The Role of Plasma Extracellular Vesicles in Remote Ischemic Conditioning and Exercise-Induced Ischemic Tolerance
Abstract
:1. Introduction
2. Results
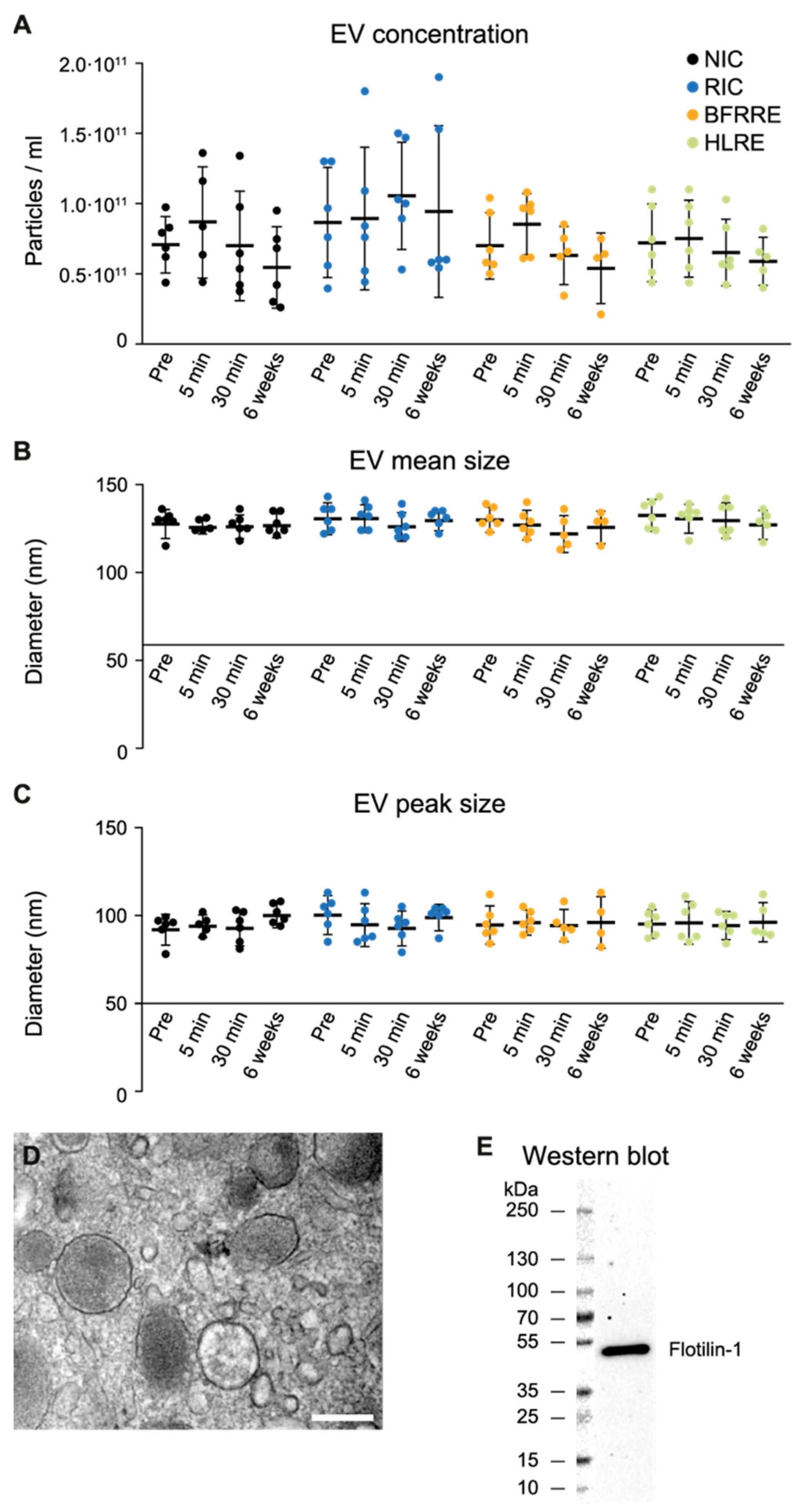
2.1. Fluctuations in Extracellular Vesicles after Single Bout Intervention
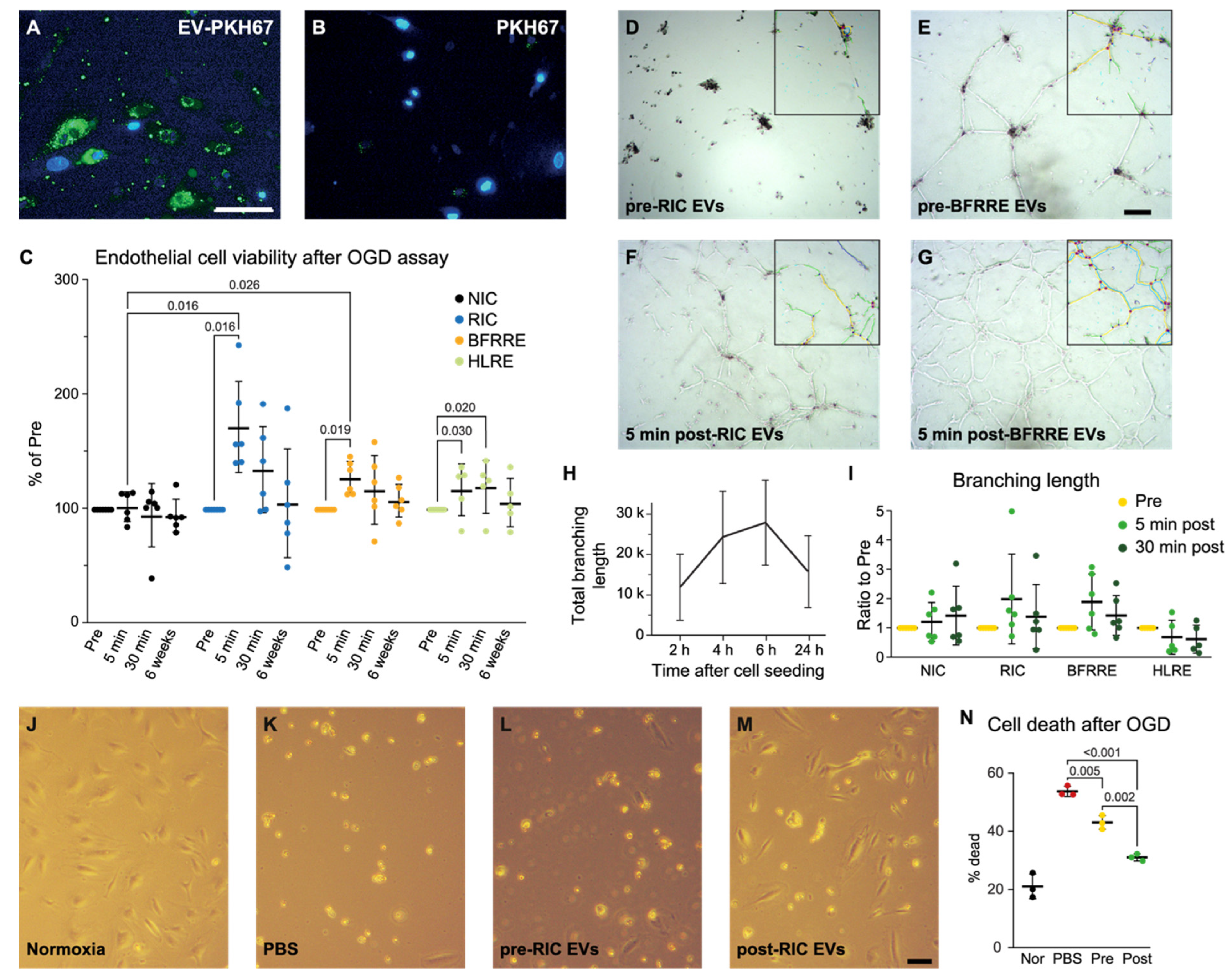
2.2. EVs Released after RIC and BFRRE Protect Endothelial Cells against Prolonged Oxygen and Glucose Deprivation
2.3. EVs Released after RIC, BFRRE, and HLRE Do Not Affect Endothelial Cell Tube Formation In Vitro
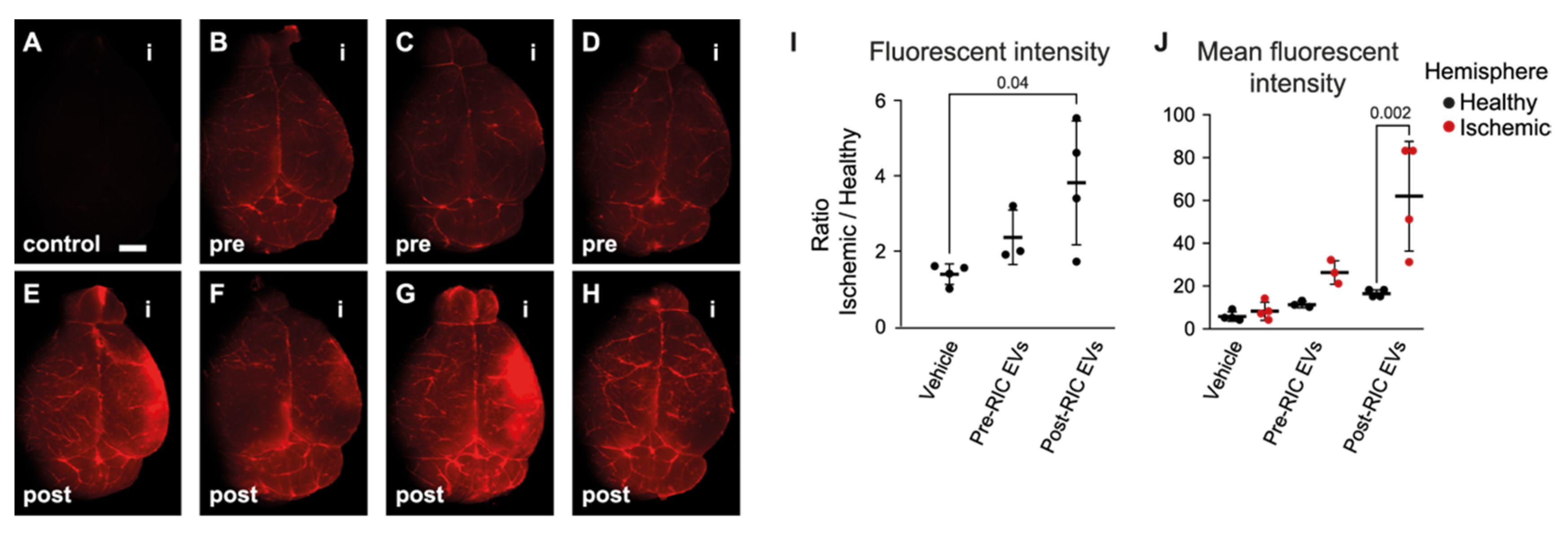
2.4. Pooled Post-RIC EVs Increase Endothelial Cell Survival In Vitro and Accumulate in the Ischemic Area of tMCAO Mice
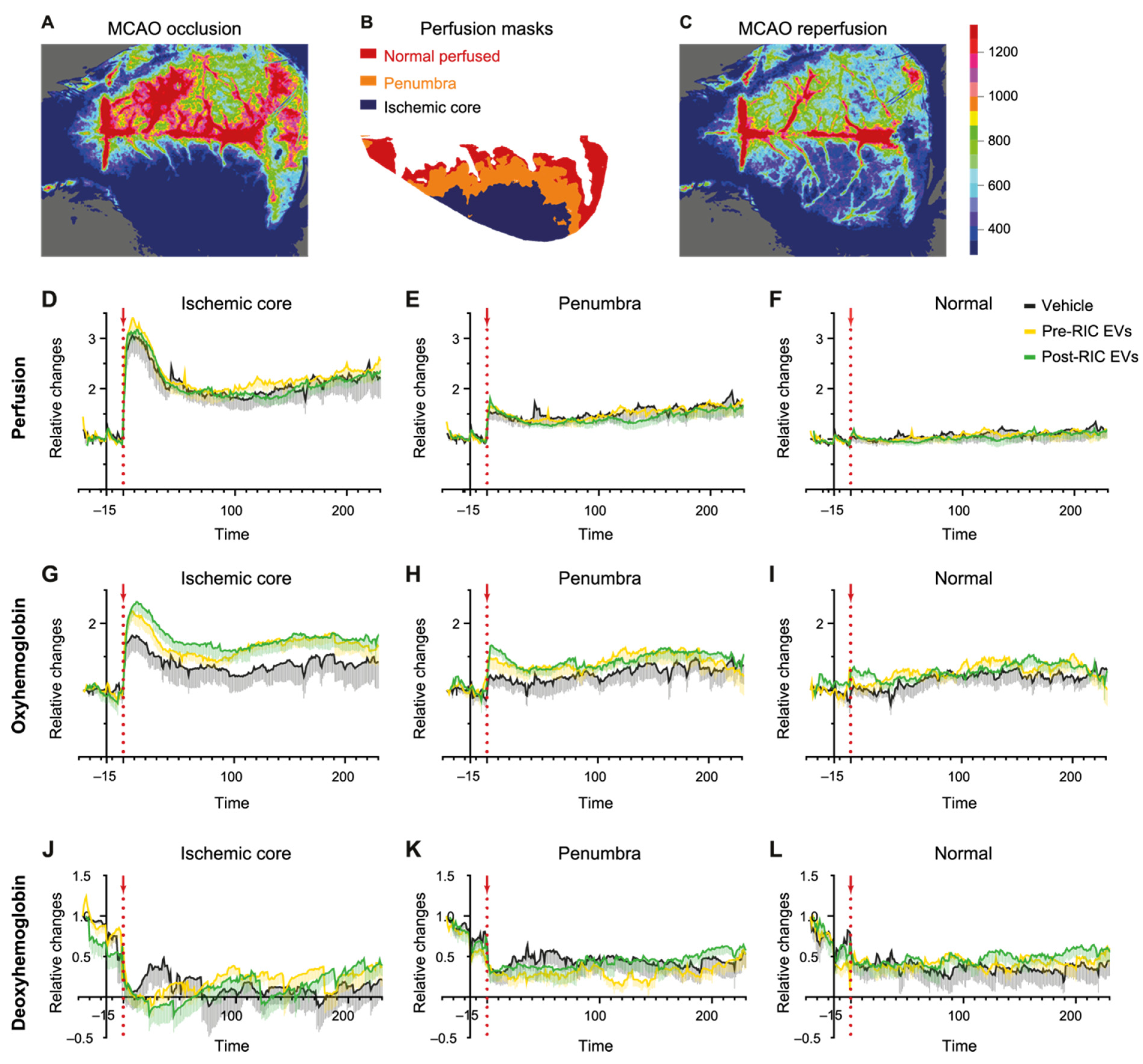
2.5. Perfusion and Hemoglobin Changes after EV Administration in the tMCAO Stroke Model
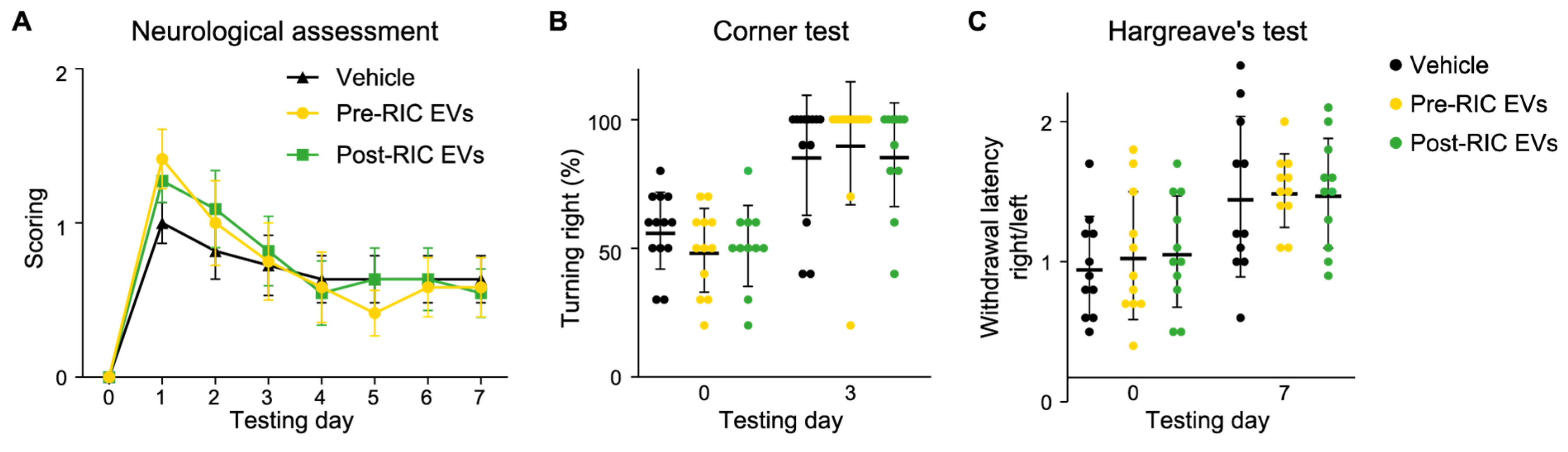
2.6. Post-RIC EVs Have No Impact on Neurological Function after Ischemic Stroke
2.7. Ischemic Core Development after EV Administration in tMCAO Mice
2.8. The Integrity of BBB at 4 h after Reperfusion
3. Discussion
4. Material and Methods
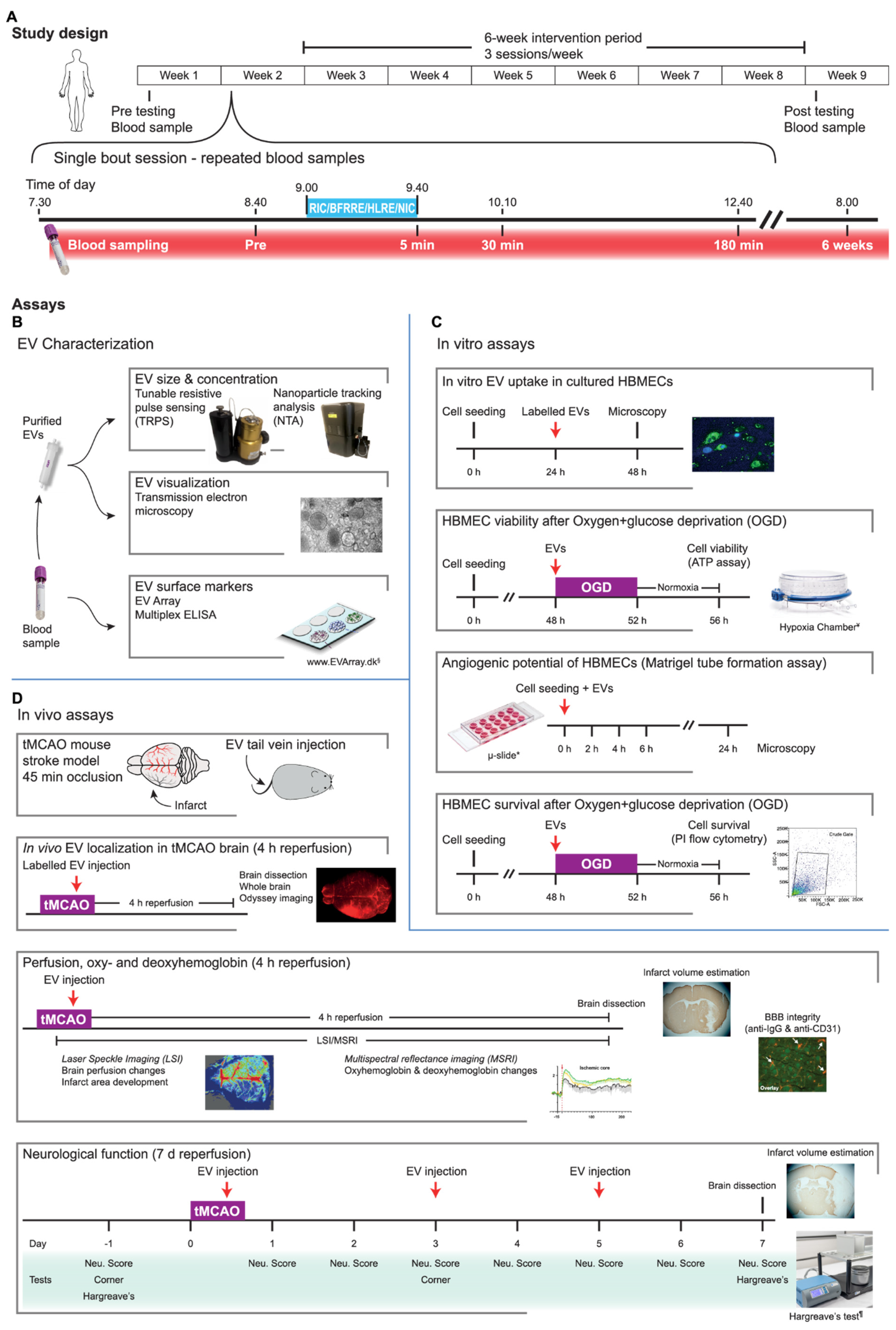
4.1. Study Design
4.2. Intervention Paradigms during Single-Treatment and Training
4.3. Blood Sampling
4.4. EV Isolation and Characterization
4.5. Assessment of EV Phenotype by EV Array
4.6. EV Normalization Strategy and Pooling
4.7. Cell Culture and Handling
4.8. EV Labeling and Uptake
4.9. Measuring Cell Viability and Cell Death after Oxygen and Glucose Deprivation (OGD)
4.10. In Vitro Angiogenesis Assay
4.11. Experimental Stroke Mouse Model
4.12. In Vivo EV Tracking
4.13. Cerebral Blood Flow (CBF) and Hemoglobin Measurement
4.14. In Vivo Image Analysis
4.15. Measuring the Effects of EVs on Neurological Function in the tMCAO Mice
4.16. Behavioral Tests
4.17. Brain Tissue Cryosectioning and Immunohistochemistry (IHC)
4.18. Infarct Volume Estimations
4.19. Imaging and Analysis of the IHC Sections
4.20. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Datta, A.; Sarmah, D.; Mounica, L.; Kaur, H.; Kesharwani, R.; Verma, G.; Veeresh, P.; Kotian, V.; Kalia, K.; Borah, A.; et al. Cell Death Pathways in Ischemic Stroke and Targeted Pharmacotherapy. Transl. Stroke Res. 2020, 11, 1185–1202. [Google Scholar] [CrossRef] [PubMed]
- Balch, M.H.H.; Nimjee, S.M.; Rink, C.; Hannawi, Y. Beyond the brain: The systemic pathophysiological response to acute ischemic stroke. J. Stroke 2020, 22, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Radak, D.; Katsiki, N.; Resanovic, I.; Jovanovic, A.; Sudar-Milovanovic, E.; Zafirovic, S.; Mousad, S.A.; Isenovic, E.R. Apoptosis and Acute Brain Ischemia in Ischemic Stroke. Curr. Vasc. Pharmacol. 2016, 15, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Khoshnam, S.E.; Winlow, W.; Farzaneh, M.; Farbood, Y.; Moghaddam, H.F. Pathogenic mechanisms following ischemic stroke. Neurol. Sci. 2017, 38, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Astrup, J.; Siesjö, B.K.; Symon, L. Thresholds in cerebral ischemia—The ischemic penumbra. Stroke 1981, 12, 723–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M.; et al. Global, regional, and national burden of stroke, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458. [Google Scholar] [CrossRef] [Green Version]
