Printable Hydrogels Based on Alginate and Halloysite Nanotubes
Abstract
:1. Introduction
2. Results
2.1. HNTs Loaded with Salicylic Acid
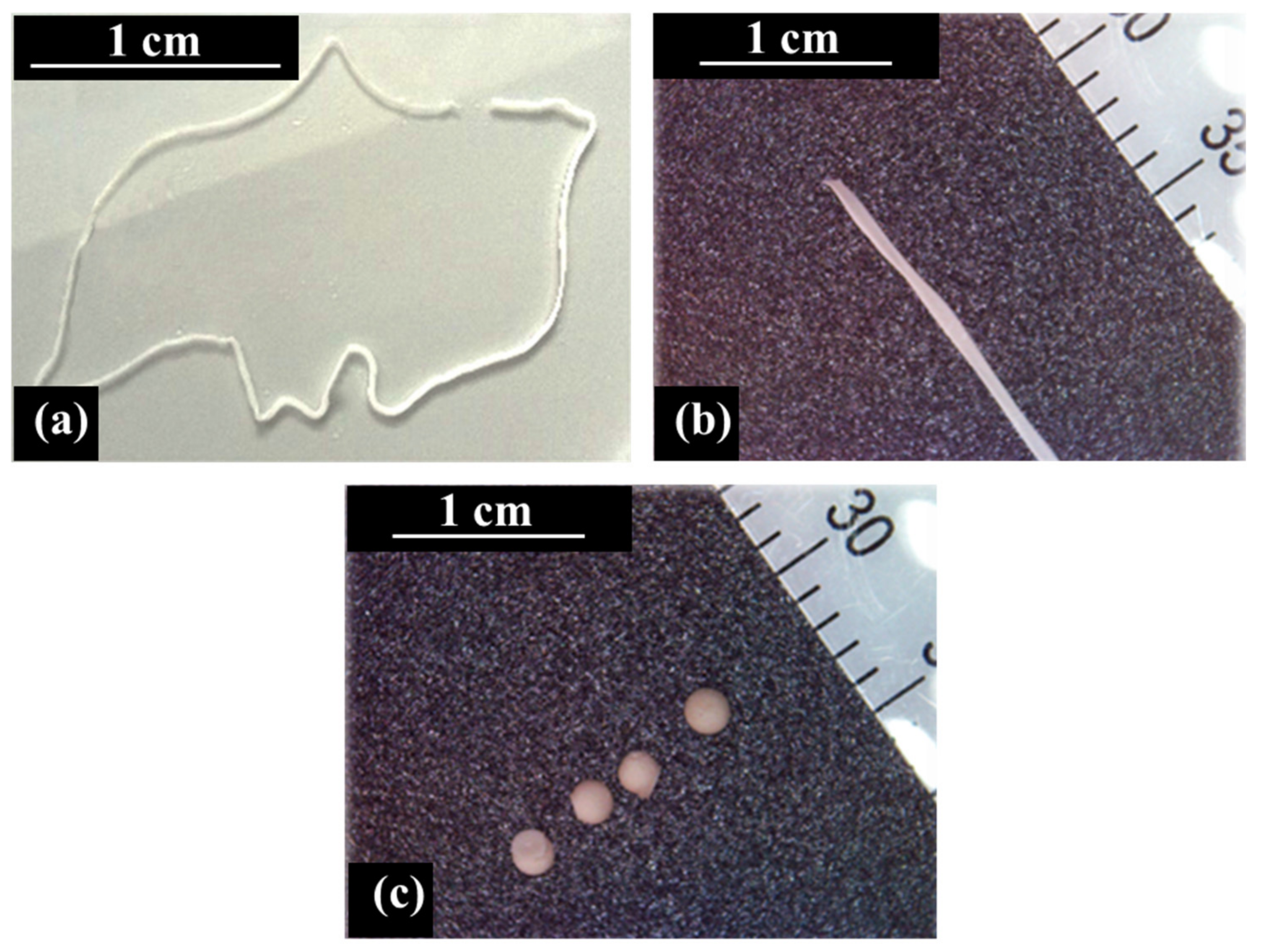
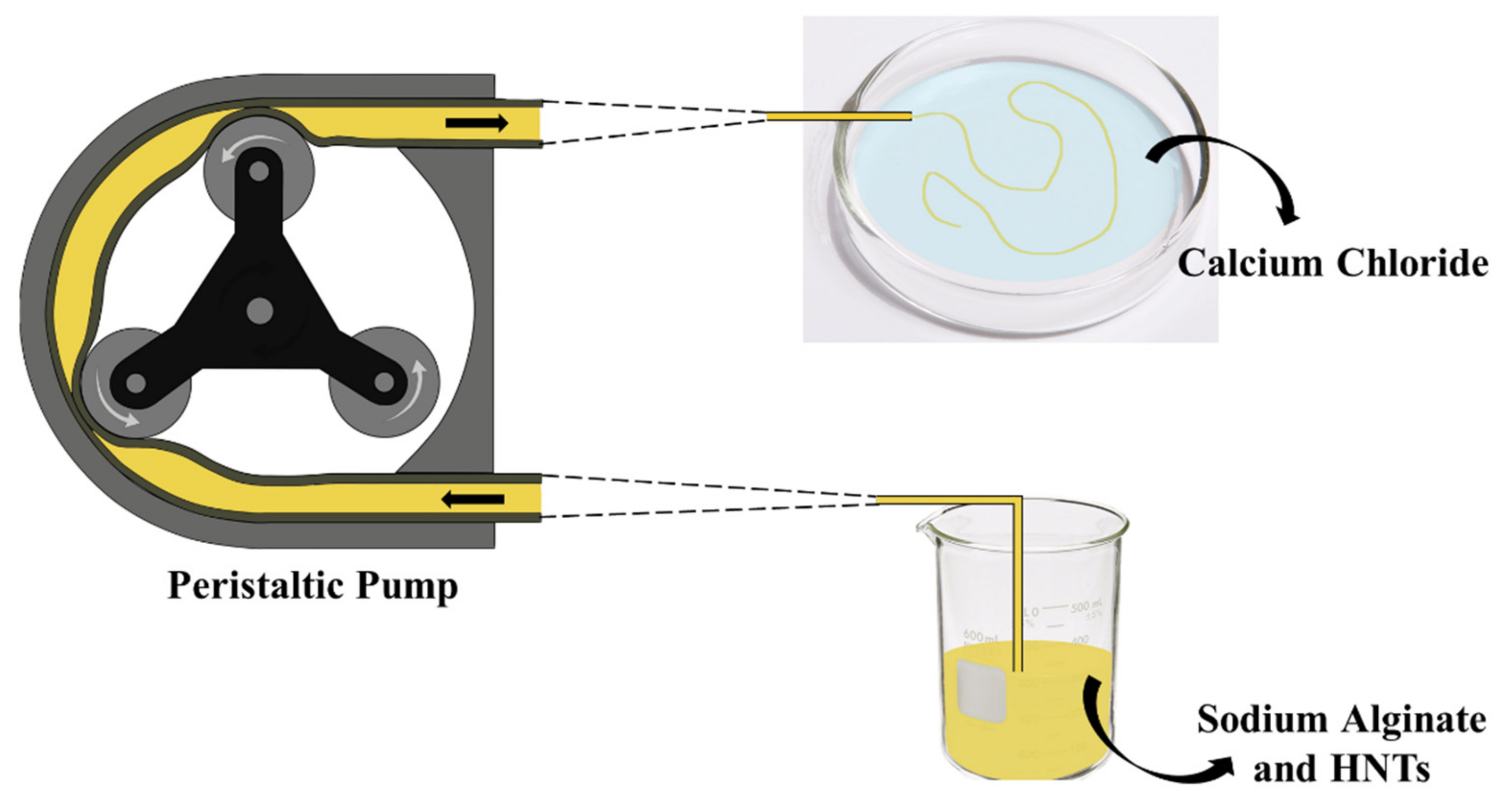
2.2. Preparation of ALG/HNTs Hydrogel Wires
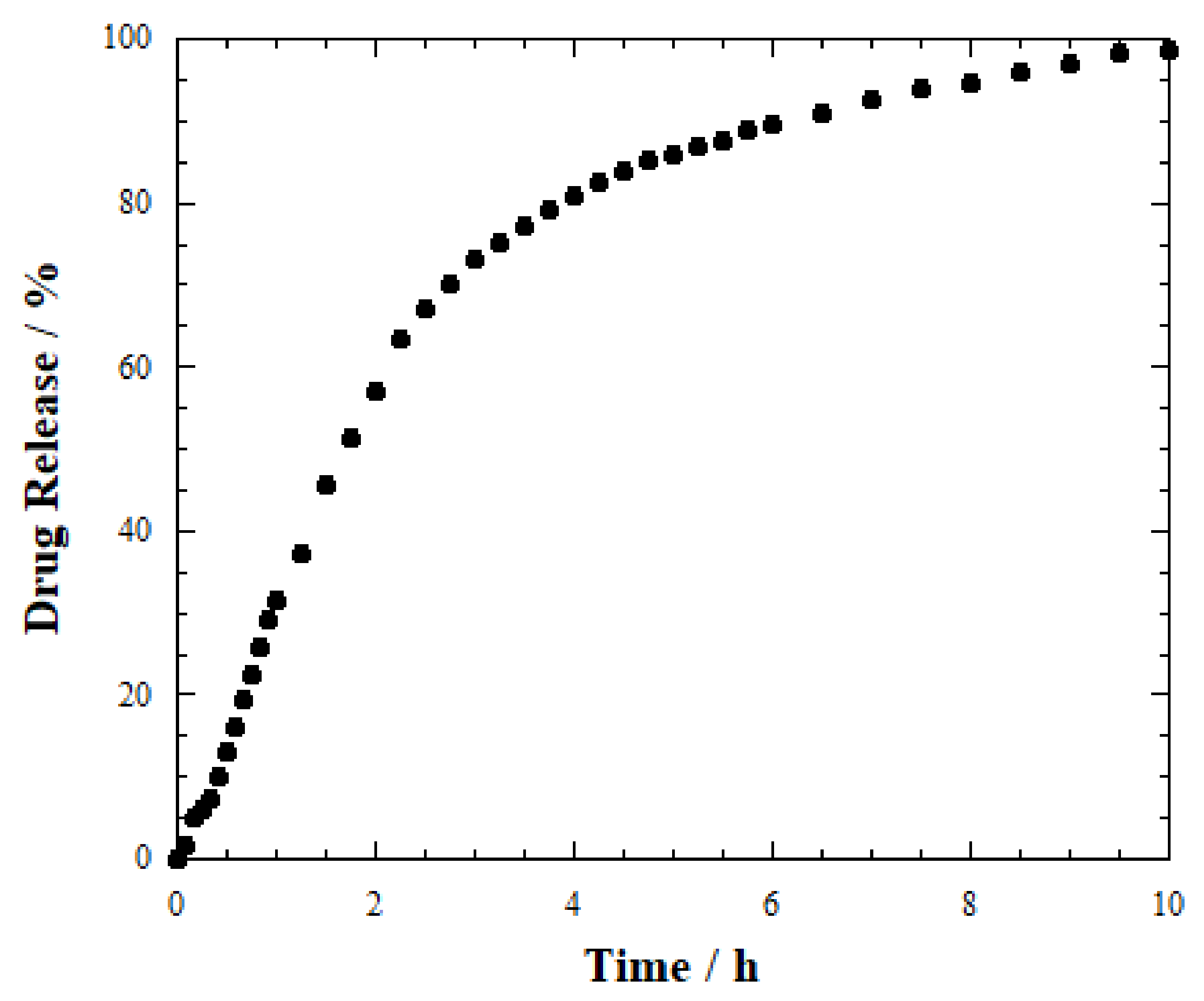
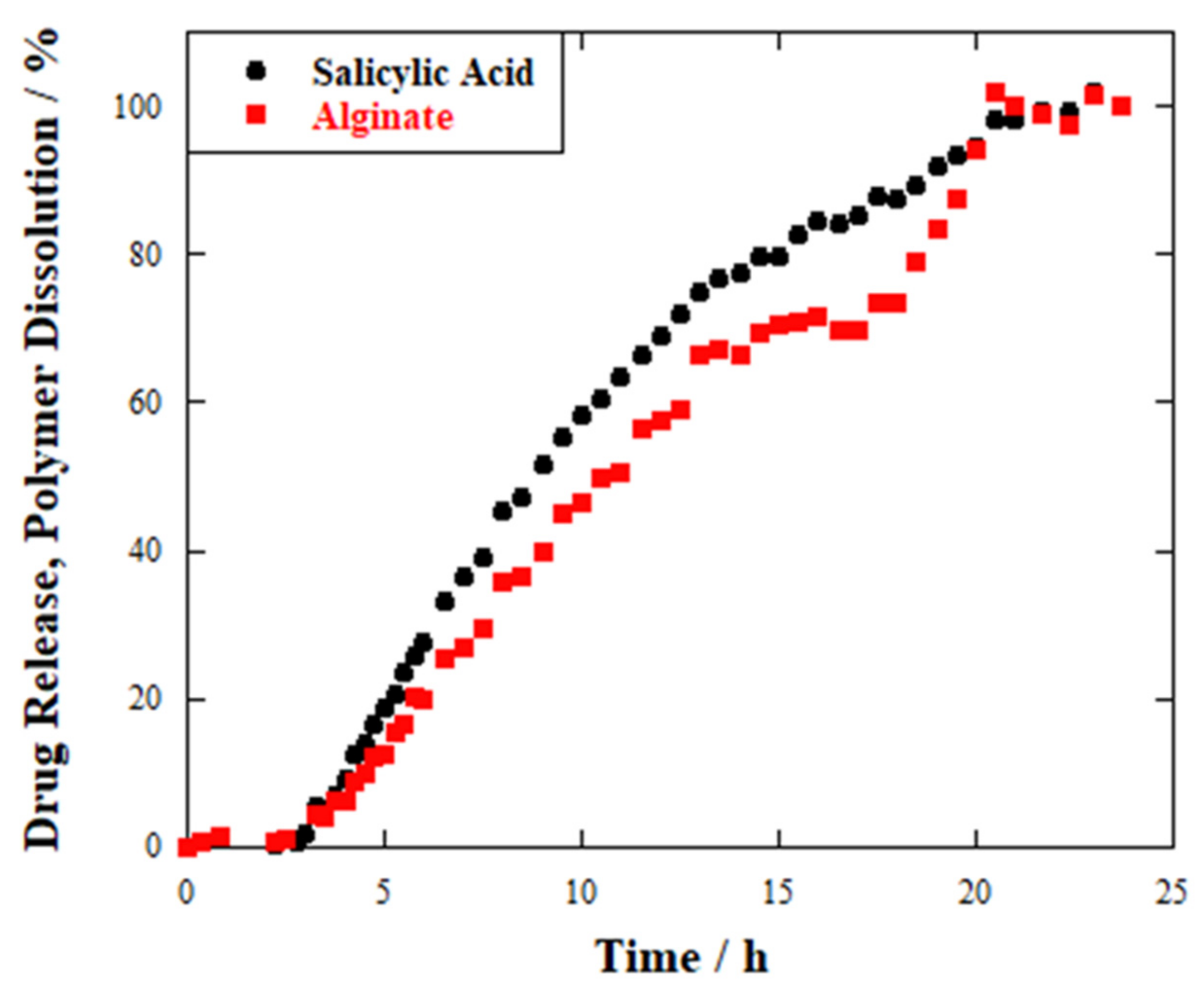
2.3. Release Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Loading of Salicylic Acid into HNTs
4.2.2. Preparation of ALG/HNTs Hydrogel Wires
4.2.3. Preparation of ALG/HNTs Hydrogel Beads
4.2.4. Release Kinetics of Salicylic Acid
4.2.5. Characterization Techniques
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as Intelligent Materials: A Brief Review of Synthesis, Properties and Applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nada, A.A.; Eckstein Andicsová, A.; Mosnáček, J. Irreversible and Self-Healing Electrically Conductive Hydrogels Made of Bio-Based Polymers. Int. J. Mol. Sci. 2022, 23, 842. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Verma, A.; Kumar, V.; Jin Yang, X.; Krishnamurthy, S.; Coulon, F.; Thakur, V.K. Cellulosic Biomass-Based Sustainable Hydrogels for Wastewater Remediation: Chemistry and Prospective. Fuel 2022, 309, 122114. [Google Scholar] [CrossRef]
- Zhang, L.; Li, K.; Xiao, W.; Zheng, L.; Xiao, Y.; Fan, H.; Zhang, X. Preparation of Collagen–Chondroitin Sulfate–Hyaluronic Acid Hybrid Hydrogel Scaffolds and Cell Compatibility in Vitro. Carbohydr. Polym. 2011, 84, 118–125. [Google Scholar] [CrossRef]
- Sikareepaisan, P.; Ruktanonchai, U.; Supaphol, P. Preparation and Characterization of Asiaticoside-Loaded Alginate Films and Their Potential for Use as Effectual Wound Dressings. Carbohydr. Polym. 2011, 83, 1457–1469. [Google Scholar] [CrossRef]
