GC-MS Metabolite and Transcriptome Analyses Reveal the Differences of Volatile Synthesis and Gene Expression Profiling between Two Apple Varieties
Abstract
:1. Introduction
2. Results
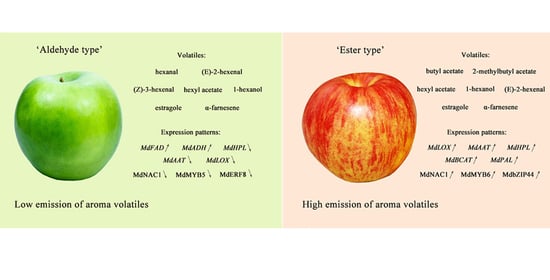
2.1. Phenotype and Physiological Traits of Granny Smith and Jonagold Apples
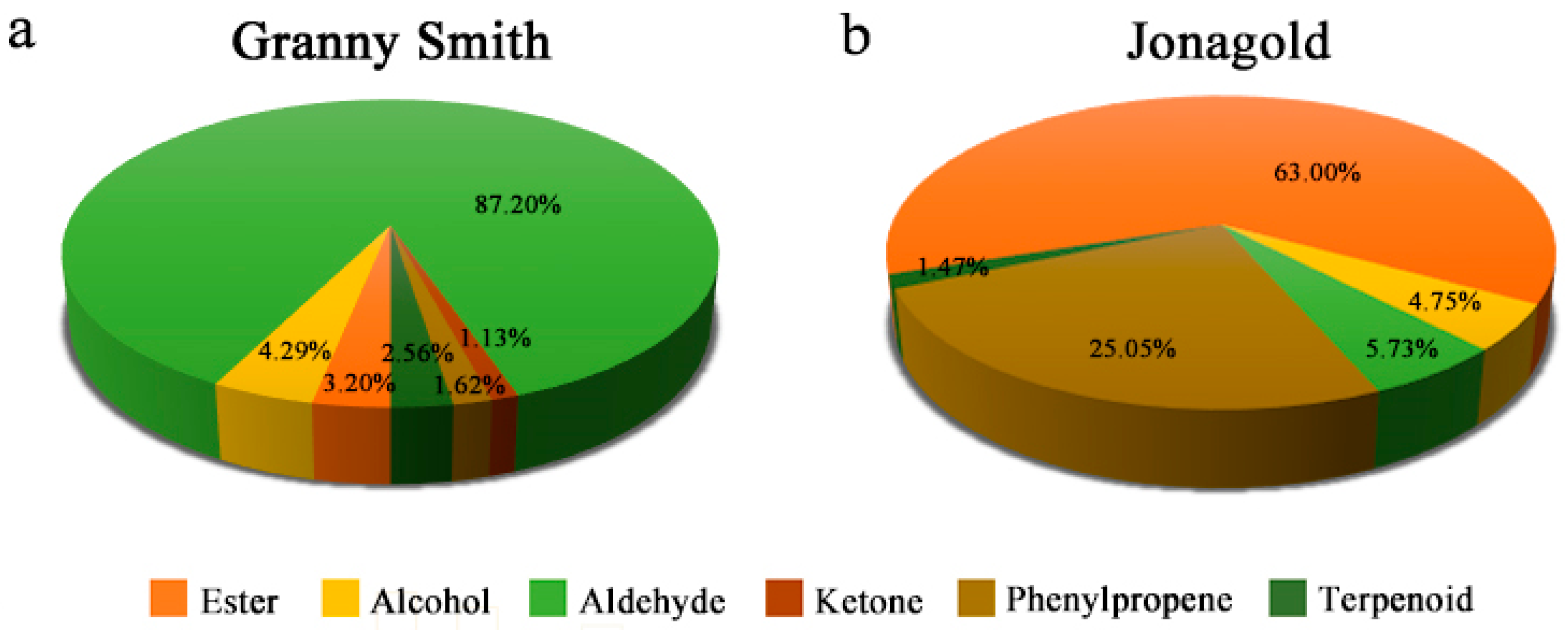
2.2. Volatile Compound Profiles in Granny Smith and Jonagold Apples
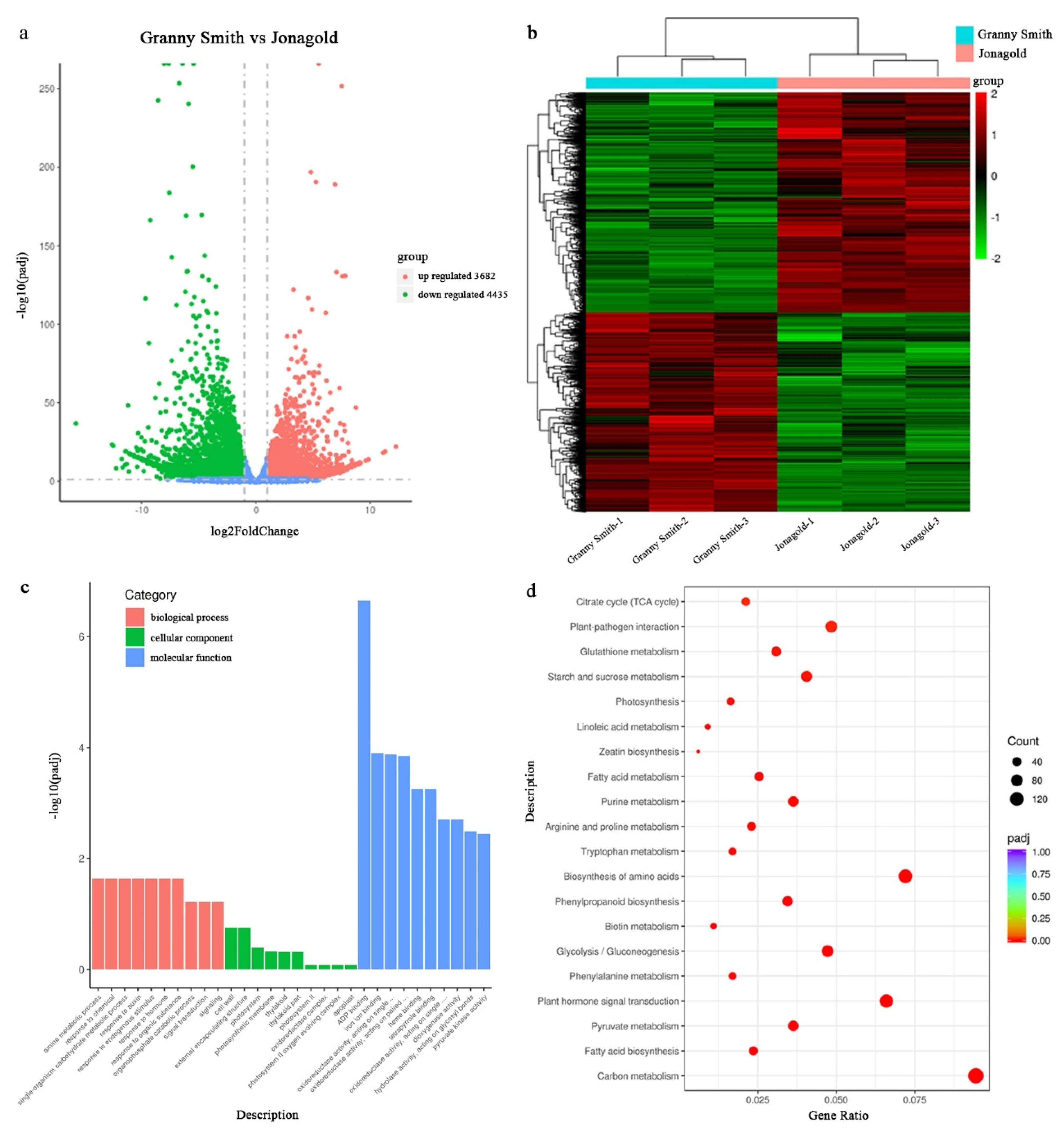
2.3. RNA-seq, Assembly and DEGs Functional Annotation
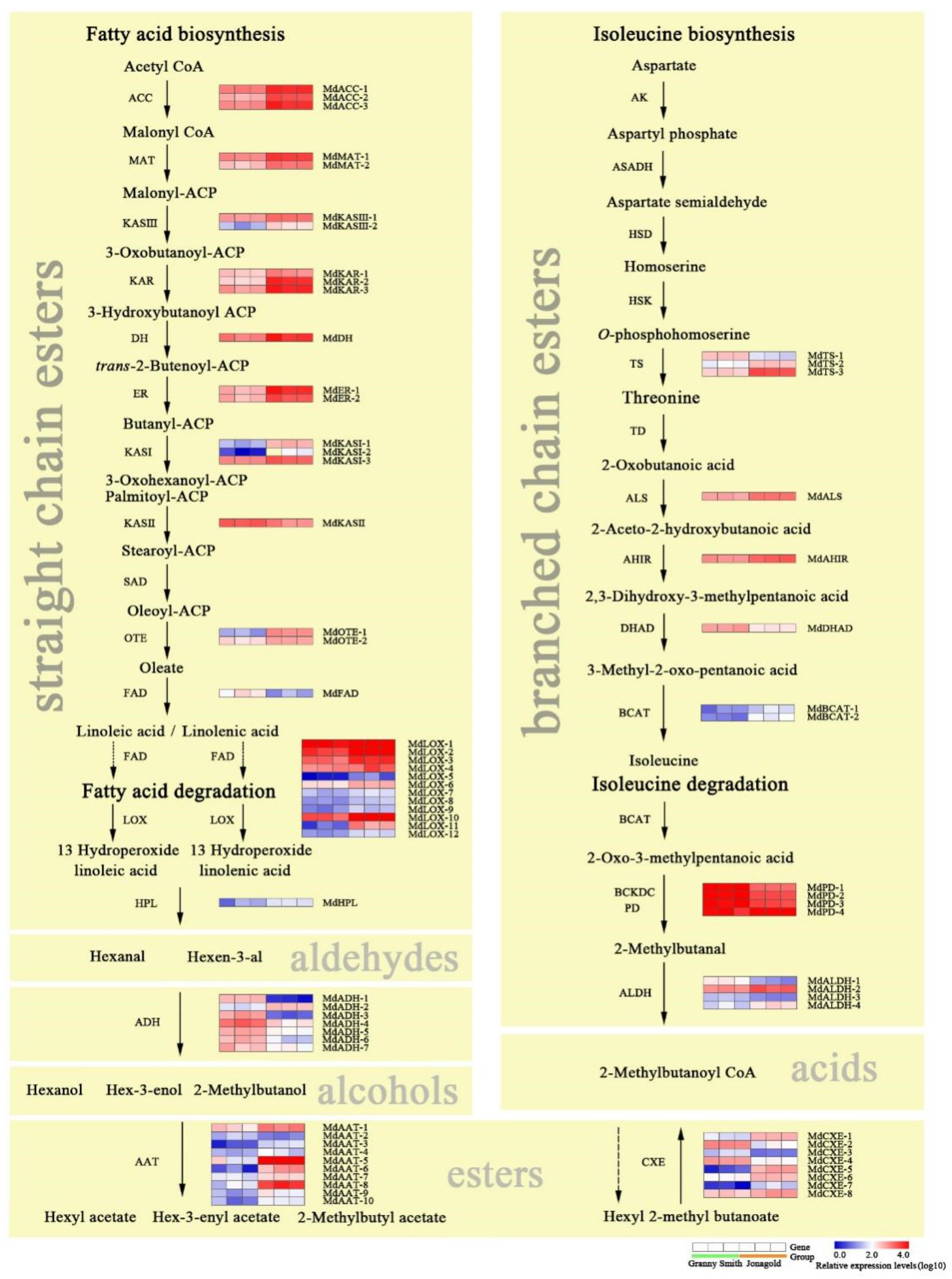
2.4. Expression Levels of Genes Involved in Fatty Acid and Isoleucine Metabolism Pathways
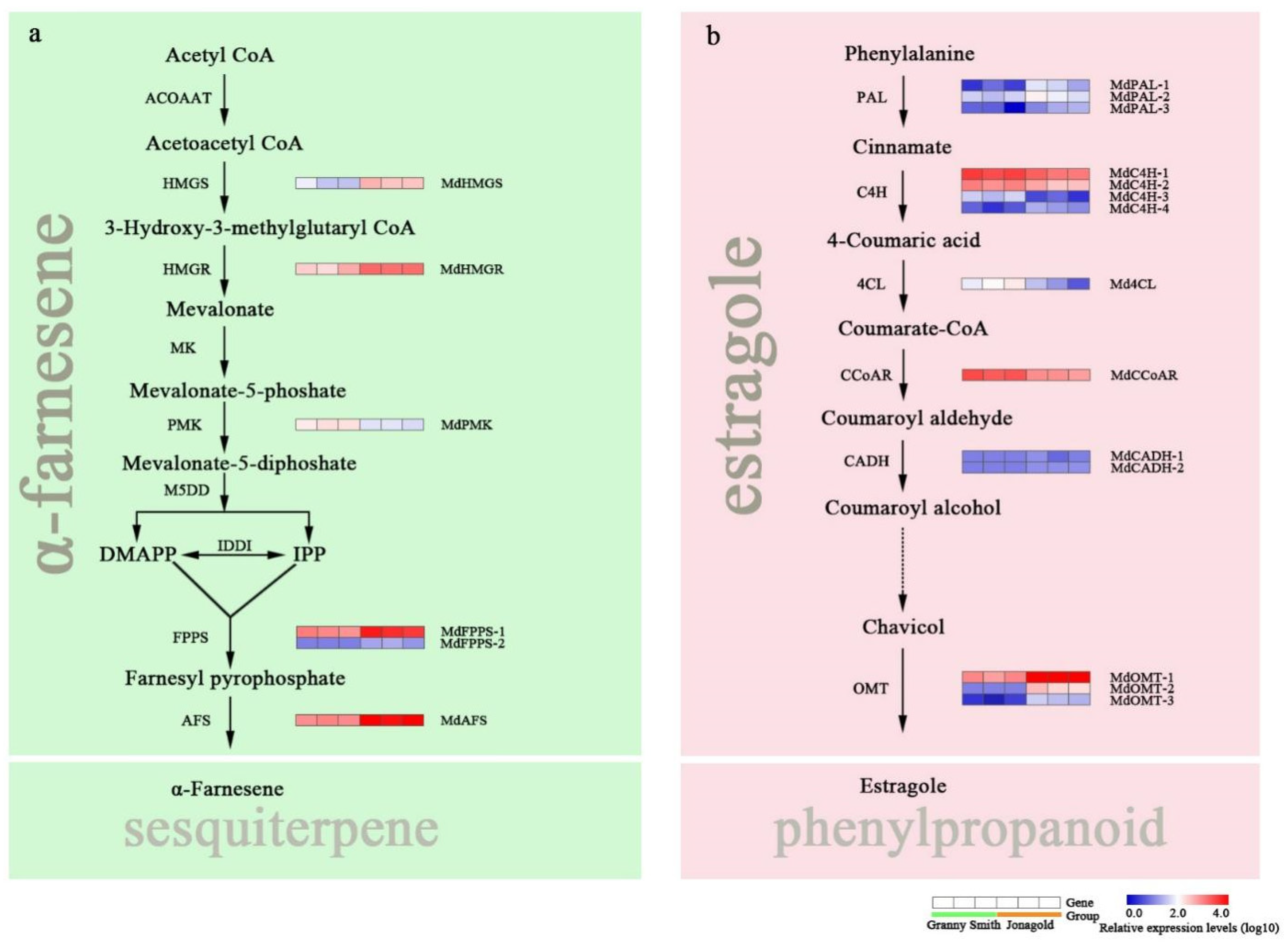
2.5. Expression Levels of Genes Related to Sesquiterpene and Phenylpropanoid Metabolism
2.6. Expression Levels of Transcription Factors Associated with Volatile Compounds
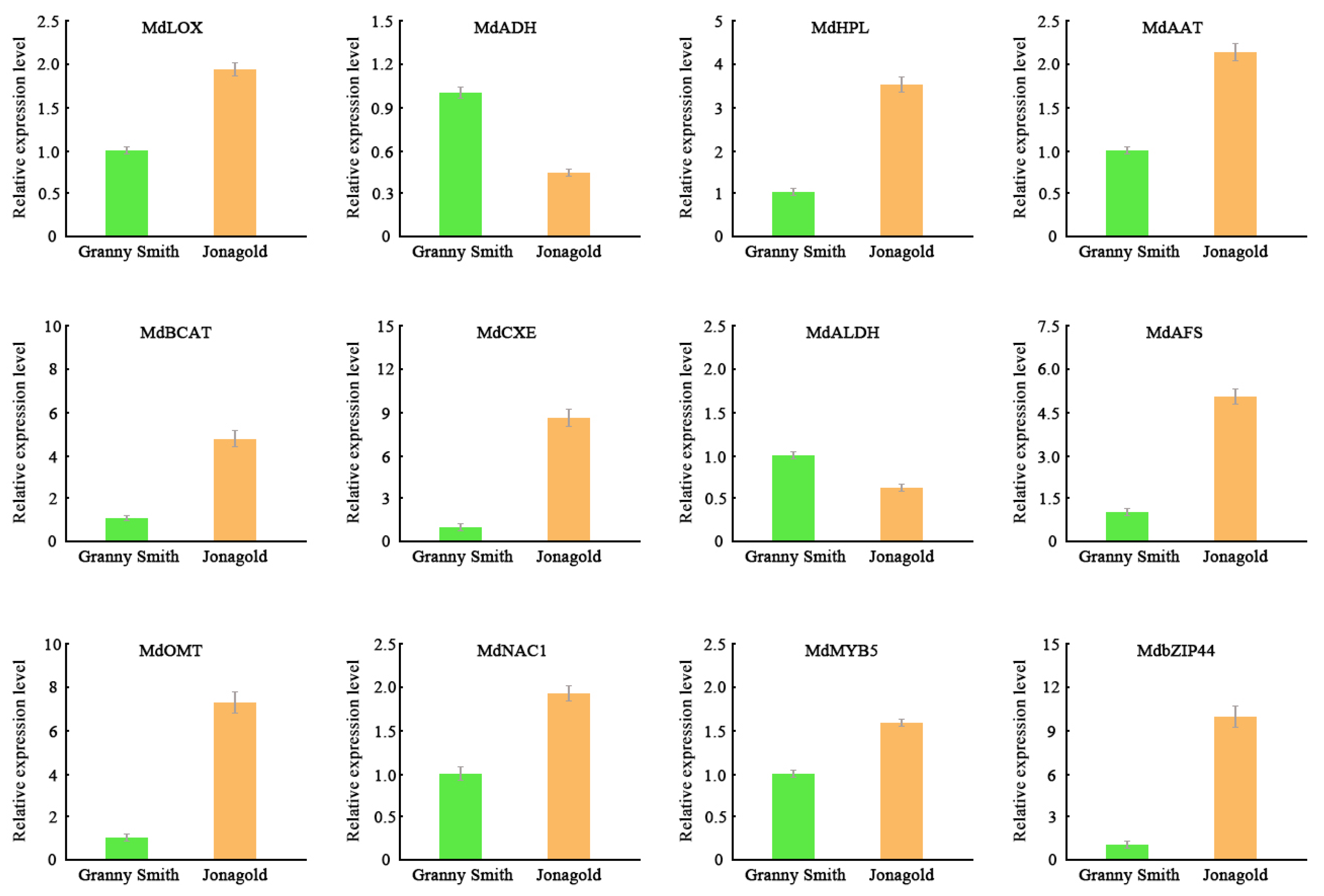
2.7. Validation of Gene Expression Levels Using qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sample Collection
4.2. Determination of Fruit Physiological Characteristics
4.3. Volatile Compounds Extraction and GC-MS Analysis
4.4. RNA Extraction, Library Preparation and Transcriptome Sequencing
4.5. Differential Expression Analysis and Functional Annotations
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aprea, E.; Gika, H.; Carlin, S.; Theodoridis, G.; Vrhovsek, U.; Mattivi, F. Metabolite profiling on apple volatile content based on solid phase microextraction and gas-chromatography time of flight mass spectrometry. J. Chromatogr. A 2011, 1218, 4517–4524. [Google Scholar] [CrossRef]
- Kader, A.A. Flavour quality of fruits and vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Farneti, B.; Khomenko, I.; Cappellin, L.; Ting, V.; Costa, G.; Biasioli, F.; Costa, F. Dynamic volatile organic compound fingerprinting of apple fruit during processing. LWT-Food Sci. Technol. 2015, 63, 21–28. [Google Scholar] [CrossRef]
- Yan, D.; Shi, J.; Ren, X.; Tao, Y.; Ma, F.; Li, R.; Liu, X.; Liu, C. Insights into the aroma profiles and characteristic aroma of ‘Honeycrisp’ apple (Malus × domestica). Food Chem. 2020, 327, 127074. [Google Scholar] [CrossRef]
