Redox Properties of 3-Iodothyronamine (T1AM) and 3-Iodothyroacetic Acid (TA1)
Abstract
:1. Introduction
2. Results
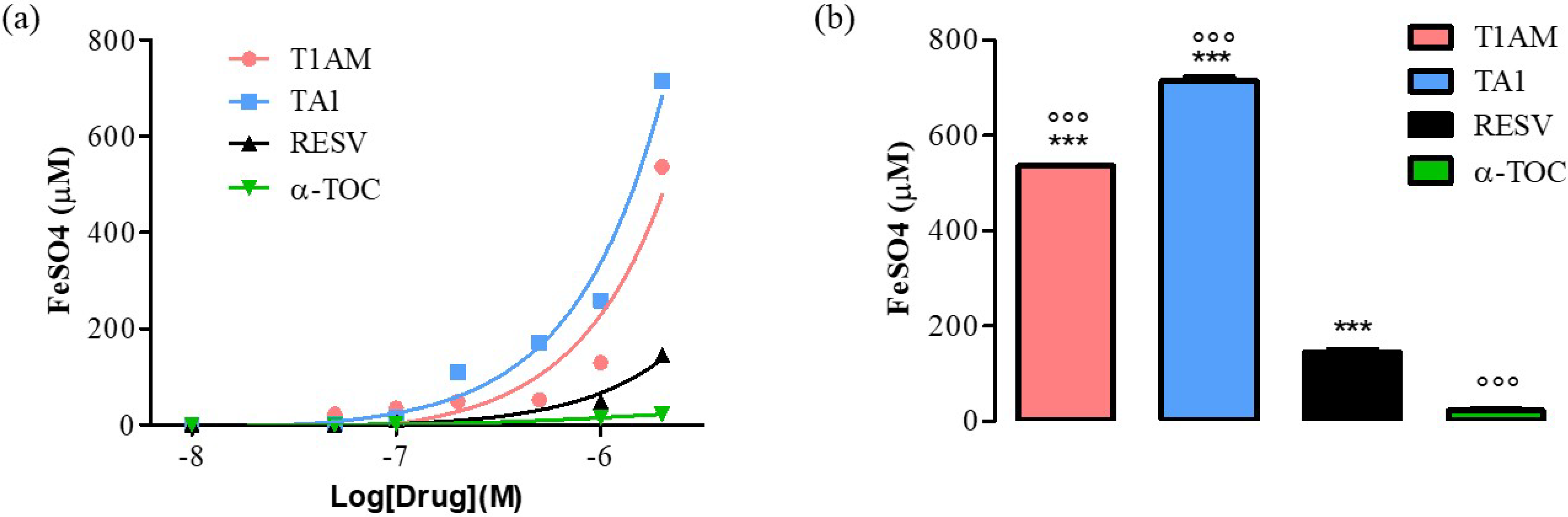
2.1. T1AM and TA1 Showed Ferric-Reducing Capacity and Superoxide and Hydroxyl Radical Scavenging Activities
2.2. T1AM and TA1 Modify the Expression and Activity Levels of Pro- and Antioxidant Cell Proteins
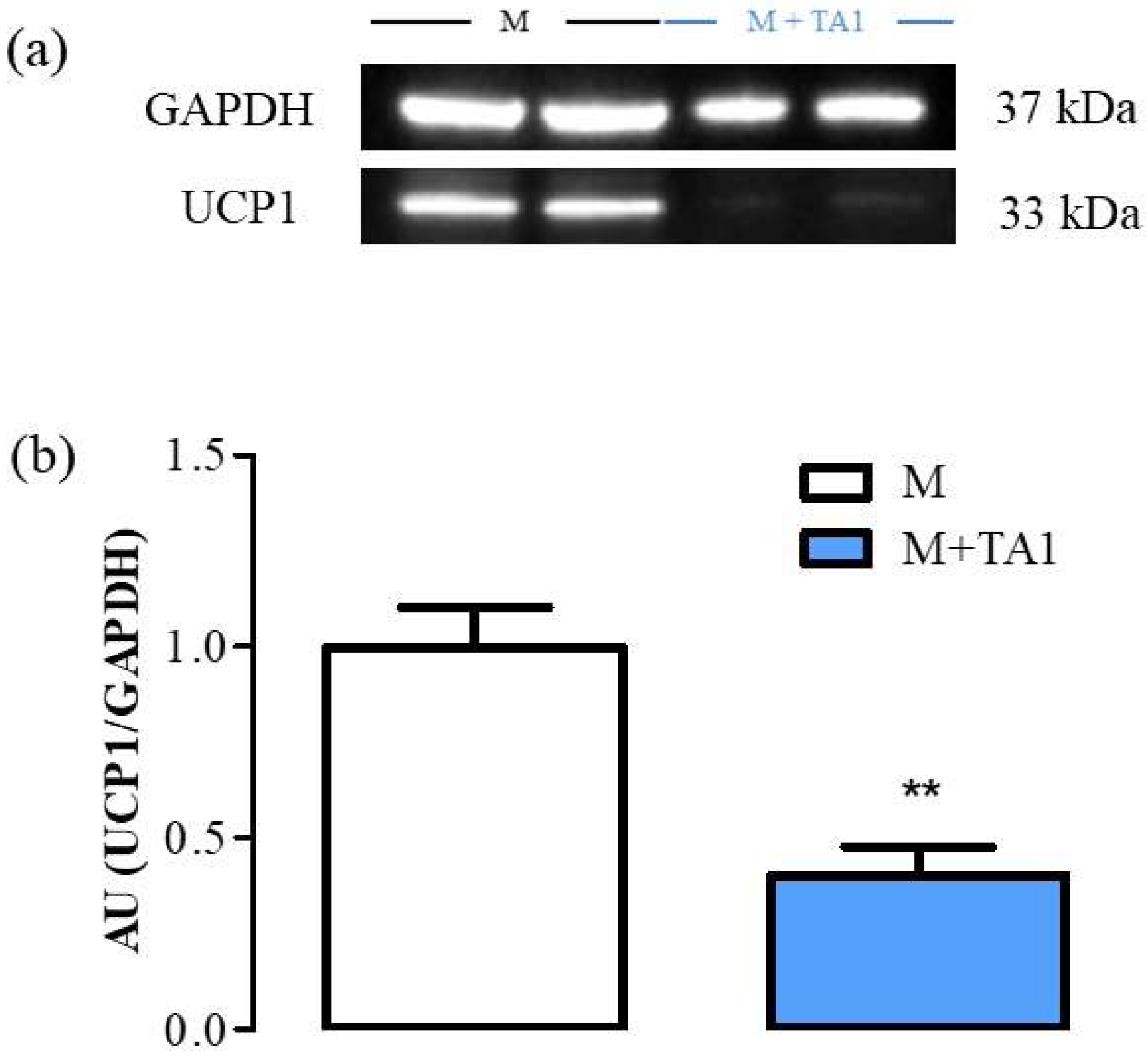
2.2.1. Evaluation of UCP-1 Protein and Amine Oxidases mRNA Levels in M + T1AM and M + TA1 Cells
2.2.2. Evaluation of mRNA Levels for MAO (A and B) and SSAO in M + T1AM and M + TA1 Cells
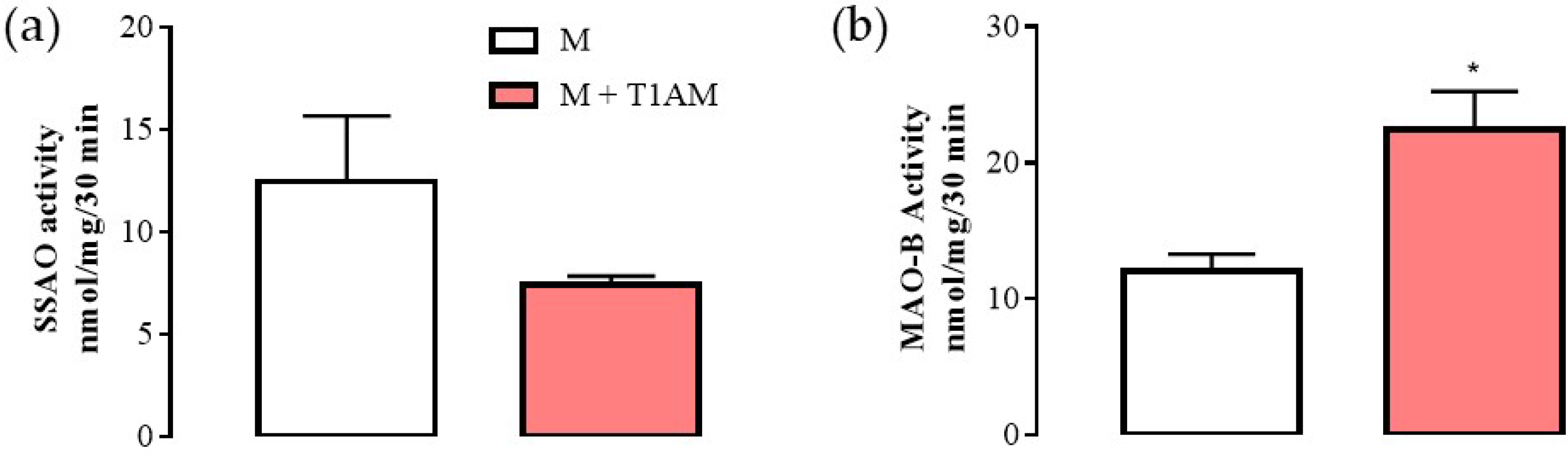
2.2.3. MAO-B Activity
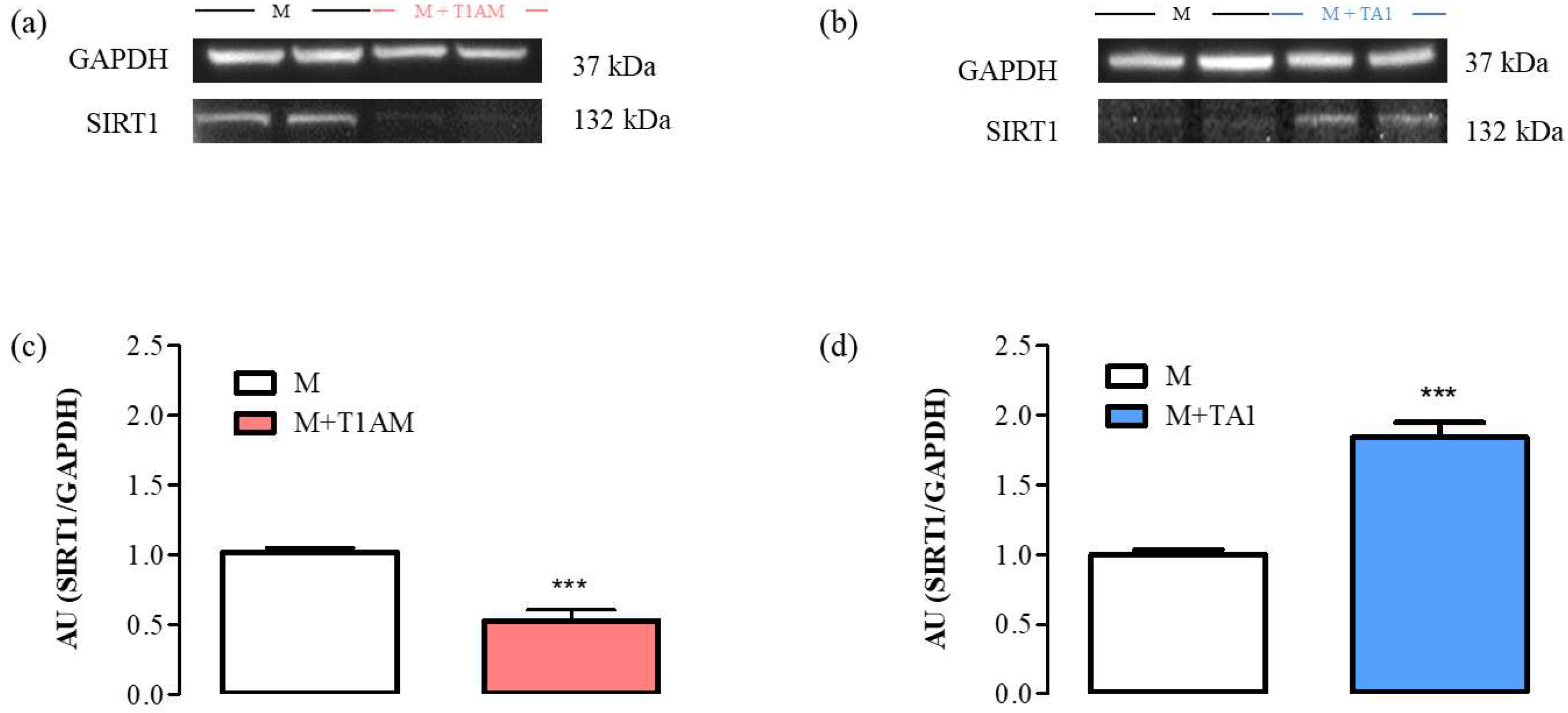
2.2.4. SIRT1 Expression and Activity in M + T1AM and M + TA1 Cells
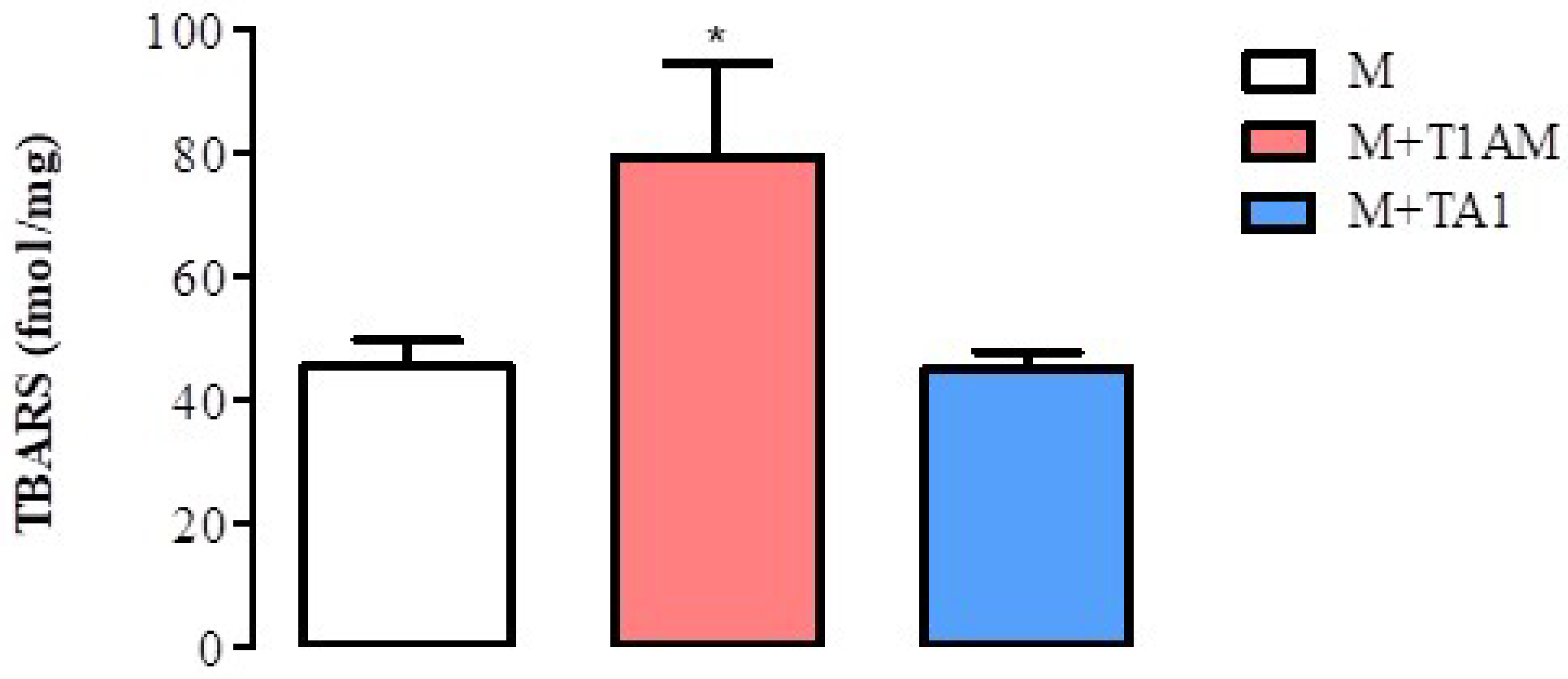
2.2.5. Lipid Peroxidation in M + T1AM and M + TA1 Cells
3. Discussion
4. Materials and Methods
4.1. Ferric-Reducing Activity Power (FRAP)
4.2. Superoxide Scavenging Activity
4.3. Hydroxyl Radicals’ Scavenging Activity
4.4. Cell Culture
4.5. Western Blot
4.6. RT-PCR
4.7. Determination of MAO and SSAO Activities (Radiochemical Assay)
4.8. Thiobarbituric Acid Reactive Substances (TBARS)
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Scanlan, T.S.; Suchland, K.L.; Hart, M.E.; Chiellini, G.; Huang, Y.; Kruzich, P.J.; Frascarelli, S.; Crossley, D.A.; Bunzow, J.R.; Ronca-Testoni, S.; et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nat. Med. 2004, 10, 638–642. [Google Scholar] [CrossRef]
