Zingerone Modulates Neuronal Voltage-Gated Na+ and L-Type Ca2+ Currents
Abstract
:1. Introduction
2. Results
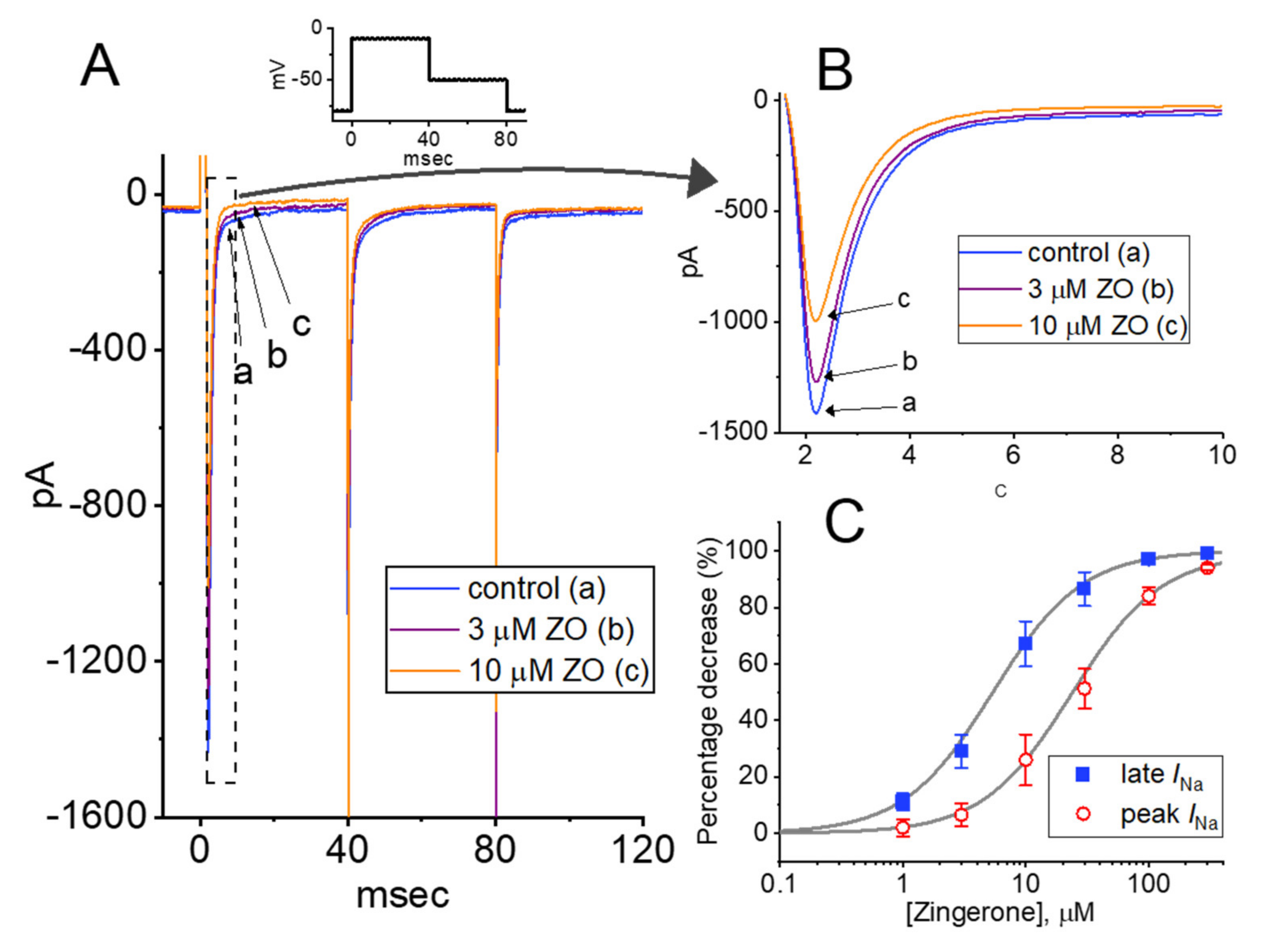
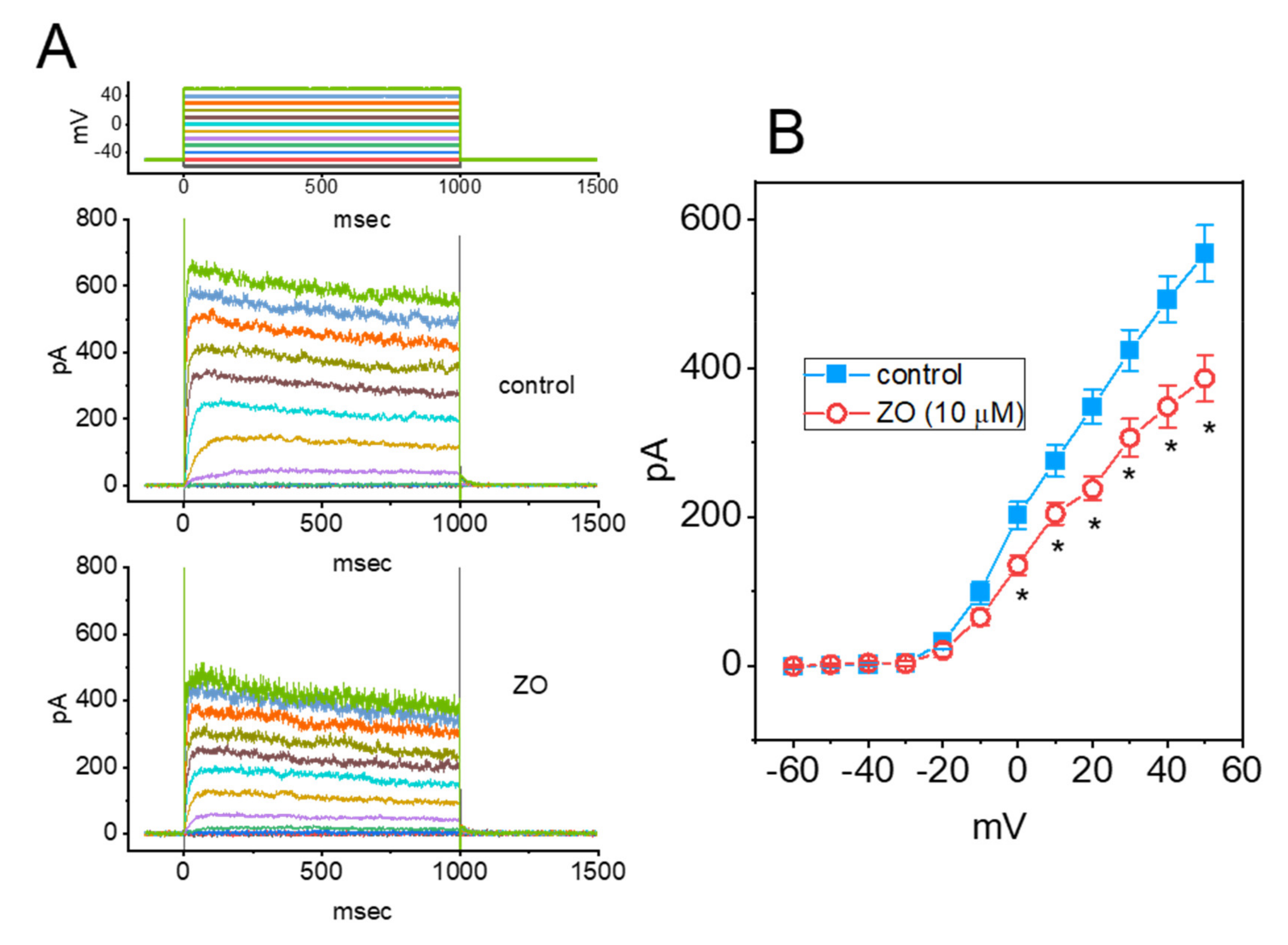
2.1. Effect of ZO on the Voltage-Gated Na+ Current (INa) Measured in GH3 Cells
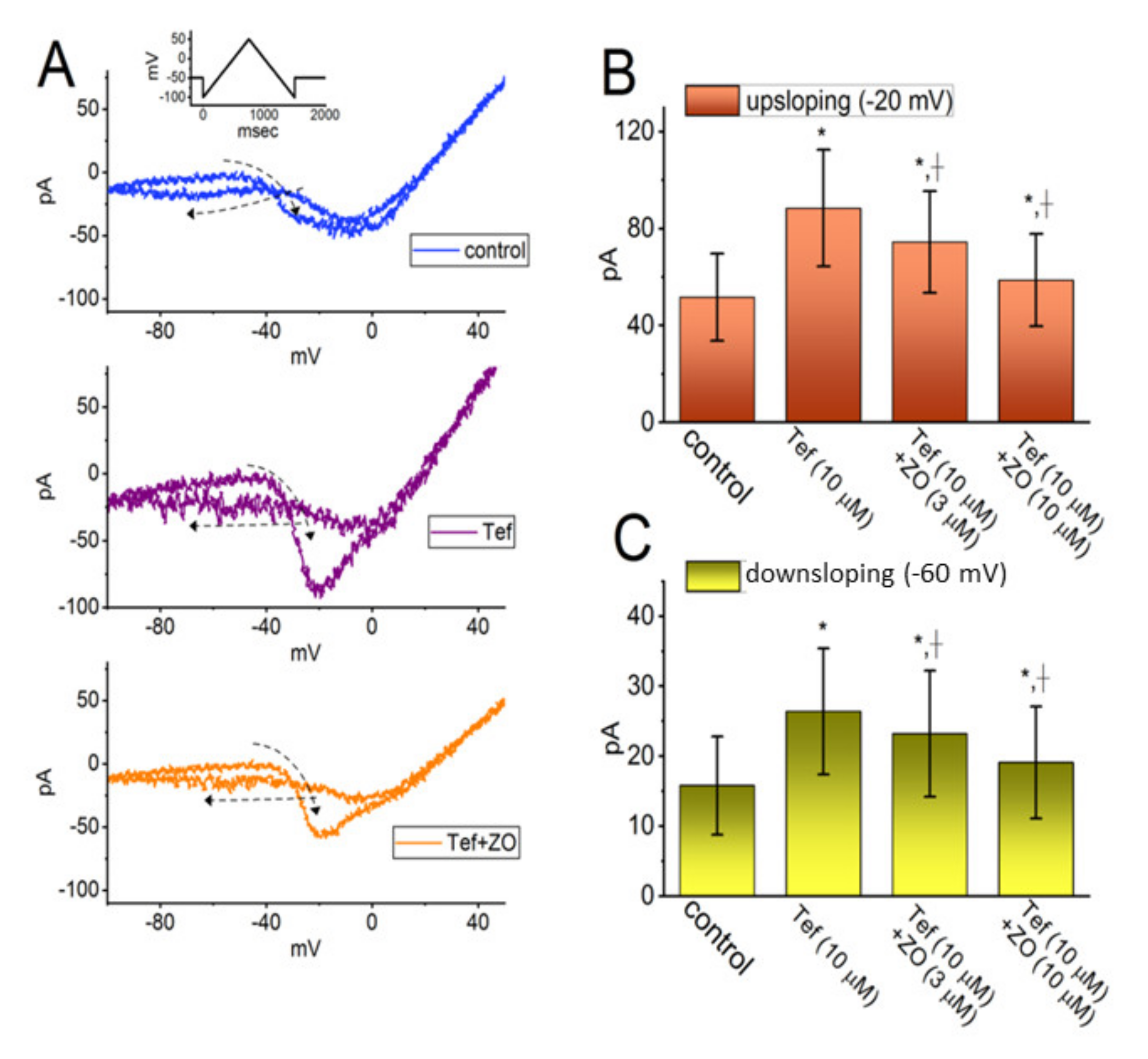
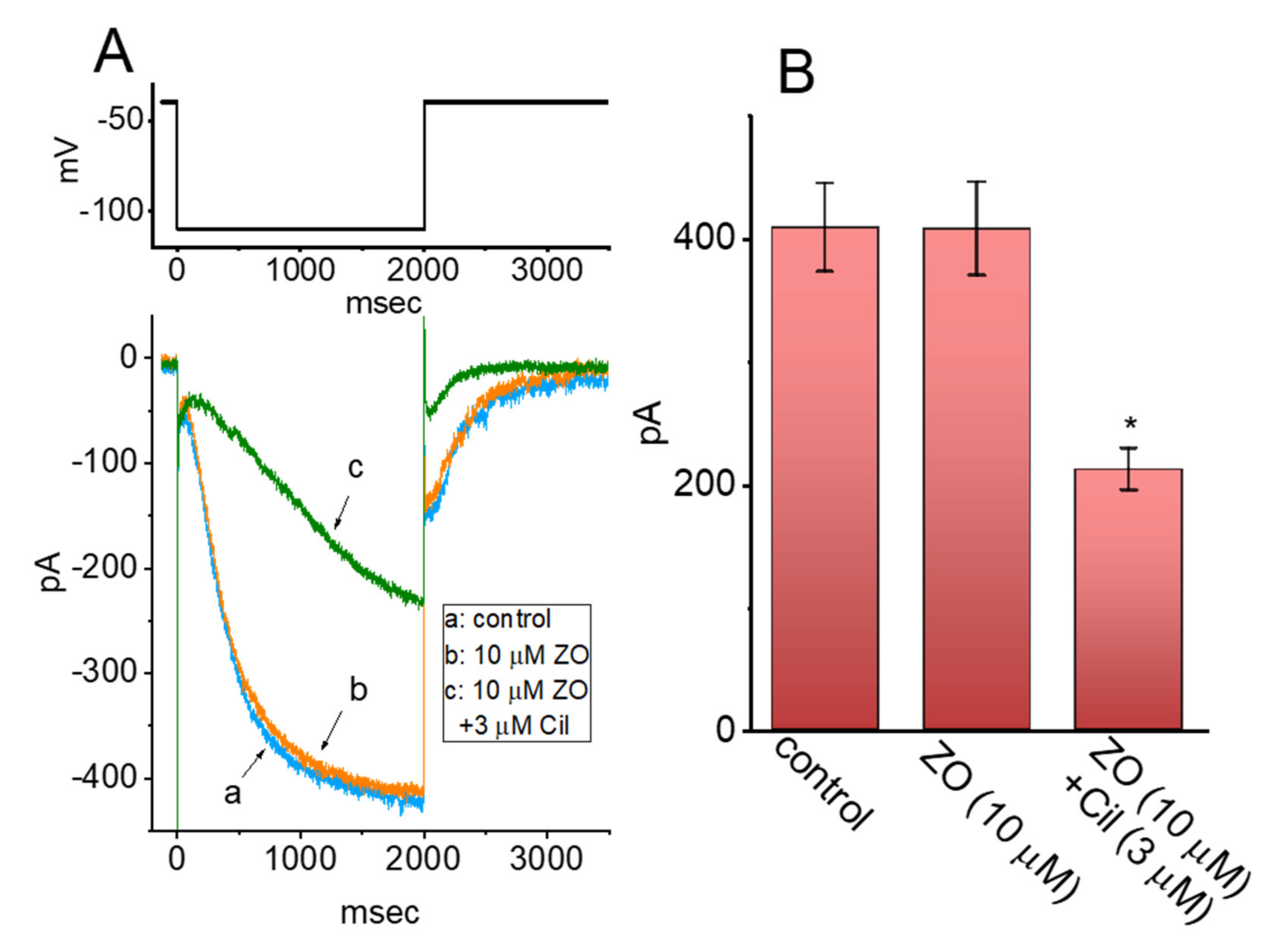
2.2. Enhanced Amplitude and Hysteresis by Tefluthrin (Tef) of Persistent Na+ Current (INa(P)) Reversed by the Addition of ZO
2.3. Effect of ZO on the L-Type Ca2+ Current (ICa,L) in GH3 Cells
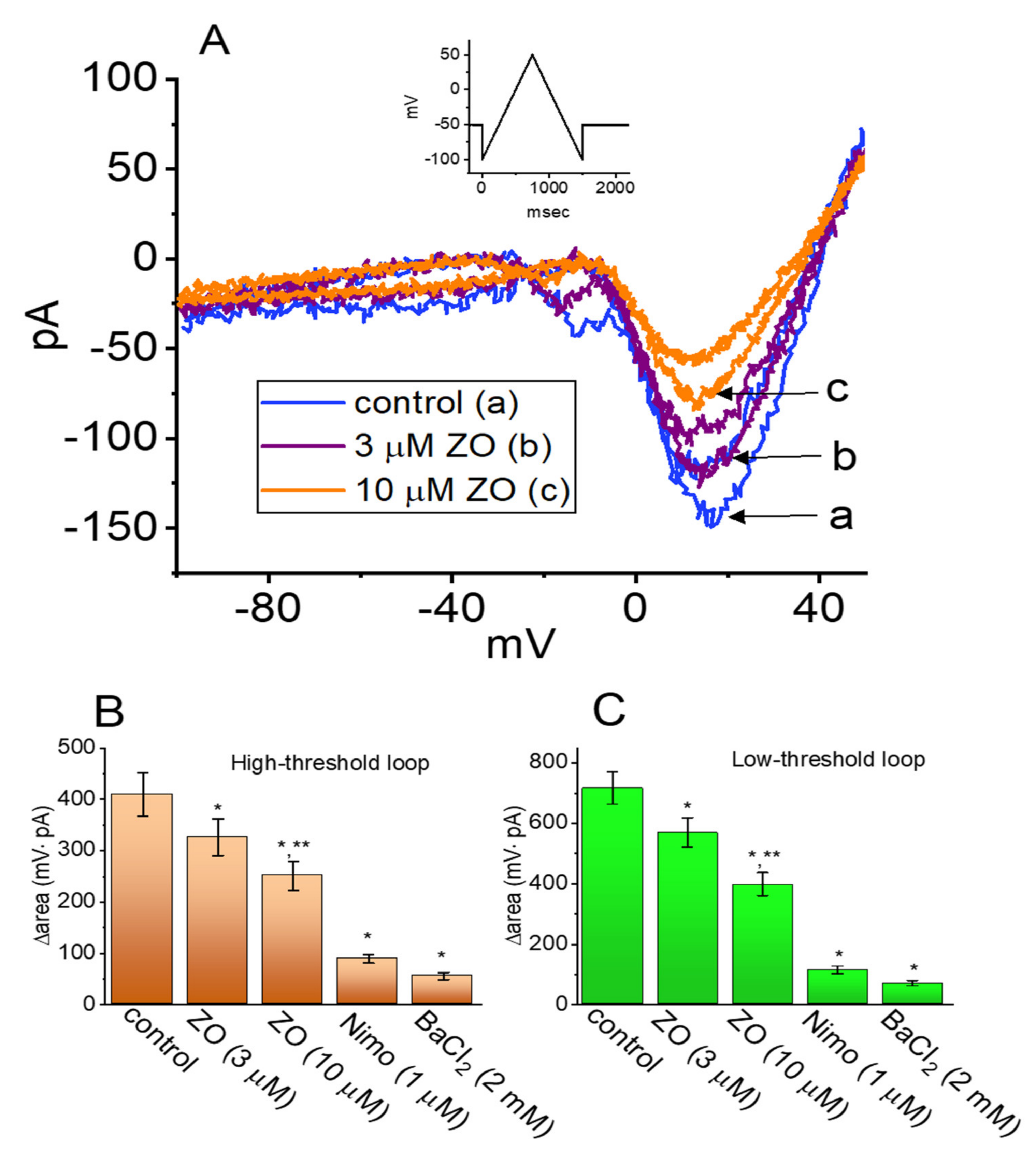
2.4. Biophysical Properties of Voltage-Dependent Hysteresis of ICa,L Activated by a Double Ramp Pulse
2.5. Effect of ZO, Nimodipine (Nimo), and BaCl2 Replacement on the Hysteresis of ICa,L
2.6. Limited Inhibition of ZO on the Hyperpolarization-Activated Cation Current (Ih) in GH3 Cells
2.7. Mild Inhibitory Effect of ZO on the Delayed-Rectifier K+ Current (IK(DR)) in GH3 Cells
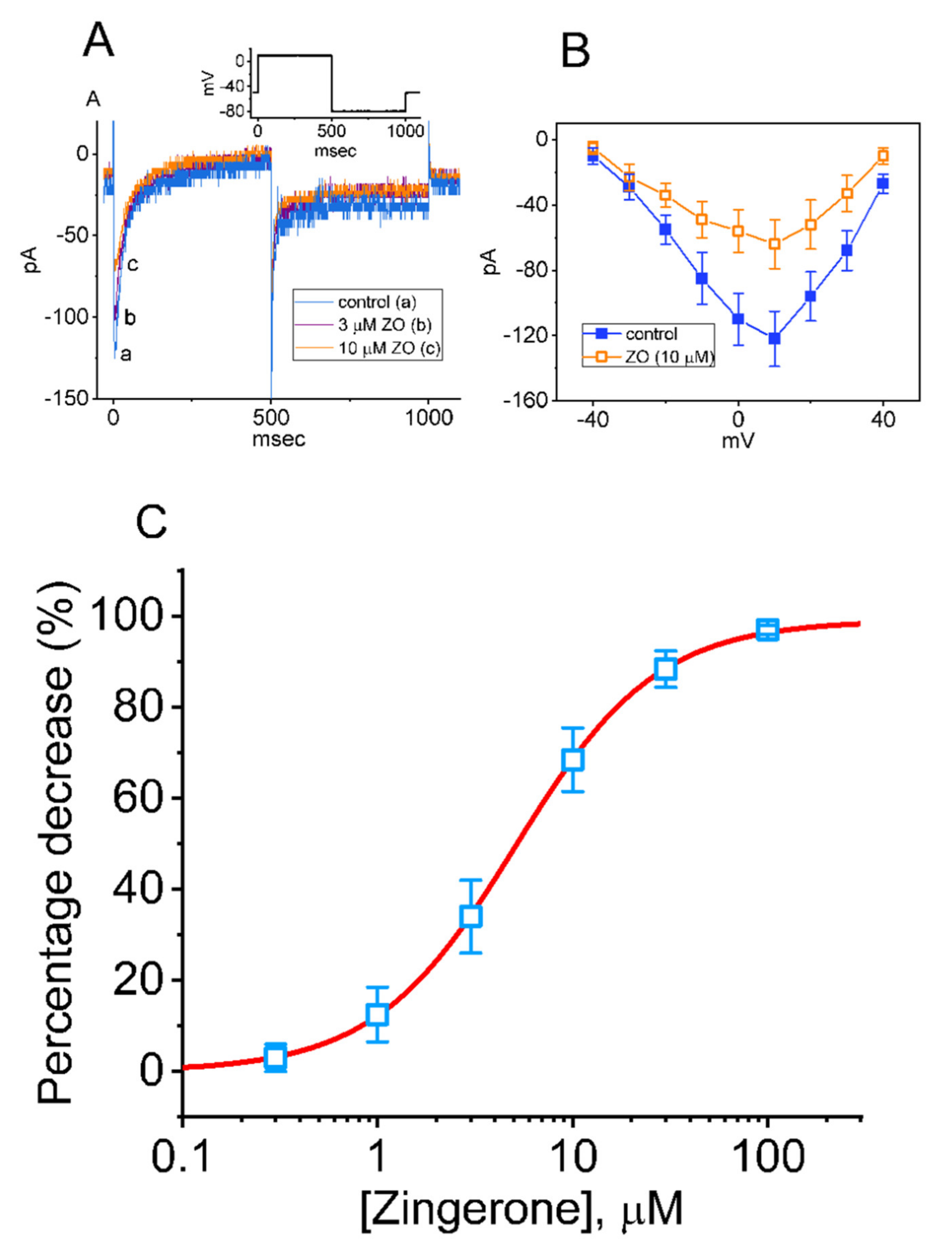
2.8. Effect of ZO on INa in Mouse Hippocampal (mHippoE-14) Neurons
3. Discussion
4. Materials and Methods
4.1. The Chemicals and Solutions Used in This Work
4.2. Cell Preparations
4.3. Electrophysiological Measurements with Data Recordings and Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, B.; Rehman, M.U.; Amin, I.; Arif, A.; Rasool, S.; Bhat, S.A.; Afzal, I.; Hussain, I.; Bilal, S.; Mir, M.U.R. A Review on Pharmacological Properties of Zingerone(4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). Sci. World J. 2015, 2015, 816364. [Google Scholar] [CrossRef] [Green Version]
