Galectin-3 in Kidney Diseases: From an Old Protein to a New Therapeutic Target
Abstract
1. Introduction
2. Gal-3: A Carbohydrate Binding Protein from the Lectin Family
2.1. The Lectin Family
2.2. Galectin-3
2.2.1. Structure
- The CRD is a polypeptide fold domain binding carbohydrates. Gal-3’s CRD interacts with various carbohydrate-containing proteins, activating different signaling pathways [19].
- A collagen-like sequence links CRD to N-link domain and is composed of nine collagen-like sequences (proline/glycine rich domain) cleavable by matrix metalloprotease [20].
2.2.2. Expression and Role
- Gal-3 is found in the plasma membrane of cells where it can modulate the interaction between epithelial cells and extracellular matrix or with other cells [28]. Its ability to bind with integrins or endothelial adhesive proteins allows adhesion with other cells or the promotion of activation of bond cells [29,30,31,32]. Gal-3 can also bind with glycoproteins to promote extra-cellular matrix binding [28,33] and create a bridge between Gal-3 and cells [34]. All these mechanisms promote a transduction cascade from membrane to intra-cellular pathways [22,35,36,37,38].
- Gal-3 is also secreted in plasma, or in organs in a soluble form, during specific damage, and acts as a damage-associated molecular pattern (DAMP) to promote an immunological response [39,40]. Many studies reported that Gal-3 plays a profibrotic role in kidneys and lungs via immune modulation of macrophage infiltration [41,42,43].
2.2.3. Galectin-3 Inhibitors
2.2.4. Galectin-3 in the Kidney
3. Gal-3 in Preclinical Models of Kidney Disease
3.1. Ischemia/Reperfusion
3.2. Toxic Injury
3.3. Glomerular Injury
3.4. Immune-Associated Renal Damage
3.4.1. Sepsis-Associated Renal Disease
3.4.2. Transplantation Model
3.5. Polycystic Model
3.6. Renal Fibrosis
3.7. Preclinical Model of Cardio-Renal Syndrome
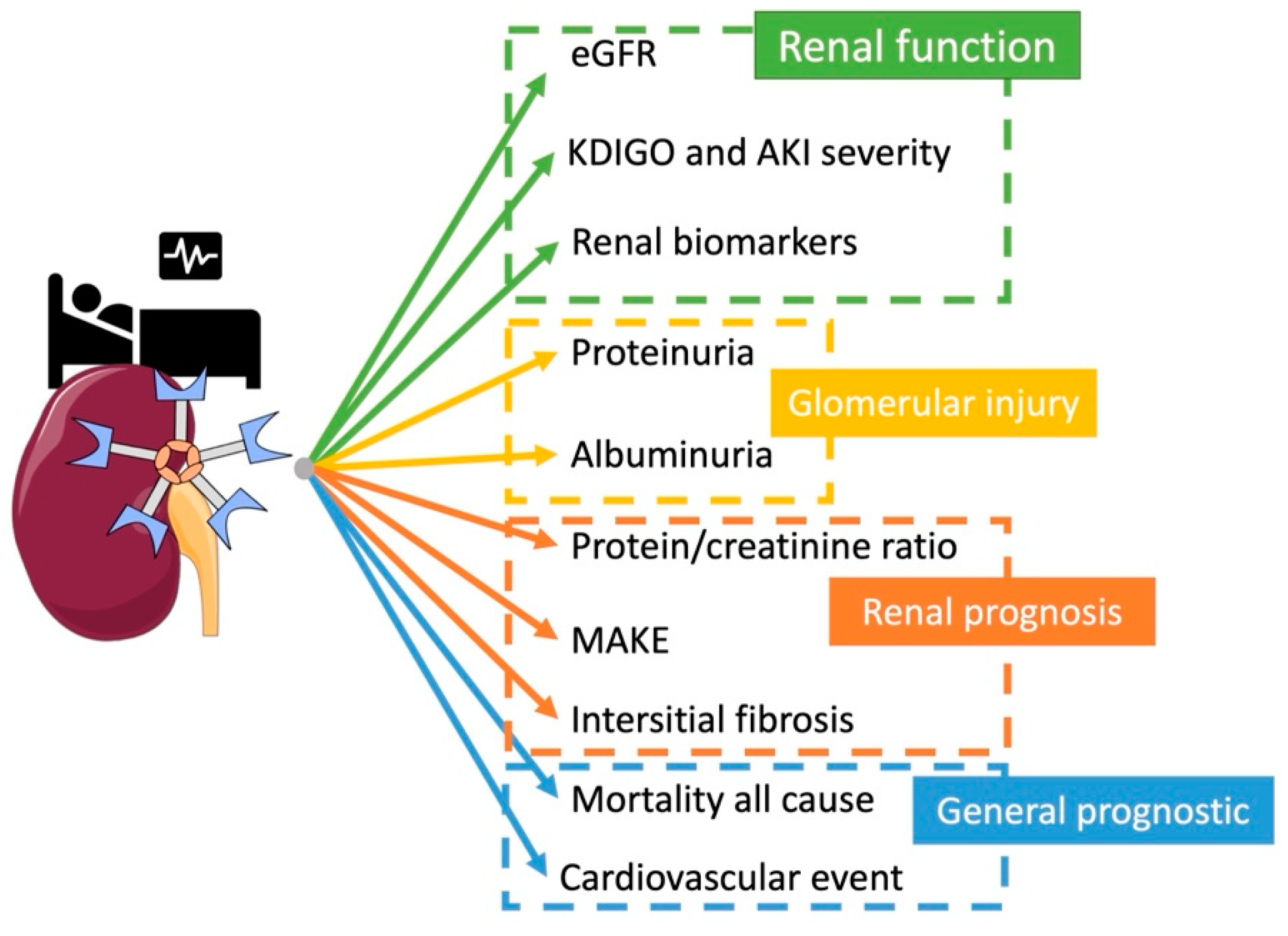
4. Gal-3 as a Biomarker
4.1. Kidney Function
4.2. Proteinuria
4.3. CKD and Renal Prognosis
4.4. Transplantation
4.5. Mortality and Poor Cardiovascular Outcome
5. Gal-3 as a Therapeutic Target and Perspective
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Slack, R.J.; Mills, R.; Mackinnon, A.C. The Therapeutic Potential of Galectin-3 Inhibition in Fibrotic Disease. Int. J. Biochem. Cell Biol. 2021, 130, 105881. [Google Scholar] [CrossRef] [PubMed]
- Besler, C.; Lang, D.; Urban, D.; Rommel, K.P.; von Roeder, M.; Fengler, K.; Blazek, S.; Kandolf, R.; Klingel, K.; Thiele, H.; et al. Plasma and Cardiac Galectin-3 in Patients with Heart Failure Reflects Both Inflammation and Fibrosis: Implications for Its Use as a Biomarker. Circ. Heart Fail. 2017, 10, e003804. [Google Scholar] [CrossRef] [PubMed]
- Van Vark, L.C.; Lesman-Leegte, I.; Baart, S.J.; Postmus, D.; Pinto, Y.M.; Orsel, J.G.; Daan Westenbrink, B.; Brunner-La Rocca, H.P.; van Miltenburg, A.J.M.; Boersma, E.; et al. Prognostic Value of Serial ST2 Measurements in Patients with Acute Heart Failure. J. Am. Coll. Cardiol. 2017, 70, 2378–2388. [Google Scholar] [CrossRef] [PubMed]
- Lakhtin, M.; Lakhtin, V.; Alyoshkin, V.; Afanasyev, S. Lectins of Beneficial Microbes: System Organisation, Functioning and Functional Superfamily. Benef. Microbes 2011, 2, 155–165. [Google Scholar] [CrossRef]
- De Schutter, K.; van Damme, E.J.M. Protein-Carbohydrate Interactions as Part of Plant Defense and Animal Immunity. Molecules 2015, 20, 9029–9053. [Google Scholar] [CrossRef]
- Feizi, T.E.N.; Haltiwanger, R.S. Editorial Overview: Carbohydrate-Protein Interactions and Glycosylation: Glycan Synthesis and Recognition: Finding the Perfect Partner in a Sugar-Coated Life. Curr. Opin. Struct. Biol. 2015, 34, 7–9. [Google Scholar] [CrossRef]
- Fortuna-Costa, A.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavão, M.S.G. Extracellular Galectin-3 in Tumor Progression and Metastasis. Front. Oncol. 2014, 4, 138. [Google Scholar] [CrossRef]
