Chitosan/Alginate Hydrogel Dressing Loaded FGF/VE-Cadherin to Accelerate Full-Thickness Skin Regeneration and More Normal Skin Repairs
Abstract
:1. Introduction
2. Results
2.1. Physical Characterization of Complex Hydrogels
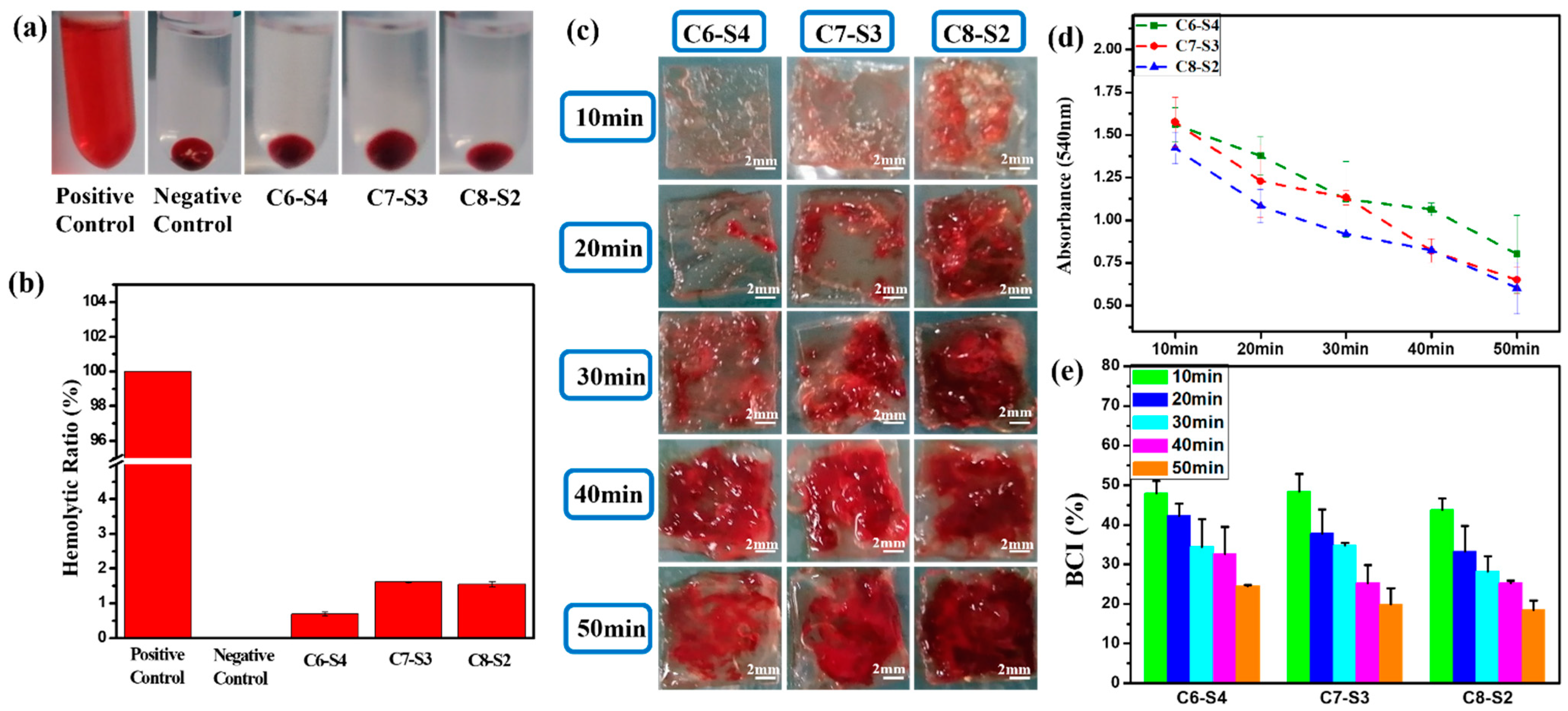
2.2. Hemolysis and Whole Blood Dynamic Coagulation Evaluation Results
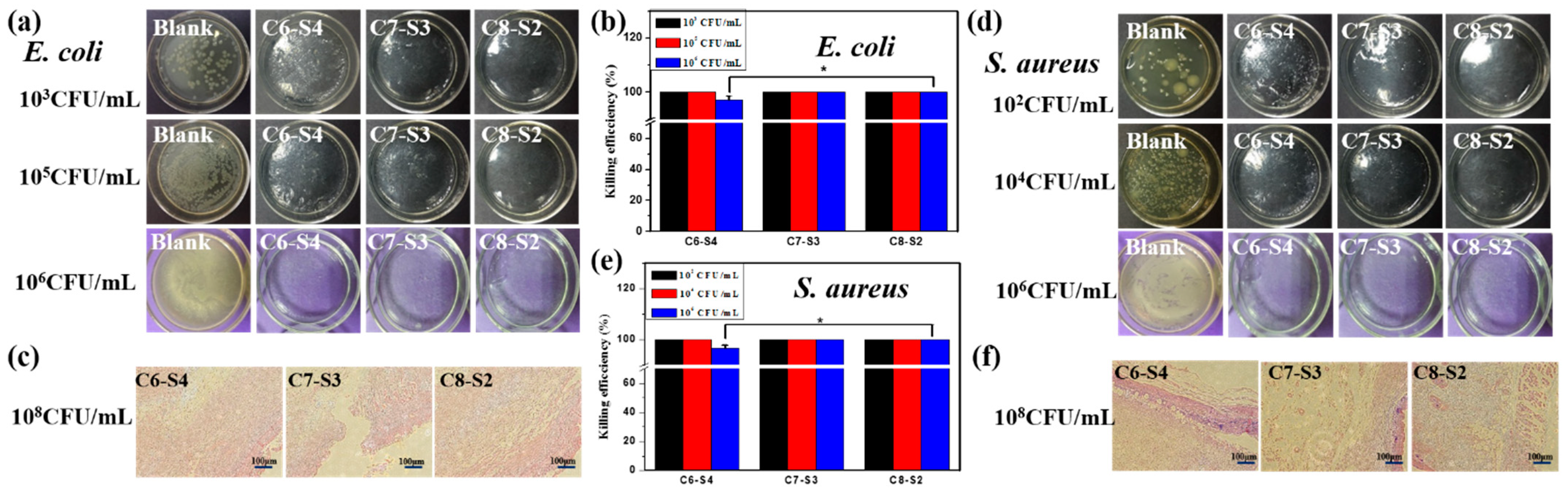
2.3. Antibacterial Activity Assessment
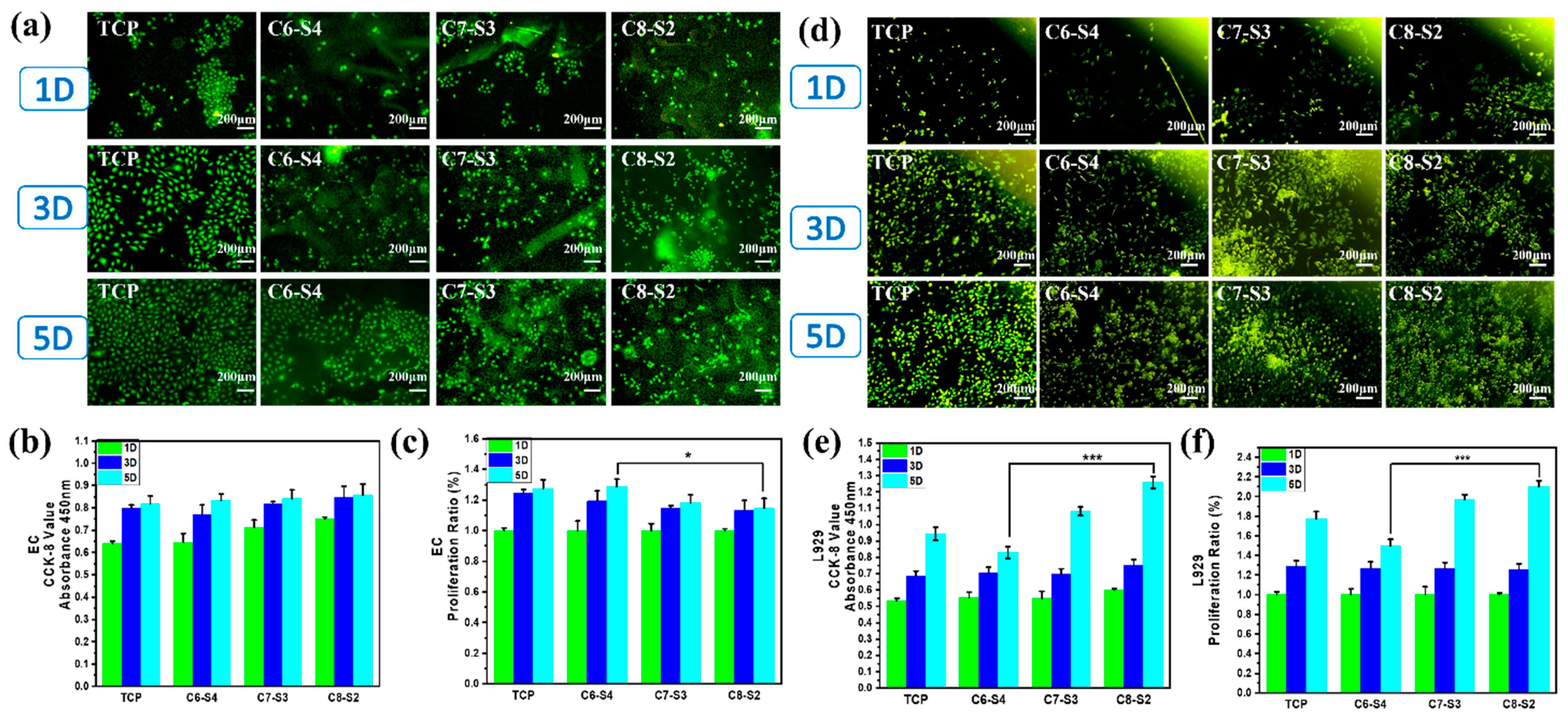
2.4. Three-Dimensional Encapsulation of Cells in Complex Hydrogels
2.5. Three-Dimensional Encapsulation of Two Factors and Two Cells in Complex Hydrogels
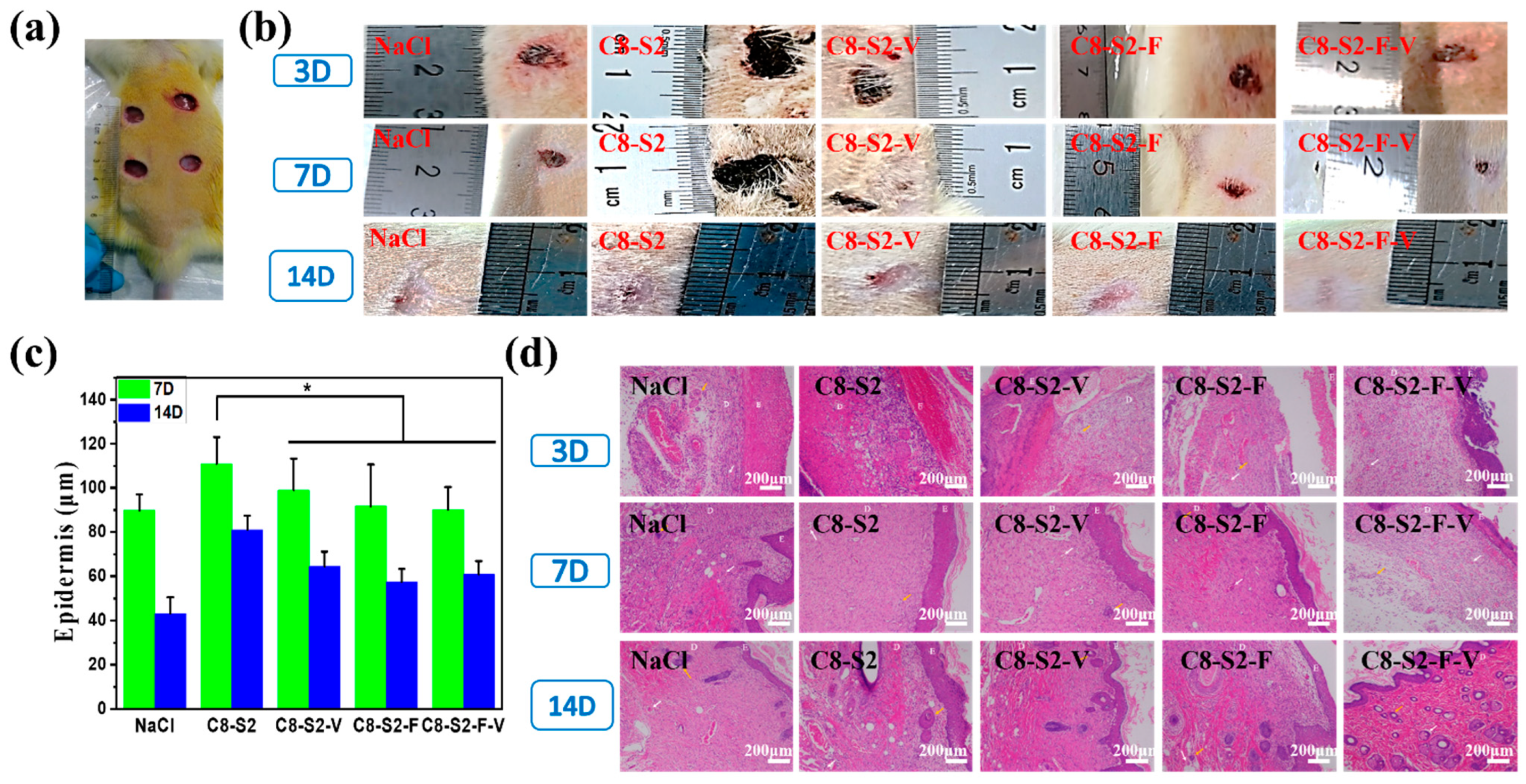
2.6. In Vivo Repair and Regeneration Evaluation
3. Discussion
3.1. The Influence of Chitosan on the Biological Properties of the Complex Hydrogels
3.2. The Influence of FGF and VE-Cadherin in 3D Hydrogels on the Cell Proliferation and Tissue Regeneration
4. Experimental and Methods
4.1. Materials and Reagents
4.2. Preparation of Complex Hydrogels (CS/SA/PEGDDA Gel)
4.3. Swelling Ratio and Degradation Ratio
4.4. Rheological Properties
4.5. Scanning Electron Microscope (SEM)
4.6. Hemolysis and Whole Blood Dynamic Coagulation Evaluation
4.7. In Vitro and In Vivo Antibacterial Activities
4.8. In Vitro Compatibility Evaluation of Epithelial Cells
4.9. In Vivo Wound Healing and Histological Analysis
4.10. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Du, X.; Liu, Y.; Wang, X.; Yan, H.; Wang, L.; Qu, L.; Kong, D.; Qiao, M.; Wang, L. Injectable hydrogel composed of hydrophobically modified chitosan/oxidized-dextran for wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109930. [Google Scholar] [CrossRef]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular matrix contribution to skin wound re-epithelialization. Matrix Biol. 2019, 75–76, 12–26. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Hu, T.; Chen, B.; Yin, Z.; Ma, P.X.; Guo, B. Adhesive Hemostatic Conducting Injectable Composite Hydrogels with Sustained Drug Release and Photothermal Antibacterial Activity to Promote Full-Thickness Skin Regeneration During Wound Healing. Small 2019, 15, e1900046. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Zare-Gachi, M.; Daemi, H.; Mohammadi, J.; Baei, P.; Bazgir, F.; Hosseini-Salekdeh, S.; Baharvand, H. Improving anti-hemolytic, antibacterial and wound healing properties of alginate fibrous wound dressings by exchanging counter-cation for infected full-thickness skin wounds. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 107, 110321. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hadisi, Z.; Ismail, A.F.; Aziz, M.; Akbari, M.; Berto, F.; Chen, X.B. In vitro and in vivo evaluation of chitosan-alginate/gentamicin wound dressing nanofibrous with high antibacterial performance. Polym. Test. 2020, 82, 106298. [Google Scholar] [CrossRef]
- Guerle-Cavero, R.; Lleal-Fontas, B.; Balfagon-Costa, A. Creation of Ionically Crosslinked Tri-Layered Chitosan Membranes to Simulate Different Human Skin Properties. Materials 2021, 14, 1807. [Google Scholar] [CrossRef]
- Chen, K.; Wang, F.; Liu, S.; Wu, X.; Xu, L.; Zhang, D. In situ reduction of silver nanoparticles by sodium alginate to obtain silver-loaded composite wound dressing with enhanced mechanical and antimicrobial property. Int. J. Biol. Macromol. 2020, 148, 501–509. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.R.; Xia, S.; Gao, G.H. An environment-stable hydrogel with skin-matchable performance for human-machine interface. Sci. China Mater. 