- Köhrle, J.; Biebermann, H. 3-Iodothyronamine-A Thyroid Hormone Metabolite with Distinct Target Profiles and Mode of Action. Endocr. Rev. 2019, 40, 602–630. [Google Scholar] [CrossRef] [PubMed]
- Laurino, A.; Gencarelli, M.; Raimondi, L. The 3-iodothyronamine (T1AM) and the 3-iodothyroacetic acid (TA1) indicate a novel connection with the histamine system for neuroprotection. Eur. J. Pharmacol. 2021, 912, 174606. [Google Scholar] [CrossRef] [PubMed]
- Duicu, O.M.; Lighezan, R.; Sturza, A.; Balica, R.; Vaduva, A.; Feier, H.; Gaspar, M.; Ionac, A.; Noveanu, L.; Borza, C.; et al. Assessment of Mitochondrial Dysfunction and Monoamine Oxidase Contribution to Oxidative Stress in Human Diabetic Hearts. Oxid. Med. Cell. Longev. 2016, 2016, 8470394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, P.; Kunduzova, O.; Masini, E.; Cambon, C.; Bani, D.; Raimondi, L.; Seguelas, M.H.; Nistri, S.; Colucci, W.; Leducq, N.; et al. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation 2005, 112, 3297–3305. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Li, J.; Wang, J.; Pu, T.; Wei, J.; Li, Q.; Wu, B.J. MAOA promotes prostate cancer cell perineural invasion through SEMA3C/PlexinA2/NRP1-cMET signaling. Oncogene 2021, 40, 1362–1374. [Google Scholar] [CrossRef]
- Accorroni, A.; Rutigliano, G.; Sabatini, M.; Frascarelli, S.; Borsò, M.; Novelli, E.; Bandini, L.; Ghelardoni, S.; Saba, A.; Zucchi, R.; et al. Exogenous 3-Iodothyronamine Rescues the Entorhinal Cortex from β-Amyloid Toxicity. Thyroid 2020, 30, 147–160. [Google Scholar] [CrossRef]