- Alsherbiny, M.A.; Abd-Elsalam, W.H.; El-Badawy, S.A.; Taher, E.; Fares, M.; Torres, A.; Chang, D.; Li, C.G. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: A comprehensive review. Food Chem. Toxicol. 2019, 123, 72–97. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R. Nutritional implications of ginger: Chemistry, biological activities and signaling pathways. J. Nutr. Biochem. 2020, 86, 108486. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Roshan, S.; Hammad, S.; Pandohee, J.; Hu, X.; Zengin, G. Ginger and its active compounds in cancer therapy: From folk uses to nano therapeutic applications. Semin. Cancer. Biol. 2021, 69, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Wali, A.F.; Rashid, S.M.; Alsaffar, R.M.; Ahmad, A.; Jan, B.L.; Paray, B.A.; Alqahtani, S.M.A.; Arafah, A.; Rehman, M.U. Zingerone targets status epilepticus by blocking hippocampal neurodegeneration via regulation of redox imbalance, inflammation and apoptosis. Pharmaceuticals 2021, 14, 146. [Google Scholar] [CrossRef]
- Su, P.; Veeraraghavan, V.P.; Mohan, S.K.; Lu, W. A ginger derivative, zingerone-a phenolic compound-induces ROS-mediated apoptosis in colon cancer cells (HCT-116). J. Biochem. Mol. Toxicol. 2019, 33, e22403. [Google Scholar] [CrossRef]
- Lu, T.L.; Lu, T.J.; Wu, S.N. Inhibitory effective perturbation of cilobradine (DK-AH269), a blocker of HCN channels, on the amplitude and gating of both hyperpolarization-activated cation and delayed-rectified potassium currents. Int. J. Mol. Sci. 2020, 21, 2416. [Google Scholar] [CrossRef] [Green Version]