2021, 64, 2313–2324. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Vatanpour, V.; Taghizadeh, A.; Taghizadeh, M.; Ganjali, M.R.; Munir, M.T.; Habibzadeh, S.; Saeb, M.R.; Ghaedi, M. Hydrogel membranes: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111023. [Google Scholar] [CrossRef]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.; Loh, E.Y.X.; Fauzi, M.B.; Ng, M.H.; Mohd Amin, M.C.I. In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Deliv. Transl. Res. 2019, 9, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, B.; Li, M.; He, J.; Yin, Z.; Guo, B. Injectable Antimicrobial Conductive Hydrogels for Wound Disinfection and Infectious Wound Healing. Biomacromolecules 2020, 21, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yuk, H.; Parada, G.A.; Wu, Y.; Liu, X.; Nabzdyk, C.S.; Youcef-Toumi, K.; Zang, J.; Zhao, X. Multifunctional “Hydrogel Skins” on Diverse Polymers with Arbitrary Shapes. Adv. Mater. 2019, 31, e1807101. [Google Scholar] [CrossRef]
- Qianqian, O.; Songzhi, K.; Yongmei, H.; Xianghong, J.; Sidong, L.; Puwang, L.; Hui, L. Preparation of nano-hydroxyapatite/chitosan/tilapia skin peptides hydrogels and its burn wound treatment. Int. J. Biol. Macromol. 2021, 181, 369–377. [Google Scholar] [CrossRef]
- Furlani, F.; Rossi, A.; Grimaudo, M.A.; Bassi, G.; Giusto, E.; Molinari, F.; Lista, F.; Montesi, M.; Panseri, S. Controlled Liposome Delivery from Chitosan-Based Thermosensitive Hydrogel for Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 894. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent advances in graft copolymerization and applications of chitosan: A review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent progress in sodium alginate based sustainable hydrogels for environmental applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Thakur, S.; Mamba, G.; Gupta, R.K.; Thakur, P.; Thakur, V.K. Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int. J. Biol. Macromol. 2020, 148, 1130–1139. [Google Scholar] [CrossRef]
- Poonguzhali, R.; Khaleel Basha, S.; Sugantha Kumari, V. Novel asymmetric chitosan/PVP/nanocellulose wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2018, 112, 1300–1309. [Google Scholar] [CrossRef]
- Deng, P.; Jin, W.; Liu, Z.; Gao, M.; Zhou, J. Novel multifunctional adenine-modified chitosan dressings for promoting wound healing. Carbohydr. Polym. 2021, 260, 117767. [Google Scholar] [CrossRef] [PubMed]
- Masood, N.; Ahmed, R.; Tariq, M.; Ahmed, Z.; Masoud, M.S.; Ali, I.; Asghar, R.; Andleeb, A.; Hasan, A. Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits. Int. J. Pharm. 2019, 559, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Hu, L.; Xiong, Y.; Zhu, X.; Wei, C.; Cao, F.; Zhou, W.; Sun, Y.; Endo, Y.; Liu, M.; et al. Quaternized chitosan-Matrigel-polyacrylamide hydrogels as wound dressing for wound repair and regeneration. Carbohydr. Polym. 2019, 226, 115302. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Baysal, K.; Aroguz, A.Z.; Adiguzel, Z.; Baysal, B.M. Chitosan/alginate crosslinked hydrogels: Preparation, characterization and application for cell growth purposes. Int. J. Biol. Macromol. 2013, 59, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Lehnert, S.; Sikorski, P. Tailoring the assembly of collagen fibers in alginate microspheres. Mater. Sci. Eng. C Mater. Biol. Appl 2021, 121, 111840. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, A.; Yuan, C.; Chen, X.; Liu, Y. Recent trends on burn wound care: Hydrogel dressings and scaffolds. Biomater. Sci. 2021, 9, 4523–4540. [Google Scholar] [CrossRef]