- Dixon, J.; Hewett, E. Factors affecting apple aroma/flavour volatile concentration: A review. N. Z. J. Crop Hortic. Sci. 2000, 28, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Schaffer, R.J.; Friel, E.N.; Souleyre, E.J.; Bolitho, K.; Thodey, K.; Ledger, S.; Bowen, J.H.; Ma, J.; Nain, B.; Cohen, D.; et al. A genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol. 2007, 144, 1899–1912. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, D.; Dunemann, F. Towards the development of molecular markers for apple volatiles. Flavour. Fragr. J. 2012, 27, 286–289. [Google Scholar] [CrossRef]
- Lopez, M.L.; Villatoro, C.; Fuentes, T.; Graell, J.; Lara, I.; Echeverria, G. Volatile compounds, quality parameters and consumer acceptance of ‘Pink Lady’ apples stored in different conditions. Postharvest Biol. Technol. 2007, 43, 55–66. [Google Scholar] [CrossRef]
- Espino-Diaz, M.; Sepulveda, D.; Gonzalez-Aguilar, G.; Olivas, G. Biochemistry of apple aroma: A review. Food Technol. Biotechnol. 2016, 54, 375–394. [Google Scholar] [CrossRef]
- Vallat, A.; Gu, H.; Dorn, S. How rainfall, relative humidity and temperature influence volatile emissions from apple trees in situ. Phytochemistry 2005, 66, 1540–1550. [Google Scholar] [CrossRef]
- Aprea, E.; Corollaro, M.L.; Betta, E.; Endrizzi, I.; Dematte, M.L.; Biasioli, F.; Gasperi, F. Sensory and instrumental profiling of 18 apple cultivars to investigate the relation between perceived quality and odour and flavour. Food Res. Int. 2012, 49, 677–686. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef]
- Rowan, D.D.; Allen, J.M.; Fielder, S.; Hunt, M.B. Biosynthesis of straight-chain ester volatiles in Red Delicious and Granny Smith apples using deuterium-labeled precursors. J. Agric. Food Chem. 1999, 47, 2553–2562. [Google Scholar] [CrossRef]
- Matich, A.; Rowan, D. Pathway analysis of branched-chain ester biosynthesis in apple using deuterium labeling and enantioselective gas chromatography-mass spectrometry. J. Agric. Food Chem. 2007, 55, 2727–2735. [Google Scholar] [CrossRef]