- Yue, H.Y.; Jiang, C.Y.; Fujita, T.; Kumamoto, E. Zingerone enhances glutamatergic spontaneous excitatory transmission by activating TRPA1 but not TRPV1 channels in the adult rat substantia gelatinosa. J. Neurophysiol. 2013, 110, 658–671. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Hong, C.S.; Lee, S.W.; Nam, J.H.; Kim, B.J. Effects of ginger and its pungent constituents on transient receptor potential channels. Int. J. Mol. Med. 2016, 38, 1905–1914. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.N.; Kim, H.J.; Kim, I.; Kim, Y.T.; Kim, B.J. The mechanism of action of zingerone in the pacemaker potentials of interstitial cells of Cajal isolated from murine small intestine. Cell Physiol. Biochem. 2018, 46, 2127–2137. [Google Scholar] [CrossRef] [Green Version]
- George, K.; Thomas, N.S.; Malathi, R. Modulatory Effect of Selected Dietary Phytochemicals on Delayed Rectifier K+ Current in Human Prostate Cancer Cells. J. Membr. Biol. 2019, 252, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Iwami, M.; Shiina, T.; Hirayama, H.; Shima, T.; Takewaki, T.; Shimizu, Y. Inhibitory effects of zingerone, a pungent component of Zingiber officinale Roscoe, on colonic motility in rats. J. Nat. Med. 2011, 65, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Iwami, M.; Shiina, T.; Hirayama, H.; Shimizu, Y. Intraluminal administration of zingerol, a non-pungent analogue of zingerone, inhibits colonic motility in rats. Biomed. Res. 2011, 32, 181–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakr, S.; Al-Amoudi, W.M. Effect of ginger extract on deltamethrin induced histomorphological and immunohistochemical changes in testes of albino rats. Life Sci. J. 2012, 9, 771–778. [Google Scholar]
