The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation
Abstract
1. Introduction
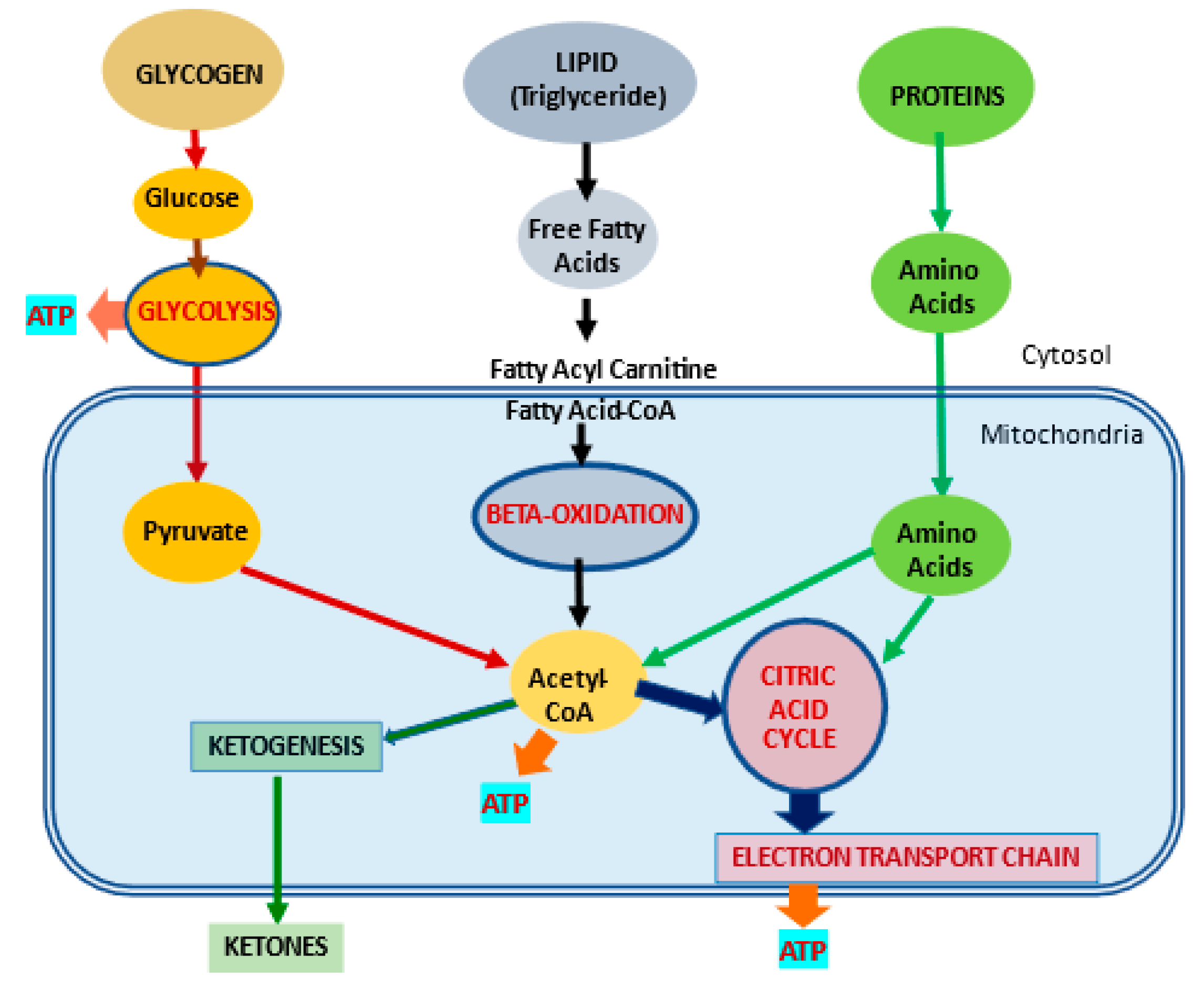
2. l-Carnitine, Mitochondria and Cellular Metabolism
2.1. l-Carnitine and Fatty Acid Metabolism
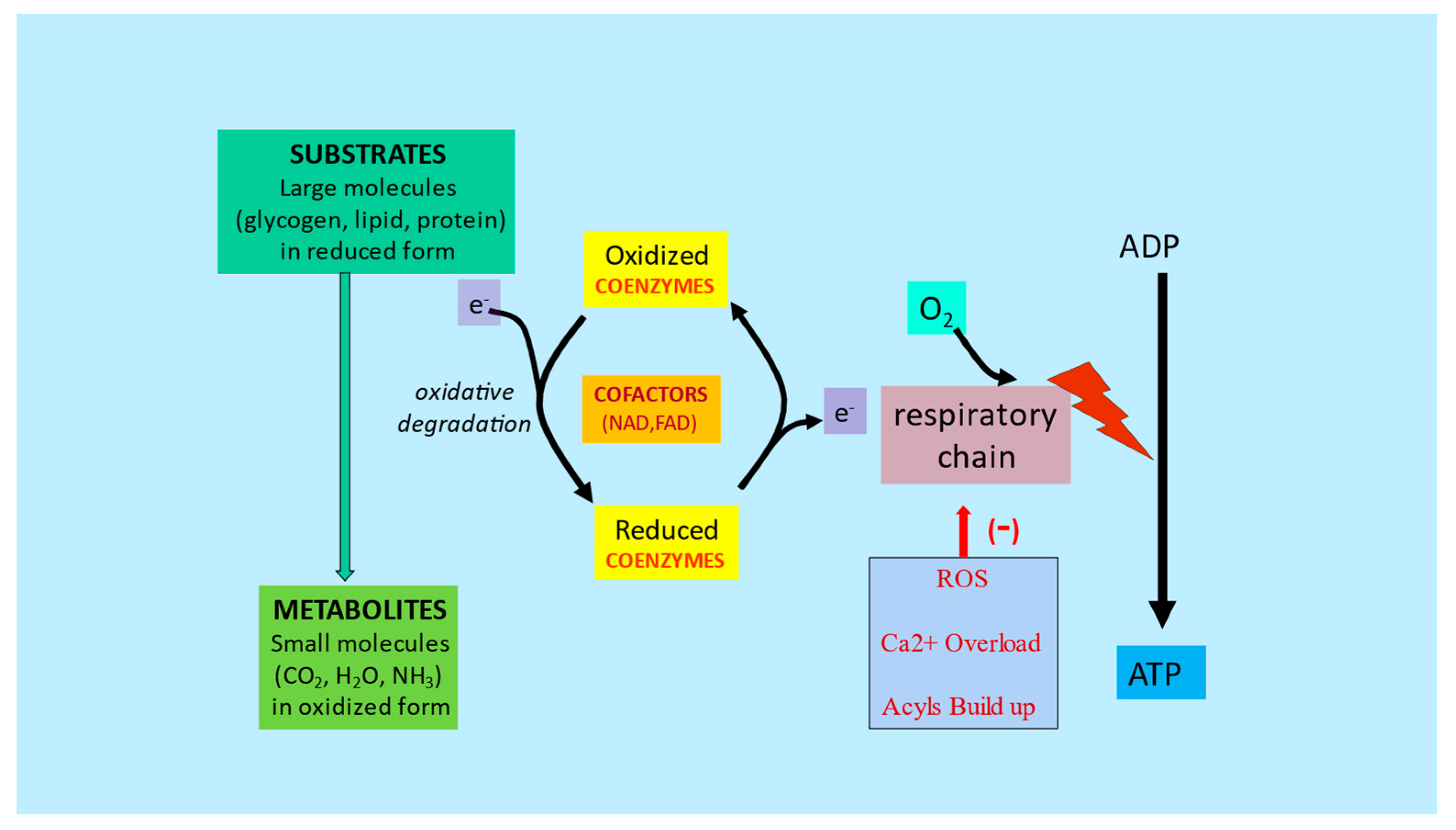
2.2. Regulation of the Mitochondrial Acetyl-CoA/CoA Ratio and Acyl-CoA/CoA Ratio
