Starch Degradation and Sucrose Accumulation of Lily Bulbs after Cold Storage
Abstract
:1. Introduction
2. Results
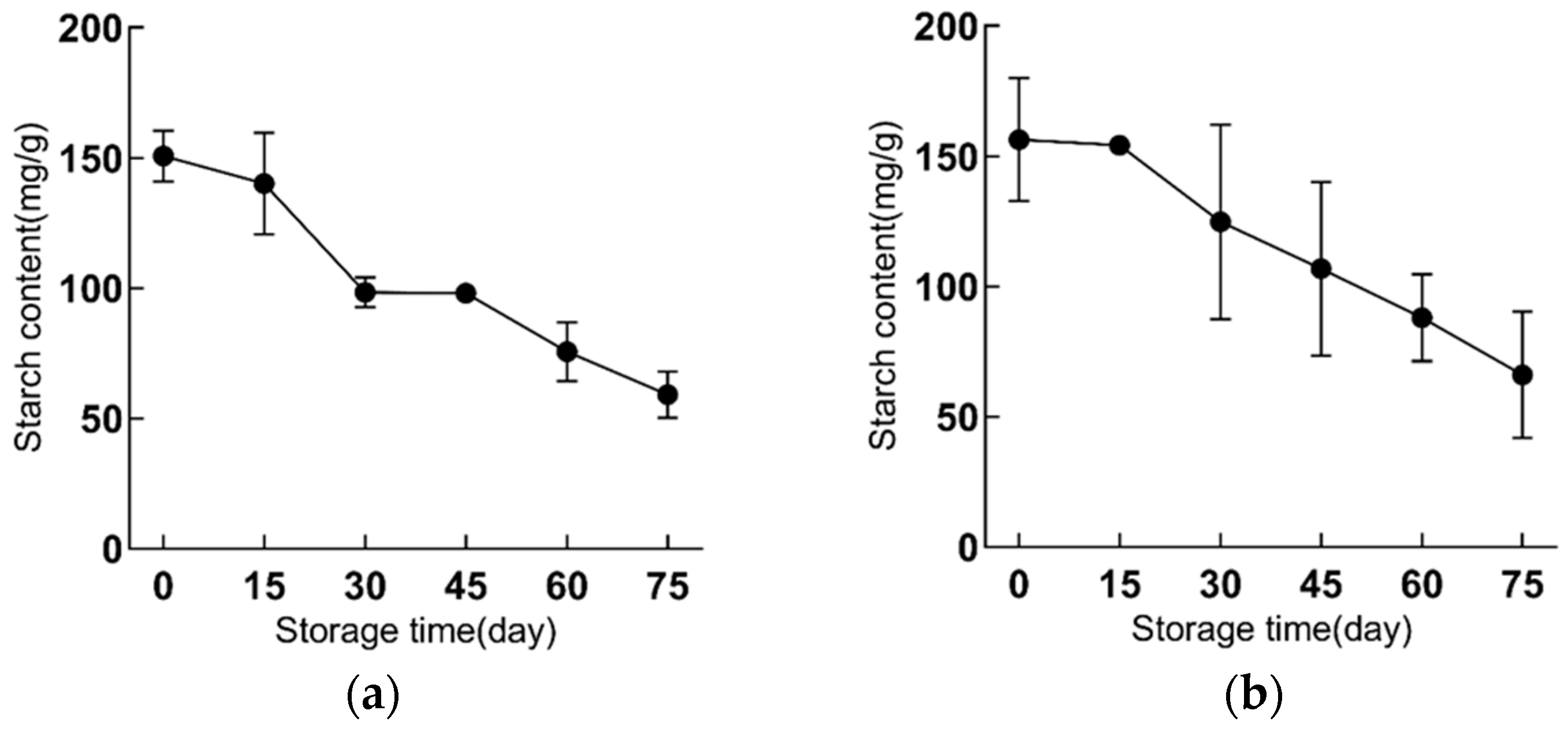
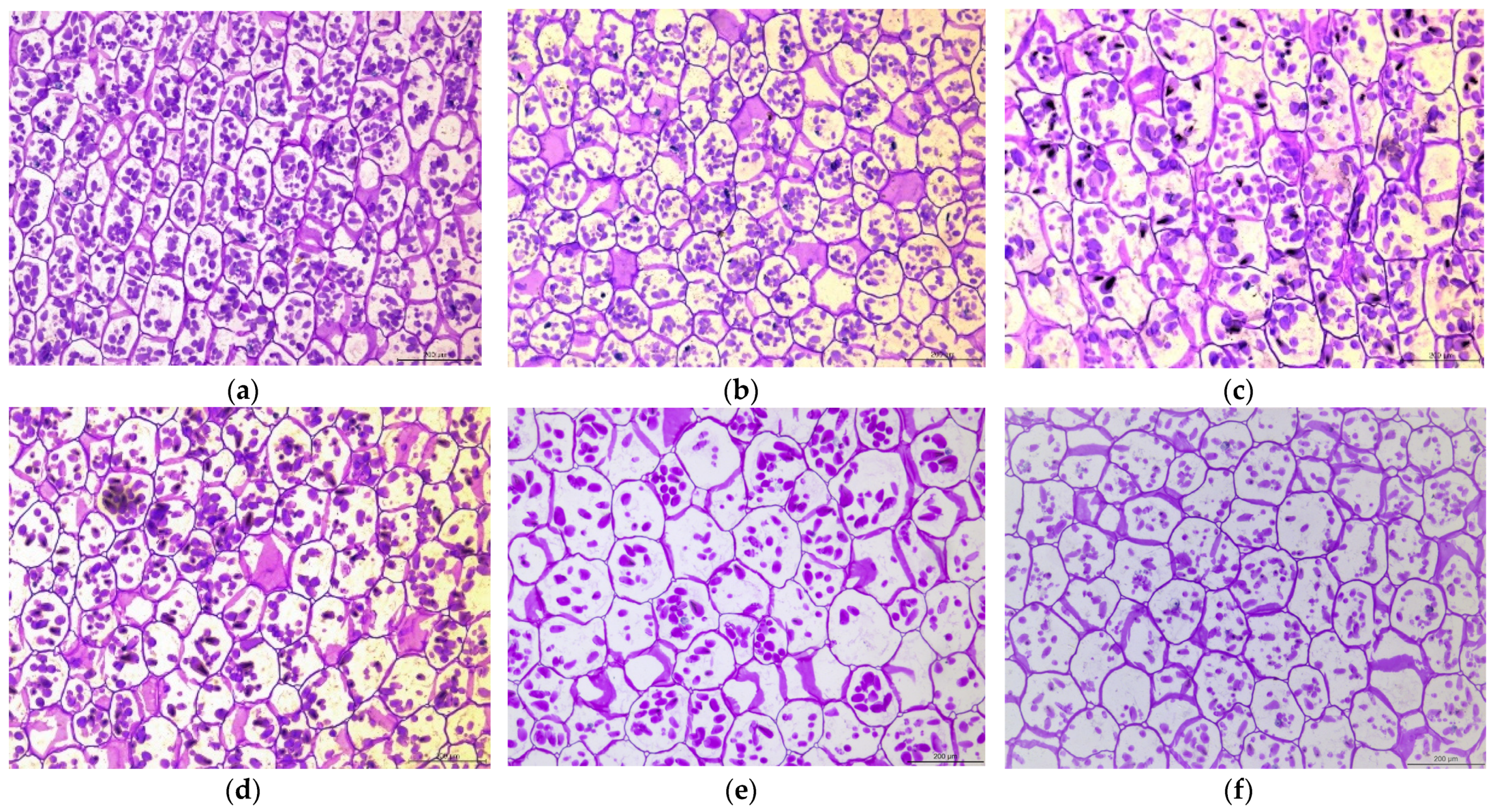
2.1. Starch Content and Starch Granules Are Gradually Reduced in Bulbs during Refrigeration
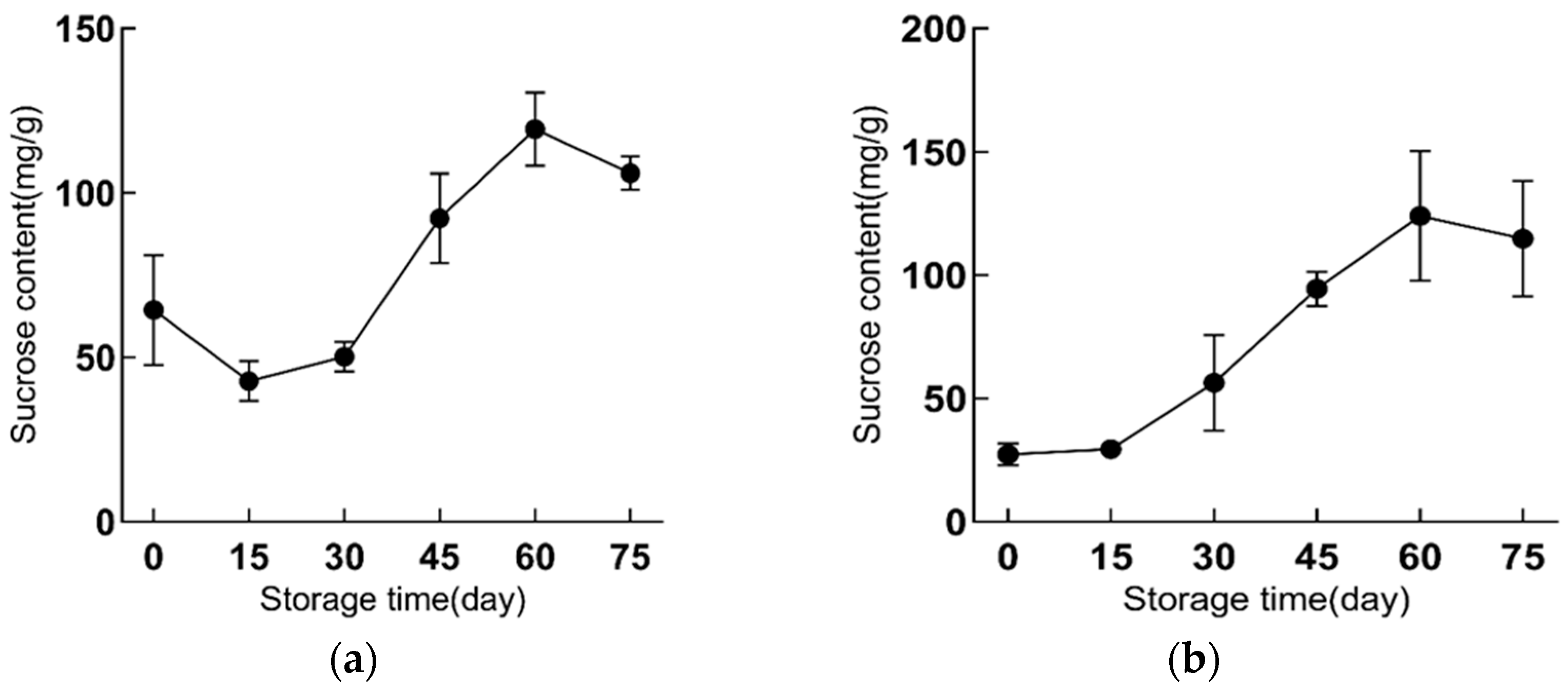
2.2. Sucrose Content Is Increased during Refrigeration
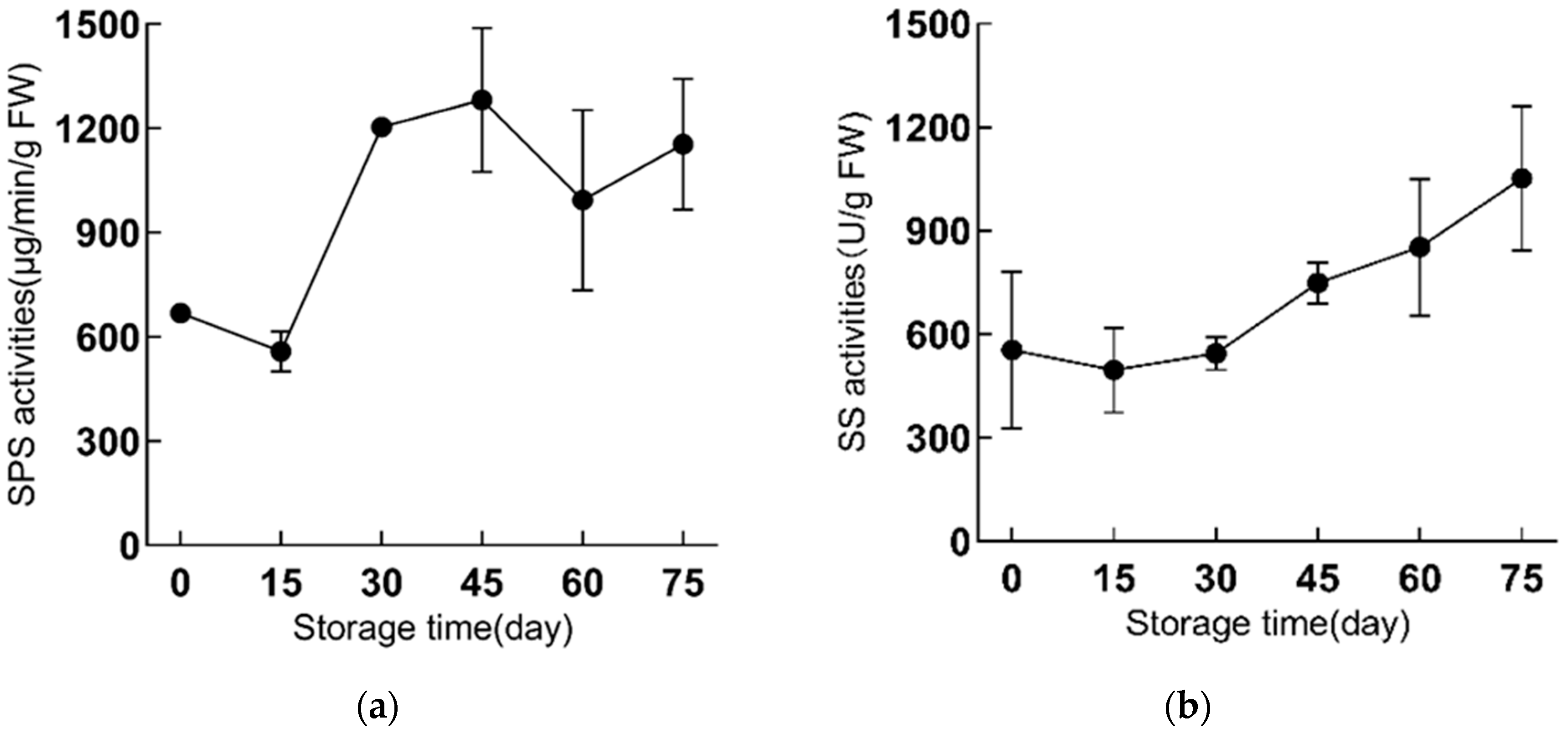
2.3. The SPS and SS Activities Are Enhanced during Refrigeration in ‘Fly Tiancheng’
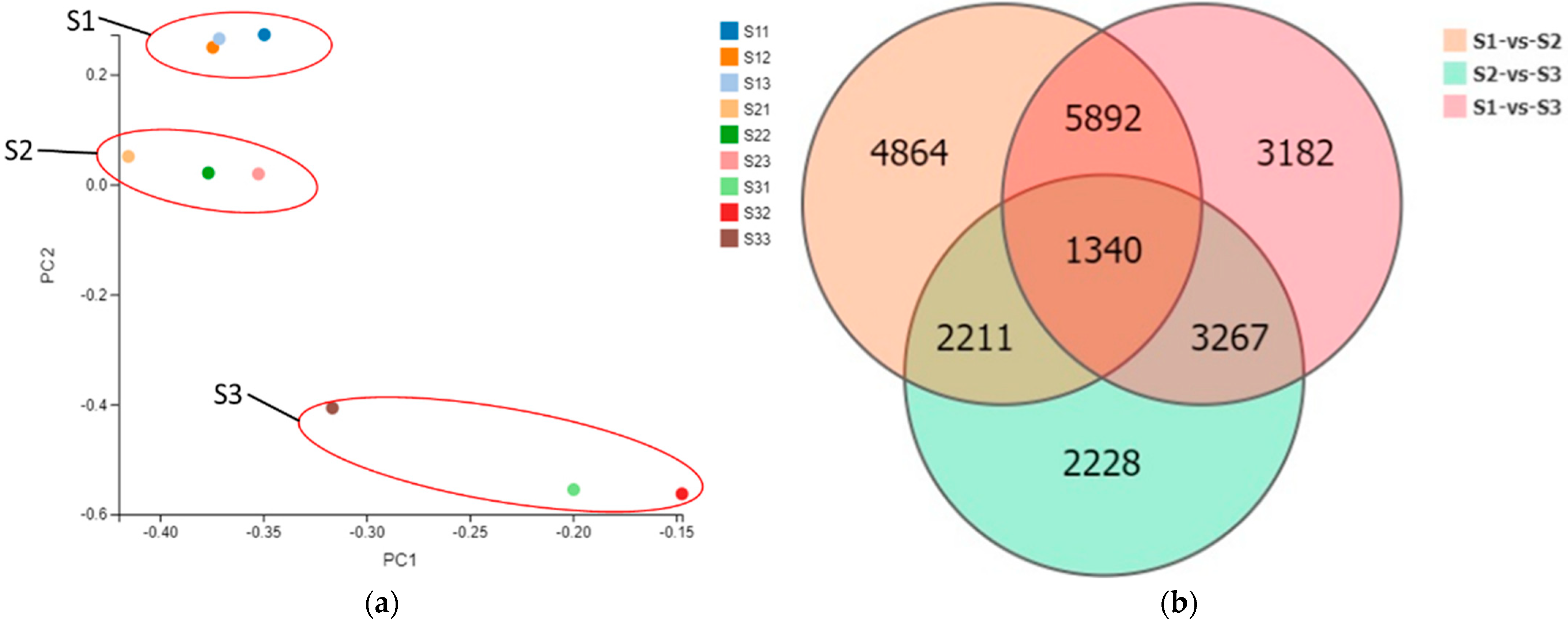
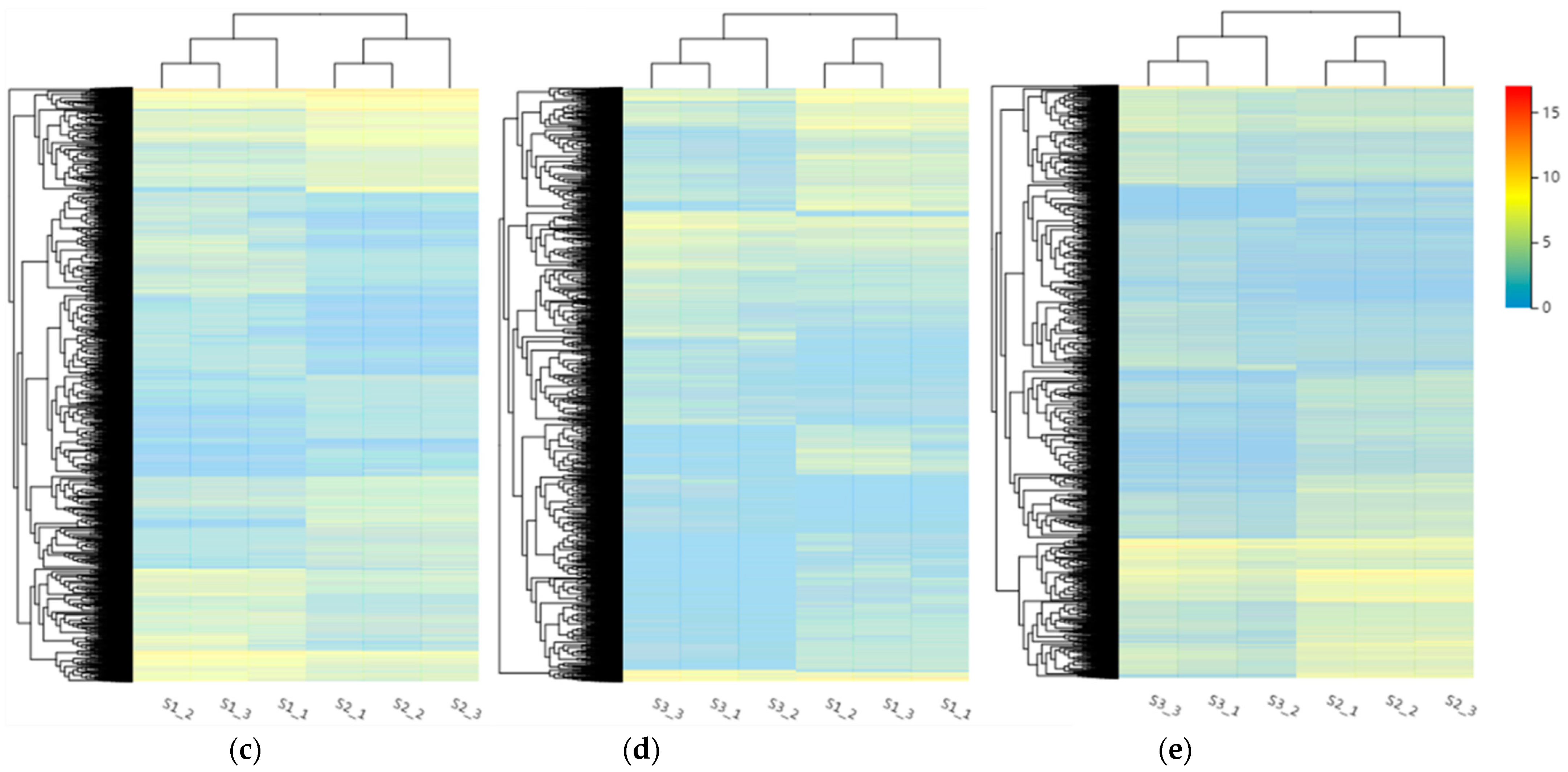
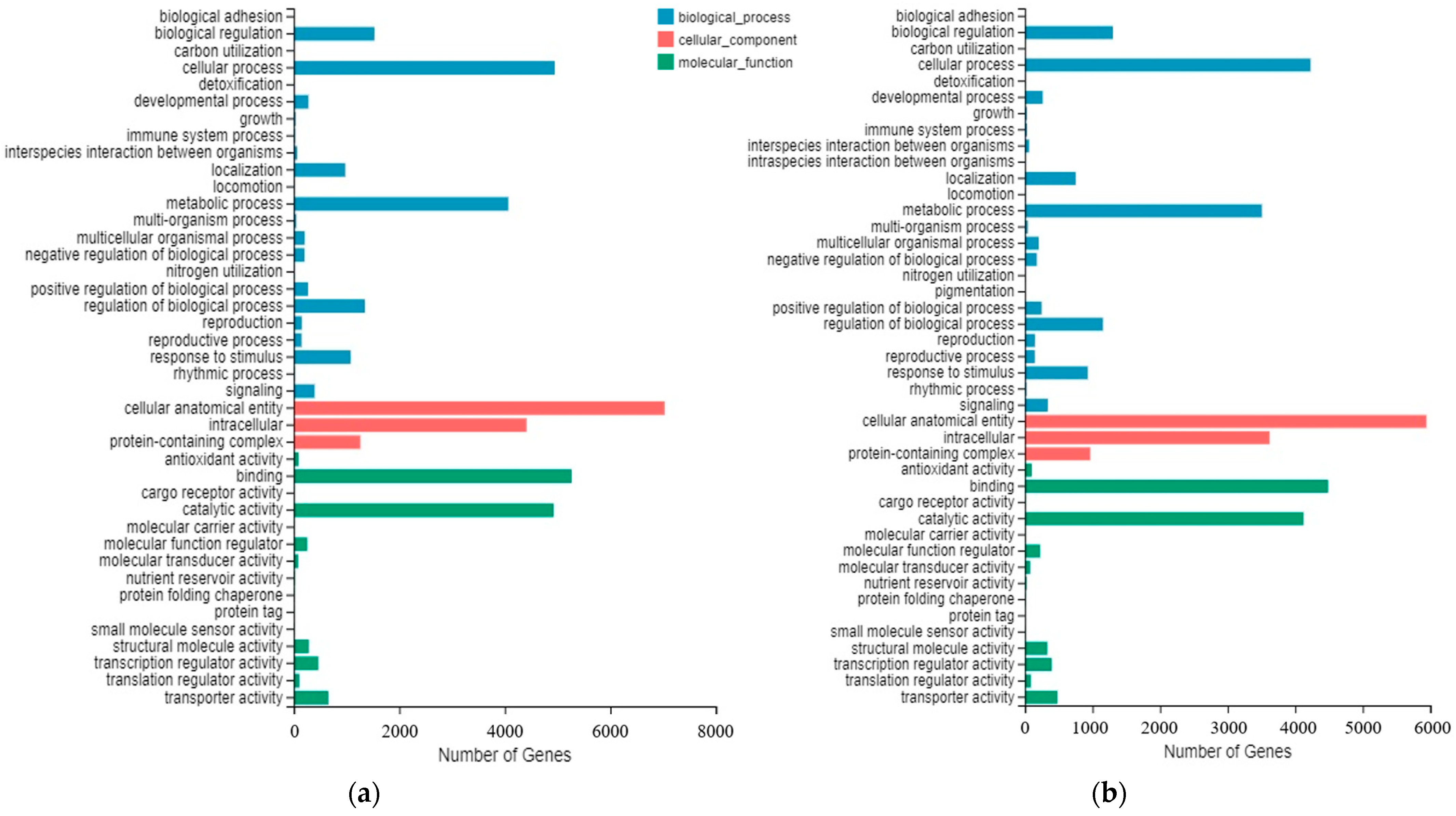
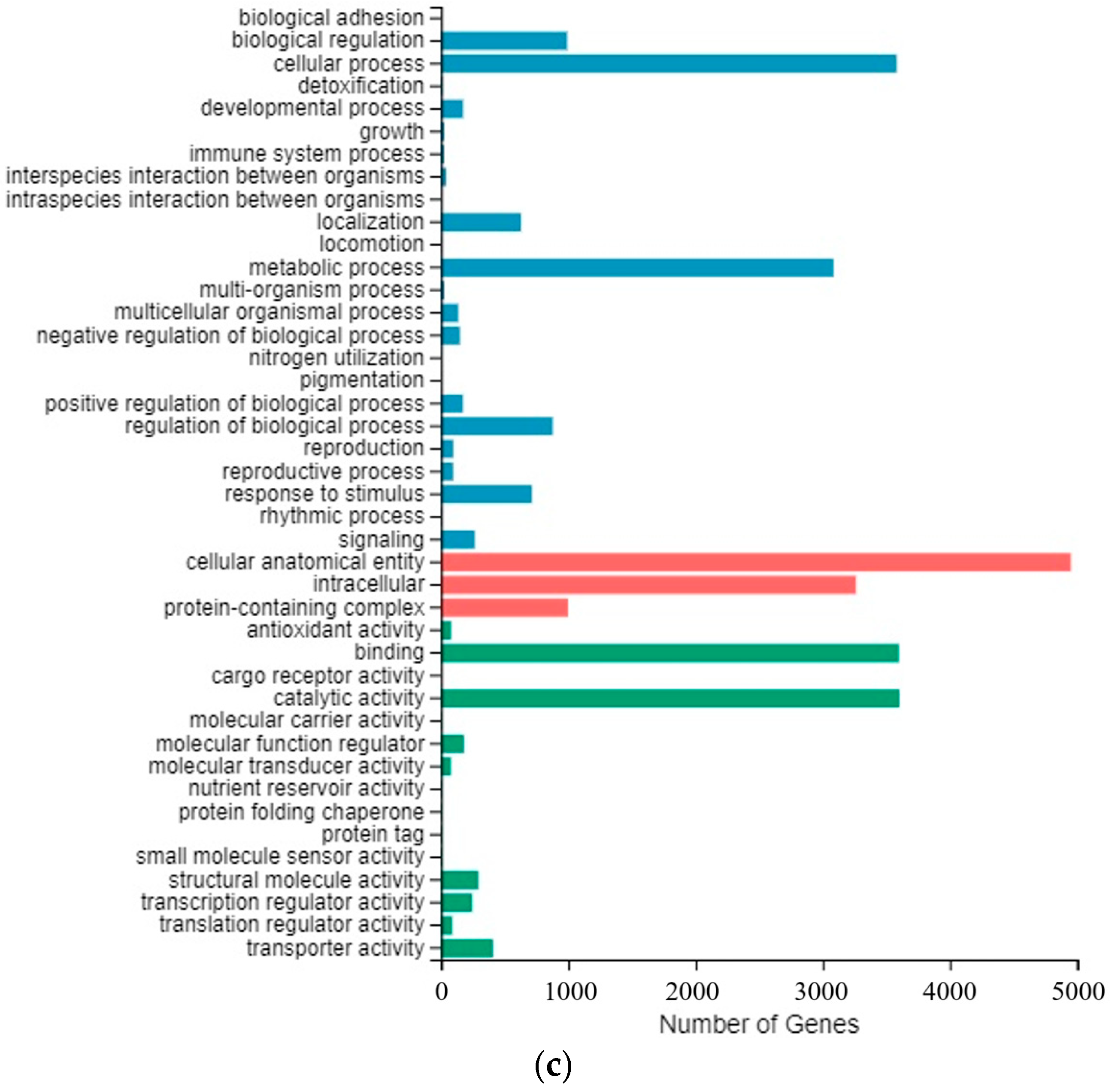
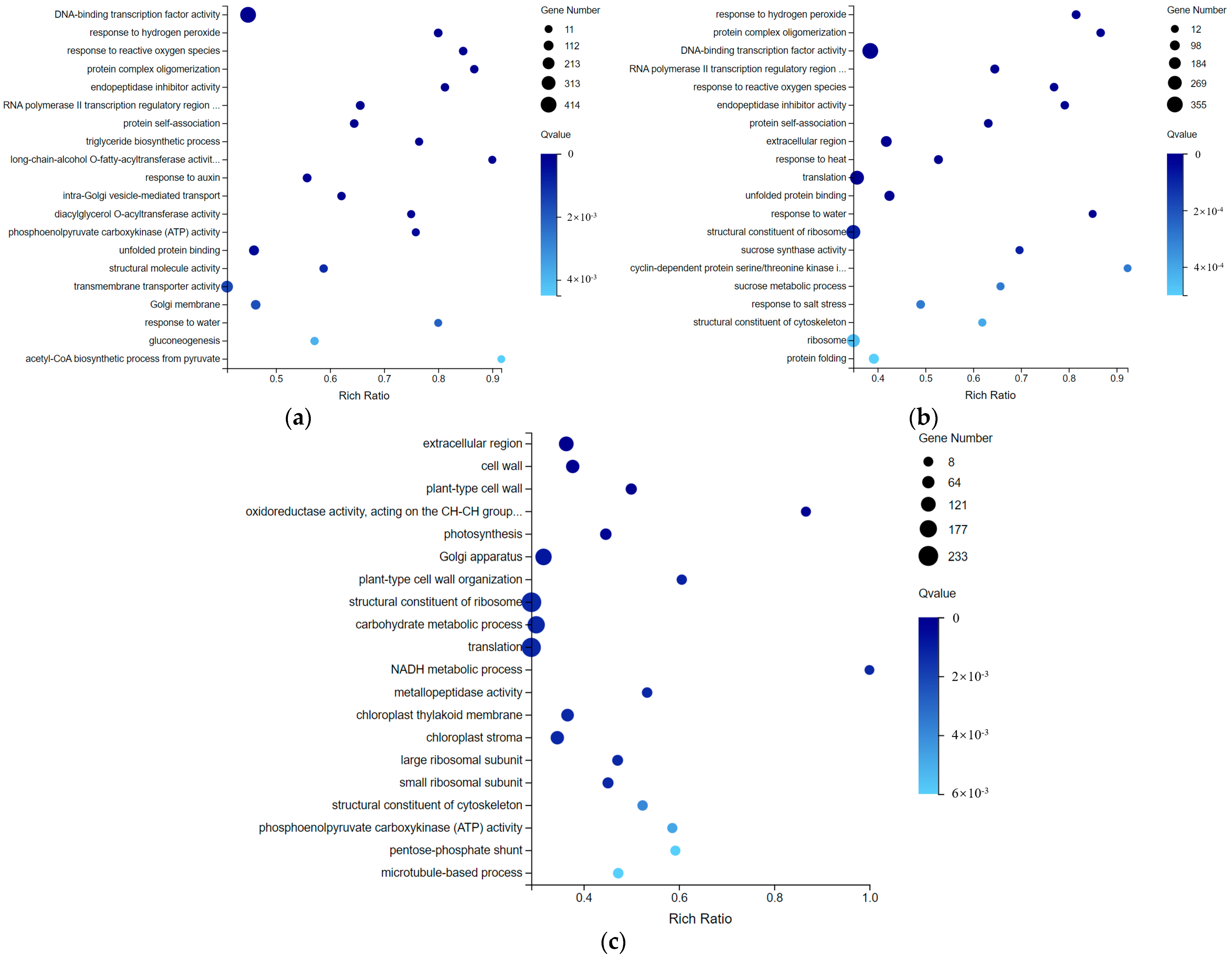
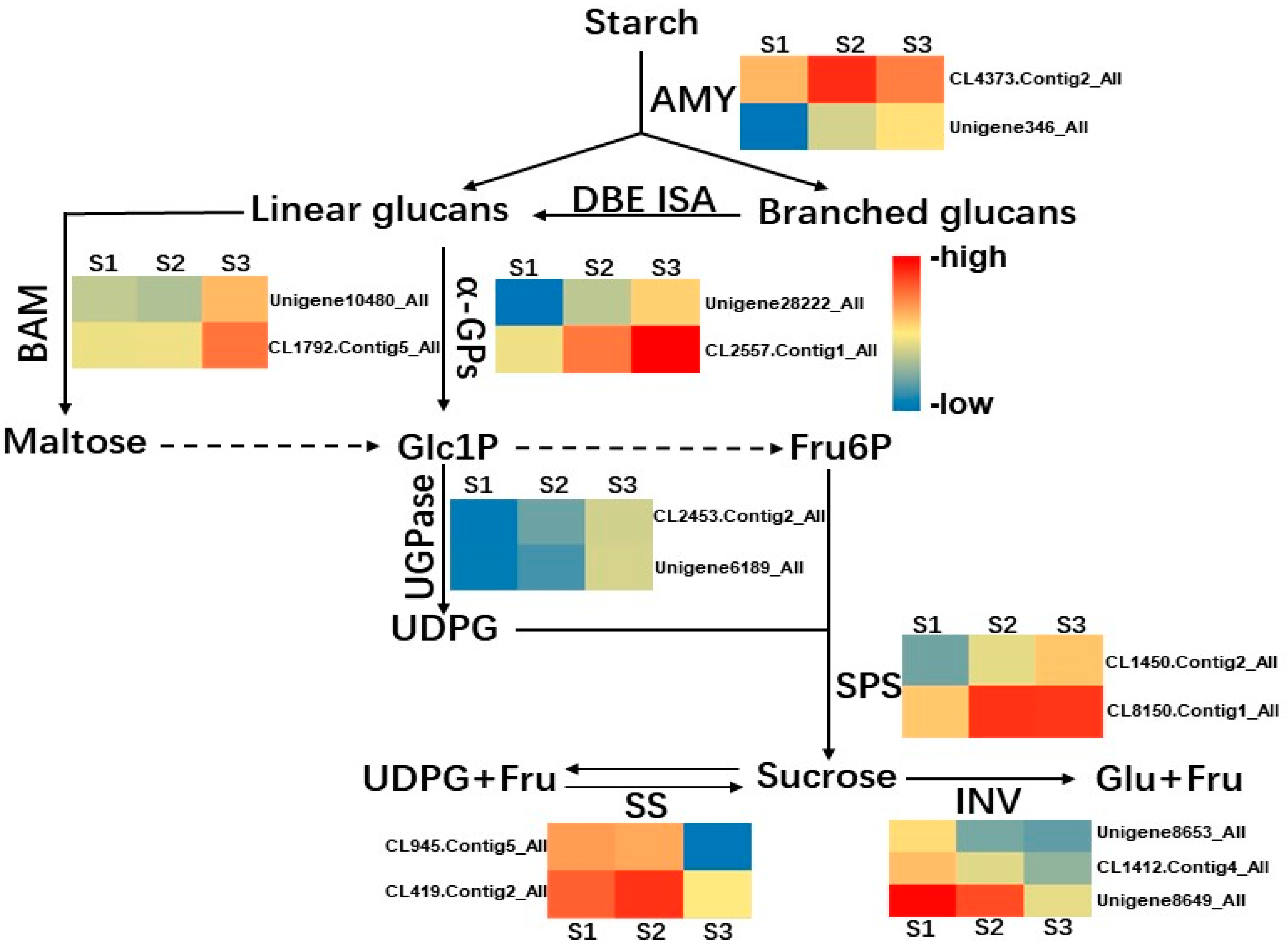
2.4. Transcriptome Data Shows Many DEGs Involving Sucrose Metabolism during Cold Storage
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Detection of Carbohydrate Contents
4.3. Histological Observation of Scales
4.4. Analysis of Sucrose Phosphate Synthase and Sucrose Synthase Activity
4.5. Total RNA Extraction
4.6. Construction of mRNA Library
4.7. De Novo Assembly and Gene Functional Annotation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Wu, Z.; Feng, J.; Yuan, G.; He, L.; Zhang, D.; Teng, N. A Novel R2R3-MYB Gene LoMYB33 From Lily Is Specifically Expressed in Anthers and Plays a Role in Pollen Development. Front. Plant Sci. 2021, 12, 730007. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, T.; Liu, X.; Yuan, G.; Hou, H.; Teng, N. A novel R2R3-MYB transcription factor LlMYB305 from Lilium longiflorum plays a positive role in thermotolerance via activating heat-protective genes. Environ. Exp. Bot. 2021, 184, 104399. [Google Scholar] [CrossRef]
