The Spectral Compositions of Light Changes Physiological Response of Chinese Cabbage to Elevated Ozone Concentration
Abstract
:1. Introduction
2. Results
2.1. Morphological Changes
2.2. Chlorophyll a Fluorescence
2.3. Gas Exchange Measurements
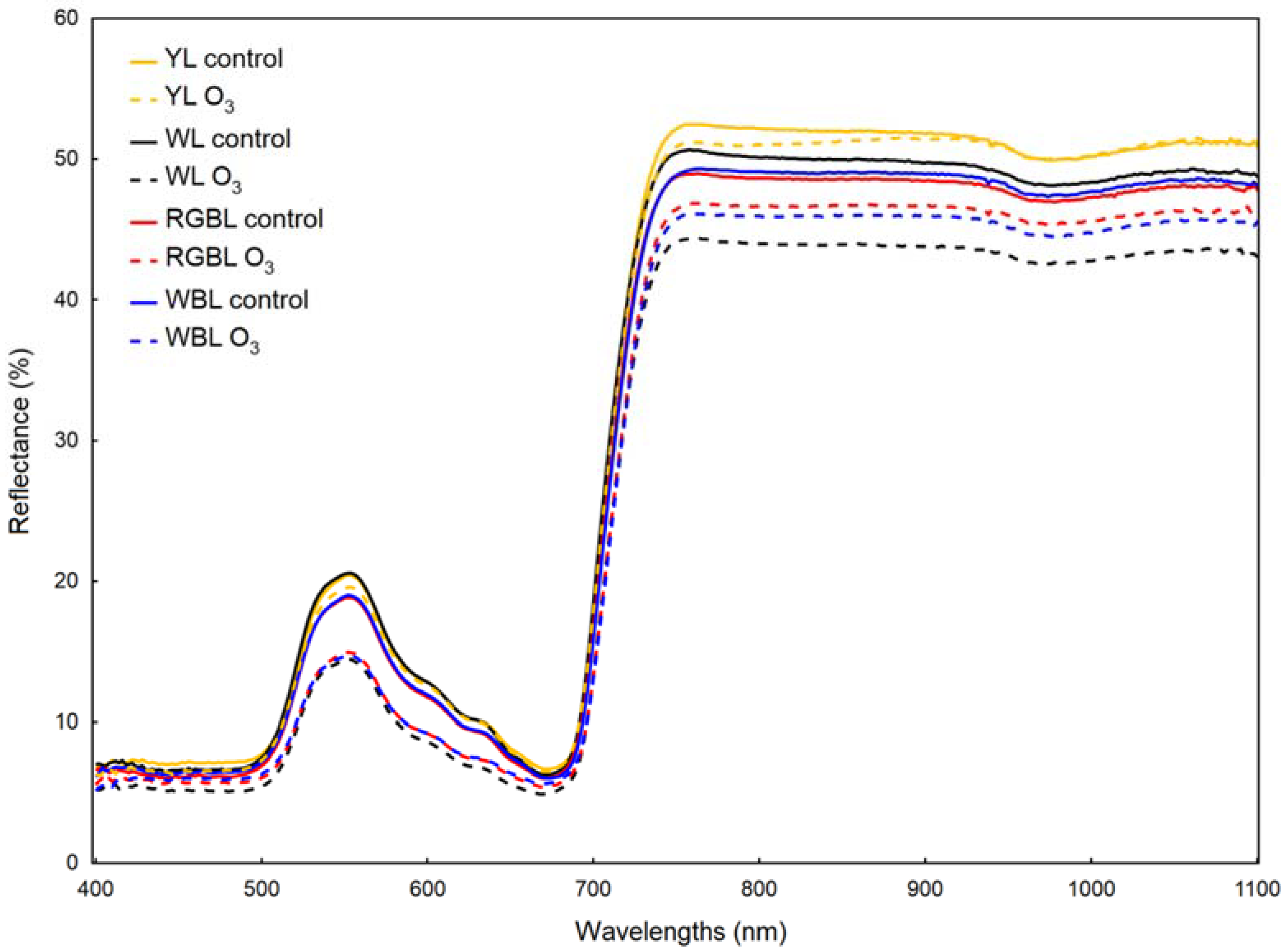
2.4. Reflectance Measurements
2.5. Chlorophyll Content
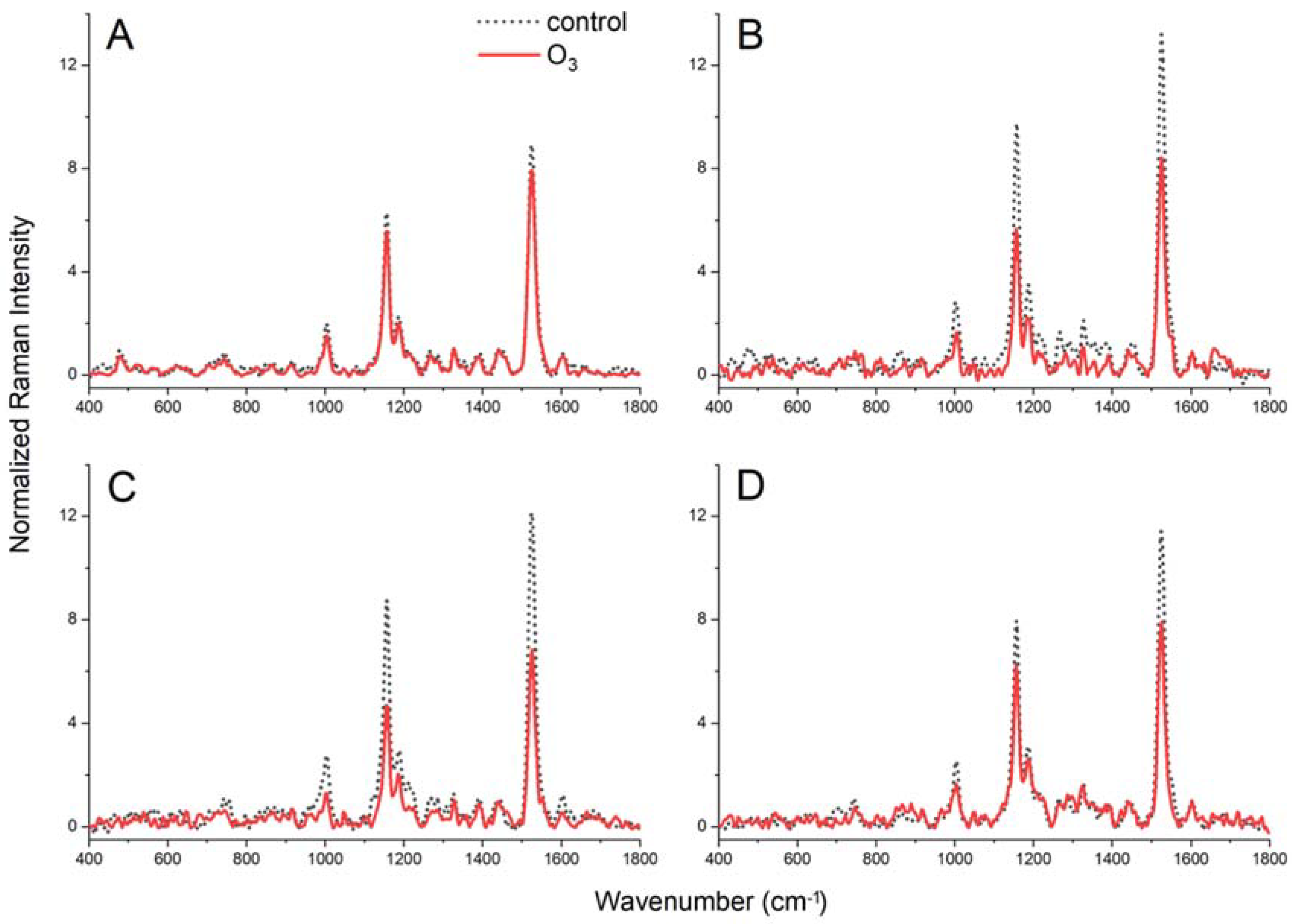
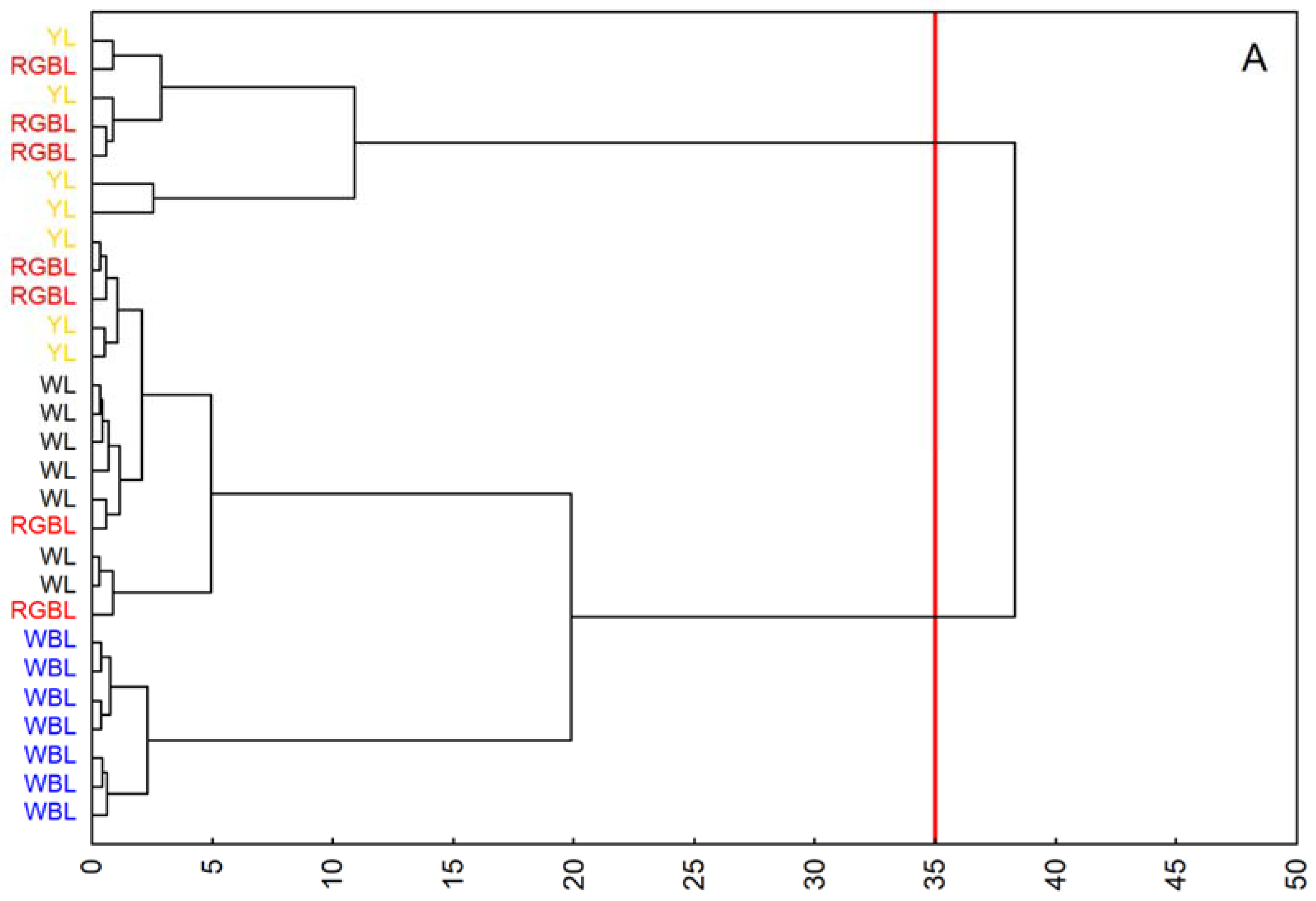
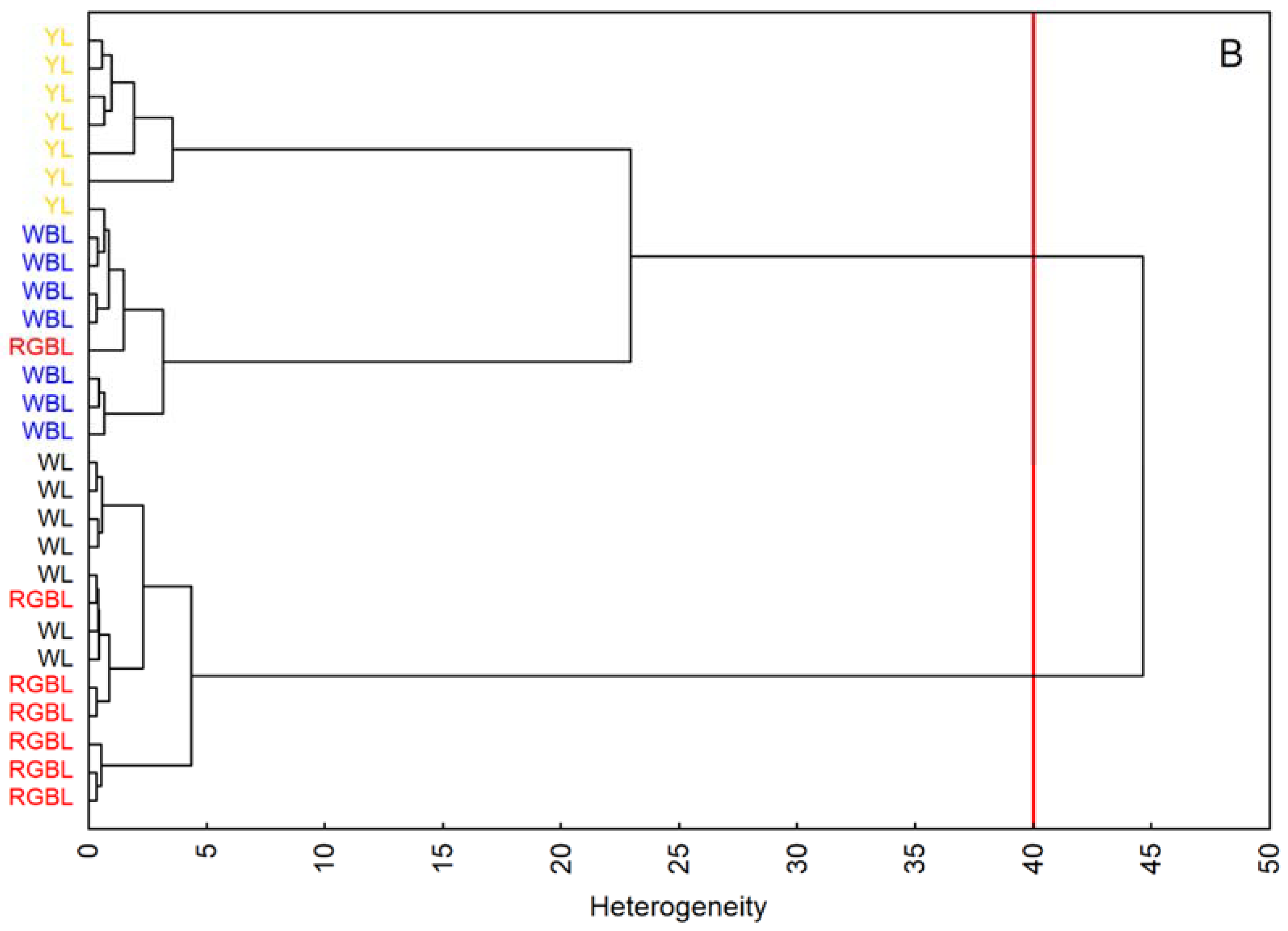
2.6. FT-Raman Spectroscopy Measurements
3. Discussion
3.1. Morphological Differences
3.2. The Physiological Effect of Ozone and Yellow LIGHT (YL) on Plants
3.3. Physiological Response of Plants to Ozone under LED Lighting
4. Materials and Methods
4.1. Plant Materials
4.2. Chlorophyll a Fluorescence
4.3. Gas Exchange Measurements
4.4. Chlorophyll Content
4.5. Reflectance Measurements
- Anthocyanin Reflectance Index (ARI2) = [(R550−1) − (R700−1)] R800, Gitelson et al. [58];
- Flavonol Reflectance Index (FRI) = [(R410)−1 − (R460)−1] R800, Merzlyak et al. [59];
- Structure-Insensitive Pigment Index (SIPI) = (R800 − R445) (R800 + R680) − 1, Peñuelas et al. [60];
- Water Band Index (WBI) = R900∙(R970)−1, Peñuelas et al. [61];where Rx—means the intensity of reflectance at a specific wavelength x.
4.6. FT-Raman Spectroscopy Measurements
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; Iperen, W.; Harbison, J. Blue light dose-responses of leaf photosynthesis, morphology andchemical composition of Cucumis sativus grown under different combinationsof red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Pedmale, U.V.; Huang, S.C.; Zander, M.; Cole, B.J.; Hetzel, J.; Ljung, K.; Reis, P.A.; Sridevi, P.; Nito, K.; Nery, J.R.; et al. Cryptochromes interact directly with PIFs to control plant growth in limiting blue light. Cell 2016, 164, 233–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vingarzan, R. A review of surface ozone background levels and trends. Atmos. Environ. 2004, 38, 3431–3442. [Google Scholar] [CrossRef]
- Mills, G.; Hayes, F.; Simpson, D.; Emberson, L.; Norris, D.; Harmens, H.; Büker, P. Evidence of widespread effects of ozone on crops and (semi-)natural vegetation in Europe (1990–2006) in relation to AOT40- and flux-based risk maps. Glob. Chang. Biol. 2011, 17, 592–613. [Google Scholar] [CrossRef] [Green Version]