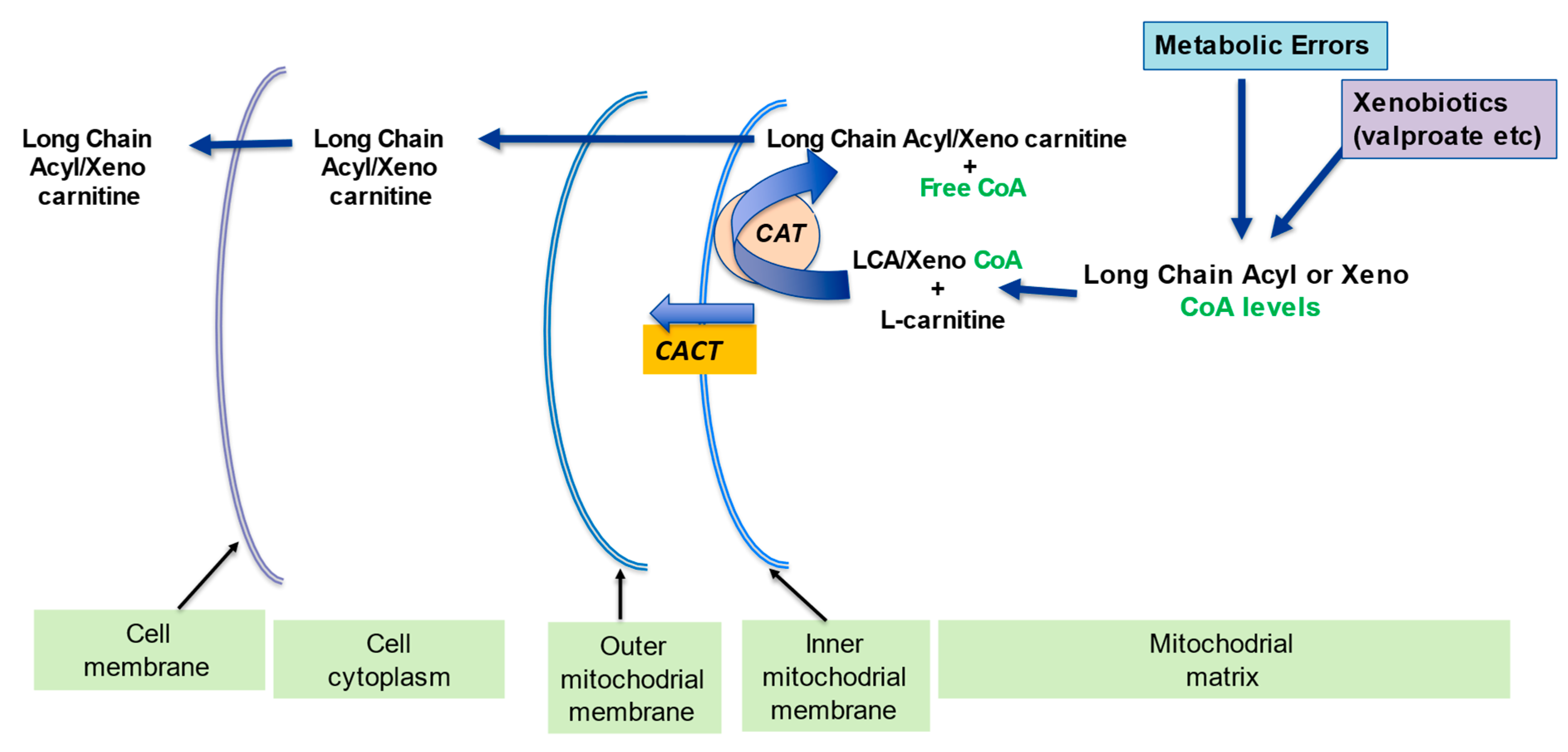
2.3. Detoxification of Toxic Metabolites
2.4. Stabilization of Cell Membranes
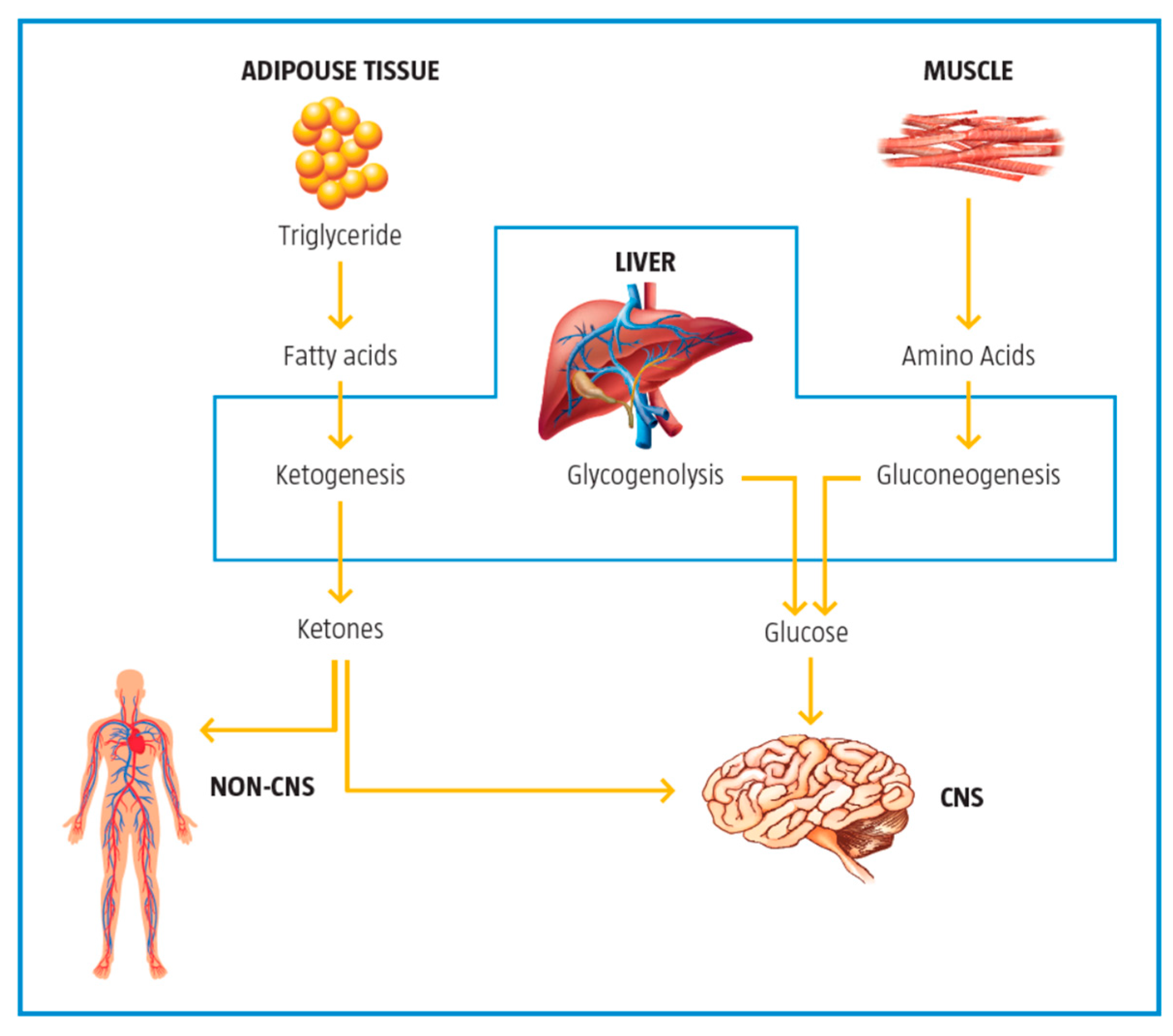
2.5. Control of Ketogenesis and Gluconeogenesis
3. Metabolic Inflexibility
4. The Role of l-Carnitine in Improving Metabolic Flexibility
4.1. Metabolic Flexibility and Acetyl-CoA/CoA Ratio
4.2. Metabolic Flexibility and Acyl-CoA/CoA Ratio
5. Role of Carnitine in Disease
5.1. l-Carnitine and Insulin Resistance
- Improved mitochondrial oxidation of the long chain acyl CoA since their accretion is linked to insulin resistance;
- Increasing the intramitochondrial acetyl-CoA/CoA which is positive for pyruvate dehydrogenase complex (PDHC) activity;
- Improving expression of enzymes in the glycolytic and gluconeogenic;
- Improved expression of genes in the insulin signaling cascade;
- Improved signaling cascade for insulin-like growth factor-1 (IGF-1) axis and IGF-1.