- Hahn, C.D.; Manlhiot, C.; Schmidt, M.R.; Nielsen, T.T.; Redington, A.N. Remote ischemic per-conditioning: A novel therapy for acute stroke? Stroke 2011, 42, 2960–2962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hess, D.C.; Blauenfeldt, R.A.; Andersen, G.; Hougaard, K.D.; Hoda, M.N.; Ding, Y.; Ji, X. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat. Rev. Neurol. 2015, 11, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Santana, M.N.S.; Souza, D.S.; Miguel-dos-Santos, R.; Rabelo, T.K.; de Vasconcelos, C.M.L.; Navia-Pelaez, J.M.; de Jesus, I.C.G.; da Silva-Neto, J.A.; Lauton-Santos, S.; dos Santos Aggum Capettini, L.; et al. Resistance exercise mediates remote ischemic preconditioning by limiting cardiac eNOS uncoupling. J. Mol. Cell. Cardiol. 2018, 125, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Ghadiri Soufi, F.; Mahmoudi Saber, M.; Ghiassie, R.; Alipour, M. Role of 12-week resistance training in preserving the heart against ischemia—Reperfusion-induced injury. Cardiol. J. 2011, 18, 140–145. [Google Scholar]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharbanda, R.K.; Mortensen, U.M.; White, P.A.; Kristiansen, S.B.; Schmidt, M.R.; Hoschtitzky, J.A.; Vogel, M.; Sorensen, K.; Redington, A.N.; MacAllister, R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 2002, 106, 2881–2883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candilio, L.; Malik, A.; Hausenloy, D.J. Protection of organs other than the heart by remote ischemic conditioning. J. Cardiovasc. Med. 2013, 14, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Su, L.; Li, X.; Di, W.; Zhang, X.; Zhang, C.; He, T.; Zhu, X.; Zhang, Y.; Li, Y. Remote limb ischemic postconditioning protects mouse brain against cerebral ischemia/reperfusion injury via upregulating expression of Nrf2, HO-1 and NQO-1 in mice. Int. J. Neurosci. 2015, 126, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Yoon, D.W.; Kim, J.H.; Lee, J.H.; Lim, C.H. Effect of remote ischemic post-conditioning on systemic inflammatory response and survival rate in lipopolysaccharide-induced systemic inflammation model. J. Inflamm. 2014, 11, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billah, M.; Ridiandries, A.; Allahwala, U.K.; Mudaliar, H.; Dona, A.; Hunyor, S.; Khachigian, L.M.; Bhindi, R. Remote Ischemic Preconditioning induces Cardioprotective Autophagy and Signals through the IL-6-Dependent JAK-STAT Pathway. Int. J. Mol. Sci. 2020, 21, 1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Thakkar, M.; Robinson, C.; Doré, S. Limb remote ischemic conditioning: Mechanisms, anesthetics, and the potential for expanding therapeutic options. Front. Neurol. 2018, 9, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severinsen, K.; Jakobsen, J.K.; Pedersen, A.R.; Overgaard, K.; Andersen, H. Effects of Resistance Training and Aerobic Training on Ambulation in Chronic Stroke. Am. J. Phys. Med. Rehabil. 2014, 93, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Murad, K.; Kitzman, D.W. Frailty and multiple comorbidities in the elderly patient with heart failure: Implications for management. Heart Fail. Rev. 2012, 17, 581–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farup, J.; de Paoli, F.; Bjerg, K.; Riis, S.; Ringgard, S.; Vissing, K. Blood flow restricted and traditional resistance training performed to fatigue produce equal muscle hypertrophy. Scand. J. Med. Sci. Sport. 