- Liu, F.T.; Rabinovich, G.A. Galectins: Regulators of Acute and Chronic Inflammation. Ann. N. Y. Acad. Sci. 2010, 1183, 158–182. [Google Scholar] [CrossRef]
- Di Lella, S.; Sundblad, V.; Cerliani, J.P.; Guardia, C.M.; Estrin, D.A.; Vasta, G.R.; Rabinovich, G.A. When Galectins Recognize Glycans: From Biochemistry to Physiology and Back Again. Biochemistry 2011, 50, 7842–7857. [Google Scholar] [CrossRef]
- Thiemann, S.; Baum, L.G. Galectins and Immune Responses-Just How Do They Do Those Things They Do? Annu. Rev. Immunol. 2016, 34, 243–264. [Google Scholar] [CrossRef]
- Arthur, C.M.; Baruffi, M.D.; Cummings, R.D.; Stowell, S.R. Evolving Mechanistic Insights into Galectin Functions. Methods Mol. Biol. 2015, 1207, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.K.; Springer, T.A. Mac-2, a Novel 32,000 Mr Mouse Macrophage Subpopulation-Specific Antigen Defined by Monoclonal Antibodies. J. Immunol. 1982, 128, 1221–1228. [Google Scholar] [PubMed]
- Hsu, D.K.; Zuberi, R.I.; Liu, F.T. Biochemical and Biophysical Characterization of Human Recombinant IgE-Binding Protein, an S-Type Animal Lectin. J. Biol. Chem. 1992, 267, 14167–14174. [Google Scholar] [CrossRef]
- Massa, S.M.; Cooper, D.N.W.; Leffler, H.; Barondes, S.H. L-29, an Endogenous Lectin, Binds to Glycoconjugate Ligands with Positive Cooperativity. Biochemistry 1993, 32, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Hughes, R.C. Binding Specificity of a Baby Hamster Kidney Lectin for H Type I and II Chains, Polylactosamine Glycans, and Appropriately Glycosylated Forms of Laminin and Fibronectin. J. Biol. Chem. 1992, 267, 6983–6990. [Google Scholar] [CrossRef]
- Sparrow, C.P.; Leffler, H.; Barondes, S.H. Multiple Soluble Beta-Galactoside-Binding Lectins from Human Lung. J. Biol. Chem. 1987, 262, 7383–7390. [Google Scholar] [CrossRef]
- Nieminen, J.; Kuno, A.; Hirabayashi, J.; Sato, S. Visualization of Galectin-3 Oligomerization on the Surface of Neutrophils and Endothelial Cells Using Fluorescence Resonance Energy Transfer. J. Biol. Chem. 2007, 282, 1374–1383. [Google Scholar] [CrossRef]
- Ahmad, N.; Gabius, H.J.; Sabesan, S.; Oscarson, S.; Brewer, C.F. Thermodynamic Binding Studies of Bivalent Oligosaccharides to Galectin-1, Galectin-3, and the Carbohydrate Recognition Domain of Galectin-3. Glycobiology 2004, 14, 817–825. [Google Scholar] [CrossRef]
- Karlsson, A.; Christenson, K.; Matlak, M.; Björstad, Å.; Brown, K.L.; Telemo, E.; Salomonsson, E.; Leffler, H.; Bylund, J. Galectin-3 Functions as an Opsonin and Enhances the Macrophage Clearance of Apoptotic Neutrophils. Glycobiology 2009, 19, 16–20. [Google Scholar] [CrossRef]
- Flores-Ibarra, A.; Vértesy, S.; Medrano, F.J.; Gabius, H.J.; Romero, A. Crystallization of a Human Galectin-3 Variant with Two Ordered Segments in the Shortened N-Terminal Tail. Sci. Rep. 2018, 8, 9835. [Google Scholar] [CrossRef]
- Huflejt, M.E.; Turck, C.W.; Lindstedt, R.; Barondes, S.H.; Leffler, H. L-29, a Soluble Lactose-Binding Lectin, Is Phosphorylated on Serine 6 and Serine 12 in Vivo and by Casein Kinase I. J. Biol. Chem. 1993, 268, 26712–26718. [Google Scholar] [CrossRef]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An Open-Ended Story. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, J.; Manninen, A.; Muller, D.J.; Helenius, J. Galectin-3 Regulates Integrin A2β1-Mediated Adhesion to Collagen-I and -IV. J. Biol. Chem. 2008, 283, 32264–32272. [Google Scholar] [CrossRef] [PubMed]
- Nio, J.; Takahashi-Iwanaga, H.; Morimatsu, M.; Kon, Y.; Iwanaga, T. Immunohistochemical and in Situ Hybridization Analysis of Galectin-3, a β-Galactoside Binding Lectin, in the Urinary System of Adult Mice. Histochem. Cell Biol. 2006, 126, 45–56. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.; Hyun, J.W.; Park, J.W.; Joo, H.; Shin, T. Expression and Immunohistochemical Localization of Galectin-3 in Various Mouse Tissues. Cell Biol. Int. 2007, 31, 655–662. [Google Scholar] [CrossRef]