- Ding, L.; Wu, Z.; Teng, R.; Xu, S.; Cao, X.; Yuan, G.; Zhang, D.; Teng, N. LlWRKY39 is involved in thermotolerance by activating LlMBF1c and interacting with LlCaM3 in lily (Lilium longiflorum). Hortic. Res. 2021, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Liu, X.; Wu, Z.; Teng, N. Transcriptome and Metabolome Analyses Provide Insights into the Stomium Degeneration Mechanism in Lily. Int. J. Mol. Sci. 2021, 22, 12124. [Google Scholar] [CrossRef]
- Zhao, K.; Xiao, Z.; Zeng, J.; Xie, H. Effects of Different Storage Conditions on the Browning Degree, PPO Activity, and Content of Chemical Components in Fresh Lilium Bulbs (Lilium brownii F.E.Brown var. viridulum Baker.). Agriculture 2021, 11, 184. [Google Scholar] [CrossRef]
- Yuee, L.; Huihui, W.; Wenbo, Z.; Haining, W.; Zhong, W. Evaluation of nutrition components in Lanzhou lily bulb by confocal Raman microscopy. Spectrochim. Acta. A 2021, 244, 118837. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Wei, H.; Zhang, Y.; Guo, Z.; Qiu, Y.; Wen, L.; Xie, Z. Structural characterization of Lanzhou lily (Lilium davidii var. unicolor) polysaccharides and determination of their associated antioxidant activity. J. Sci. Food Agric. 2020, 100, 5603–5616. [Google Scholar] [CrossRef]
- Munafo, J.J.; Gianfagna, T.J. Quantitative analysis of steroidal glycosides in different organs of Easter lily (Lilium longiflorum Thunb.) by LC-MS/MS. J. Agric. Food Chem. 2011, 59, 995–1004. [Google Scholar] [CrossRef]
- Munafo, J.J.; Ramanathan, A.; Jimenez, L.S.; Gianfagna, T.J. Isolation and structural determination of steroidal glycosides from the bulbs of easter lily (Lilium longiflorum Thunb.). J. Agric. Food Chem. 2010, 58, 8806–8813. [Google Scholar] [CrossRef]
- Wang, T.; Huang, H.; Zhang, Y.; Li, X.; Li, H.; Jiang, Q.; Gao, W. Role of effective composition on antioxidant, anti-inflammatory, sedative-hypnotic capacities of 6 common edible Lilium varieties. J. Food Sci. 2015, 80, H857–H868. [Google Scholar] [CrossRef]