5.2. l-Carnitine and Endocrine Imbalances
5.3. l-Carnitine and Fatty Liver Disease
5.4. l-Carnitine in Neurodegenerative Diseases
- Augmented excitotoxicity;
- Diminished energy production;
- Reduced antioxidant potential.
5.5. Potential Use of l-Carnitine as a Therapeutic Agent
6. Summary—Disease Initiation and the Metabolic Approach
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mynatt, R.L. Carnitine and type 2 diabetes. Diabetes Metab. Res. Rev. 2009, 25, S45–S49. [Google Scholar] [CrossRef]
- Virmani, A.; Pinto, L.; Bauermann, O.; Zerelli, S.; Diedenhofen, A.; Binienda, Z.K.; Ali, S.F.; van der Leij, F.R. The carnitine palmitoyl transferase (CPT) system and possible relevance for neuropsychiatric and neurological conditions. Mol. Neurobiol. 2015, 52, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jiang, W.; Chen, G. l-carnitine treatment of insulin resistance: A systematic review and meta-analysis. Adv. Clin. Exp. Med. 2017, 26, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Liu, Y.Y.; Liu, G.H.; Lu, H.B.; Mao, C.Y. l-Carnitine and heart disease. Life Sci. 2018, 194, 88–97. [Google Scholar] [CrossRef]
- Takashima, H.; Maruyama, T.; Abe, M. Significance of Levocarnitine Treatment in Dialysis Patients. Nutrients 2021, 13, 1219. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Mao, Y.Z.; He, S.M.; Yang, Y.; Chen, X. Quantitative efficacy of l-carnitine supplementation on glycemic control in type 2 diabetes mellitus patients. Expert Rev. Clin. Pharmacol. 2021, 14, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.L.; Abney, T.O.; Lapp, D.F. Biosynthesis and metabolism of carnitine. J. Child Neurol. 1995, 10 (Suppl. 2), S3–S7. [Google Scholar] [CrossRef]
- Longo, N.; Amat di San Filippo, C.; Pasquali, M. Disorders of carnitine transport and the carnitine cycle. Am. J. Med. Genet. C Semin. Med. Genet. 2006, 15, 77–85. [Google Scholar] [CrossRef]
- Bieber, L.L. Carnitine. Annu. Rev. Biochem. 1988, 57, 261–283. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Visser, G.; Ferdinandusse, S.; Vaz, F.M.; Houtkooper, R.H. Mitochondrial fatty acid oxidation disorders: Laboratory diagnosis, pathogenesis, and the complicated route to treatment. J. Lipid Atheroscler. 2020, 9, 313–333. [Google Scholar] [CrossRef]
- Indiveri, C.; Iacobazzi, V.; Tonazzi, A.; Giangregorio, N.; Infantino, V.; Convertini, P. The mitochondrial carnitine/acylcarnitine carrier: Function, structure and physiopathology. Mol. Asp. Med. 2011, 32, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Kerner, J.; Hoppel, C. Fatty acid import into mitochondria. Biochim. Biophys. Acta 2000, 1486, 1–17. [Google Scholar] [CrossRef]
- Vaz, F.M.; Wanders, R.J. Carnitine biosynthesis in mammals. Biochem. J. 2002, 1, 417–429. [Google Scholar] [CrossRef]
- Fingerhut, R.; Ensenauer, R.; Röschinger, W.; Arnecke, R.; Olgemöller, B.; Roscher, A.A. Stability of acylcarnitines and free carnitine in dried blood samples: Implications for retrospective diagnosis of inborn errors of metabolism and neonatal screening for carnitine transporter deficiency. Anal. Chem. 2009, 1, 3571–3575. [Google Scholar] [CrossRef] [PubMed]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Houten, S.M.; Violante, S.; Ventura, F.V.; Wanders, R.J. The Biochemistry and Physiology of Mitochondrial Fatty Acid β-Oxidation and Its Genetic Disorders. Annu. Rev. Physiol. 2016, 78, 23–44. [Google Scholar] [CrossRef]
- Drosatos, K.; Schulze, P.C. Cardiac lipotoxicity: Molecular pathways and therapeutic implications. Curr. Heart Fail. Rep. 2013, 10, 109–121. [Google Scholar] [CrossRef]
- Ferreira, G.C.; McKenna, M.C. l-carnitine and acetyl-l-carnitine roles and neuroprotection in developing brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef]
- Hülsmann, W.C.; Dubelaar, M.L.; Lamers, J.M.; Maccari, F. Protection by acyl-carnitines and phenylmethylsulfonyl fluoride of rat heart subjected to ischemia and reperfusion. Biochim. Biophys. Acta 1985, 30, 62–66. [Google Scholar] [CrossRef]
- Virmani, A.; Binienda, Z. Role of carnitine esters in brain neuropathology. Mol. Aspects Med. 2004, 25, 533–549. [Google Scholar] [CrossRef]
- Foster, D.W. The role of the carnitine system in human metabolism. Ann. N. Y. Acad. Sci. 2004, 1033, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, K.K.; Gupta, S. Biochemistry, Ketogenesis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK493179/ (accessed on 28 January 2022).