- Nangia-Makker, P.; Nakahara, S.; Hogan, V.; Raz, A. Galectin-3 in Apoptosis, a Novel Therapeutic Target. J. Bioenerg. Biomembr. 2007, 39, 79. [Google Scholar] [CrossRef] [PubMed]
- Davidson, P.J.; Davis, M.J.; Patterson, R.J.; Ripoche, M.A.; Poirier, F.; Wang, J.L. Shuttling of Galectin-3 between the Nucleus and Cytoplasm. Glycobiology 2002, 12, 329–337. [Google Scholar] [CrossRef]
- Ochieng, J.; Furtak, V.; Lukyanov, P. Extracellular Functions of Galectin-3. Glycoconj. J. 2002, 19, 527–535. [Google Scholar] [CrossRef]
- Almkvist, J.; Karlsson, A. Galectins as Inflammatory Mediators. Glycoconj. J. 2002, 19, 575–581. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Baum, L.G.; Tinari, N.; Paganelli, R.; Natoli, C.; Liu, F.T.; Iacobelli, S. Galectins and Their Ligands: Amplifiers, Silencers or Tuners of the Inflammatory Response? Trends Immunol. 2002, 23, 313–320. [Google Scholar] [CrossRef]
- Fukushi, J.I.; Makagiansar, I.T.; Stallcup, W.B. NG2 Proteoglycan Promotes Endothelial Cell Motility and Angiogenesis via Engagement of Galectin-3 and A3β1 Integrin. Mol. Biol. Cell 2004, 15, 3580. [Google Scholar] [CrossRef] [PubMed]
- Sedlář, A.; Trávníčková, M.; Bojarová, P.; Vlachová, M.; Slámová, K.; Křen, V.; Bačáková, L. Interaction between Galectin-3 and Integrins Mediates Cell-Matrix Adhesion in Endothelial Cells and Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 5144. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.C. Galectins as Modulators of Cell Adhesion. Biochimie 2001, 83, 667–676. [Google Scholar] [CrossRef]
- Kuwabara, I.; Liu, F.T. Galectin-3 Promotes Adhesion of Human Neutrophils to Laminin. J. Immunol. 1996, 156, 3939–3944. [Google Scholar] [PubMed]
- Yang, R.Y.; Hsu, D.K.; Liu, F.T. Expression of Galectin-3 Modulates T-Cell Growth and Apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 6737. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, S.; Oka, N.; Raz, A. On the Role of Galectin-3 in Cancer Apoptosis. Apoptosis 2005, 10, 267–275. [Google Scholar] [CrossRef]
- Akahani, S.; Nangia-Makker, P.; Inohara, H.; Kim, H.-R.C.; Raz, A. Galectin-3: A Novel Antiapoptotic Molecule with A Functional BH1 (NWGR) Domain of Bcl-2 Family. Cancer Res. 1997, 57, 5272–5276. [Google Scholar]
- Sharma, U.C.; Pokharel, S.; van Brakel, T.J.; van Berlo, J.H.; Cleutjens, J.P.M.; Schroen, B.; André, S.; Crijns, H.J.G.M.; Gabius, H.J.; Maessen, J.; et al. Galectin-3 Marks Activated Macrophages in Failure-Prone Hypertrophied Hearts and Contributes to Cardiac Dysfunction. Circulation 2004, 110, 3121–3128. [Google Scholar] [CrossRef]
- Sato, S.; St-Pierre, C.; Bhaumik, P.; Nieminen, J. Galectins in Innate Immunity: Dual Functions of Host Soluble β-Galactoside-Binding Lectins as Damage-Associated Molecular Patterns (DAMPs) and as Receptors for Pathogen-Associated Molecular Patterns (PAMPs). Immunol. Rev. 2009, 230, 172–187. [Google Scholar] [CrossRef]
- Díaz-Alvarez, L.; Ortega, E. The Many Roles of Galectin-3, a Multifaceted Molecule, in Innate Immune Responses against Pathogens. Mediat. Inflamm. 2017, 2017, 9247574. [Google Scholar] [CrossRef]
- Jiang, J.X.; Chen, X.; Hsu, D.K.; Baghy, K.; Serizawa, N.; Scott, F.; Takada, Y.; Takada, Y.; Fukada, H.; Chen, J.; et al. Galectin-3 Modulates Phagocytosis-Induced Stellate Cell Activation and Liver Fibrosis in Vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G439. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; MacKinnon, A.; Marson, L.P.; Sethi, T. Tubular Atrophy and Interstitial Fibrosis after Renal Transplantation Is Dependent on Galectin-3. Transplantation 2012, 93, 477–484. [Google Scholar] [CrossRef] [PubMed]
- MacKinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of Transforming Growth Factor-Β1–Driven Lung Fibrosis by Galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537. [Google Scholar] [CrossRef]