- Pengyu, W.; Jian, L.; Fatma, A.K.A.; Wenyi, K.; Jinfeng, W.; Zhenhua, L.; Changqin, L. A critical review on chemical constituents and pharmacological effects of Lilium. Food Sci. Hum. Well. 2019, 8, 330–336. [Google Scholar] [CrossRef]
- Hong, X.-X.; Luo, J.-G.; Guo, C.; Kong, L.-Y. New steroidal saponins from the bulbs of Lilium brownii var. viridulum. Carbohyd. Res. 2012, 361, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, L.; Kong, L. Preparative separation of phenylpropenoid glycerides from the bulbs of Lilium lancifolium by high-speed counter-current chromatography and evaluation of their antioxidant activities. Food Chem. 2011, 131, 1056–1062. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, N.; Qiu, F.; Hai, W.; Tang, H.; Zhang, Y.; Wen, A. Triterpenoid saponins from the roots of Clematis argentilucida and their cytotoxic activity. Planta Med. 2014, 80, 942–948. [Google Scholar] [CrossRef]
- Lee, E.; Yun, N.; Jang, Y.P.; Kim, J. Lilium lancifolium Thunb. extract attenuates pulmonary inflammation and air space enlargement in a cigarette smoke-exposed mouse model. J. Ethnopharmacol. 2013, 149, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Luo, J.; Lv, H.; Kong, L. Determination of anti-hyperglycaemic activity in steroidal glycoside rich fraction of lily bulbs and characterization of the chemical profiles by LC-Q-TOF-MS/MS. J. Funct. Foods 2014, 6, 585–597. [Google Scholar] [CrossRef]
- Hou, Y.; Jiang, J. Origin and concept of medicine food homology and its application in modern functional foods. Food Funct. 2013, 4, 1727–1741. [Google Scholar] [CrossRef]
- Bao, J.; Liu, C. Study on the Development of Agricultural Products Cold Chain Logistics. In Proceedings of the 2014 International Conference on Mechatronics, Electronic, Industrial and Control Engineering, Shenyang, China, 15–17 November 2014; pp. 1667–1670. [Google Scholar]