- Al-Amoudi, W.M. Toxic effects of Lambda-cyhalothrin, on the rat thyroid: Involvement of oxidative stress and ameliorative effect of ginger extract. Toxicol. Rep. 2018, 5, 728–736. [Google Scholar] [CrossRef]
- Yousef, D.M.; El-Fatah, S.S.A.; Hegazy, A.A. Protective effect of ginger extract against alterations of rat thyroid structure induced by cypermethrin administration. J. Exp. Med. Biol. 2019, 1, 19–25. [Google Scholar]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 2010, 31, 845–915. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.C.; Lee, H.F.; Chi, C.S. SCN8A Encephalopathy: Case Report and Literature Review. Neurol. Int. 2021, 13, 14. [Google Scholar] [CrossRef]
- Huang, C.W.; Chow, J.C.; Tsai, J.J.; Wu, S.N. Characterizing the effects of Eugenol on neuronal ionic currents and hyperexcitability. Psychopharmacology 2012, 221, 575–587. [Google Scholar] [CrossRef]
- Wu, S.N.; Wu, Y.H.; Chen, B.S.; Lo, Y.C.; Liu, Y.C. Underlying mechanism of actions of tefluthrin, a pyrethroid insecticides, on voltage-gated ion currents and on action currents in pituitary tumor (GH3) cells and GnRH-secreting (GT1-7) neurons. Toxicology 2009, 258, 70–77. [Google Scholar] [CrossRef]
- So, E.C.; Wu, S.N.; Lo, Y.C.; Su, K. Differential regulation of tefluthrin and telmisartan on the gating charges of INa activation and inactivation as well as on resurgent and persistent INa in a pituitary cell line (GH3). Toxicol. Lett. 2018, 285, 104–112. [Google Scholar] [CrossRef]