2015, 25, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Groennebaek, T.; Sieljacks, P.; Nielsen, R.; Pryds, K.; Jespersen, N.R.; Wang, J.; Carlsen, C.R.; Schmidt, M.R.; De Paoli, F.V.; Miller, B.F.; et al. Effect of Blood Flow Restricted Resistance Exercise and Remote Ischemic Conditioning on Functional Capacity and Myocellular Adaptations in Patients with Heart Failure. Circ. Hear. Fail. 2019, 12, e006427. [Google Scholar] [CrossRef] [PubMed]
- Groennebaek, T.; Jespersen, N.R.; Jakobsgaard, J.E.; Sieljacks, P.; Wang, J.; Rindom, E.; Musci, R.V.; Bøtker, H.E.; Hamilton, K.L.; Miller, B.F.; et al. Skeletal Muscle Mitochondrial Protein Synthesis and Respiration Increase with Low-Load Blood Flow Restricted as Well as High-Load Resistance Training. Front. Physiol. 2018, 9, 1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieljacks, P.; Wang, J.; Groennebaek, T.; Rindom, E.; Jakobsgaard, J.E.; Herskind, J.; Gravholt, A.; Møller, A.B.; Musci, R.V.; De Paoli, F.V.; et al. Six weeks of low-load blood flow restricted and high-load resistance exercise training produce similar increases in cumulative myofibrillar protein synthesis and ribosomal biogenesis in healthy males. Front. Physiol. 2019, 10, 649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Buzás, E.I.; De Kleijn, D.P.V.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S.; et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panteleev, M.A.; Abaeva, A.A.; Balandina, A.N.; Belyaev, A.V.; Nechipurenko, D.Y.; Obydennyi, S.I.; Sveshnikova, A.N.; Shibeko, A.M.; Ataullakhanov, F.I. Extracellular vesicles of blood plasma: Content, origin, and properties. Biochem. Suppl. Ser. A Membr. Cell Biol. 2017, 11, 187–192. [Google Scholar] [CrossRef]
- Guescini, M.; Canonico, B.; Lucertini, F.; Maggio, S.; Annibalini, G.; Barbieri, E.; Luchetti, F.; Papa, S.; Stocchi, V. Muscle Releases Alpha-Sarcoglycan Positive Extracellular Vesicles Carrying miRNAs in the Bloodstream. PLoS ONE 2015, 10, e0125094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfì, E.; Thairi, C.; Femminò, S.; Alloatti, G.; Moccia, F.; Brizzi, M.F.; Pagliaro, P.; Penna, C. Extracellular vesicles (EVs) in ischemic conditioning and angiogenesis: Focus on endothelial derived EVs. Vascul. Pharmacol. 2021, 140, 106873. [Google Scholar] [CrossRef] [PubMed]
- Nederveen, J.P.; Warnier, G.; Di Carlo, A.; Nilsson, M.I.; Tarnopolsky, M.A. Extracellular Vesicles and Exosomes: Insights from Exercise Science. Front. Physiol. 2021, 11, 1757. [Google Scholar] [CrossRef] [PubMed]
- Just, J.; Yan, Y.; Farup, J.; Sieljacks, P.; Sloth, M.; Venø, M.; Gu, T.; de Paoli, F.V.; Nyengaard, J.R.; Bæk, R.; et al. Blood flow-restricted resistance exercise alters the surface profile, miRNA cargo and functional impact of circulating extracellular vesicles. Sci. Rep. 2020, 10, 5835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minghua, W.; Zhijian, G.; Chahua, H.; Qiang, L.; Minxuan, X.; Luqiao, W.; Weifang, Z.; Peng, L.; Biming, Z.; Lingling, Y.; et al. Plasma exosomes induced by remote ischaemic preconditioning attenuate myocardial ischaemia/reperfusion injury by transferring MIR-24 article. Cell Death Dis. 2018, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Lassen, T.R.; Just, J.; Hjortbak, M.V.; Jespersen, N.R.; Stenz, K.T.; Gu, T.; Yan, Y.; Su, J.; Hansen, J.; Bæk, R.; et al. Cardioprotection by remote ischemic conditioning is transferable by plasma and mediated by extracellular vesicles. Basic Res. Cardiol. 