- Schneider, G.F.; Cheesman, A.W.; Winter, K.; Turner, B.L.; Sitch, S.; Kursar, T.A. Current ambient concentrations of ozone in Panama modulate the leaf chemistry of the tropical tree Ficus insipida. Chemosphere 2017, 172, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Degl’Innocenti, E.; Guidi, L.; Soldatini, G.F. Characterisation of the photosynthetic response of tobacco leaves toozone: CO2 assimilation and chlorophyll fluorescence. J. Plant Physiol. 2002, 159, 845–853. [Google Scholar] [CrossRef]
- Tetteh, R.; Yamaguchi, M.; Wadac, Y.; Funadad, R.; Izutad, T. Effects of ozoneon growth, net photosynthesis and yield of two African varieties of Vigna unguiculata. Environ. Pollut. 2015, 196, 230–238. [Google Scholar] [CrossRef]
- Emberson, L.; Pleijel, H.; Ainsworth, E.; Van Den Berg, M.; Ren, W.; Osborne, S.; Mills, G.; Pandey, D.; Dentener, F.; Bueker, P.; et al. Ozone effects on crops and consideration in crop models. Eur. J. Agron. 2018, 100, 19–34. [Google Scholar] [CrossRef]
- Booker, F.; Muntifering, R.; McGrath, M.; Burkey, K.; Decoteau, D.; Fiscus, E.; Manning, W.; Krupa, S.; Chappelka, A.; Grantz, D. The ozone component of global change: Potential effects on agricultural and horticultural plant yield, product quality and interactions with invasive species. J. Integr. Plant Biol. 2009, 51, 337–351. [Google Scholar] [CrossRef]
- Bergmann, E.; Bender, J.; Weigel, H.J. Impact of tropospheric ozone on terrestrial biodiversity: A literature analysis to identify ozone sensitive taxa. J. Appl. Bot. Food Qual. 2017, 90, 83–105. [Google Scholar] [CrossRef]
- Fiscus, E.L.; Miller, J.E.; Booker, F.L.; Heagle, A.S.; Reid, C.D. The impact of ozone and other limitations on the crop productivity response to CO2. Technology 2002, 8, 181–192. [Google Scholar]
- Guidi, L.; Degl’Innocenti, E. Ozone effects on high light-induced photoinhibition in Phaseolus vulgaris. Plant Sci. 2008, 174, 590–596. [Google Scholar] [CrossRef]
- Kleiber, T.; Borowiak, K.; Schroeter-Zakrzewska, A. Effect of ozone treatment and light colour on photosynthesis andyield of lettuce. Sci. Hortic. 2017, 217, 130–136. [Google Scholar] [CrossRef]
- Rozpądek, P.; Ślesak, I.; Cebula, S.; Waligorski, P.; Dziurka, M.; Skoczowski, A.; Miszalski, Z. Ozone fumigation results in accelerated growth and persistent changes in the antioxidant system of Brassica oleracea L. var. capitata f. alba. J. Plant Physiol. 2013, 170, 1259–1266. [Google Scholar] [CrossRef]