- Farcuh, M.; Copes, B.; Le-Navenec, G.; Marroquin, J.; Cantu, D.; Bradford, K.J.; Guinard, J.; Deynze, A.V. Sensory, physicochemical and volatile compound analysis of short and long shelf-life melon (Cucumis melo L.) genotypes at harvest and after postharvest storage. Food Chem. X 2020, 8, 100107. [Google Scholar] [CrossRef]
- Martinez-Romero, D.; Valero, D.; Serrano, M.; Burlo, F.; Carbonell, A.; Burgos, L.; Riquelme, F. Exogenous polyamines and gibberellic acid effects on peach (Prunus persica L.) storability improvement. J. Food Sci. 2000, 65, 288–294. [Google Scholar] [CrossRef]
- Nunes, M.C.N.; Emond, J.-P.; Brecht, J.K. Quality Curves for Highbush Blueberries as a Function of the Storage Temperature. Small Fruits Rev. 2004, 3, 423–440. [Google Scholar] [CrossRef]
- Cheng, S.; Wei, B.; Zhou, Q.; Tan, D.; Ji, S. 1-Methylcyclopropene alleviates chilling injury by regulating energy metabolism and fatty acid content in ‘Nanguo’ pears. Postharvest Biol. Technol. 2015, 109, 130–136. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, C.; Cheng, S.; Wei, B.; Liu, X.; Ji, S. Changes in energy metabolism accompanying pitting in blueberries stored at low temperature. Food Chem. 2014, 164, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Moshchenskaya, Y.L.; Galibina, N.A.; Novitskaya, L.L.; Nikerova, K.M. The Role of Sucrose Synthase in Sink Organs of Woody Plants. Russ. J. Plant Physiol. 2019, 66, 10–21. [Google Scholar] [CrossRef]
- Wan, H.; Wu, L.; Yang, Y.; Zhou, G.; Ruan, Y. Evolution of Sucrose Metabolism: The Dichotomy of Invertases and Beyond. Trends Plant Sci. 2018, 23, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Xie, Z.K.; Wang, Y.J.; An, L.P. Changes in Carbohydrate Metabolism and Bulb Growth as Induced by Low-Temperature Release of Dormancy in Lily Bulbs. Philipp. Agric. Sci. 2011, 94, 149–154. [Google Scholar]