- Landucci, E.; Gencarelli, M.; Mazzantini, C.; Laurino, A.; Pellegrini-Giampietro, D.E.; Raimondi, L. N-(3-Ethoxy-phenyl)-4-pyrrolidin-1-yl-3-trifluoromethyl-benzamide (EPPTB) prevents 3-iodothyronamine (T1AM)-induced neuroprotection against kainic acid toxicity. Neurochem. Int. 2019, 129, 104460. [Google Scholar] [CrossRef]
- Doyle, K.P.; Suchland, K.L.; Ciesielski, T.M.; Lessov, N.S.; Grandy, D.K.; Scanlan, T.S.; Stenzel-Poore, M.P. Novel thyroxine derivatives, thyronamine and 3-iodothyronamine, induce transient hypothermia and marked neuroprotection against stroke injury. Stroke 2007, 38, 2569–2576. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Hu, B.; Liu, X. Thyroid Hormone Metabolite 3-Iodothyronamine (T1AM) Alleviates Hypoxia/Reoxygenation-Induced Cardiac Myocyte Apoptosis via Akt/FoxO1 Pathway. Med. Sci. Monit. 2020, 26, e923195. [Google Scholar] [CrossRef]
- Ghanian, Z.; Maleki, S.; Reiland, H.; Bütz, D.E.; Chiellini, G.; Assadi-Porter, F.M.; Ranji, M. Optical imaging of mitochondrial redox state in rodent models with 3-iodothyronamine. Exp. Biol. Med. 2014, 239, 151–158, Erratum in Exp. Biol. Med. 2014, 239, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacripanti, G.; Lorenzini, L.; Bandini, L.; Frascarelli, S.; Zucchi, R.; Ghelardoni, S. 3-Iodothyronamine and 3,5,3′-triiodo-L-thyronine reduce SIRT1 protein expression in the HepG2 cell line. Horm. Mol. Biol. Clin. Investig. 2020, 41, 20190045. [Google Scholar] [CrossRef] [PubMed]