- Almannai, M.; Alfadhel, M.; El-Hattab, A.W. Carnitine inborn errors of metabolism. Molecules 2019, 24, 3251. [Google Scholar] [CrossRef] [PubMed]
- Muoio, D.M. Metabolic inflexibility: When mitochondrial indecision leads to metabolic gridlock. Cell 2014, 159, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Goodpaster, B.H.; Sparks, L.M. Metabolic flexibility in health and disease. Cell Metab. 2017, 25, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Virmani, A.; Pinto, L.; Binienda, Z.; Ali, S. Food, nutrigenomics, and neurodegeneration—Neuroprotection by what you eat! Mol. Neurobiol. 2013, 48, 353–362. [Google Scholar] [CrossRef]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Berezhnov, A.V.; Fedotova, E.I.; Nenov, M.N.; Kasymov, V.A.; Pimenov, O.Y.; Dynnik, V.V. Dissecting cellular mechanisms of long-chain acylcarnitines-driven cardiotoxicity: Disturbance of calcium homeostasis, activation of Ca2+-dependent phospholipases, and mitochondrial energetics collapse. Int. J. Mol. Sci. 2020, 10, 7461. [Google Scholar] [CrossRef]
- Waterson, M.J.; Horvath, T.L. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015, 22, 962–970. [Google Scholar] [CrossRef]
- Roh, E.; Song, D.K.; Kim, M.S. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism. Exp. Mol. Med. 2016, 48, e216. [Google Scholar] [CrossRef]
- Haigh, J.L.; New, L.E.; Filippi, B.M. Mitochondrial Dynamics in the Brain Are Associated with Feeding, Glucose Homeostasis, and Whole-Body Metabolism. Front. Endocrinol. 2020, 11, 580879. [Google Scholar] [CrossRef]
- van de Weijer, T.; Sparks, L.M.; Phielix, E. Relationships between mitochondrial function and metabolic flexibility in type 2 diabetes mellitus. PLoS ONE 2013, 8, e51648. [Google Scholar] [CrossRef]
- Stark, R.; Reichenbach, A.; Andrews, Z.B. Hypothalamic carnitine metabolism integrates nutrient and hormonal feedback to regulate energy homeostasis. Mol. Cell. Endocrinol. 2015, 418 Pt 1, 9–16. [Google Scholar] [CrossRef]
- Wesselink, E.; Koekkoek, W.A.C.; Grefte, S.; Witkamp, R.F.; van Zanten, A.R.H. Feeding mitochondria: Potential role of nutritional components to improve critical illness convalescence. Clin. Nutr. 2019, 38, 982–995. [Google Scholar] [CrossRef]
- Theurey, P.; Rieusset, J. Mitochondria-Associated Membranes Response to Nutrient Availability and Role in Metabolic Diseases. Trends Endocrinol. Metab. 2017, 28, 32–45. [Google Scholar] [CrossRef]
- Bruls, Y.M.; de Ligt, M.; Lindeboom, L. Carnitine supplementation improves metabolic flexibility and skeletal muscle acetylcarnitine formation in volunteers with impaired glucose tolerance: A randomised controlled trial. EBioMedicine 2019, 49, 318–330. [Google Scholar] [CrossRef]
- Muoio, D.M.; Noland, R.C.; Kovalik, J.P. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012, 15, 764–777. [Google Scholar] [CrossRef]
- Noland, R.C.; Koves, T.R.; Seiler, S.E. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J. Biol. Chem. 2009, 284, 22840–22852. [Google Scholar] [CrossRef]
- Power, R.A.; Hulver, M.W.; Zhang, J.Y. Carnitine revisited: Potential use as adjunctive treatment in diabetes. Diabetologia 2007, 50, 824–832. [Google Scholar] [CrossRef]
