis. Arch. Immunol. Ther. Exp. 2018, 66, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Viglietta, V.; Baecher-Allan, C.; Weiner, H.L.; Hafler, D.A. Loss of Functional Suppression by CD4+CD25+ Regulatory T Cells in Patients with Multiple Sclerosis. J. Exp. Med. 2004, 199, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mexhitaj, I.; Nyirenda, M.H.; Li, R.; O’mahony, J.; Rezk, A.; Rozenberg, A.; Moore, C.S.; Johnson, T.; Sadovnick, D.; Collins, D.L.; et al. Abnormal effector and regulatory T cell subsets in paediatric-onset multiple sclerosis. Brain 2019, 142, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Maltos, M.; Treviño-Frenk, I.; García-González, H.; Rosetti, M.; Barriga-Maldonado, V.; Morales-Ramírez, F.; López-Hernández, D.; Rosetti, F.; Crispín, J. Identification of regulatory T cell molecules associated with severity of multiple sclerosis. Mult. Scler. J. 2020, 27, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Frisullo, G.; Nociti, V.; Iorio, R.; Patanella, A.K.; Caggiula, M.; Marti, A.; Sancricca, C.; Angelucci, F.; Mirabella, M.; Tonali, P.A.; et al. Regulatory T cells fail to suppress CD4+ T-bet+ T cells in relapsing multiple sclerosis patients. Immunology 2009, 127, 418–428. [Google Scholar] [CrossRef]
- Dummer, C.D.; Carpio, V.N.; Gonçalves, L.F.S.; Manfro, R.C.; Veronese, F.V. FOXP3+ regulatory T cells: From suppression of rejection to induction of renal allograft tolerance. Transpl. Immunol. 2012, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Renavikar, P.S.; Sinha, S.; Brate, A.A.; Borcherding, N.; Crawford, M.P.; Steward-Tharp, S.M.; Karandikar, N.J. IL-12-Induced Immune Suppressive Deficit During CD8+ T-Cell Differentiation. Front. Immunol. 2020, 11, 568630. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Yang, L.; Qiao, G.; Li, Z.; Zhang, L.; Yin, F.; Xie, N.; Zhang, J. Cutting Edge: CTLA-4–B7 Interaction Suppresses Th17 Cell Differentiation. J. Immunol. 2010, 185, 1375–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noori-Zadeh, A.; Mesbah-Namin, S.A.; Bistoon-Beigloo, S.; Bakhtiyari, S.; Abbaszadeh, H.-A.; Darabi, S.; Rajabibazl, M.; Abdanipour, A. Regulatory T cell number in multiple sclerosis patients: A meta-analysis. Mult. Scler. Relat. Disord. 2016, 5, 73–76. [Google Scholar] [CrossRef]
- Rubtsov, Y.P.; Niec, R.E.; Josefowicz, S.; Li, L.; Darce, J.; Mathis, D.; Benoist, C.; Rudensky, A.Y. Stability of the Regulatory T Cell Lineage In Vivo. Science 2010, 329, 1667–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, K.A.; Bhamidipati, K.; Rubin, S.; Kipp, L.; Robinson, W.H.; Lanz, T.V. Immunomodulatory receptors are differentially expressed in B and T cell subsets relevant to autoimmune disease. Clin. Immunol. 2019, 209, 108276. [Google Scholar] [CrossRef]
- Kim, H.-J.; Barnitz, R.A.; Kreslavsky, T.; Brown, F.D.; Moffett, H.; Lemieux, M.E.; Kaygusuz, Y.; Meissner, T.; Holderried, T.A.W.; Chan, S.; et al. Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science 2015, 350, 334–339. [Google Scholar] [CrossRef] [Green Version]
- McGrath, M.M.; Najafian, N. The Role of Coinhibitory Signaling Pathways in Transplantation and Tolerance. Front. Immunol. 2012, 3, 47. [Google Scholar] [CrossRef] [Green Version]