- Schuster, J.; Binder, S. The mitochondrial branched-chain aminotransferase (AtBCAT-1) is capable to initiate degradation of leucine, isoleucine and valine in almost all tissues in Arabidopsis thaliana. Plant Mol. Biol. 2005, 57, 241–254. [Google Scholar] [CrossRef]
- Fraternale, D.; Ricci, D.; Flamini, G.; Giomaro, G. Volatile profile of red apple from Marche region (Italy). Rec. Nat. Prod. 2011, 53, 202–207. [Google Scholar]
- Yauk, Y.K.; Chagne, D.; Tomes, S.; Matich, A.J.; Wang, M.Y.; Chen, X.; Maddumage, R.; Hunt, M.B.; Rowan, D.D.; Atkinsonet, R.G. The O-methyltransferase gene MdoOMT1 is required for biosynthesis of methylated phenylpropenes in ripe apple fruit. Plant J. 2015, 82, 937–950. [Google Scholar] [CrossRef]
- Young, J.C.; Chu, C.; Lu, X.; Zhu, H. Ester variability in apple varieties as determined by solid-phase microextraction and gas chromatography-mass spectrometry. J. Agric. Food Chem. 2004, 52, 8086–8093. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, X.; Wei, L.; Cao, X.; Ge, Y.; Li, J. Collaborative analysis on difference of apple fruits flavour using electronic nose and electronic tongue. Sci. Hortic. 2020, 260, 108879. [Google Scholar] [CrossRef]
- Abbott, J.A.; Saftner, R.A.; Gross, K.C.; Vinyard, B.T.; Janick, J. Consumer evaluation and quality measurement of fresh-cut slices of ‘Fuji,’ ‘Golden Delicious,’ ‘GoldRush,’ and ‘Granny Smith’ apples. Postharvest Biol. Technol. 2004, 33, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Souleyre, E.; Chagne, D.; Chen, X.; Tomes, S.; Turner, R.; Wang, M.; Maddumage, R.; Hunt, M.; Winz, R.; Wiedow, C.; et al. The AAT1 locus is critical for the biosynthesis of esters contributing to ‘ripe apple’ flavour in ‘Royal Gala’ and ‘Granny Smith’ apples. Plant J. 2014, 78, 903–915. [Google Scholar] [CrossRef]
- Girard, B.; Lau, O.L. Effect of maturity and storage on quality and volatile production of ‘Jonagold’ apples. Food Res. Int. 1995, 28, 465–471. [Google Scholar] [CrossRef]