- Stegmayr, J.; Zetterberg, F.; Carlsson, M.C.; Huang, X.; Sharma, G.; Kahl-Knutson, B.; Schambye, H.; Nilsson, U.J.; Oredsson, S.; Leffler, H. Extracellular and Intracellular Small-Molecule Galectin-3 Inhibitors. Sci. Rep. 2019, 9, 2186. [Google Scholar] [CrossRef] [PubMed]
- Glinsky, V.V.; Raz, A. Modified Citrus Pectin Anti-Metastatic Properties: One Bullet, Multiple Targets. Carbohydr. Res. 2009, 344, 1788. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhi, Y.; Sun, L.; Peng, X.; Zhang, T.; Xue, H.; Tai, G.; Zhou, Y. The Inhibitory Effects of a Rhamnogalacturonan Ι (RG-I) Domain from Ginseng Pectin on Galectin-3 and Its Structure-Activity Relationship. J. Biol. Chem. 2013, 288, 33953. [Google Scholar] [CrossRef]
- Cotter, F.; Smith, D.A.; Boyd, T.E.; Richards, D.A.; Alemany, C.; Loesch, D.; Salogub, G.; Tidmarsh, G.F.; Gammon, G.M.; Gribben, J. Single-Agent Activity of GCS-100, a First-in-Class Galectin-3 Antagonist, in Elderly Patients with Relapsed Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2009, 27, 7006. [Google Scholar] [CrossRef]
- Demotte, N.; Wieërs, G.; van der Smissen, P.; Moser, M.; Schmidt, C.; Thielemans, K.; Squifflet, J.L.; Weynand, B.; Carrasco, J.; Lurquin, C.; et al. A Galectin-3 Ligand Corrects the Impaired Function of Human CD4 and CD8 Tumor-Infiltrating Lymphocytes and Favors Tumor Rejection in Mice. Cancer Res. 2010, 70, 7476–7488. [Google Scholar] [CrossRef]
- Traber, P.G.; Zomer, E. Therapy of Experimental NASH and Fibrosis with Galectin Inhibitors. PLoS ONE 2013, 8, e83481. [Google Scholar] [CrossRef]
- Blanchard, H.; Yu, X.; Collins, P.M.; Bum-Erdene, K. Galectin-3 Inhibitors: A Patent Review (2008-Present). Expert Opin. Ther. Pat. 2014, 24, 1053–1065. [Google Scholar] [CrossRef]
- Cumpstey, I.; Salomonsson, E.; Sundin, A.; Leffler, H.; Nilsson, U.J. Double Affinity Amplification of Galectin–Ligand Interactions through Arginine–Arene Interactions: Synthetic, Thermodynamic, and Computational Studies with Aromatic Diamido Thiodigalactosides. Chem. A Eur. J. 2008, 14, 4233–4245. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Sanz, R.; Garcia-Fuentes, L.; Vargas-Berenguel, A. Human Galectin-3 Selective and High Affinity Inhibitors. Present State and Future Perspectives. Curr. Med. Chem. 2013, 20, 2979–2990. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.; Kumar, R.; Stenström, O.; Verma, P.; Verma, P.R.; Håkansson, M.; Kahl-Knutsson, B.; Zetterberg, F.; Leffler, H.; Akke, M.; et al. Systematic Tuning of Fluoro-Galectin-3 Interactions Provides Thiodigalactoside Derivatives with Single-Digit NM Affinity and High Selectivity. J. Med. Chem. 2018, 61, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Winyard, P.J.D.; Bao, Q.; Hughes, R.C.; Woolf, A.S. Epithelial Galectin-3 during Human Nephrogenesis and Childhood Cystic Diseases. J. Am. Soc. Nephrol. 1997, 8, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Hughes, R.C. Galectin-3 Expression and Effects on Cyst Enlargement and Tubulogenesis in Kidney Epithelial MDCK Cells Cultured in Three-Dimensional Matrices in Vitro. J. Cell Sci. 1995, 108, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Bichara, M.; Attmane-Elakeb, A.; Brown, D.; Essig, M.; Karim, Z.; Muffat-Joly, M.; Micheli, L.; Eude-Le Parco, I.; Cluzeaud, F.; Peuchmaur, M.; et al. Exploring the Role of Galectin 3 in Kidney Function: A Genetic Approach. Glycobiology 2006, 16, 36–45. [Google Scholar] [CrossRef]
- Herrmann, J.; Turck, C.W.; Atchison, R.E.; Huflejt, M.E.; Poulter, L.; Gitt, M.A.; Burlingame, A.L.; Barondes, S.H.; Leffler, H. Primary Structure of the Soluble Lactose Binding Lectin L-29 from Rat and Dog and Interaction of Its Non-Collagenous Proline, Glycine-, Tyrosine-Rich Sequence with Bacterial and Tissue Collagenase. J. Biol. Chem. 1993, 268, 26704–26711. [Google Scholar] [CrossRef]