2021, 116, 16. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Stewart, T.; Banks, W.A.; Zhang, J. The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr. Pharm. Des. 2018, 23, 6206–6214. [Google Scholar] [CrossRef] [PubMed]
- Bunggulawa, E.J.; Wang, W.; Yin, T.; Wang, N.; Durkan, C.; Wang, Y.; Wang, G. Recent advancements in the use of exosomes as drug delivery systems 06 Biological Sciences 0601 Biochemistry and Cell Biology. J. Nanobiotechnol. 2018, 16, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome theranostics: Biology and translational medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wernbom, M.; Augustsson, J.; Raastad, T. Ischemic strength training: A low-load alternative to heavy resistance exercise? Scand. J. Med. Sci. Sports 2008, 18, 401–416. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sport. Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Vissing, K.; Andersen, J.L.; Schjerling, P. Are exercise-induced genes induced by exercise? FASEB J. 2005, 19, 94–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, K.; Neerukonda, S.N.; Katneni, U.; Buehler, P.W. Extracellular vesicles from red blood cells and their evolving roles in health, coagulopathy and therapy. Int. J. Mol. Sci. 2021, 22, 1–25. [Google Scholar]
- Annibalini, G.; Contarelli, S.; Lucertini, F.; Guescini, M.; Maggio, S.; Ceccaroli, P.; Gervasi, M.; Ferri Marini, C.; Fardetti, F.; Grassi, E.; et al. Muscle and Systemic Molecular Responses to a Single Flywheel Based Iso-Inertial Training Session in Resistance-Trained Men. Front. Physiol. 2019, 10, 554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frühbeis, C.; Helmig, S.; Tug, S.; Simon, P.; Krämer-Albers, E.M. Physical exercise induces rapid release of small extracellular vesicles into the circulation. J. Extracell. Vesicles 2015, 4, 28239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansford, K.A.; Shill, D.D.; Dicks, A.B.; Marshburn, M.P.; Southern, W.M.; Jenkins, N.T. Effect of acute exercise on circulating angiogenic cell and microparticle populations. Exp. Physiol. 2016, 101, 155–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitham, M.; Parker, B.L.; Friedrichsen, M.; Hingst, J.R.; Hjorth, M.; Hughes, W.E.; Egan, C.L.; Cron, L.; Watt, K.I.; Kuchel, R.P.; et al. Extracellular Vesicles Provide a Means for Tissue Crosstalk during Exercise. Cell Metab. 2018, 27, 237–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, P.M.; Jäger, B.; Piackova, E.; Sztulman, L.; Wegberger, C.; Wojta, J.; Gyöngyösi, M.; Kiss, A.; Podesser, B.K.; Spittler, A.; et al. Changes in circulating extracellular vesicles in patients with st-elevation myocardial infarction and potential effects of remote ischemic conditioning-a randomized controlled trial. Biomedicines 2020, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Stranska, R.; Gysbrechts, L.; Wouters, J.; Vermeersch, P.; Bloch, K.; Dierickx, D.; Andrei, G.; Snoeck, R. Comparison of membrane affinity-based method with size-exclusion chromatography for isolation of exosome-like vesicles from human plasma. J. Transl. Med. 2018, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, A.; Neuberger, E.; Esch-Heisser, L.; Haller, N.; Jorgensen, M.M.; Baek, R.; Möbius, W.; Simon, P.; Krämer-Albers, E.M. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J. Extracell. Vesicles 2019, 8, 1615820. [Google Scholar] [CrossRef] [PubMed]