- Holland, D.; Larkov, O.; Bar-yaakov, I.; Bar, E.; Zax, A.; Brandeis, E.; Ravid, U.; Lewinsohn, E. Developmental and varietal differences in volatile ester formation and acetyl-coA: Alcohol acetyl transferase activities in apple (Malus domestica Borkh.) fruit. J. Agric. Food Chem. 2005, 53, 7198–7203. [Google Scholar] [CrossRef]
- Ting, V.; Soukoulis, C.; Silcock, P.; Cappellin, L.; Romano, A.; Aprea, E.; Bremer, P.; Mark, T.; Gasperi, F.; Biasioli, F. In Vitro and In Vivo flavor release from intact and fresh-cut apple in relation with genetic, textural, and physicochemical parameters. J. Food Sci. 2012, 77, 1226–1233. [Google Scholar] [CrossRef]
- Vara-Ubol, S.; Chambers, E.; Kongpensook, V.; Oupadissakoon, C.; Yenket, R.; Retiveau, A. Determination of the sensory characteristics of rose apples cultivated in Thailand. J. Food Sci. 2006, 71, 547–552. [Google Scholar] [CrossRef]
- Schneider, C.; Pratt, D.; Porter, N.; Brash, A. Control of oxygenation in lipoxygenase and cyclooxygenase catalysis. Chem. Biol. 2007, 14, 473–488. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Hao, N.; Feng, R.; Meng, Z.; Li, Y.; Zhao, Z. Transcriptome and metabolite profiling analyses provide insight into volatile compounds of the apple cultivar ‘Ruixue’ and its parents during fruit development. BMC Plant Biol. 2021, 21, 231. [Google Scholar] [CrossRef]
- Yang, S.; Meng, Z.; Fan, J.; Yan, L.; Yang, Y.; Zhao, Z. Evaluation of the volatile profiles in pulp of 85 apple cultivars (Malus domestica) by HS-SPME combined with GC-MS. J. Food Meas. Charact. 2021, 15, 4215–4225. [Google Scholar] [CrossRef]
- Harker, F.R.; Kupferman, E.M.; Marin, A.B.; Gunson, F.A.; Triggs, C.M. Eating quality standards for apples based on consumer preferences. Postharvest Biol. Technol. 2008, 50, 70–78. [Google Scholar] [CrossRef]
- Yang, S.; Hao, N.; Meng, Z.; Li, Y.; Zhao, Z. Identification, comparison and classification of volatile compounds in peels of 40 apple cultivars by HS-SPME with GC-MS. Foods 2021, 10, 1051. [Google Scholar] [CrossRef]
- Rowan, D.D.; Hunt, M.B.; Dimouro, A.; Alspach, P.A.; Chagne, D. Profiling fruit volatiles in the progeny of a ‘Royal Gala’ × ‘Granny Smith’ apple (Malus × domestica) cross. J. Agric. Food Chem. 2009, 57, 7953–7961. [Google Scholar] [CrossRef]