- Meijers, W.C.; van der Velde, A.R.; Ruifrok, W.P.; Schroten, N.F.; Dokter, M.M.; Damman, K.; Assa, S.; Franssen, C.F.; Gansevoort, R.T.; van Gilst, W.H.; et al. Renal Handling of Galectin-3 in the General Population, Chronic Heart Failure, and Hemodialysis. J. Am. Heart Assoc. 2014, 3, e000962. [Google Scholar] [CrossRef]
- Nishiyama, J.; Kobayashi, S.; Ishida, A.; Nakabayashi, I.; Tajima, O.; Miura, S.; Katayama, M.; Nogami, H. Up-Regulation of Galectin-3 in Acute Renal Failure of the Rat. Am. J. Pathol. 2000, 157, 815. [Google Scholar] [CrossRef]
- Fernandes Bertocchi, A.P.; Campanhole, G.; Wang, P.H.M.; Gonçalves, G.M.; Damião, M.J.; Cenedeze, M.A.; Beraldo, F.C.; de Paula Antunes Teixeira, V.; dos Reis, M.A.; Mazzali, M.; et al. A Role for Galectin-3 in Renal Tissue Damage Triggered by Ischemia and Reperfusion Injury. Transpl. Int. 2008, 21, 999–1007. [Google Scholar] [CrossRef]
- Prud’homme, M.; Coutrot, M.; Michel, T.; Boutin, L.; Genest, M.; Poirier, F.; Launay, J.-M.; Kane, B.; Kinugasa, S.; Prakoura, N.; et al. Acute Kidney Injury Induces Remote Cardiac Damage and Dysfunction Through the Galectin-3 Pathway. JACC: Basic Transl. Sci. 2019, 4, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Vansthertem, D.; Cludts, S.; Nonclercq, D.; Gossiaux, A.; Saussez, S.; Legrand, A.; Gabius, H.J.; Toubeau, G. Immunohistochemical Localization of Galectins-1 and -3 and Monitoring of Tissue Galectin-Binding Sites during Tubular Regeneration after Renal Ischemia Reperfusion in the Rat. Histol. Histopathol. 2010, 25, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Kipari, T.; Haslett, C.; Iredale, J.P.; Liu, F.T.; Hughes, J.; Sethi, T. Galectin-3 Expression and Secretion Links Macrophages to the Promotion of Renal Fibrosis. Am. J. Pathol. 2008, 172, 288. [Google Scholar] [CrossRef] [PubMed]
- Kolatsi-Joannou, M.; Price, K.L.; Winyard, P.J.; Long, D.A. Modified Citrus Pectin Reduces Galectin-3 Expression and Disease Severity in Experimental Acute Kidney Injury. PLoS ONE 2011, 6, e18683. [Google Scholar] [CrossRef]
- Li, H.Y.; Yang, S.; Li, J.C.; Feng, J.X. Galectin 3 Inhibition Attenuates Renal Injury Progression in Cisplatin-Induced Nephrotoxicity. Biosci. Rep. 2018, 38, 20181803. [Google Scholar] [CrossRef]
- Markovic, B.S.; Jankovic, M.G.; Djokovic, B.; Jovicic, N.; Randall Harrell, C.; Fellabaum, C.; Djonov, V.; Arsenijevic, N.; Lukic, M.L.; Volarevic, V. Galectin 3 Protects from Cisplatin-Induced Acute Kidney Injury by Promoting TLR-2-Dependent Activation of IDO1/Kynurenine Pathway in Renal DCs. Theranostics 2019, 9, 5976–6001. [Google Scholar] [CrossRef]
- Pugliese, G.; Pricci, F.; Leto, G.; Amadio, L.; Iacobini, C.; Romeo, G.; Lenti, L.; Sale, P.; Gradini, R.; Liu, F.T.; et al. The Diabetic Milieu Modulates the Advanced Glycation End Product-Receptor Complex in the Mesangium by Inducing or Upregulating Galectin-3 Expression. Diabetes 2000, 49, 1249–1257. [Google Scholar] [CrossRef][Green Version]
- Pugliese, G.; Pricci, F.; Iacobini, C.; Leto, G.; Amadio, L.; Barsotti, P.; Frigeri, L.; Hsu, D.K.; Vlasara, H.; Liu, F.-T.; et al. Accelerated Diabetic Glomerulopathy in Galectin-3/AGE Receptor 3 Knockout Mice. FASEB J. 2001, 15, 2471–2479. [Google Scholar] [CrossRef]
- Iacobini, C.; Menini, S.; Oddi, G.; Ricci, C.; Amadio, L.; Pricci, F.; Olivieri, A.; Sorcini, M.; Di Mario, U.; Pesce, C.; et al. Galectin-3/AGE-Receptor 3 Knockout Mice Show Accelerated AGE-Induced Glomerular Injury: Evidence for a Protective Role of Galectin-3 as an AGE Receptor. FASEB J. 2004, 18, 1773–1775. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Peng, R.; Chen, W.; Fu, X.; Zhang, L.; Peng, H.; Zhang, Z. Long Non-Coding RNA Rpph1 Promotes Inflammation and Proliferation of Mesangial Cells in Diabetic Nephropathy via an Interaction with Gal-3. Cell Death Dis. 2019, 10, 526. [Google Scholar] [CrossRef]