- Mi, Y.; Han, J.; Zhu, J.; Jin, T. Role of the PD-1/PD-L1 Signaling in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis: Recent Insights and Future Directions. Mol. Neurobiol. 2021, 58, 6249–6271. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Ibañez-Vega, J.; Vilchez, C.; Jimenez, K.; Guevara, C.; Burgos, P.I.; Naves, R. Cellular and molecular regulation of the programmed death-1/programmed death ligand system and its role in multiple sclerosis and other autoimmune diseases. J. Autoimmun. 2021, 123, 102702. [Google Scholar] [CrossRef] [PubMed]
- Parry, R.V.; Chemnitz, J.M.; Frauwirth, K.A.; Lanfranco, A.R.; Braunstein, I.; Kobayashi, S.V.; Linsley, P.S.; Thompson, C.B.; Riley, J.L. CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Mol. Cell. Biol. 2005, 25, 9543–9553. [Google Scholar] [CrossRef] [Green Version]
- Mohammadzadeh, A.; Rad, I.A.; Ahmadi-Salmasi, B. CTLA-4, PD-1 and TIM-3 expression predominantly downregulated in MS patients. J. Neuroimmunol. 2018, 323, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Javan, M.R.; Aslani, S.; Zamani, M.R.; Rostamnejad, J.; Asadi, M.; Farhoodi, M.; Nicknam, M.H. Downregulation of Immunosuppressive Molecules, PD-1 and PD-L1 but not PD-L2, in the Patients with Multiple Sclerosis. Iran. J. Allergy Asthma Immunol. 2016, 15, 296–302. [Google Scholar] [PubMed]
- Trabattoni, D.; Saresella, M.; Pacei, M.; Marventano, I.; Mendozzi, L.; Rovaris, M.; Caputo, D.; Borelli, M.; Clerici, M. Costimulatory Pathways in Multiple Sclerosis: Distinctive Expression of PD-1 and PD-L1 in Patients with Different Patterns of Disease. J. Immunol. 2009, 183, 4984–4993. [Google Scholar] [CrossRef] [Green Version]
- Sage, P.T.; Schildberg, F.A.; Sobel, R.A.; Kuchroo, V.K.; Freeman, G.J.; Sharpe, A.H. Dendritic Cell PD-L1 Limits Autoimmunity and Follicular T Cell Differentiation and Function. J. Immunol. 2018, 200, 2592–2602. [Google Scholar] [CrossRef]
- Cai, J.; Wang, D.; Zhang, G.; Guo, X. The Role of PD-1/PD-L1 Axis in Treg Development and Function: Implications for Cancer Immunotherapy. OncoTargets Ther. 2019, 12, 8437–8445. [Google Scholar] [CrossRef] [Green Version]
- Scotto, L.; Naiyer, A.J.; Galluzzo, S.; Rossi, P.; Manavalan, J.S.; Kim-Schulze, S.; Fang, J.; Favera, R.D.; Cortesini, R.; Suciu-Foca, N. Overlap between molecular markers expressed by naturally occurring CD4+CD25+ regulatory T cells and antigen specific CD4+CD25+ and CD8+CD28- T suppressor cells. Hum. Immunol. 2004, 65, 1297–1306. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, B.; Rui, K.; Wang, S. The Role of GITR/GITRL Interaction in Autoimmune Diseases. Front. Immunol. 2020, 11, 588682. [Google Scholar] [CrossRef]
- Szewczak, L.; Donskow-Łysoniewska, K. Cytokines and Transgenic Matrix in Autoimmune Diseases: Similarities and Differences. Biomedicines 2020, 8, 559. [Google Scholar] [CrossRef]
- Abarca-Zabalía, J.; García, M.I.; Ros, A.L.; Marín-Jiménez, I.; Martínez-Ginés, M.L.; López-Cauce, B.; Martín-Barbero, M.L.; Salvador-Martín, S.; Sanjurjo-Saez, M.; García-Domínguez, J.M.; et al. Differential Expression of SMAD Genes and S1PR1 on Circulating CD4+ T Cells in Multiple Sclerosis and Crohn’s Disease. Int. J. Mol. Sci. 2020, 21, 676. [Google Scholar] [CrossRef] [Green Version]