- Bussotti, F.; Strasser, R.J.; Schaub, M. Photosynthetic behavior of woody species under high ozone exposure probed with the JIP-Test—A review. Environ. Pollut. 2007, 147, 430–437. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Neill, S.O.; Gould, K.S. Anthocyanins in leaves: Light attenuators or antioxidants? Funct. Plant Biol. 2003, 30, 865–873. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, M.H.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J.; et al. The use of chlorophyll fluorescence kinetics analysis to study the performance of photosynthetic machinery in plants. Emerg. Technol. Manag. Crop Stress Toler. 2014, 2, 347–384. [Google Scholar] [CrossRef]
- Rapacz, M.; Sasal, M.; Kalaji, H.M.; Kościelniak, J. Is the OJIP test a reliable indicator of winter hardiness and freezing tolerance of common wheat and Triticale under variable winter environments? PLoS ONE 2015, 10, e0134820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Żurek, G.; Rybka, K.; Pogrzeba, M.; Krzyżak, J.; Prokopiuk, K. Chlorophyll a fluorescence in evaluation of the effect of heavy metal soil contamination on perennial grasses. PLoS ONE 2014, 9, e91475. [Google Scholar] [CrossRef]
- Kalaji, M.H.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant 2016, 38, 102. [Google Scholar] [CrossRef] [Green Version]
- Bussotti, F.; Desotgiu, R.; Cascio, C.; Pollastrini, M.; Gravano, E.; Gerosa, G.; Marzuoli, R.; Nali, C.; Lorenzini, G.; Salvatori, E.; et al. Ozone stress in woody plants assessed with chlorophyll a fluorescence. A criticalreassessment of existing data. Environ. Exp. Bot. 2011, 73, 19–30. [Google Scholar] [CrossRef]
- Goumenaki, E.; Taybi, T.; Borland, A.; Barnes, J. Mechanisms underlying the impacts of ozone on photosynthetic performance. Environ. Exp. Bot. 2010, 69, 259–266. [Google Scholar] [CrossRef]
- Gottardini, E.; Cristofori, A.; Cristofolini, F.; Nali, C.; Pellegrini, E.; Bussotti, F.; Ferretti, M. Chlorophyll-related indicators are linked to visible ozone symptoms: Evidence from a field study on native Viburnum lantana L. plants innorthern Italy. Ecol. Indic. 2014, 39, 65–74. [Google Scholar] [CrossRef]
- Oliwa, J.; Skoczowski, A. The high red/far-red ratio supports the acclimation of fern Platycerium bifurcatum to high light. Biol. Futur. 2019, 70, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Oliwa, J.; Kornas, A.; Skoczowski, A. Morphogenesis of sporotrophophyll leaves in Platycerium bifurcatum depends on the red/far-red ratio in the light spectrum. Acta Physiol. Plant 2016, 38, 247. [Google Scholar] [CrossRef] [Green Version]
- Solovchenko, A. (Ed.) Quantification of screening pigments and their efficiency in situ. In Photoprotection in Plants; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Rys, M.; Pociecha, E.; Oliwa, J.; Ostrowska, A.; Jurczyk, B.; Saja, D.; Janeczko, A. Deacclimation of winter oilseed rape: Insight into physiological changes. Agronomy 2020, 10, 1565. [Google Scholar] [CrossRef]