- Yong, Y.B.; Li, W.Q.; Wang, J.M.; Zhang, Y.; Lu, Y.M. Identification of gene co-expression networks involved in cold resistance of Lilium lancifolium. Biol. Plantarum 2018, 62, 287–298. [Google Scholar] [CrossRef]
- Xu, R.; Niimi, Y.; Han, D. Changes in endogenous abscisic acid and soluble sugars levels during dormancy-release in bulbs of Lilium rubellum. Sci. Hortic. 2006, 111, 68–72. [Google Scholar] [CrossRef]
- Shin, K.S.; Chakrabarty, D.; Paek, K.Y. Sprouting rate, change of carbohydrate contents and related enzymes during cold treatment of lily bulblets regenerated in vitro. Sci. Hortic. 2002, 96, 195–204. [Google Scholar] [CrossRef]
- Yang, C.; Li, Q.; Jiang, X.; Fan, Y.; Gao, J.; Zhang, C. Dynamic changes in alpha- and beta-amylase activities and gene expression in bulbs of the Oriental hybrid lily ‘Siberia’ during dormancy release. J. Hortic. Sci. Biotechnol. 2015, 90, 753–759. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Shammai, A.; Petreikov, M.; Yeselson, Y.; Faigenboim, A.; Moy-Komemi, M.; Cohen, S.; Cohen, D.; Besaulov, E.; Efrati, A.; Houminer, N.; et al. Natural genetic variation for expression of a SWEET transporter among wild species of Solanum lycopersicum (tomato) determines the hexose composition of ripening tomato fruit. Plant J. 2018, 96, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Saleem, B.A.; Malik, A.U.; Pervez, M.A.; Khan, A.S.; Khan, M.N. Spring application of growth regulators affects fruit quality of ‘blood red’ sweet orange. Pak. J. Bot. 2008, 40, 1013–1023. [Google Scholar]
- Gomez, M.; Lajolo, F.; Cordenunsi, B. Evolution of soluble sugars during ripening of papaya fruit and its relation to sweet taste. J. Food Sci. 2002, 67, 442–447. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C. Starch: A Flexible, Adaptable Carbon Store Coupled to Plant Growth. Annu. Rev. Plant Biol. 2020, 71, 217–245. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, J.; Kaur, L.; Sodhi, N.S.; Gill, B.S. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Sikora, M.; Kowalski, S.; Tomasik, P. Binary hydrocolloids from starches and xanthan gum. Food Hydrocoll. 2008, 22, 943–952. [Google Scholar] [CrossRef]