- Prudovsky, I. Cellular Mechanisms of FGF-Stimulated Tissue Repair. Cells 2021, 10, 1830. [Google Scholar] [CrossRef] [PubMed]
- Firoozi, N.; Kang, Y. Immobilization of FGF on Poly(xylitol dodecanedioic Acid) Polymer for Tissue Regeneration. Sci. Rep. 2020, 10, 10419. [Google Scholar] [CrossRef]
- Karimi, M.; Maghsoud, Z.; Halabian, R. Effect of Preconditioned Mesenchymal Stem Cells with Nisin Prebiotic on the Expression of Wound Healing Factors Such as TGF-β1, FGF-2, IL-1, IL-6, and IL-10. Regen. Eng. Transl. Med. 2021, 7, 30–40. [Google Scholar] [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef]
- Mossahebi-Mohammadi, M.; Quan, M.; Zhang, J.S.; Li, X. FGF Signaling Pathway: A Key Regulator of Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 79. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Yang, C.; Huang, J.; Liu, L.; Zhang, Y.; Li, S.; Zhang, L.; Sun, Q.; Yang, P. Conditioned medium derived from FGF-2-modified GMSCs enhances migration and angiogenesis of human umbilical vein endothelial cells. Stem Cell Res. Ther. 2020, 11, 68. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, J.I.; Zhang, Z.; Oh, M.; Pobocik, A.M.; Imazato, S.; Shi, S.; Nor, J.E. VE-Cadherin and Anastomosis of Blood Vessels Formed by Dental Stem Cells. J. Dent. Res. 2020, 99, 437–445. [Google Scholar] [CrossRef]
- Duong, C.N.; Vestweber, D. Mechanisms Ensuring Endothelial Junction Integrity Beyond VE-Cadherin. Front. Physiol. 2020, 11, 519. [Google Scholar] [CrossRef]