- Okamura, T.; Morita, K.; Iwasaki, Y.; Inoue, M.; Komai, T.; Fujio, K.; Yamamoto, K. Role of TGF-α3 in the regulation of immune responses. Clin. Exp. Rheumatol. 2015, 33, S63–S69. [Google Scholar]
- Hurst, S.M.; Wilkinson, T.; McLoughlin, R.; Jones, S.; Horiuchi, S.; Yamamoto, N.; Rose-John, S.; Fuller, G.M.; Topley, N.; Jones, S.A. IL-6 and Its Soluble Receptor Orchestrate a Temporal Switch in the Pattern of Leukocyte Recruitment Seen during Acute Inflammation. Immunity 2001, 14, 705–714. [Google Scholar] [CrossRef] [Green Version]
- Lublin, F.D.; Reingold, S.C. Defining the clinical course of multiple sclerosis: Results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [Green Version]
- Kamali-Sarvestani, E.; Nikseresht, A.; Aflaki, E.; Sarvari, J.; Gharesi-Fard, B. TNF-α, TNF-β and IL-4 gene polymorphisms in Iranian patients with multiple sclerosis. Acta Neurol. Scand. 2007, 115, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Głaczynska, M.; Machcinska, M.; Donskow-Lysoniewska, K. Effects of Different Media on Human T Regulatory Cells Phenotype. In Vivo 2021, 35, 283–289. [Google Scholar] [CrossRef] [PubMed]


| Population | Age (Increase) | Gender (Female) | RRMS | EDSS (Increase) |
|---|---|---|---|---|
| CD8+ CD8+CD122+ | − | |||
| CD8+CD122+Helios+ | + | |||
| CD8+CD25+ CD8+CD25+FoxP3+ | + | + | ||
| CD4+ | ||||
| CD4+CD25+ | + | − | − | |
| CD4+CD25+FoxP3+ |
| Characteristics | RRMS Patients (n = 15) | Healthy Controls (n = 35) | p-Value |
|---|---|---|---|
| Gender. n (%) | |||
| Female | 13 (86.7) | 12 (33.3) | 0.0083 |
| Male | 2 (13.3) | 24 (66.7) | |
| Age. years | |||
| Median | 30 | 38.5 | 0.0004 |
| Range | 19.0–47.0 | 19.0–57.0 | |
| Treatment | No | No | - |
| EDSS score | |||
| Median | 1.5 | - | - |
| Range | (1.0–3.0) | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machcińska, M.; Kierasińska, M.; Michniowska, M.; Maruszewska-Cheruiyot, M.; Szewczak, L.; Rola, R.; Karlińska, A.; Stear, M.; Donskow-Łysoniewska, K. Reduced Expression of PD-1 in Circulating CD4+ and CD8+ Tregs Is an Early Feature of RRMS. Int. J. Mol. Sci. 2022, 23, 3185. https://doi.org/10.3390/ijms23063185
Machcińska M, Kierasińska M, Michniowska M, Maruszewska-Cheruiyot M, Szewczak L, Rola R, Karlińska A, Stear M, Donskow-Łysoniewska K. Reduced Expression of PD-1 in Circulating CD4+ and CD8+ Tregs Is an Early Feature of RRMS. International Journal of Molecular Sciences. 2022; 23(6):3185. https://doi.org/10.3390/ijms23063185
Chicago/Turabian StyleMachcińska, Maja, Magdalena Kierasińska, Martyna Michniowska, Marta Maruszewska-Cheruiyot, Ludmiła Szewczak, Rafał Rola, Anna Karlińska, Michael Stear, and Katarzyna Donskow-Łysoniewska. 2022. "Reduced Expression of PD-1 in Circulating CD4+ and CD8+ Tregs Is an Early Feature of RRMS" International Journal of Molecular Sciences 23, no. 6: 3185. https://doi.org/10.3390/ijms23063185
APA StyleMachcińska, M., Kierasińska, M., Michniowska, M., Maruszewska-Cheruiyot, M., Szewczak, L., Rola, R., Karlińska, A., Stear, M., & Donskow-Łysoniewska, K. (2022). Reduced Expression of PD-1 in Circulating CD4+ and CD8+ Tregs Is an Early Feature of RRMS. International Journal of Molecular Sciences, 23(6), 3185. https://doi.org/10.3390/ijms23063185