- Irving, D.E.; Hurst, P.L.; Ragg, J.S. Changes in carbohydrates and carbohydrate metabolizing enzymes during the development, maturation, and ripening of buttercup squash (Cucurbita maxima D ‘Delica’). J. Am. Soc. Hortic. Sci. 1997, 122, 310–314. [Google Scholar] [CrossRef]
- Harvey, W.J.; Grant, D.G.; Lammerink, J.P. Physical and sensory changes during the development and storage of buttercup squash. N. Z. J. Crop Hortic. Sci. 1997, 25, 341–351. [Google Scholar] [CrossRef]
- Phillips, T.G. Changes in the Composition of Squash During Storage. Plant Physiol. 1946, 21, 533–541. [Google Scholar] [CrossRef]
- Dijk, C.V.; Fischer, M.; Holm, J.; Beekhuizen, J.G.; Stolle-Smits, T.; Boeriu, C. Texture of cooked potatoes (Solanum tuberosum). 1. Relationships between dry matter content, sensory-perceived texture, and near-infrared spectroscopy. J. Agric. Food Chem. 2002, 50, 5082–5088. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, H.; Liu, J.; Reid, S.; Liu, T.; Xu, S.; Tian, Z.; Sonnewald, U.; Song, B.; Xie, C. Amylases StAmy23, StBAM1 and StBAM9 regulate cold-induced sweetening of potato tubers in distinct ways. J. Exp. Bot. 2017, 68, 2317–2331. [Google Scholar] [CrossRef] [PubMed]
- Malone, J.G.; Mittova, V.; Ratcliffe, R.G.; Kruger, N.J. The response of carbohydrate metabolism in potato tubers to low temperature. Plant Cell Physiol. 2006, 47, 1309–1322. [Google Scholar] [CrossRef] [PubMed]
- Weber, A. Solute transporters as connecting elements between cytosol and plastid stroma. Curr. Opin. Plant Biol. 2004, 7, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Geigenberger, P.; Stitt, M. Sucrose synthase catalyses a readily reversible reaction in vivo in developing potato tubers and other plant tissues. Planta 1993, 189, 329–339. [Google Scholar] [CrossRef]