- Assadi-Porter, F.M.; Reiland, H.; Sabatini, M.; Lorenzini, L.; Carnicelli, V.; Rogowski, M.; Selen Alpergin, E.S.; Tonelli, M.; Ghelardoni, S.; Saba, A.; et al. Metabolic Reprogramming by 3-Iodothyronamine (T1AM): A New Perspective to Reverse Obesity through Co-Regulation of Sirtuin 4 and 6 Expression. Int. J. Mol. Sci. 2018, 19, 1535. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.; Escande, C.; Denicola, A. Potential Modulation of Sirtuins by Oxidative Stress. Oxid. Med. Cell. Longev. 2016, 2016, 9831825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Zhang, H.; Ji, S.; Jia, P.; Chen, Y.; Li, Y.; Wang, T. Resveratrol and its derivative pterostilbene attenuate oxidative stress-induced intestinal injury by improving mitochondrial redox homeostasis and function via SIRT1 signaling. Free Radic. Biol. Med. 2021, 177, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Q.; Yan, H.; Jiao, G.; Wang, H.; Chi, H.; Zhou, H.; Chen, L.; Shan, Y.; Chen, Y. Resveratrol Protects Osteoblasts against Dexamethasone-Induced Cytotoxicity through Activation of AMP-Activated Protein Kinase. Drug Des. Dev. Ther. 2020, 14, 4451–4463. [Google Scholar] [CrossRef]
- Gencarelli, M.; Laurino, A.; Landucci, E.; Buonvicino, D.; Mazzantini, C.; Chiellini, G.; Raimondi, L. 3-Iodothyronamine Affects Thermogenic Substrates’ Mobilization in Brown Adipocytes. Biology 2020, 9, 95. [Google Scholar] [CrossRef]