- Rys, M.; Szaleniec, M.; Skoczowski, A.; Stawoska, I.; Janeczko, A. FT-Raman spectroscopy as a tool in evaluation the response of plants to drought stress. Open Chemistry 2015, 13, 1091–1100. [Google Scholar] [CrossRef]
- Skoczowski, A.; Rys, M. Isothermal calorimetry and Raman spectroscopy to study response of plants to abiotic and biotic stress. In Molecular Stress Physiology of Plants; Rout, G.R., Das, A.B., Eds.; Springer: New Delhi, India, 2013; pp. 263–288. [Google Scholar]
- Rys, M.; Saja-Garbarz, D.; Skoczowski, A. Phytotoxic effects of selected herbal extracts on the germination, growth and metabolism of mustard and oilseed rape. Agronomy 2022, 12, 110. [Google Scholar] [CrossRef]
- Schrader, B.; Klump, H.H.; Schenzel, K.; Schulz, H. Non-destructive NIR FT Raman analysis of plants. J. Mol. Struct. 1999, 509, 201–212. [Google Scholar] [CrossRef]
- Schulz, H. Qualitative and quantitative FT-Raman analysis of plants. In Optical Spectroscopy and Computational Methods in Biology and Medicine; Baranska, M., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 253–278. [Google Scholar]
- Vitek, P.; Novotna, K.; Hodanova, P.; Rapantova, B.; Klem, K. Detection of herbicide effects on pigment composition and PSII photochemistry in Helianthus annuus by Raman spectroscopy and chlorophyll a fluorescence. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 170, 234–241. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M.; Baranski, R. Potential of NIR-FT-Raman spectroscopy in natural carotenoid analysis. Biopolymers 2005, 77, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Baranska, M.; Schulz, H.; Baranski, R.; Nothnagel, T.; Christensen, L.P. In Situ Simultaneous Analysis of Polyacetylenes, Carotenoids and Polysaccharides in Carrot Roots. J. Agric. Food Chem. 2005, 53, 6565–6571. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benitez, J.J.; Dominguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muik, B.; Lendl, B.; Molina-Diaz, A.; Ayora-Canada, M.J. Direct monitoring of lipid oxidation in edible oils by Fourier transform Raman spectroscopy. Chem. Phys. Lipids. 2005, 134, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, L.G.; Lokke, M.M.; Micklander, E.; Engelsen, S.B. Vibrational microspectroscopy of food. Raman vs. FT-IR. Trends Food Sci. Technol. 2003, 14, 50–57. [Google Scholar] [CrossRef]