- Ruan, Y.; Jin, Y.; Yang, Y.; Li, G.; Boyer, J.S. Sugar Input, Metabolism, and Signaling Mediated by Invertase: Roles in Development, Yield Potential, and Response to Drought and Heat. Mol. Plant 2010, 3, 942–955. [Google Scholar] [CrossRef]
- Su, J.; Yao, Y.; Liu, Y.; Han, Q.; Zhang, W. Function, structure and catalytic mechanism of sucrose phosphate synthase: A review. Chin. J. Biotechnol. 2021, 37, 1858–1868. [Google Scholar] [CrossRef]
- Wang, L.; Cui, N.; Zhang, K.; Fan, H.; Li, T. Research Advance of Sucrose Phosphate Synthase (SPS) in Higher Plant. Int. J. Agric. Biol. 2013, 15, 1221–1226. [Google Scholar]
- Winter, H.; Huber, S.C. Regulation of sucrose metabolism in higher plants: Localization and regulation of activity of key enzymes. Crit. Rev. Biochem. Mol. 2000, 35, 253–289. [Google Scholar] [CrossRef]
- Huber, S.C.; Huber, J.L. Role and regulation of sucrose-phosphate synthase in higher plants. Annu. Rev. Plant Biol. 1996, 47, 431–444. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Huang, Z.; Mi, L.; Xu, K.; Wu, J.; Fan, Y.; Ma, S.; Jiang, D. Effects of Low Temperature at Booting Stage on Sucrose Metabolism and Endogenous Hormone Contents in Winter Wheat Spikelet. Front. Plant Sci. 2019, 10, 498. [Google Scholar] [CrossRef]
- Nemati, F.; Ghanati, F.; Gavlighi, H.A.; Sharifi, M. Comparison of sucrose metabolism in wheat seedlings during drought stress and subsequent recovery. Biol. Plant. 2018, 62, 595–599. [Google Scholar] [CrossRef]
- Solis-Guzman, M.G.; Arguello-Astorga, G.; Lopez-Bucio, J.; Ruiz-Herrera, L.F.; Lopez-Meza, J.E.; Sanchez-Calderon, L.; Carreon-Abud, Y.; Martinez-Trujillo, M. Arabidopsis thaliana sucrose phosphate synthase (sps) genes are expressed differentially in organs and tissues, and their transcription is regulated by osmotic