- Gao, C.; Zhang, Y.; Xie, J.H.; Wang, X.P.; Cao, L.; Chen, G.Q.; Mao, H.L.; Bi, X.D.; Gu, Z.W.; Yang, J. VE-cadherin functionalized injectable PAMAM/HA hydrogel promotes endothelial differentiation of hMSCs and vascularization. Appl. Mater. Today 2020, 20, 100690. [Google Scholar] [CrossRef]
- Huang, L.X.; Abdalla, A.M.E.; Xiao, L.; Yang, G. Biopolymer-Based Microcarriers for Three-Dimensional Cell Culture and Engineered Tissue Formation. Int. J. Mol. Sci. 2020, 21, 1895. [Google Scholar] [CrossRef] [Green Version]
- Chou, T.C.; Fu, E.; Wu, C.J.; Yeh, J.H. Chitosan enhances platelet adhesion and aggregation. Biochem. Biophys. Res. Commun. 2003, 302, 480–483. [Google Scholar] [CrossRef]
- Zhong, Y.; Hu, H.; Min, N.; Wei, Y.; Li, X.; Li, X. Application and outlook of topical hemostatic materials: A narrative review. Ann. Transl. Med. 2021, 9, 577. [Google Scholar] [CrossRef]
- Rondon, E.P.; Benabdoun, H.A.; Vallières, F.; Petrônio, M.S.; Tiera, M.J.; Benderdour, M.; Fernandes, J.C. Evidence Supporting the Safety of Pegylated Diethylaminoethyl-Chitosan Polymer as a Nanovector for Gene Therapy Applications. Int. J. Nanomed. 2020, 15, 6183–6200. [Google Scholar] [CrossRef]
- Chen, K.Y.; Chen, Y.C.; Lin, T.H.; Yang, C.Y.; Kuo, Y.W.; Lei, U. Hemostatic Enhancement via Chitosan Is Independent of Classical Clotting Pathways—A Quantitative Study. Polymers 2020, 12, 2391. [Google Scholar] [CrossRef]
- Li, J.H.; Zhuang, S.L. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Lampugnani, M.G.; Dejana, E.; Giampietro, C. Vascular Endothelial (VE)-Cadherin, Endothelial Adherens Junctions, and Vascular Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029322. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Malinova, T.S.; Angulo-Urarte, A.; Nuchel, J.; Tauber, M.; van der Stoel, M.M.; Janssen, V.; de Haan, A.; Groenen, A.G.; Tebbens, M.; Graupera, M.; et al. A junctional PACSIN2/EHD4/MICAL-L1 complex coordinates VE-cadherin trafficking for endothelial migration and angiogenesis. Nat. Commun. 2021, 12, 2610. [Google Scholar] [CrossRef]