- Xu, D.F.; Liu, Y.J.; Mao, Y.F.; Wang, Y.; Xu, C.F.; Zhu, X.Y.; Jiang, L. Elevated angiotensin II induces platelet apoptosis through promoting oxidative stress in an AT1R-dependent manner during sepsis. J. Cell. Mol. Med. 2021, 25, 4124–4135. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, K.W.; Liang, Y.; Ip, M.S.; Mak, J.C. Inhibition of monoamine oxidase-B by selegiline reduces cigarette smoke-induced oxidative stress and inflammation in airway epithelial cells. Toxicol. Lett. 2017, 268, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R. Molecular aspects of monoamine oxidase B. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 69, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Libert, S.; Pointer, K.; Bell, E.L.; Das, A.; Cohen, D.E.; Asara, J.M.; Kapur, K.; Bergmann, S.; Preisig, M.; Otowa, T.; et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell 2011, 147, 1459–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef] [PubMed]
- Rogowski, M.; Gollahon, L.; Chellini, G.; Assadi-Porter, F.M. Uptake of 3-iodothyronamine hormone analogs inhibits the growth and viability of cancer cells. FEBS Open Bio 2017, 7, 587–601, Erratum in FEBS Open Bio 2017, 7, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinderman-Maman, E.; Cohen, K.; Moskovich, D.; Hercbergs, A.; Werner, H.; Davis, P.J.; Ellis, M.; Ashur-Fabian, O. Thyroid hormones derivatives reduce proliferation and induce cell death and DNA damage in ovarian cancer. Sci. Rep. 2017, 7, 16475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bour, S.; Daviaud, D.; Gres, S.; Lefort, C.; Prévot, D.; Zorzano, A.; Wabitsch, M.; Saulnier-Blache, J.S.; Valet, P.; Carpéné, C. Adipogenesis-related increase of semicarbazide-sensitive amine oxidase and monoamine oxidase in human adipocytes. Biochimie 2007, 89, 916–925. [Google Scholar] [CrossRef]