- Wei, L.; Tan, J.; Li, L.; Wang, H.; Liu, S.; Chen, J.; Weng, Y.; Liu, T. Chitosan/Alginate Hydrogel Dressing Loaded FGF/VE-Cadherin to Accelerate Full-Thickness Skin Regeneration and More Normal Skin Repairs. Int. J. Mol. Sci. 2022, 23, 1249. [Google Scholar] [CrossRef]
- Ou, K.-L.; Huang, C.-F.; Lan, W.-C.; Huang, B.-H.; Pan, H.-A.; Shen, Y.-K.; Saito, T.; Tsai, H.-Y.; Cho, Y.-C.; Hung, K.-S.; et al. An Innovative Customized Biomimetic Hydrogel for Drug Screening Application Potential: Biocompatibility and Cell Invasion Ability. Int. J. Mol. Sci. 2022, 23, 1488. [Google Scholar] [CrossRef]
- Dehshahri, A.; Kumar, A.; Madamsetty, V.S.; Uzieliene, I.; Tavakol, S.; Azedi, F.; Fekri, H.S.; Zarrabi, A.; Mohammadinejad, R.; Thakur, V.K. New Horizons in Hydrogels for Methotrexate Delivery. Gels 2021, 7, 2. [Google Scholar] [CrossRef]
- Melnyk, Y.; Stetsyshyn, Y.; Skorokhoda, V.; Nastishin, Y. Polyvinylpyrrolidone-Graft-Poly(2-Hydroxyethylmethacrylate) Hydrogel Membranes for Encapsulated Forms of Drugs. J. Polym. Res. 2020, 27, 354. [Google Scholar] [CrossRef]
- Saxena, A.K. Synthetic Biodegradable Hydrogel (PleuraSeal) Sealant for Sealing of Lung Tissue after Thoracoscopic Resection. J. Thorac. Cardiovasc. Surg. 2010, 139, 496–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furlani, F.; Rossi, A.; Grimaudo, M.A.; Bassi, G.; Giusto, E.; Molinari, F.; Lista, F.; Montesi, M.; Panseri, S. Controlled Liposome Delivery from Chitosan-Based Thermosensitive Hydrogel for Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 894. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Madry, H.; Cucchiarini, M. Application of Alginate Hydrogels for Next-Generation Articular Cartilage Regeneration. Int. J. Mol. Sci. 2022, 23, 1147. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Jia, Y.; Chen, X.; Li, X.; An, L. A Multiphasic Model for the Volume Change of Polyelectrolyte Hydrogels. J. Chem. Phys. 2010, 133, 114904. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Barroso, I.A.; Brunet, M.Y.; Peacock, B.; Federici, A.S.; Hoey, D.A.; Cox, S.C. Controlled Release of Epigenetically-Enhanced Extracellular Vesicles from a GelMA/Nanoclay Composite Hydrogel to Promote Bone Repair. Int. J. Mol. Sci. 2022, 23, 832. [Google Scholar] [CrossRef]
- Huang, B.; Liu, M.; Zhou, C. Chitosan Composite Hydrogels Reinforced with Natural Clay Nanotubes. Carbohydr. Polym. 2017, 175, 689–698. [Google Scholar] [CrossRef]
- Lvov, Y.M.; DeVilliers, M.M.; Fakhrullin, R.F. The Application of Halloysite Tubule Nanoclay in Drug Delivery. Expert Opin. Drug Deliv. 2016, 13, 977–986. [Google Scholar] [CrossRef]
- Lvov, Y.; Aerov, A.; Fakhrullin, R. Clay Nanotube Encapsulation for Functional Biocomposites. Adv. Colloid Interface Sci. 2014, 207, 189–198. [Google Scholar] [CrossRef]
- Emmanuel, E.; Yong, L.L.; Anggraini, V.; Pasbakhsh, P. Can Halloysite Nanotubes Be Used to Remediate Zinc and Lead-Contaminated Marine Clay? A Solidification/Stabilization Approach. Appl. Clay Sci. 2020, 186, 105441. [Google Scholar] [CrossRef]
- Panchal, A.; Rahman, N.; Konnova, S.; Fakhrullin, R.; Zhang, D.; Blake, D.; John, V.; Ivanov, E.; Lvov, Y. Clay Nanotube Liquid Marbles Enhanced with Inner Biofilm Formation for the Encapsulation and Storage of Bacteria at Room Temperature. ACS Appl. Nano Mater. 2020, 3, 1263–1271. [Google Scholar] [CrossRef]
- Lvov, Y.; Panchal, A.; Fu, Y.; Fakhrullin, R.; Kryuchkova, M.; Batasheva, S.; Stavitskaya, A.; Glotov, A.; Vinokurov, V. Interfacial Self-Assembly in Halloysite Nanotube Composites. Langmuir 2019, 35, 8646–8657. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Yuan, P.; Liu, D.; Annabi-Bergaya, F.; Zhou, J.; Chen, F.; Liu, Z. Effects of Microstructure of Clay Minerals, Montmorillonite, Kaolinite and Halloysite, on Their Benzene Adsorption Behaviors. Appl. Clay Sci. 2017, 143, 184–191. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Pickering Emulsions Stabilized by Halloysite Nanotubes: From General Aspects to Technological Applications. Adv. Mater. Interfaces 2022. [Google Scholar] [CrossRef]
- Pasbakhsh, P.; Churchman, G.J.; Keeling, J.L. Characterisation of Properties of Various Halloysites Relevant to Their Use as Nanotubes and Microfibre Fillers. Appl. Clay Sci. 2013, 74, 47–57. [Google Scholar] [CrossRef]
- Zhao, Y.; Kong, W.; Jin, Z.; Fu, Y.; Wang, W.; Zhang, Y.; Liu, J.; Zhang, B. Storing Solar Energy within Ag-Paraffin@Halloysite Microspheres as a Novel Self-Heating Catalyst. Appl. Energy 2018, 222, 180–188. [Google Scholar] [CrossRef]
- Bugatti, V.; Viscusi, G.; Naddeo, C.; Gorrasi, G. Nanocomposites Based on PCL and Halloysite Nanotubes Filled with Lysozyme: Effect of Draw Ratio on the Physical Properties and Release Analysis. Nanomaterials 2017, 7, 213. [Google Scholar] [CrossRef] [Green Version]
- Abdullayev, E.; Lvov, Y. Clay Nanotubes for Corrosion Inhibitor Encapsulation: Release Control with End Stoppers. J. Mater. Chem. 2010, 20, 6681–6687. [Google Scholar] [CrossRef]
- Huang, B.; Liu, M.; Zhou, C. Cellulose–Halloysite Nanotube Composite Hydrogels for Curcumin Delivery. Cellulose 2017, 24, 2861–2875. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, C.; Song, H.; Bai, L.; Cheng, Y.; Ba, X.; Wu, Y. A Facile One-Step Grafting of Polyphosphonium onto Halloysite Nanotubes Initiated by Ce(IV). Chem. Commun. 2019, 55, 1040–1043. [Google Scholar] [CrossRef]
- Członka, S.; Kairytė, A.; Miedzińska, K.; Strąkowska, A. Polyurethane Composites Reinforced with Walnut Shell Filler Treated with Perlite, Montmorillonite and Halloysite. Int. J. Mol. Sci. 2021, 22, 7304. [Google Scholar] [CrossRef]