- Hulsmann, W.C.; Peschechera, A.; Serafini, F.; Ferrari, L.E. Release of ischemia in paced rat Langendorff hearts by supply of l-carnitine: Role of endogenous long-chain acylcarnitine. Mol. Cell. Biochem. 1996, 156, 87–91. [Google Scholar] [CrossRef]
- Liepinsh, E.; Makrecka-Kuka, M.; Makarova, E. Decreased acylcarnitine content improves insulin sensitivity in experimental mice models of insulin resistance. Pharmacol. Res. 2016, 113, 788–795. [Google Scholar] [CrossRef]
- Várnagy, A.; Bene, J.; Sulyok, E.; Kovács, G.L.; Bódis, J.; Melegh, B. Acylcarnitine esters profiling of serum and follicular fluid in patients undergoing in vitro fertilization. Reprod. Biol. Endocrinol. 2013, 11, 67. [Google Scholar] [CrossRef]
- Arrigoni-Martelli, E.; Caso, V. Carnitine protects mitochondria and removes toxic acyls from xenobiotics. Drugs Exp. Clin. Res. 2001, 27, 27–49. [Google Scholar]
- Rebouche, C.J.; Seim, H. Carnitine metabolism and its regulation in microorganisms and mammals. Annu. Rev. Nutr. 1998, 18, 39–61. [Google Scholar] [CrossRef]
- Holme, E.; Jodal, U.; Linstedt, S.; Nordin, I. Effects of pivalic acid-containing prodrugs on carnitine homeostasis and on response to fasting in children. Scand. J. Clin. Lab. Investig. 1992, 52, 361–372. [Google Scholar] [CrossRef]
- Bene, J.; Csiky, B.; Komlosi, K.; Sulyok, E.; Melegh, B. Dynamic adaptive changes of the serum carnitine esters during and after l-carnitine supplementation in patients with maintenance haemodialysis. Scand. J. Clin. Lab. Investig. 2011, 71, 280–286. [Google Scholar] [CrossRef]
- Bene, J.; Szabo, A.; Komlósi, K.; Melegh, B. Mass Spectrometric Analysis of l-carnitine and its Esters: Potential Biomarkers of Disturbances in Carnitine Homeostasis. Curr. Mol. Med. 2020, 20, 336–354. [Google Scholar] [CrossRef]
- Bene, J.; Hadzsiev, K.; Melegh, B. Role of carnitine and its derivatives in the development and management of type 2 diabetes. Nutr. Diabetes 2018, 8, 8. [Google Scholar] [CrossRef]
- Ringseis, R.; Keller, J.; Eder, K. Role of carnitine in the regulation of glucose homeostasis and insulin sensitivity: Evidence from in vivo and in vitro studies with carnitine supplementation and carnitine deficiency. Eur. J. Nutr. 2012, 51, 1–18. [Google Scholar] [CrossRef]
- Molfino, A.; Cascino, A.; Conte, C.; Ramaccini, C.; Rossi Fanelli, F.; Laviano, A. Caloric restriction and l-carnitine administration improves insulin sensitivity in patients with impaired glucose metabolism. JPEN J. Parenter. Enteral. Nutr. 2010, 34, 295–299. [Google Scholar] [CrossRef]
- Malaguarnera, M.; Gargante, M.P.; Russo, C. l-carnitine supplementation to diet: A new tool in treatment of nonalcoholic steatohepatitis—A randomized and controlled clinical trial. Am. J. Gastroenterol. 2010, 105, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Dumollard, R.; Duchen, M.; Carroll, J. The role of mitochondrial function in the oocyte and embryo. Curr. Top. Dev. Biol. 2007, 77, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Kim, S.; Peng, W.; Krainc, D. Regulation and function of mitochondria-lysosome membrane contact sites in cellular homeostasis. Trends Cell. Biol. 2019, 29, 500–513. [Google Scholar] [CrossRef]
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of sperm mitochondrial function: A key organelle for sperm motility. J. Clin. Med. 2020, 9, 363. [Google Scholar] [CrossRef]