- Raimondi, L.; Pirisino, R.; Banchelli, G.; Ignesti, G.; Conforti, L.; Buffoni, F. Cultured preadipocytes produce a semicarbazide-sensitive amine oxidase (SSAO) activity. J. Neural Transm. Suppl. 1990, 32, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, J. Esculetin Inhibits Adipogenesis and Increases Antioxidant Activity during Adipocyte Differentiation in 3T3-L1 Cells. Prev. Nutr. Food Sci. 2017, 22, 118–123. [Google Scholar] [CrossRef]
- Laurino, A.; Landucci, E.; Cinci, L.; Gencarelli, M.; De Siena, G.; Bellusci, L.; Chiellini, G.; Raimondi, L. Brain Histamine Modulates the Antidepressant-Like Effect of the 3-Iodothyroacetic Acid (TA1). Front. Cell. Neurosci. 2019, 13, 176. [Google Scholar] [CrossRef]
- Laurino, A.; Landucci, E.; Resta, F.; De Siena, G.; Pellegrini-Giampietro, D.E.; Masi, A.; Mannaioni, G.; Raimondi, L. Anticonvulsant and Neuroprotective Effects of the Thyroid Hormone Metabolite 3-Iodothyroacetic Acid. Thyroid 2018, 28, 1387–1397. [Google Scholar] [CrossRef]
- Lodovici, M.; Bigagli, E.; Tarantini, F.; Di Serio, C.; Raimondi, L. Losartan reduces oxidative damage to renal DNA and conserves plasma antioxidant capacity in diabetic rats. Exp. Biol. Med. 2015, 240, 1500–1504. [Google Scholar] [CrossRef] [Green Version]