- von Klitzing, R.; Stehl, D.; Pogrzeba, T.; Schomäcker, R.; Minullina, R.; Panchal, A.; Konnova, S.; Fakhrullin, R.; Koetz, J.; Möhwald, H.; et al. Halloysites Stabilized Emulsions for Hydroformylation of Long Chain Olefins. Adv. Mater. Interfaces 2016, 4, 1600435–1600443. [Google Scholar] [CrossRef]
- Sadjadi, S. Halloysite-Based Hybrids/Composites in Catalysis. Appl. Clay Sci. 2020, 189, 105537. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Hueckel, T.; Cavallaro, G.; Sacanna, S.; Lazzara, G. Pickering Emulsions Based on Wax and Halloysite Nanotubes: An Ecofriendly Protocol for the Treatment of Archeological Woods. ACS Appl. Mater. Interfaces 2021, 13, 1651–1661. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G. Dispersion of Halloysite Loaded with Natural Antimicrobials into Pectins: Characterization and Controlled Release Analysis. Carbohydr. Polym. 2015, 127, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ren, T.; Ji, Y.; Han, L.; Wu, Y.; Song, H.; Bai, L.; Ba, X. Selective Modification of Halloysite Nanotubes with 1-Pyrenylboronic Acid: A Novel Fluorescence Probe with Highly Selective and Sensitive Response to Hyperoxide. ACS Appl. Mater. Interfaces 2015, 7, 23805–23811. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Abdullayev, E.; Vasiliev, A.; Lvov, Y. Halloysite Nanotubule Clay for Efficient Water Purification. J. Colloid Interface Sci. 2013, 406, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes as Nanoreactors for Heterogeneous Micellar Catalysis. J. Colloid Interface Sci. 2022, 608, 424–434. [Google Scholar] [CrossRef]