- Massa, G.D.; Kim, H.H.; Wheeler, R.M.; Mitchell, C.A. Plant Productivity in Response to LED Lighting. Hortic. Sci. 2008, 43, 1951–1956. [Google Scholar] [CrossRef]
- Paul, N.D.; Moore, J.P. Manipulation of light spectrum for crop growth regulation. Acta Hortic. 2006, 711, 357–362. [Google Scholar] [CrossRef]
- Bacarin, M.A.; Deuner, S.; da Silva, F.S.; Cassol, D.; Silva, D.M. Chlorophyll a fluorescence as indicative of the salt stress on Brassica napus L. Braz. J. Plant Physiol. 2011, 23, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Novak, K.; Schaub, M.; Fuhrer, J.; Skelly, J.M.; Hug, C.; Landolt, W.; Bleuler, P.; Kräuchi, N. Seasonal trends in reduced leaf gas exchange and ozone-induced foliar injury in three ozone sensitive woody plants species. Environ. Pollut. 2005, 136, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Kitao, M.; Löw, M.; Heerdt, C.; Grams, T.E.E.; Häberle, K.H.; Matyssek, R. Effects of chronic elevated ozone exposure on gas exchange responses of adult beech trees (Fagus sylvatica) as related to the within–canopy light gradient. Environ. Pollut. 2009, 157, 537–544. [Google Scholar] [CrossRef]
- Bąba, W.; Kalaji, H.M.; Kompała-Bąba, A.; Goltsev, V. Acclimatization of photosynthetic apparatus of tor grass (Brachypodiumpinnatum) during expansion. PLoS ONE 2016, 11, e0156201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, E.; Hoshika, Y.; Dusart, N.; Cotrozzi, L.; Gérard, J.; Nali, C.; Vaultier, M.-N.; Jolivet, Y.; Lorenzini, G.; Paoletti, E. Antioxidative responses of three oak species under ozone and water stress conditions. Sci. Total Environ. 2019, 647, 390–399. [Google Scholar] [CrossRef]
- Brunetti, C.; Guidi, L.; Sebastiani, F.; Tattini, M. Isoprenoids and phenylpropanoids are key components of the antioxidant defense system of plants facing severe excess light stress. Environ. Exp. Bot. 2015, 119, 54–62. [Google Scholar] [CrossRef]
- Long, S.P.; Naidu, S.L. Effects of oxidants at the biochemical, cell and physiological levels, with particular reference to ozone. In Air Pollution and Plant Life, 2nd ed.; Bell, J.N.B., Treshow, M., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2002; pp. 69–88. [Google Scholar]
- Fuhrer, J.; Booker, F.L. Ecological issues related to ozone: Agricultural issues. Environ. Int. 2003, 29, 141–154. [Google Scholar] [CrossRef]
- Gitelson, A.; Chivkunova, O.; Merzlyak, M. Nondestructive estimation of anthocyanins and chlorophylls in anthocyanic leaves. Am. J. Bot. 2009, 96, 1861–1868. [Google Scholar] [CrossRef]