- Weber, N.C.; Riedemann, I.; Smit, K.F.; Zitta, K.; van de Vondervoort, D.; Zuurbier, C.J.; Hollmann, M.W.; Preckel, B.; Albrecht, M. Plasma from human volunteers subjected to remote ischemic preconditioning protects human endothelial cells from hypoxia–induced cell damage. Basic Res. Cardiol. 2015, 110, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Zhang, M.; Zhang, X.; Li, J.; Wang, Y.; Fan, Y.; Shi, R. Limb Ischemic Preconditioning Protects Endothelium from Oxidative Stress by Enhancing Nrf2 Translocation and Upregulating Expression of Antioxidases. PLoS ONE 2015, 10, e0128455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Zhang, L.; Pu, H.; Mao, L.; Hu, X.; Jiang, X.; Xu, N.; Stetler, R.A.; Zhang, F.; Liu, X.; et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat. Commun. 2016, 7, 10523. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Li, N.; Li, S.; Han, R.; Huang, Q.; Hu, J.; Jin, K.; Ji, X. Limb ischemic conditioning improved cognitive deficits via eNOS-dependent augmentation of angiogenesis after chronic cerebral hypoperfusion in rats. Aging Dis. 2018, 9, 869–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, K.A.; MacNeil, R.G.; Dirain, M.; Sandesara, B.; Manini, T.M.; Buford, T.W. Blood flow restriction enhances post-resistance exercise angiogenic gene expression. Med. Sci. Sports Exerc. 2012, 44, 2077–2083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, R.A.; Hunt, J.E.A.; Lewis, M.P.; Martin, N.R.W.; Player, D.J.; Stangier, C.; Taylor, C.W.; Turner, M.C. The acute angiogenic signalling response to low-load resistance exercise with blood flow restriction. Eur. J. Sport Sci. 2018, 18, 397–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webb, R.L.; Kaiser, E.E.; Scoville, S.L.; Thompson, T.A.; Fatima, S.; Pandya, C.; Sriram, K.; Swetenburg, R.L.; Vaibhav, K.; Arbab, A.S.; et al. Human Neural Stem Cell Extracellular Vesicles Improve Tissue and Functional Recovery in the Murine Thromboembolic Stroke Model. Transl. Stroke Res. 2018, 9, 530–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betzer, O.; Perets, N.; Angel, A.; Motiei, M.; Sadan, T.; Yadid, G.; Offen, D.; Popovtzer, R. In Vivo Neuroimaging of Exosomes Using Gold Nanoparticles. ACS Nano 2017, 11, 10883–10893. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Stewart, T.; Sheng, L.; Li, N.; Bullock, K.; Song, N.; Shi, M.; Banks, W.A.; Zhang, J. Transmission of α-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: Another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol. Commun. 2017, 5, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Flannagan, R.S.; Canton, J.; Furuya, W.; Glogauer, M.; Grinstein, S. The phosphatidylserine receptor TIM4 utilizes integrins as coreceptors to effect phagocytosis. Mol. Biol. Cell 2014, 25, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic, M.M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 32. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.P.; Tian, T.; Wang, J.Y.; He, J.N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.X.; Qiu, X.T.; Li, C.C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ren, C.; Li, H.; Jiang, F.; Wang, L.; Xia, C.; Ji, X. Role of exosomes induced by remote ischemic preconditioning in neuroprotection against cerebral ischemia. Neuroreport 2019, 30, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Inoh, S.; Asano, T.; Sano, K.; Kubota, M.; Shimazaki, H.; Ueta, N. Effect of transient ischemia on free fatty acids and phospholipids in the gerbil brain. Lipid peroxidation as a possible cause of postischemic injury. J. Neurosurg. 1980, 53, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion-from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitagawa, K.; Saitoh, M.; Ishizuka, K.; Shimizu, S. Remote Limb Ischemic Conditioning during Cerebral Ischemia Reduces Infarct Size through Enhanced Collateral Circulation in Murine Focal Cerebral Ischemia. J. Stroke Cerebrovasc. Dis. 2018, 27, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ma, Y.; Dong, B.; Bandet, M.V.; Shuaib, A.; Winship, I.R. Prevention of the collapse of pial collaterals by remote ischemic perconditioning during acute ischemic stroke. J. Cereb. Blood Flow Metab. 2017, 37, 3001–3014. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Li, S.; Wang, B.; Han, R.; Li, N.; Gao, J.; Li, X.; Jin, K.; Ji, X. Limb remote ischemic conditioning increases Notch signaling activity and promotes arteriogenesis in the ischemic rat brain. Behav. Brain Res. 2018, 340, 87–93. [Google Scholar] [CrossRef] [PubMed]
- England, T.J.; Hedstrom, A.; O’Sullivan, S.; Donnelly, R.; Barrett, D.A.; Sarmad, S.; Sprigg, N.; Bath, P.M. RECAST (Remote Ischemic Conditioning after Stroke Trial): A Pilot Randomized Placebo Controlled Phase II Trial in Acute Ischemic Stroke. Stroke 2017, 48, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liang, K.; Zhang, L.; Hu, Y.; Ge, Y.; Zhao, J. Upper Limb Ischemic Postconditioning as Adjunct Therapy in Acute Stroke Patients: A Randomized Pilot. J. Stroke Cerebrovasc. Dis. 2018, 27, 3328–3335. [Google Scholar] [CrossRef] [PubMed]
- Pico, F.; Lapergue, B.; Ferrigno, M.; Rosso, C.; Meseguer, E.; Chadenat, M.-L.; Bourdain, F.; Obadia, M.; Hirel, C.; Duong, D.L.; et al. Effect of In-Hospital Remote Ischemic Perconditioning on Brain Infarction Growth and Clinical Outcomes in Patients With Acute Ischemic Stroke. JAMA Neurol. 2020, 77, 725. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ren, C.; Li, Y.; Gao, C.; Li, N.; Li, H.; Wu, D.; He, X.; Xia, C.; Ji, X. Remote ischemic conditioning enhances oxygen supply to ischemic brain tissue in a mouse model of stroke: Role of elevated 2,3-biphosphoglycerate in erythrocytes. J. Cereb. Blood Flow Metab. 2020, 41, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Gedik, N.; Maciel, L.; Schulte, C.; Skyschally, A.; Heusch, G.; Kleinbongard, P. Cardiomyocyte mitochondria as targets of humoral factors released by remote ischemic preconditioning. Arch. Med. Sci. 2017, 13, 448–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Badawi, M.; Pomeroy, S.; Sutaria, D.S.; Xie, Z.; Baek, A.; Jiang, J.; Elgamal, O.A.; Mo, X.; Perle, K.L.; et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J. Extracell. Vesicles 2017, 6, 1324730. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.F.; Lázaro-Ibáñez, E.; Forsgard, M.A.M.; Shatnyeva, O.; Osteikoetxea, X.; Karlsson, F.; Heath, N.; Ingelsten, M.; Rose, J.; Harris, J.; et al. Extracellular vesicles induce minimal hepatotoxicity and immunogenicity. Nanoscale 2019, 11, 6990–7001. [Google Scholar] [CrossRef] [PubMed]