- Chiew, C.S.C.; Gourich, W.; Pasbakhsh, P.; Poh, P.E.; Tey, B.T.; Song, C.P.; Chan, E.-S. Life Cycle Assessment on Alginate-Based Nanocomposite Beads for the Removal of Lead(II) from Aqueous Solutions. J. Water Process Eng. 2022, 45, 102531. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; Gorrasi, G. Design of a Hybrid Bio-Adsorbent Based on Sodium Alginate/Halloysite/Hemp Hurd for Methylene Blue Dye Removal: Kinetic Studies and Mathematical Modeling. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127925. [Google Scholar] [CrossRef]
- Akbari, A.; Padervand, M.; Jalilian, E.; Seidi, F. Sodium Alginate-Halloysite Nanotube Gel Beads as Potential Delivery System for Sunitinib Malate Anticancer Compound. Mater. Lett. 2020, 274, 128038. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Li, W.; Liu, Z.; Liu, Y.; Wei, H.; Li, J. Synthesis and Characterization of Double-Network Hydrogels Based on Sodium Alginate and Halloysite for Slow Release Fertilizers. Int. J. Biol. Macromol. 2020, 164, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, F.; Abbas, G.; Ameer, N.; Akhtar, M.F.; Shah, S.; Hanif, M. Preparation and Characterization of Halloysite Nanotubes Containing Hydrogels for Controlled Release Drug Delivery of Cetirizine Dihydrochloride. Polym. Bull. 2021. [Google Scholar] [CrossRef]
- Glukhova, S.A.; Molchanov, V.S.; Lokshin, B.V.; Rogachev, A.V.; Tsarenko, A.A.; Patsaev, T.D.; Kamyshinsky, R.A.; Philippova, O.E. Printable Alginate Hydrogels with Embedded Network of Halloysite Nanotubes: Effect of Polymer Cross-Linking on Rheological Properties and Microstructure. Polymers 2021, 13, 4130. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tan, C.; Li, L. Review of 3D Printable Hydrogels and Constructs. Mater. Des. 2018, 159, 20–38. [Google Scholar] [CrossRef]
- Wesołowski, M. Thermal Decomposition of Salicylic Acid and Its Salts. Thermochim. Acta 1979, 31, 133–146. [Google Scholar] [CrossRef]