- Lopez, M.L.; Lavilla, M.T.; Riba, M.; Vendrell, M. Comparison of volatile compounds in two seasons in apples: Golden Delicious and Granny Smith. J. Food Qual. 1998, 21, 155–166. [Google Scholar] [CrossRef]
- Roth, E.; Berna, A.; Beullens, K.; Yarramraju, S.; Lammertyn, J.; Schenk, A.; Nicolai, B. Postharvest quality of integrated and organically produced apple fruit. Postharvest Biol. Technol. 2007, 45, 11–19. [Google Scholar] [CrossRef]
- Rowan, D.D.; Lane, H.P.; Allen, J.M.; Fielder, S.; Hunt, M.B. Biosynthesis of 2-methylbutyl, 2-methyl-2-butenyl, and 2-methylbutanoate esters in Red Delicious and Granny Smith apples using deuterium-labeled substrates. J. Agric. Food Chem. 1996, 44, 3276–3285. [Google Scholar] [CrossRef]
- Gang, D.R.; Wang, J.H.; Dudareva, N.; Nam, K.H.; Simon, J.E.; Lewinsohn, E.; Pichersky, E. An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol. 2001, 125, 539–555. [Google Scholar] [CrossRef] [Green Version]
- Echeverria, G.; Graell, J.; Lopez, M.L.; Lara, I. Volatile production, quality and aroma-related enzyme activities during maturation of ‘Fuji’ apples. Postharvest Biol. Technol. 2004, 31, 217–227. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Kader, A.A.; Dandekar, A.M. Apple aroma: Alcohol acyltransferase, a rate limiting step for ester biosynthesis, is regulated by ethylene. Plant Sci. 2005, 168, 1199–1210. [Google Scholar] [CrossRef]
- Vogt, J.; Schiller, D.; Ulrich, D.; Schwab, W.; Dunemann, F. Identifcation of lipoxygenase (LOX) genes involved in fruit favour formation in apple (Malus × domestica). Tree Genet. Genomes 2013, 9, 1493–1511. [Google Scholar] [CrossRef]
- Souleyre, E.; Greenwood, D.; Friel, E.; Karunairetnam, S.; Newcomb, R. An alcohol acyl transferase from apple (cv. Royal Gala), MpAAT1, produces esters involved in apple fruit flavor. FEBS J. 2005, 272, 3132–3144. [Google Scholar] [CrossRef]
- Green, S.; Friel, E.N.; Matich, A.; Beuning, L.L.; Cooney, J.M.; Rowan, D.D.; MacRae, E. Unusual features of a recombinant apple alphafarnesene synthase. Phytochemistry 2007, 68, 176–188. [Google Scholar] [CrossRef]