- Frenay, A.R.S.; Yu, L.; van der Velde, A.R.; Vreeswijk-Baudoin, I.; López-Andrés, N.; van Goor, H.; Silljé, H.H.; Ruifrok, W.P.; de Boer, R.A. Pharmacological Inhibition of Galectin-3 Protects against Hypertensive Nephropathy. Am. J. Physiol. Ren. Physiol. 2015, 308, F500–F509. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Bao, Q.; Hughes, R.C. Galectin-3 Modulates Rat Mesangial Cell Proliferation and Matrix Synthesis during Experimental Glomerulonephritis Induced by Anti-Thy1.1 Antibodies. J. Pathol. 1999, 187, 481–489. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, H.; Eliaz, A.; Kellum, J.A.; Peng, Z.; Eliaz, I. Galectin-3 in Septic Acute Kidney Injury: A Translational Study. Crit. Care 2021, 25, 109. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.G.; Johnson, T.M.; Woolf, A.S.; Dahm-Vicker, E.M.; Long, D.A.; Guay-Woodford, L.; Hillman, K.A.; Bawumia, S.; Venner, K.; Hughes, R.C.; et al. Galectin-3 Associates with the Primary Cilium and Modulates Cyst Growth in Congenital Polycystic Kidney Disease. Am. J. Pathol. 2006, 169, 1925. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, V.; Desmedt, S.; Delanghe, J.R.; Speeckaert, R.; Speeckaert, M.M. Galectin-3 in Renal Pathology: More Than Just an Innocent Bystander? Am. J. Nephrol. 2016, 43, 305–317. [Google Scholar] [CrossRef]
- Nishi, Y.; Sano, H.; Kawashima, T.; Okada, T.; Kuroda, T.; Kikkawa, K.; Kawashima, S.; Tanabe, M.; Goto, T.; Matsuzawa, Y.; et al. Role of Galectin-3 in Human Pulmonary Fibrosis. Allergol. Int. 2007, 56, 57–65. [Google Scholar] [CrossRef]
- Okamura, D.M.; Pasichnyk, K.; Lopez-Guisa, J.M.; Collins, S.; Hsu, D.K.; Liu, F.T.; Eddy, A.A. Galectin-3 Preserves Renal Tubules and Modulates Extracellular Matrix Remodeling in Progressive Fibrosis. Am. J. Physiol. Ren. Physiol. 2011, 300, 245–253. [Google Scholar] [CrossRef]
- Gasparitsch, M.; Arndt, A.-K.; Pawlitschek, F.; Oberle, S.; Keller, U.; Kasper, M.; Bierhaus, A.; Schaefer, F.; Weber, L.T.; Lange-Sperandio, B. RAGE-Mediated Interstitial Fibrosis in Neonatal Obstructive Nephropathy Is Independent of NF-JB Activation. Kidney Int. 2013, 84, 911–919. [Google Scholar] [CrossRef]
- Martinez-Martinez, E.; Ibarrola, J.; Calvier, L.; Fernandez-Celis, A.; Leroy, C.; Cachofeiro, V.; Rossignol, P.; Lopez-Andres, N. Galectin-3 Blockade Reduces Renal Fibrosis in Two Normotensive Experimental Models of Renal Damage. PLoS ONE 2016, 11, e0166272. [Google Scholar] [CrossRef]
- Boutin, L.; Dépret, F.; Samuel, J.L.; Legrand, M.; Mebazaa, A.; Gayat, E.; Chadjichristos, C. Impact of Galectin-3 Tissue Deletion in Renal Damage and Type-3 Cardio-Renal Syndrome. Néphrologie Thérapeutique 2021, 17, 284. [Google Scholar] [CrossRef]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Drechsler, C.; Delgado, G.; Wanner, C.; Blouin, K.; Pilz, S.; Tomaschitz, A.; Kleber, M.E.; Dressel, A.; Willmes, C.; Krane, V.; et al. Galectin-3, Renal Function, and Clinical Outcomes: Results from the Luric and 4D Studies. J. Am. Soc. Nephrol. 2015, 26, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Wyler Von Ballmoos, M.; Likosky, D.S.; Rezaee, M.; Lobdell, K.; Alam, S.; Parker, D.; Owens, S.; Thiessen-Philbrook, H.; MacKenzie, T.; Brown, J.R. Elevated Preoperative Galectin-3 Is Associated with Acute Kidney Injury after Cardiac Surgery. BMC Nephrol. 2018, 19, 280. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.C.B.; Cheung, C.L.; Lee, A.C.H.; Lam, J.K.Y.; Wong, Y.; Shiu, S.W.M. Galectin-3 Is Independently Associated with Progression of Nephropathy in Type 2 Diabetes Mellitus. Diabetologia 2018, 61, 1212–1219. [Google Scholar] [CrossRef]
- Boutin, L.; Legrand, M.; Sadoune, M.; Mebazaa, A.; Gayat, E.; Chadjichristos, C.E.; Dépret, F. Elevated Plasma Galectin-3 Is Associated with Major Adverse Kidney Events and Death after ICU Admission. Crit. Care 2022, 26, 13. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Kobayashi, S.; Hemmi, N.; Ikee, R.; Hyodo, N.; Saigusa, T.; Namikoshi, T.; Yamada, M.; Suzuki, S.; Miura, S. Galectin-3-Positive Cell Infiltration in Human Diabetic Nephropathy. Nephrol. Dial. Transplant. 2004, 19, 602–607. [Google Scholar] [CrossRef]