- Wu, S.N.; Li, H.F.; Jan, C.R. Regulation of Ca2+-activated nonselective cationic currents in rat pituitary GH3 cells: Involvement in L-type Ca2+ current. Brain Res. 1999, 812, 133–141. [Google Scholar] [CrossRef]
- Lo, Y.K.; Wu, S.N.; Lee, C.T.; Li, H.F.; Chiang, H.T. Characterization of action potential waveform-evoked L-type calcium currents in pituitary GH3 cells. Pflugers Arch. 2001, 442, 547–557. [Google Scholar] [CrossRef]
- Wang, Y.J.; Lin, M.W.; Lin, A.A.; Peng, H.; Wu, S.N. Evidence for state-dependent block of DPI201-106, a synthetic inhibitor of Na+ channel inactivation, on delayed-rectifier K+ current in pituitary tumor (GH3) cells. J. Physiol. Pharmacol. 2008, 59, 409–423. [Google Scholar] [PubMed]
- So, E.C.; Foo, N.P.; Ko, S.Y.; Wu, S.N. Bisoprolol, Known to Be a Selective β1-Receptor Antagonist, Differentially but Directly Suppresses IK(M) and IK(erg) in Pituitary Cells and Hippocampal Neurons. Int. J. Mol. Sci. 2019, 20, 657. [Google Scholar] [CrossRef] [Green Version]
- Benarroch, E.E. HCN channels: Function and clinical implications. Neurology 2013, 80, 304–310. [Google Scholar] [CrossRef]
- Chang, W.T.; Wu, S.N. Characterization of Direct Perturbations on Voltage-Gated Sodium Current by Esaxerenone, a Nonsteroidal Mineralocorticoid Receptor Blocker. Biomedicines 2021, 9, 549. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Wu, S.N. Effectiveness of Columbianadin, a Bioactive Coumarin Derivative, in Perturbing Transient and Persistent INa. Int. J. Mol. Sci. 2021, 22, 621. [Google Scholar] [CrossRef]