- Gaudinier, A.; Brady, S.M. Mapping transcriptional networks in plants: Data-driven discovery of novel biological mechanisms. Annu. Rev. Plant Biol. 2016, 67, 575–594. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Zhang, Y.L.; Shan, W.; Cai, Y.J.; Liang, S.M.; Chen, J.Y.; Lu, W.J.; Kuang, J.F. Identification of two transcriptional activators MabZIP4/5 in controlling aroma biosynthetic genes during banana ripening. J. Agric. Food Chem. 2018, 66, 6142–6150. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Luo, Z.; Wang, L.; Liu, W.; Li, D.; Belwal, T.; Xu, Y.; Li, L. FaMYB9 is involved in the regulation of C6 volatile biosynthesis in strawberry. Plant Sci. 2020, 293, 110422. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wei, C.; Duan, W.; Gao, Y.; Kuang, J.; Liu, M.; Chen, K.; Klee, H.; Zhang, B. Transcriptional and epigenetic analysis reveals that NAC transcription factors regulate fruit flavor ester biosynthesis. Plant J. 2021, 106, 785–800. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Shen, S.; Yin, X.; Klee, H.; Zhang, B.; Chen, K.; Hancock, R. Transcription factor CitERF71 activates the terpene synthase gene CitTPS16 involved in the synthesis of E-geraniol in sweet orange fruit. J. Exp. Bot. 2017, 68, 4929–4938. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Han, S.; Jiang, L.; Yu, M.; Ma, R.; Yu, Z. 1-MCP treatment affects peach fruit aroma metabolism as revealed by transcriptomics and metabolite analyses. Food Res. Int. 2019, 122, 573–584. [Google Scholar] [CrossRef]
- Contreras, C.; Beaudry, R. Lipoxygenase-associated apple volatiles and their relationship with aroma perception during ripening. Postharvest Biol. Technol. 2013, 82, 28–38. [Google Scholar] [CrossRef]
- Qin, G.; Tao, S.; Cao, Y.; Wu, J.; Zhang, H.; Huang, W.; Zhang, S. Evaluation of the volatile profile of 33 Pyrus ussuriensis cultivars by HS-SPME with GC-MS. Food Chem. 2012, 134, 2367–2382. [Google Scholar] [CrossRef]

| Trait | Granny Smith | Jonagold |
|---|---|---|
| Fresh weight (g) | 205 ± 15 | 240 ± 18 |
| Height (mm) | 50.05 ± 1.23 | 56.20 ± 1.85 |