- Kang, E.H.; Moon, K.C.; Lee, E.Y.; Lee, Y.J.; Lee, E.B.; Ahn, C.; Song, Y.W. Renal Expression of Galectin-3 in Systemic Lupus Erythematosus Patients with Nephritis. Lupus 2009, 18, 22–28. [Google Scholar] [CrossRef]
- Ostalska-Nowicka, D.; Nowicki, M.; Kondraciuk, B.; Partyka, M.; Samulak, D.; Witt, M. Expression of Galectin-3 in Nephrotic Syndrome Glomerulopaties in Children. Folia Histochem. Cytobiol. 2009, 47, 315–322. [Google Scholar] [CrossRef][Green Version]
- Yilmaz, H.; Inan, O.; Darcin, T.; Bilgic, M.A.; Akcay, A. Serum Galectin-3 Levels Were Associated with Proteinuria in Patients with Familial Mediterranean Fever. Clin. Exp. Nephrol. 2015, 19, 436–442. [Google Scholar] [CrossRef]
- Hussain, S.; Habib, A.; Hussain, M.S.; Najmi, A.K. Potential Biomarkers for Early Detection of Diabetic Kidney Disease. Diabetes Res. Clin. Pract. 2020, 161, 108082. [Google Scholar] [CrossRef]
- Alam, M.L.; Katz, R.; Bellovich, K.A.; Bhat, Z.Y.; Brosius, F.C.; de Boer, I.H.; Gadegbeku, C.A.; Gipson, D.S.; Hawkins, J.J.; Himmelfarb, J.; et al. Soluble ST2 and Galectin-3 and Progression of CKD. Kidney Int. Rep. 2019, 4, 103. [Google Scholar] [CrossRef] [PubMed]
- Rebholz, C.M.; Selvin, E.; Liang, M.; Ballantyne, C.M.; Hoogeveen, R.C.; Aguilar, D.; McEvoy, J.W.; Grams, M.E.; Coresh, J. Plasma Galectin-3 Levels Are Associated with the Risk of Incident Chronic Kidney Disease. Kidney Int. 2018, 93, 252. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.J.; Ro, H.; Kim, H.; Chang, J.H.; Lee, H.H.; Chung, W.; Jung, J.Y. Soluble ST2 and Galectin-3 as Predictors of Chronic Kidney Disease Progression and Outcomes. Am. J. Nephrol. 2021, 52, 119–130. [Google Scholar] [CrossRef]
- Ou, S.M.; Tsai, M.T.; Chen, H.Y.; Li, F.A.; Tseng, W.C.; Lee, K.H.; Chang, F.P.; Lin, Y.P.; Yang, R.B.; Tarng, D.C. Identification of Galectin-3 as Potential Biomarkers for Renal Fibrosis by RNA-Sequencing and Clinicopathologic Findings of Kidney Biopsy. Front. Med. 2021, 8, 2123. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor, C.G.; Velde-Keyzer, C.A.T.; Diepstra, A.; van Londen, M.; Pol, R.A.; Post, A.; Gans, R.O.B.; Nolte, I.M.; Slart, R.H.J.A.; de Borst, M.H.; et al. Galectin-3 and Risk of Late Graft Failure in Kidney Transplant Recipients: A 10-Year Prospective Cohort Study. Transplantation 2021, 105, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Hogas, S.; Schiller, A.; Voroneanu, L.; Constantinescu, D.; Timar, R.; Cianga, P.; Siriopol, D.; Bob, F.; Cianga, C.; Onofriescu, M.; et al. Predictive Value for Galectin 3 and Cardiotrophin 1 in Hemodialysis Patients. Angiology 2016, 67, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wan, Y.; Chata, R.; Brazzale, A.; Atherton, J.J.; Kostner, K.; Dimeski, G.; Punyadeera, C. A Pilot Study to Demonstrate Diagnostic Potential of Galectin-3 Levels in Saliva. J. Clin. Pathol. 2016, 69, 1100–1104. [Google Scholar] [CrossRef]
- Hara, A.; Niwa, M.; Kanayama, T.; Noguchi, K.; Niwa, A.; Matsuo, M.; Kuroda, T.; Hatano, Y.; Okada, H.; Tomita, H. Galectin-3: A Potential Prognostic and Diagnostic Marker for Heart Disease and Detection of Early Stage Pathology. Biomolecules 2020, 10, 1277. [Google Scholar] [CrossRef]
- A Phase 2a Study of Weekly Doses of GCS-100 in Patients with Chronic Kidney Disease—Full Text View—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01843790 (accessed on 19 January 2022).
- La Jolla Pharmaceutical Company Reports Positive, Top-Line Results from Phase 2 Clinical Trial of GCS-100 in Chronic Kidney Disease. Press Release Data Mar 10. Available online: https://www.sec.gov/Archives/edgar/data/920465/000092046514000012/pressreleasedatamar10.htm (accessed on 25 January 2022).