- Tan, J.Y.; Li, L.; Wang, H.R.; Wei, L.; Gao, X.L.; Zeng, Z.; Liu, S.N.; Fan, Y.H.; Liu, T.; Chen, J.Y. Biofunctionalized fibrin gel co-embedded with BMSCs and VEGF for accelerating skin injury repair. Mater. Sci. Eng. C Mater. 2021, 121, 111749. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Tan, J.; Li, L.; Wang, H.; Liu, S.; Chen, J.; Weng, Y.; Liu, T. Chitosan/Alginate Hydrogel Dressing Loaded FGF/VE-Cadherin to Accelerate Full-Thickness Skin Regeneration and More Normal Skin Repairs. Int. J. Mol. Sci. 2022, 23, 1249. https://doi.org/10.3390/ijms23031249
Wei L, Tan J, Li L, Wang H, Liu S, Chen J, Weng Y, Liu T. Chitosan/Alginate Hydrogel Dressing Loaded FGF/VE-Cadherin to Accelerate Full-Thickness Skin Regeneration and More Normal Skin Repairs. International Journal of Molecular Sciences. 2022; 23(3):1249. https://doi.org/10.3390/ijms23031249
Chicago/Turabian StyleWei, Lai, Jianying Tan, Li Li, Huanran Wang, Sainan Liu, Junying Chen, Yajun Weng, and Tao Liu. 2022. "Chitosan/Alginate Hydrogel Dressing Loaded FGF/VE-Cadherin to Accelerate Full-Thickness Skin Regeneration and More Normal Skin Repairs" International Journal of Molecular Sciences 23, no. 3: 1249. https://doi.org/10.3390/ijms23031249
APA StyleWei, L., Tan, J., Li, L., Wang, H., Liu, S., Chen, J., Weng, Y., & Liu, T. (2022). Chitosan/Alginate Hydrogel Dressing Loaded FGF/VE-Cadherin to Accelerate Full-Thickness Skin Regeneration and More Normal Skin Repairs. International Journal of Molecular Sciences, 23(3), 1249. https://doi.org/10.3390/ijms23031249