- Steele, M.; Gitelson, A.; Rundquist, D.; Merzlyak, M. Nondestructive estimation of anthocyanin content in grapevine leaves. Am. J. Enol. Viticult. 2009, 60, 87–92. [Google Scholar]
- Baroli, I.; Melis, A. Photoinhibitory damage is modulated by the rate of photosynthesis and by the photosystem II lightharvesting chlorophyll antenna size. Planta 1998, 205, 288–296. [Google Scholar] [CrossRef]
- Burritt, D.J.; MacKenzie, S. Antioxidant metabolism during acclimation of Begonia × erythrophylla to high light levels. Ann. Bot. 2003, 91, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanism Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK, 2000; pp. 443–480. [Google Scholar]
- Knapp, A.K.; Carter, G.A. Variability in leaf optical properties among 26 species from a broad range of habitats. Am. J. Bot. 1998, 85, 940–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gitelson, A.A.; Merzylak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 71, 38–45. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Smagin, A.I.; Gitelson, A.A. Apple flavonols during fruit adaptation to solar radiation: Spectral features and technique for non-destructive assessment. J. Plant Physiol. 2005, 162, 151–160. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Baret, F. Semiempirical indices to assess carotenoids/chlorophyll a ratio from leaf spectra reflectance. Photosynthetica 1995, 31, 221–230. [Google Scholar]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Save, R. The Reflectance at the 950–970 region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Naumann, D. FT-infrared and FT-Raman spectroscopy in biomedical research. Appl. Spectrosc. Rev. 2001, 36, 239–298. [Google Scholar] [CrossRef]
- Farber, C.; Shires, M.; Ong, K.; Byrne, D.; Kurouski, D. Raman spectroscopy as an early detection tool for rose rosette infection. Planta 2019, 250, 1247–1254. [Google Scholar] [CrossRef]
- Andreev, G.; Schrader, B.; Schulz, H.; Fuchs, R.; Popov, S.; Handjieva, N. Non-destructive NIR-FT-Raman analyses in practice. Part 1. Analyses of plants and historic textiles. Fresenius J. Anal. Chem. 2001, 371, 1009–1017. [Google Scholar]
- Baranski, R.; Baranska, M.; Schulz, H. Changes in carotenoid content and distribution in living plant tissue can be observed and mapped in situ using NIR-FT-Raman spectroscopy. Planta 2005, 222, 448–457. [Google Scholar] [CrossRef]