- Tao, H.; Zhou, J.; Wu, T.; Cheng, Z. High-throughput superoxide anion radical scavenging capacity assay. J. Agric. Food Chem. 2014, 62, 9266–9272. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, T.; Peiretti, F.; Defoort, C.; Borel, P.; Govers, R. 2′,7′-dichlorofluorescin-based analysis of Fenton chemistry reveals auto-amplification of probe fluorescence and albumin as catalyst for the detection of hydrogen peroxide. Biochem. J. 2020, 20, BCJ20200602. [Google Scholar] [CrossRef] [PubMed]
- Laurino, A.; Spinelli, V.; Gencarelli, M.; Balducci, V.; Dini, L.; Diolaiuti, L.; Ghionzoli, M.; Messineo, A.; Mugelli, A.; Cerbai, E.; et al. Angiotensin-II Drives Human Satellite Cells toward Hypertrophy and Myofibroblast Trans-Differentiation by Two Independent Pathways. Int. J. Mol. Sci. 2019, 20, 4912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luceri, C.; Bigagli, E.; Agostiniani, S.; Giudici, F.; Zambonin, D.; Scaringi, S.; Ficari, F.; Lodovici, M.; Malentacchi, C. Analysis of Oxidative Stress-Related Markers in Crohn’s Disease Patients at Surgery and Correlations with Clinical Findings. Antioxidants 2019, 8, 378. [Google Scholar] [CrossRef] [Green Version]



| Name Product | Dilution | Supplier |
|---|---|---|
| Anti-UCP1 | 1:500 (WB) | Merk-Millipore, Darmstadt, Germany (U6382) |
| Anti-Histone H3 (acetyl K9 + K14 + K18 + K23 + K27) | 1:1000 (WB) | Abcam, Cambridge Biomedical Campus, UK (ab47915) |
| Anti-SIRT1 | 1:1000 (WB) | St John’s Laboratory Ltd. London, UK (STJ95667) |
| Anti-acetyl-p53 Antibody (Lys373, Lys382) | 1:1000 (WB) | Merk-Millipore, Darmstadt, Germany (06-758) |