| Diameter (mm) | 57.80 ± 2.10 | 61.55 ± 2.35 |
| L* value | 63.52 ± 0.65 | 58.62 ± 1.67 |
| a* value | −17.62 ± 1.85 | 13.38 ± 2.30 |
| b* value | 37.95 ± 1.52 | 29.13 ± 2.58 |
| TSS (°Brix) | 13.7 ± 0.2 | 14.2 ± 0.2 |
| TA (%) | 0.40 ± 0.04 | 0.32 ± 0.03 |
| Firmness (kg/cm2) | 7.05 ± 0.15 | 6.58 ± 0.12 |
| Internal ethylene (μL/L) | 5 ± 1 | 62 ± 8 |
| CO2 production (μmol/kg·s) | 70 ± 8 | 48 ± 5 |
| Class | Compounds | CAS No | RT | RI | Granny Smith | Jonagold |
|---|---|---|---|---|---|---|
| Ester | Propyl acetate | 109-60-4 | 10.67 | 982 | – | 7.99 ± 0.21 |
| Isobutyl acetate | 110-19-0 | 11.72 | 1020 | – | 2.40 ± 0.11 | |
| Propyl propionate | 106-36-5 | 12.57 | 1050 | – | 1.40 ± 0.02 | |
| Butyl acetate | 123-86-4 | 13.40 | 1074 | - | 213.44 ± 15.74 | |
| 2-Methylbutyl acetate | 624-41-9 | 14.84 | 1126 | – | 85.03 ± 6.17 | |
| Isobutyl butanoate | 539-90-2 | 15.29 | 1155 | – | 1.19 ± 0.03 | |
| Butyl propionate | 590-01-2 | 15.41 | 1157 | – | 8.87 ± 0.24 | |
| Amyl acetate | 628-63-7 | 16.37 | 1178 | – | 14.65 ± 1.35 | |
| Butyl butanoate | 109-21-7 | 17.69 | 1240 | – | 5.75 ± 0.17 | |
| Butyl 2-methylbutanoate | 15706-73-7 | 18.08 | 1243 | – | 19.21 ± 1.08 | |
| Hexyl acetate | 142-92-7 | 19.28 | 1274 | 1.25 ± 0.06 | 885.38 ± 25.44 | |
| (E)-2-Hexenyl acetate | 2497-18-9 | 21.01 | 1338 | – | 1.84 ± 0.06 | |
| Hexyl propanoate | 2445-76-3 | 21.14 | 1347 | – | 8.40 ± 0.54 | |
| Heptyl acetate | 112-06-1 | 22.09 | 1386 | – | 3.16 ± 0.26 | |
| Butyl hexanoate | 626-82-4 | 23.17 | 1410 | – | 1.75 ± 0.10 | |
| Hexyl butanoate | 2639-63-6 | 23.23 | 1423 | 0.35 ± 0.02 | 27.19 ± 1.58 | |
| Hexyl 2-methylbutyrate | 10032-15-2 | 23.53 | 1438 | 0.46 ± 0.03 | 31.95 ± 2.13 | |
| Heptyl formate | 112-23-2 | 24.15 | 1455 | – | 1.59 ± 0.08 | |
| Octyl acetate | 112-14-1 | 24.81 | 1483 | – | 5.56 ± 0.42 | |
| Hexyl hexanoate | 6378-65-0 | 28.07 | 1593 | – | 6.60 ± 0.38 | |
| Alcohol | 1-Butanol | 71-36-3 | 15.35 | 1156 | – | 19.42 ± 1.02 |
| 2-Methyl-1-butanol | 137-32-6 | 17.20 | 1210 | 0.91 ± 0.05 | 6.42 ± 0.38 | |
| 2-Hexyn-1-ol | 764-60-3 | 17.41 | 1225 | 0.85 ± 0.05 | – | |