- Lau, E.S.; Liu, E.; Paniagua, S.M.; Sarma, A.A.; Zampierollo, G.; López, B.; Díez, J.; Wang, T.J.; Ho, J.E. Galectin-3 Inhibition with Modified Citrus Pectin in Hypertension. JACC Basic Transl. Sci. 2021, 6, 12. [Google Scholar] [CrossRef]
- Hirani, N.; MacKinnon, A.C.; Nicol, L.; Ford, P.; Schambye, H.; Pedersen, A.; Nilsson, U.J.; Leffler, H.; Sethi, T.; Tantawi, S.; et al. Target-Inhibition of Galectin-3 by Inhaled TD139 in Patients with Idiopathic Pulmonary Fibrosis. Eur. Respir. J. 2020, 57, 2002559. [Google Scholar] [CrossRef]
- Friedman, L.A.; Ring, K.L.; Mills, A.M. LAG-3 and GAL-3 in Endometrial Carcinoma: Emerging Candidates for Immunotherapy. Int. J. Gynecol. Pathol. 2020, 39, 203–212. [Google Scholar] [CrossRef] [PubMed]



| Structure | Pharmacokinetic and Pharmacodynamics | Clinical Evidence | Administrative | References | |
| CRD Based Multivalent | |||||
| Modified citrus pectin (MCP) | Polypeptide formed with anhydro-galacturonic acid and galactose with shorter carbohydrate chains modified by pH and temperature | Gal-3 antagonist, soluble protein binding with Gal-3 CRD. Kd: 143 nM | Cancer: Phase II, single-center, open label, trial evaluating the safety and efficacy of MCP on PSA kinetics in prostate cancer (NCT01681823) Cardiac fibrosis: Phase III, randomized study, single-center trial evaluating the efficacy of MCP treatment to reduce cardiac fibrosis in patients with hypertension. (NCT01960946) | Approved by FDA, from Econugenics (Santa Rosa, CA, USA) | [45,46] |
| GBC590/GCS100 | A combination of purified MCP (polymerized) | Gal-3 antagonist, soluble protein binding with CRD Unknown Kd. | Renal disease: -Phase I, open label study, evaluated the security of weekly doses of GCS-100 in patients with chronic kidney disease. (NCT01717248) -Phase IIa, placebo-controlled, randomized, single-blind study evaluated of weekly doses of GCS-100 in patients with chronic kidney disease and eGFR change. (NCT01843790) Cancer: -Phase II trials evaluated the reduction of metastasis and stabilized colorectal carcinomas during outcompeting Gal-3 in binding to its receptors. (NCT00110721) | Approver by FDA from La Jolla Pharmaceuticals (San Diego, CA, USA) | [47,48] |
| RG 1–4 | Polypeptide formed with rhamnogalacturonan I (RG-I)-rich fragment | Link to Gal-3 CRD and limit Gal-3 oigomerization. Kd: 22 nM | No clinical data | No FDA approved, from Galectin Therapeutics (Norcross, GA, USA) | [46] |
| Davanat and Belapectin | A natural galactomannan polysaccharide | Multivalent binding with Gal-3 CRD Kd of Davanatt: 200–300 µM Kd of Belapectin: 2.9 µM | Liver fibrosis: Phases I, II, and III study in a multi-center, study, to evaluate the safety and pharmacokinetic of modified Davanat in subjects with non-alcoholic steatohepatitis (NASH) with advanced hepatic fibrosis to improve portal hypertension and oesophagial varice (NCT02462967, NCT04365868) Cancer: Phase Ib study of a galectin inhibitor (GR-MD-02) and ipilimumab in patients with metastatic melanoma (NCT02117362) | FDA approved, from Galectin Therapeutics (Norcross, GA, USA) | [44,49,50] |
| Small-molecule carbohydrate-based inhibitors | |||||
| Lactose/LacNac or modified LacNac | N-Aceetyl-D-lactosamine / or modified with a Arg144 guanidino group for modified LacNac | Natural ligant of Gal-3 with rich Galactomannan domain. Modified LacNac Kd: 320 nM | No clinical study and clinical implication as their affinity for Gal-3 are weak. | No FDA approval as a drug | [50,51] |
| Small size synthetic monovalent inhibitor | Thiodigalactoside scaffold (TD139/ GB0139 and GB1211) | Specific binding monovalent to subsite C and D of Gal-3 CRD GB0139 Kd: 2.3 nM GB1211 Kd: 30–55 nM | Pulmonary fibrosis: -Phase IIb, randomized, double-blind, multicenter, parallel, placebo-controlled study in subjects with idiopathic pulmonary fibrosis (IPF) investigating the efficacy and safety of GB0139. (NCT03832946) -Phase IIb, randomized controlled trial patient with HIV investigating the safety, tolerability and pharmacokinetic of TD139. (NCT02257177) -Phase IIb, single center, open label to assess the safety, tolerability and pharmacokinetics of GB1211 in participants with hepatic Impairment (Child Pugh B & C). (NCT05009680) | GB0139: FDA approved from Galecto inc (Ole Maaloes, Copenhagen, Denmark) GB1211: FDA approved from Galecto inc (Ole Maaloes, Copenhagen, Denmark) | [50,52,53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutin, L.; Dépret, F.; Gayat, E.; Legrand, M.; Chadjichristos, C.E. Galectin-3 in Kidney Diseases: From an Old Protein to a New Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 3124. https://doi.org/10.3390/ijms23063124
Boutin L, Dépret F, Gayat E, Legrand M, Chadjichristos CE. Galectin-3 in Kidney Diseases: From an Old Protein to a New Therapeutic Target. International Journal of Molecular Sciences. 2022; 23(6):3124. https://doi.org/10.3390/ijms23063124
Chicago/Turabian StyleBoutin, Louis, François Dépret, Etienne Gayat, Matthieu Legrand, and Christos E. Chadjichristos. 2022. "Galectin-3 in Kidney Diseases: From an Old Protein to a New Therapeutic Target" International Journal of Molecular Sciences 23, no. 6: 3124. https://doi.org/10.3390/ijms23063124
APA StyleBoutin, L., Dépret, F., Gayat, E., Legrand, M., & Chadjichristos, C. E. (2022). Galectin-3 in Kidney Diseases: From an Old Protein to a New Therapeutic Target. International Journal of Molecular Sciences, 23(6), 3124. https://doi.org/10.3390/ijms23063124