- Villalba-Galea, C.A. Hysteresis in voltage-gated channels. Channels 2017, 11, 140–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Börjesson, S.I.; Elinder, F. Structure, function, and modification of the voltage sensor in voltage-gated ion channels. Cell. Biochem. Biophys. 2008, 52, 149–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.G.; Kim, S.Y.; Jeong, M.; Oh, M.S. Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacol. Ther. 2018, 182, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, C.M.; Bezanilla, F. Currents related to movement of the gating particles of the sodium channels. Nature 1973, 242, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Madhvani, R.V.; Angelini, M.; Xie, Y.; Pantazis, A.; Suriany, S.; Borgstrom, N.P.; Garfinkel, A.; Qu, Z.; Weiss, J.N.; Olcese, R. Targeting the late component of the cardiac L-type Ca2+ current to suppress early afterdepolarizations. J. Gen. Physiol. 2015, 145, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Zhu, Q.; Wang, Q.L.; Adu-Frimpong, M.; Wei, C.M.; Weng, W.; Bao, R.; Wang, Y.P.; Yu, J.N.; Xu, X.M. Improvement of Oral Bioavailability and Anti-Tumor Effect of Zingerone Self-Microemulsion Drug Delivery System. J. Pharm. Sci. 2021, 110, 2718–2727. [Google Scholar] [CrossRef]
- Wu, S.N.; Chang, H.D. Diethyl pyrocarbonate, a histidine-modifying agent, directly stimulates activity of ATP-sensitive potassium channels in pituitary GH3 cells. Biochem. Pharmacol. 