| 1-Hexanol | 111-27-3 | 21.40 | 1361 | 1.00 ± 0.04 | 74.69 ± 4.81 | |
| Aldehyde | Hexanal | 66-25-1 | 13.76 | 1090 | 7.95 ± 0.58 | 23.93 ± 1.84 |
| (Z)-3-Hexenal | 6789-80-6 | 15.60 | 1161 | 3.32 ± 0.27 | – | |
| (E)-2-Hexenal | 6728-26-3 | 17.93 | 1240 | 40.24 ± 3.62 | 86.21 ± 6.92 | |
| (Z)-2-Heptenal | 57266-86-1 | 21.03 | 1339 | 1.42 ± 0.08 | – | |
| 1-Nonanal | 124-19-6 | 22.79 | 1401 | 0.50 ± 0.03 | 1.66 ± 0.20 | |
| (E)-2-Octenal | 2548-87-0 | 23.89 | 1443 | 1.90 ± 0.10 | 2.12 ± 0.06 | |
| (Z)-2-Nonenal | 60784-31-8 | 26.63 | 1531 | 0.81 ± 0.06 | 3.47 ± 0.12 | |
| (E)-2-Decenal | 3913-81-3 | 29.08 | 1655 | – | 3.88 ± 0.23 | |
| Ketone | 1-Octen-3-one | 4312-99-6 | 20.22 | 1305 | 0.73 ± 0.05 | – |
| phenylpropene | Estragole | 140-67-0 | 29.66 | 1687 | 1.04 ± 0.10 | 530.16 ± 47.66 |
| Terpenoid | α-Farnesene | 502-61-4 | 30.75 | 1754 | 1.65 ± 0.11 | 31.15 ± 2.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Li, D.; Li, S.; Yang, H.; Zhao, Z. GC-MS Metabolite and Transcriptome Analyses Reveal the Differences of Volatile Synthesis and Gene Expression Profiling between Two Apple Varieties. Int. J. Mol. Sci. 2022, 23, 2939. https://doi.org/10.3390/ijms23062939
Yang S, Li D, Li S, Yang H, Zhao Z. GC-MS Metabolite and Transcriptome Analyses Reveal the Differences of Volatile Synthesis and Gene Expression Profiling between Two Apple Varieties. International Journal of Molecular Sciences. 2022; 23(6):2939. https://doi.org/10.3390/ijms23062939
Chicago/Turabian StyleYang, Shunbo, Dongmei Li, Shanshan Li, Huijuan Yang, and Zhengyang Zhao. 2022. "GC-MS Metabolite and Transcriptome Analyses Reveal the Differences of Volatile Synthesis and Gene Expression Profiling between Two Apple Varieties" International Journal of Molecular Sciences 23, no. 6: 2939. https://doi.org/10.3390/ijms23062939
APA StyleYang, S., Li, D., Li, S., Yang, H., & Zhao, Z. (2022). GC-MS Metabolite and Transcriptome Analyses Reveal the Differences of Volatile Synthesis and Gene Expression Profiling between Two Apple Varieties. International Journal of Molecular Sciences, 23(6), 2939. https://doi.org/10.3390/ijms23062939