| Anti-GAPDH | 1:2000 (WB) | Cell Signaling Technology, Leiden, The Netherlands (D16H11) |
| Gene | Forward Primer 5′ ≥ 3′ | Reverse Primer 3′ ≥ 5′ | Size (Bp) |
|---|---|---|---|
| MAO-A | GAACCCGAGTCCAAGGATGT | ATGGCCCAAACCATAGGCTG | 239 |
| MAO-B | GATGGGCCAAGAGATTCCCAG | CATGGGTCTCCGCAGTTACA | 164 |
| SSAO | AATGTGAGCGAGTTGGTGGT | TGTGTCCTGGCGTTTGTAG | 211 |
| 18s | AAACGGCTACCACATCCAAG | CCTCCAATGGATCCTCGTTA | 155 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gencarelli, M.; Lodovici, M.; Bellusci, L.; Raimondi, L.; Laurino, A. Redox Properties of 3-Iodothyronamine (T1AM) and 3-Iodothyroacetic Acid (TA1). Int. J. Mol. Sci. 2022, 23, 2718. https://doi.org/10.3390/ijms23052718
Gencarelli M, Lodovici M, Bellusci L, Raimondi L, Laurino A. Redox Properties of 3-Iodothyronamine (T1AM) and 3-Iodothyroacetic Acid (TA1). International Journal of Molecular Sciences. 2022; 23(5):2718. https://doi.org/10.3390/ijms23052718
Chicago/Turabian StyleGencarelli, Manuela, Maura Lodovici, Lorenza Bellusci, Laura Raimondi, and Annunziatina Laurino. 2022. "Redox Properties of 3-Iodothyronamine (T1AM) and 3-Iodothyroacetic Acid (TA1)" International Journal of Molecular Sciences 23, no. 5: 2718. https://doi.org/10.3390/ijms23052718
APA StyleGencarelli, M., Lodovici, M., Bellusci, L., Raimondi, L., & Laurino, A. (2022). Redox Properties of 3-Iodothyronamine (T1AM) and 3-Iodothyroacetic Acid (TA1). International Journal of Molecular Sciences, 23(5), 2718. https://doi.org/10.3390/ijms23052718