- Eravuchira, P.J.; El-Abassy, R.M.; Deshpande, S.; Matei, M.F.; Mishra, S.; Tandon, P.; Kuhnert, N.; Materny, A. Raman spectroscopic characterization of different regioisomers of monoacyl and diacyl chlorogenic acid. Vib. Spectrosc. 2012, 61, 10–16. [Google Scholar] [CrossRef]
- Oliwa, J.; Stawoska, I.; Janeczko, A.; Oklestkova, J.; Skoczowski, A. Response of the photosynthetic apparatus in the tropical fern Platycerium bifurcatum to increased ozone concentration. Photosynthetica 2019, 57, 1119–1129. [Google Scholar] [CrossRef] [Green Version]
- Withnall, R.; Chowdhry, B.Z.; Silver, J.; Edwards, H.G.M.; de Oliveira, L.F.C. Raman spectra of carotenoids in natural products. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2207–2212. [Google Scholar] [CrossRef]

| Chlorophyll a Fluorescence Parameter | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| YL | WL | RGBL | WBL | |||||
| Control | O3 | Control | O3 | Control | O3 | Control | O3 | |
| Fv/Fm | 0.845 a ± 0.014 | 0.826 ab ± 0.012 | 0.823 bc ± 0.009 | 0.841 a ± 0.014 | 0.819 c ± 0.013 | 0.840 a ± 0.009 | 0.803 d ± 0.009 | 0.817 c ± 0.011 |
| PIABS | 2.600 bc ± 0.259 | 2.130 de ± 0.208 | 1.781 ef ± 0.212 | 2.773 b ± 0.232 | 1.803 ef ± 0.190 | 3.395 a ± 0.266 | 1.575 f ± 0.214 | 2.387 cd ± 0.228 |
| RC/CSm | 1271 a ± 46 | 1095 c ± 41 | 1056 cd ± 33 | 1103 bc ± 42 | 1027 de ± 40 | 1166 b ± 41 | 992 de ± 44 | 964 e ± 38 |
| ABS/CSm | 3359 a ± 84 | 2988 d ± 74 | 3092 bc ± 85 | 3114 b ± 90 | 2868 de ± 76 | 2966 d ± 80 | 2813 ef ± 79 | 2730 f ± 72 |
| TR0/CSm | 2837 a ± 74 | 2492 c ± 78 | 2543 bc ± 82 | 2619 b ± 81 | 2348 d ± 84 | 2491 c ± 79 | 2261 d ± 76 | 2239 d ± 69 |
| ET0/CSm | 1561 a ± 62 | 1326 b ± 70 | 1324 b ± 59 | 1553 a ± 66 | 1223 bc ± 70 | 1541 a ± 64 | 1146 c ± 68 | 1292 b ± 73 |
| DI0/CSm | 521 bc ± 18 | 495 cd ± 20 | 549 ab ± 21 | 494 cd ± 18 | 519 c ± 20 | 474 d ± 18 | 551 a ± 14 | 490 cd ± 19 |
| Parameter | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| YL | WL | RGBL | WBL | |||||
| Control | O3 | Control | O3 | Control | O3 | Control | O3 | |
| PN (µmol CO2 m−2 s−1) | 8.73 a ± 0.80 | 7.57 b ± 0.82 | 2.99 e ± 0.77 | 5.20 cd ± 0.89 | 7.64 b ± 0.94 | 6.25 bc ± 1.73 | 4.57 c ± 0.69 | 3.77 de ± 0.85 |
| E (mol H2O m−2 s−1) | 0.97 c ± 0.24 | 0.56 d ± 0.22 | 0.52 d ± 0.19 | 1.81 b ± 0.49 | 2.20 b ± 0.43 | 3.24 a ± 0.28 | 0.70 cd ± 0.27 | 2.34 b ± 0.56 |
| gs (mmol H2O m−2 s−1) | 0.12 bc ± 0.02 | 0.07 d ± 0.01 | 0.02 e ± 0.00 | 0.09 cd ± 0.02 | 0.10 bc ± 0.02 | 0.20 a ± 0.03 | 0.03 e ± 0.01 | 0.15 ab ± 0.03 |
| Ci (µmol CO2 mol) | 254 c ± 24 | 171 d ± 23 | 159 d ± 26 | 282 bc ± 23 | 249 c ± 20 | 309 ab ± 26 | 146 d ± 23 | 333 a ± 24 |