- Blauenfeldt, R.A.; Hjort, N.; Gude, M.F.; Behrndtz, A.B.; Fisher, M.; Valentin, J.B.; Kirkegaard, H.; Johnsen, S.P.; Hess, D.C.; Andersen, G. A multicentre, randomised, sham-controlled trial on REmote iSchemic conditioning in patients with acute STroke (RESIST)—Rationale and study design. Eur. Stroke J. 2020, 5, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antes, T.J.; Middleton, R.C.; Luther, K.M.; Ijichi, T.; Peck, K.A.; Liu, W.J.; Valle, J.; Echavez, A.K.; Marbán, E. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J. Nanobiotechnol. 2018, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sieljacks, P.; Knudsen, L.; Wernbom, M.; Vissing, K. Body position influences arterial occlusion pressure: Implications for the standardization of pressure during blood flow restricted exercise. Eur. J. Appl. Physiol. 2018, 118, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.S.; Soon, P.S.; Marsh, D.J. Comparison of methodologies to detect low levels of hemolysis in serum for accurate assessment of serum microRNAs. PLoS ONE 2016, 11, e0153200. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.; Bæk, R.; Pedersen, S.; Søndergaard, E.K.L.; Kristensen, S.R.; Varming, K. Extracellular Vesicle (EV) array: Microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J. Extracell. Vesicles 2013, 2, 20920. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, G.; Martinelli, M.; Courty, J.; Cascone, I. Angiogenesis Analyzer for ImageJ. In Proceedings of the 4th ImageJ User and Developer Conference, Mondorf les Bains, Luxembourg, 24–26 October 2012. [Google Scholar]
- Engel, O.; Kolodziej, S.; Dirnagl, U.; Prinz, V. Modeling stroke in mice—Middle cerebral artery occlusion with the filament model. J. Vis. Exp. 2011, 47, 2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, A.K.; Bolay, H.; Moskowitz, M.A.; Boas, D.A. Dynamic imaging of cerebral blood flow using laser speckle. J. Cereb. Blood Flow Metab. 2001, 21, 195–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, A.K.; Devor, A.; Bolay, H.; Andermann, M.L.; Moskowitz, M.A.; Dale, A.M.; Boas, D.A. Simultaneous imaging of total cerebral hemoglobin concentration, oxygenation, and blood flow during functional activation. Opt. Lett. 2003, 28, 28–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postnov, D.D.; Tang, J.; Erdener, S.E.; Kılıç, K.; Boas, D.A. Dynamic light scattering imaging. Sci. Adv. 2020, 6, eabc4628. [Google Scholar] [CrossRef] [PubMed]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.; Guo, R.M.; Yang, M.; Wen, X.H.; Shen, J. A stable focal cerebral ischemia injury model in adult mice: Assessment using 7T MR imaging. Am. J. Neuroradiol. 2012, 33, 935–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Schallert, T.; Zhang, Z.G.; Jiang, Q.; Arniego, P.; Li, Q.; Lu, M.; Chopp, M. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J. Neurosci. Methods 2002, 117, 207–214. [Google Scholar] [CrossRef]