- Abbas, A.; Hussain, M.A.; Amin, M.; Tahir, M.N.; Jantan, I.; Hameed, A.; Bukhari, S.N.A. Multiple Cross-Linked Hydroxypropylcellulose–Succinate–Salicylate: Prodrug Design, Characterization, Stimuli Responsive Swelling–Deswelling and Sustained Drug Release. RSC Adv. 2015, 5, 43440–43448. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes Filled with MgO for Paper Reinforcement and Deacidification. Appl. Clay Sci. 2021, 213, 106231. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Pasbakhsh, P.; Milioto, S.; Lazzara, G. Why Does Vacuum Drive to the Loading of Halloysite Nanotubes? The Key Role of Water Confinement. J. Colloid Interface Sci. 2019, 547, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Effects of Halloysite Content on the Thermo-Mechanical Performances of Composite Bioplastics. Appl. Clay Sci. 2020, 185, 105416. [Google Scholar] [CrossRef] [Green Version]
- Lisuzzo, L.; Cavallaro, G.; Parisi, F.; Milioto, S.; Fakhrullin, R.; Lazzara, G. Core/Shell Gel Beads with Embedded Halloysite Nanotubes for Controlled Drug Release. Coatings 2019, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Lisuzzo, L.; Wicklein, B.; Dico, G.L.; Lazzara, G.; del Real, G.; Aranda, P.; Ruiz-Hitzky, E. Functional Biohybrid Materials Based on Halloysite, Sepiolite and Cellulose Nanofibers for Health Applications. Dalton Trans. 2020, 49, 3830–3840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Halloysite Nanotubes Filled with Salicylic Acid and Sodium Diclofenac: Effects of Vacuum Pumping on Loading and Release Properties. J. Nanostruct. Chem. 2021, 11, 663–673. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Layered Composite Based on Halloysite and Natural Polymers: A Carrier for the PH Controlled Release of Drugs. New J. Chem. 2019, 43, 10887–10893. [Google Scholar] [CrossRef] [Green Version]