| Reflectance Parameter | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| YL | WL | RGBL | WBL | |||||
| Control | O3 | Control | O3 | Control | O3 | Control | O3 | |
| ARI2 | 0.555 d ± 0.021 | 0.728 a ± 0.002 | 0.686 b ± 0.001 | 0.748 a ± 0.001 | 0.628 c ± 0.003 | 0.595 cd ± 0.002 | 0.486 e ± 0.005 | 0.588 d ± 0.004 |
| SIPI | 0.763 a ± 0.012 | 0.771 a ± 0.002 | 0.767 a ± 0.001 | 0.788 a ± 0.022 | 0.774 a ± 0.017 | 0.781 a ± 0.004 | 0.772 a ± 0.024 | 0.770 a ± 0.006 |
| FRI | 0.799 d ± 0.011 | 1.051 b ± 0.002 | 0.393 g ± 0.005 | 0.493 f ± 0.003 | 0.657 e ± 0.003 | 0.957 c ± 0.011 | 0.725 de ± 0.022 | 1.815 a ± 0.027 |
| WBI | 1.036 a ± 0.006 | 1.030 a ± 0.001 | 1.032 a ± 0.002 | 1.029 a ± 0.011 | 1.030 a ± 0.018 | 1.027 a ± 0.011 | 1.031 a ± 0.013 | 1.032 a ± 0.013 |
| Leaf Side | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| YL | WL | RGBL | WBL | |||||
| Control | O3 | Control | O3 | Control | O3 | Control | O3 | |
| Upper | 36.63 ab ± 1.72 | 35.19 b ± 1.74 | 35.56 b ± 1.77 | 36.75 ab ± 1.66 | 38.50 a ± 1.73 | 38.42 a ± 1.35 | 35.24 b ± 1.56 | 36.28 ab ± 1.51 |
| Bottom | 37.10 abc ± 1.76 | 34.22 d ± 1.67 | 35.74 bcd ± 1.65 | 36.98 abc ± 1.70 | 38.15 ab ± 1.78 | 38.70 a ± 1.74 | 34.66 cd ± 1.42 | 36.90 abc ± 1.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoczowski, A.; Oliwa, J.; Stawoska, I.; Rys, M.; Kocurek, M.; Czyczyło-Mysza, I. The Spectral Compositions of Light Changes Physiological Response of Chinese Cabbage to Elevated Ozone Concentration. Int. J. Mol. Sci. 2022, 23, 2941. https://doi.org/10.3390/ijms23062941
Skoczowski A, Oliwa J, Stawoska I, Rys M, Kocurek M, Czyczyło-Mysza I. The Spectral Compositions of Light Changes Physiological Response of Chinese Cabbage to Elevated Ozone Concentration. International Journal of Molecular Sciences. 2022; 23(6):2941. https://doi.org/10.3390/ijms23062941
Chicago/Turabian StyleSkoczowski, Andrzej, Jakub Oliwa, Iwona Stawoska, Magdalena Rys, Maciej Kocurek, and Ilona Czyczyło-Mysza. 2022. "The Spectral Compositions of Light Changes Physiological Response of Chinese Cabbage to Elevated Ozone Concentration" International Journal of Molecular Sciences 23, no. 6: 2941. https://doi.org/10.3390/ijms23062941
APA StyleSkoczowski, A., Oliwa, J., Stawoska, I., Rys, M., Kocurek, M., & Czyczyło-Mysza, I. (2022). The Spectral Compositions of Light Changes Physiological Response of Chinese Cabbage to Elevated Ozone Concentration. International Journal of Molecular Sciences, 23(6), 2941. https://doi.org/10.3390/ijms23062941