- Balkaya, M.; Kröber, J.M.; Rex, A.; Endres, M. Assessing post-stroke behavior in mouse models of focal ischemia. J. Cereb. Blood Flow Metab. 2013, 33, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheah, M.; Fawcett, J.; Andrews, M. Assessment of Thermal Pain Sensation in Rats and Mice Using the Hargreaves Test. Bio-Protocol 2017, 7, e2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grønhøj, M.H.; Clausen, B.H.; Fenger, C.D.; Lambertsen, K.L.; Finsen, B. Beneficial potential of intravenously administered IL-6 in improving outcome after murine experimental stroke. Brain. Behav. Immun. 2017, 65, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Clausen, B.H.; Lambertsen, K.L.; Dagnæs-Hansen, F.; Babcock, A.A.; von Linstow, C.U.; Meldgaard, M.; Kristensen, W.B.; Deierborg, T.; Finsen, B. Cell therapy centered on IL-1Ra is neuroprotective in experimental stroke. Acta Neuropathol. 2016, 3, 775–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommer, C. Histology and infarct volume determination in rodent models of stroke. In Rodent Models of Stroke; Humana Press Inc.: New York, NY, USA, 2016; Volume 120, pp. 263–277. [Google Scholar]
- Gundersen, H.J.G.; Bagger, P.; Bendtsen, T.F.; Evans, S.M.; Korbo, L.; Marcussen, N.; Moller, A.; Nielsen, K.; Nyengaard, J.R.; Pakkenberg, B.; et al. The new stereological tools: Disector, fractionator, mucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 1988, 96, 857–881. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, H.J.G. The nucleator. J. Microsc. 1988, 151, 3–21. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, T.; Just, J.; Stenz, K.T.; Yan, Y.; Sieljacks, P.; Wang, J.; Groennebaek, T.S.; Jakobsgaard, J.E.; Rindom, E.; Herskind, J.; et al. The Role of Plasma Extracellular Vesicles in Remote Ischemic Conditioning and Exercise-Induced Ischemic Tolerance. Int. J. Mol. Sci. 2022, 23, 3334. https://doi.org/10.3390/ijms23063334
Gu T, Just J, Stenz KT, Yan Y, Sieljacks P, Wang J, Groennebaek TS, Jakobsgaard JE, Rindom E, Herskind J, et al. The Role of Plasma Extracellular Vesicles in Remote Ischemic Conditioning and Exercise-Induced Ischemic Tolerance. International Journal of Molecular Sciences. 2022; 23(6):3334. https://doi.org/10.3390/ijms23063334
Chicago/Turabian StyleGu, Tingting, Jesper Just, Katrine Tang Stenz, Yan Yan, Peter Sieljacks, Jakob Wang, Thomas Skjaerlund Groennebaek, Jesper Emil Jakobsgaard, Emil Rindom, Jon Herskind, and et al. 2022. "The Role of Plasma Extracellular Vesicles in Remote Ischemic Conditioning and Exercise-Induced Ischemic Tolerance" International Journal of Molecular Sciences 23, no. 6: 3334. https://doi.org/10.3390/ijms23063334
APA StyleGu, T., Just, J., Stenz, K. T., Yan, Y., Sieljacks, P., Wang, J., Groennebaek, T. S., Jakobsgaard, J. E., Rindom, E., Herskind, J., Gravholt, A., Lassen, T. R., Jørgensen, M., Bæk, R., Gutiérrez-Jiménez, E., Iversen, N. K., Rasmussen, P. M., Nyengaard, J. R., Jørgensen, M. M., ... Drasbek, K. R. (2022). The Role of Plasma Extracellular Vesicles in Remote Ischemic Conditioning and Exercise-Induced Ischemic Tolerance. International Journal of Molecular Sciences, 23(6), 3334. https://doi.org/10.3390/ijms23063334