- Blanco, I.; Abate, L.; Bottino, F.A.; Bottino, P. Thermal Behaviour of a Series of Novel Aliphatic Bridged Polyhedral Oligomeric Silsesquioxanes (POSSs)/Polystyrene (PS) Nanocomposites: The Influence of the Bridge Length on the Resistance to Thermal Degradation. Polym. Degrad. Stab. 2014, 102, 132–137. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
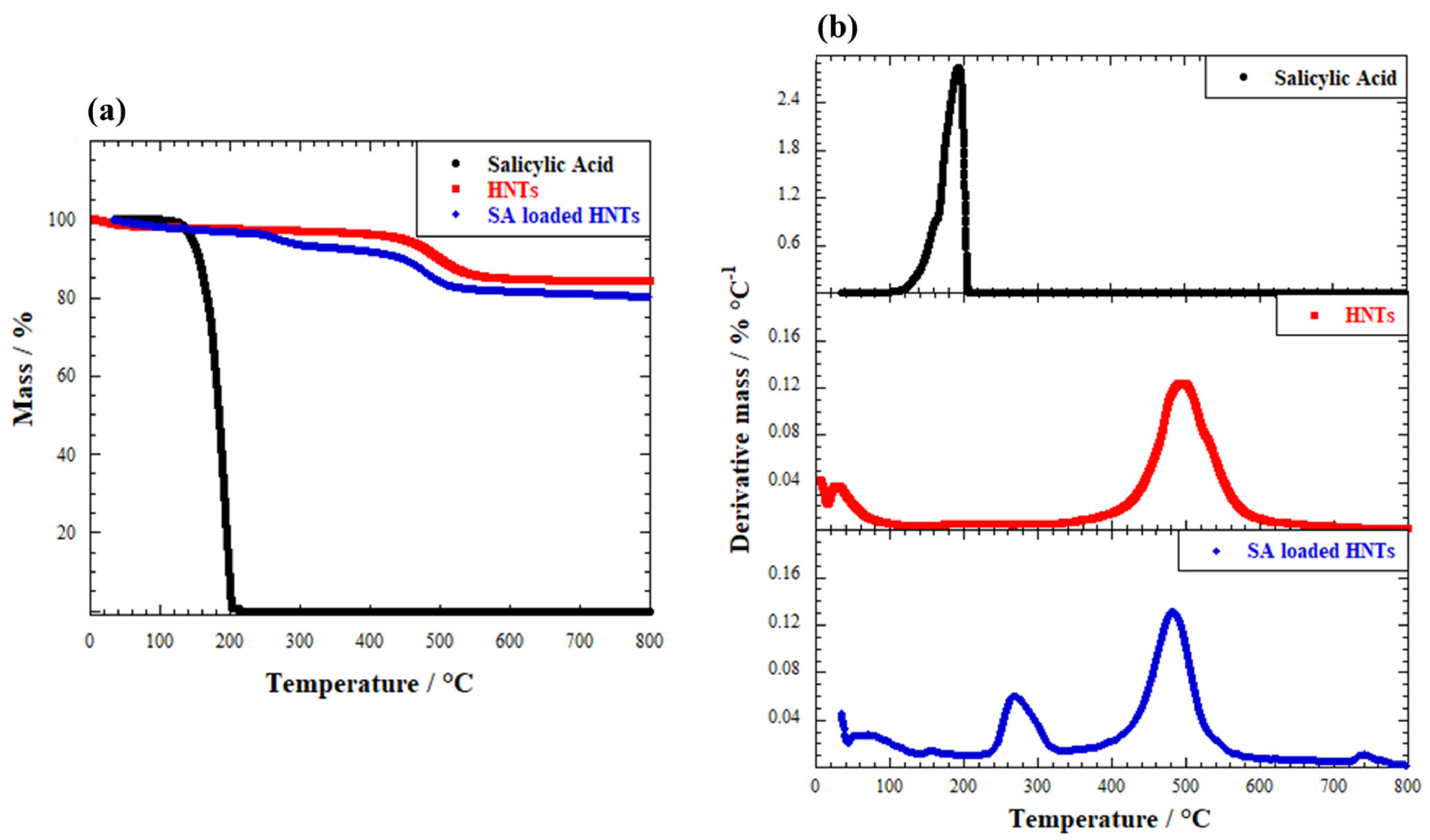


| Sample | ML150 (wt%) | MR800 (wt%) |
|---|---|---|
| Salicylic acid | 6.80 ± 0.11 | 0 |
| HNTs | 2.17 ± 0.04 | 84.1 ± 1.2 |
| SA-loaded HNTs | 2.49 ± 0.04 | 80.4 ± 1.1 |
| Sample | Tube Diameter (mm) | Average Diameter (mm) |
|---|---|---|
| Hydrogel beads | 0.38 | 1.26 ± 0.06 |
| Hydrogel wires_1 | 0.38 | 0.19 ± 0.03 |
| Hydrogel wires_2 | 0.76 | 0.47 ± 0.15 |
| Sample | T50% (h) | Induction Period (h) |
|---|---|---|
| HNTs-SA tablet | 2 | 0 |
| Hydrogel wires_1 | 6 | 0 |
| Hydrogel wires_2 | 10 | 2 |
| Hydrogel beads | 9 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavallaro, G.; Lisuzzo, L.; Lazzara, G.; Milioto, S. Printable Hydrogels Based on Alginate and Halloysite Nanotubes. Int. J. Mol. Sci. 2022, 23, 3294. https://doi.org/10.3390/ijms23063294
Cavallaro G, Lisuzzo L, Lazzara G, Milioto S. Printable Hydrogels Based on Alginate and Halloysite Nanotubes. International Journal of Molecular Sciences. 2022; 23(6):3294. https://doi.org/10.3390/ijms23063294
Chicago/Turabian StyleCavallaro, Giuseppe, Lorenzo Lisuzzo, Giuseppe Lazzara, and Stefana Milioto. 2022. "Printable Hydrogels Based on Alginate and Halloysite Nanotubes" International Journal of Molecular Sciences 23, no. 6: 3294. https://doi.org/10.3390/ijms23063294
APA StyleCavallaro, G., Lisuzzo, L., Lazzara, G., & Milioto, S. (2022). Printable Hydrogels Based on Alginate and Halloysite Nanotubes. International Journal of Molecular Sciences, 23(6), 3294. https://doi.org/10.3390/ijms23063294