2006, 71, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.Y.; Huang, C.W.; Wu, S.N. High ability of zileuton ((±)-1-(1-benzo[b]thien-2-ylethyl)-1-hydroxyurea) to stimulate IK(Ca) but suppress IK(DR) and IK(M). independently of 5-lipoxygenase inhibition. Eur. J. Pharmacol. 2020, 887, 173482. [Google Scholar] [CrossRef]
- Ortner, N.J.; Striessnig, J. L-type calcium channels as drug targets in CNS disorders. Channels 2016, 10, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.C.; Wu, S.N.; Huang, C.W. The specific effects of OD-1, a peptide activator, on voltage-gated sodium current and seizure susceptibility. Int. J. Mol. Sci. 2020, 21, 8254. [Google Scholar] [CrossRef]
- Simeone, K.A.; Sabesan, S.; Kim, D.Y.; Kerrigan, J.F.; Rho, J.M.; Simeone, T.A. L-Type calcium channel blockade reduces network activity in human epileptic hypothalamic hamartoma tissue. Epilepsia 2011, 52, 531–540. [Google Scholar] [CrossRef]
- Chang, W.T.; Liu, P.Y.; Gao, Z.H.; Lee, S.W.; Lee, W.K.; Wu, S.N. Evidence for the effectiveness of remdesivir (GS-5734), a nucleoside-analog antiviral drug in the inhibition of IK(M) or IK(DR) and in the stimulation of IMEP. Front. Pharmacol. 2020, 1091. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Wu, S.N.; Huang, C.W. Telmisartan, an antagonist of angiotensin II receptors, accentuates voltage-gated Na+ currents and hippocampal neuronal excitability. Front. Neurosci. 2020, 14, 902. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.Y.; Wu, S.N.; Huang, C.W. The Integrated Effects of Brivaracetam, a Selective Analog of Levetiracetam, on Ionic Currents and Neuronal Excitability. Biomedicines 2021, 9, 369. [Google Scholar] [CrossRef]