stress. Gene Expr. Patterns 2017, 25, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Baroja-Fernandez, E.; Munoz, F.J.; Montero, M.; Etxeberria, E.; Sesma, M.T.; Ovecka, M.; Bahaji, A.; Ezquer, I.; Li, J.; Prat, S.; et al. Enhancing Sucrose Synthase Activity in Transgenic Potato (Solanum tuberosum L.) Tubers Results in Increased Levels of Starch, ADPglucose and UDPglucose and Total Yield. Plant Cell Physiol. 2009, 50, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, R.; Salanoubat, M.; Willmitzer, L.; Sonnewald, U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). Plant J. 1995, 7, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Touchette, B.W.; Burkholder, J.M. Overview of the physiological ecology of carbon metabolism in seagrasses. J. Exp. Mar. Biol. Ecol. 2000, 250, 169–205. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 354–357. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Xu, S.; Liu, X.; Li, T.; Zhang, D.; Teng, N.; Wu, Z. Starch Degradation and Sucrose Accumulation of Lily Bulbs after Cold Storage. Int. J. Mol. Sci. 2022, 23, 4366. https://doi.org/10.3390/ijms23084366
Yu J, Xu S, Liu X, Li T, Zhang D, Teng N, Wu Z. Starch Degradation and Sucrose Accumulation of Lily Bulbs after Cold Storage. International Journal of Molecular Sciences. 2022; 23(8):4366. https://doi.org/10.3390/ijms23084366
Chicago/Turabian StyleYu, Junpeng, Sujuan Xu, Xinyue Liu, Ting Li, Dehua Zhang, Nianjun Teng, and Ze Wu. 2022. "Starch Degradation and Sucrose Accumulation of Lily Bulbs after Cold Storage" International Journal of Molecular Sciences 23, no. 8: 4366. https://doi.org/10.3390/ijms23084366
APA StyleYu, J., Xu, S., Liu, X., Li, T., Zhang, D., Teng, N., & Wu, Z. (2022). Starch Degradation and Sucrose Accumulation of Lily Bulbs after Cold Storage. International Journal of Molecular Sciences, 23(8), 4366. https://doi.org/10.3390/ijms23084366