- Hung, T.Y.; Chu, F.L.; Wu, D.C.; Wu, S.N.; Huang, C.W. The protective role of peroxisome proliferator-activated receptor-gamma in seizure and neuronal excitotoxicity. Mol. Neurobiol. 2019, 56, 5497–5506. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Tzeng, R.C.; Huang, C.W.; Wu, S.N. The Novel Direct Modulatory Effects of Perampanel, an Antagonist of AMPA Receptors, on Voltage-Gated Sodium and M-type Potassium Currents. Biomolecules 2019, 9, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milton, R.L.; Caldwell, J.H. How do patch clamp seals form? A lipid bleb model. Pflugers Arch. 1990, 416, 758–762. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, M.-C.; Wu, S.-N.; Huang, C.-W. Zingerone Modulates Neuronal Voltage-Gated Na+ and L-Type Ca2+ Currents. Int. J. Mol. Sci. 2022, 23, 3123. https://doi.org/10.3390/ijms23063123
Lai M-C, Wu S-N, Huang C-W. Zingerone Modulates Neuronal Voltage-Gated Na+ and L-Type Ca2+ Currents. International Journal of Molecular Sciences. 2022; 23(6):3123. https://doi.org/10.3390/ijms23063123
Chicago/Turabian StyleLai, Ming-Chi, Sheng-Nan Wu, and Chin-Wei Huang. 2022. "Zingerone Modulates Neuronal Voltage-Gated Na+ and L-Type Ca2+ Currents" International Journal of Molecular Sciences 23, no. 6: 3123. https://doi.org/10.3390/ijms23063123
APA StyleLai, M.-C., Wu, S.-N., & Huang, C.-W. (2022). Zingerone Modulates Neuronal Voltage-Gated Na+ and L-Type Ca2+ Currents. International Journal of Molecular Sciences, 23(6), 3123. https://doi.org/10.3390/ijms23063123