- Di Emidio, G.; Rea, F.; Placidi, M.; Virmani, A.; Macchiarelli, G.; Palmerini, M.G.; D’Alessandro, A.M.; Artini, P.G.; Tatone, C. Regulatory Functions of l-carnitine, acetyl, and propionyl l-carnitine in a PCOS mouse model: Focus on antioxidant/antiglycative molecular pathways in the ovarian microenvironment. Antioxidants 2020, 9, 867. [Google Scholar] [CrossRef]
- Park, Y.J.; Pang, M.G. Mitochondrial functionality in male fertility: From spermatogenesis to fertilization. Antioxidants 2021, 10, 98. [Google Scholar] [CrossRef]
- Demain, L.A.; Conway, G.S.; Newman, W.G. Genetics of mitochondrial dysfunction and infertility. Clin. Genet. 2017, 91, 199–207. [Google Scholar] [CrossRef]
- Fenkci, S.M.; Fenkci, V.; Oztekin, O.; Rota, S.; Karagenc, N. Serum total l-carnitine levels in non-obese women with polycystic ovary syndrome. Hum. Reprod. 2008, 23, 1602–1606. [Google Scholar] [CrossRef]
- Cavallini, G.; Magli, M.C.; Crippa, A.; Ferraretti, A.P.; Gianaroli, L. Reduction in sperm aneuploidy levels in severe oligoasthenoteratospermic patients after medical therapy: A preliminary report. Asian J. Androl. 2012, 14, 591–598. [Google Scholar] [CrossRef]
- Petrillo, T.; Battipaglia, C.; Virmani, M.A.; Genazzani, A.R.; Genazzani, A.D. Neuroendocrine effects of carnitines on reproductive impairments. Int. J. Mol. Sci. 2021, 22, 10781. [Google Scholar] [CrossRef]
- Samimi, M.; Jamilian, M.; Ebrahimi, F.A.; Rahimi, M.; Tajbakhsh, B.; Asemi, Z. Oral carnitine supplementation reduces body weight and insulin resistance in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Clin. Endocrinol. 2016, 84, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.M.; Hamed, A.H.; Saso, S.; Thabet, H.H. Adding l-carnitine to clomiphene resistant PCOS women improves the quality of ovulation and the pregnancy rate. A randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 180, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Jamilian, M.; Foroozanfard, F.; Kavossian, E. Effects of chromium and carnitine co-supplementation on body weight and metabolic profiles in overweight and obese women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. Biol. Trace Elem. Res. 2020, 193, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, S.; Nazari, L.; Hoseini, S.; Moghaddam, P.B.; Gachkar, L. Effects of l-carnitine on polycystic ovary syndrome. JBRA Assist. Reprod. 2019, 23, 392–395. [Google Scholar] [CrossRef]
- Virmani, M.A.; Krsmanovic, L.Z.; Stojilkovic, S.S.; Catt, K.J. Stimulatory effects of L-acetylcarnitine on the pituitary-gonadal axis in female rats. In Major Advances in Human Female Reproduction; Adashi, E.Y., Mancuso, S., Eds.; Raven Press: New York, NY, USA, 1991; Volume 73, pp. 291–296. [Google Scholar]
- Vitullo, P.; Cossetti, C.; Virmani, M. Effect of specific nutrients on ovulation, oocytes development, gene expression and coupling success in mice. Int. J. Clin. Med. 2018, 9, 660–674. [Google Scholar] [CrossRef][Green Version]
- Kalhori, Z.; Mehranjani, M.S.; Azadbakht, M.; Shariatzadeh, M.A. l-Carnitine improves endocrine function and folliculogenesis by reducing inflammation, oxidative stress and apoptosis in mice following induction of polycystic ovary syndrome. Reprod. Fertil. Dev. 2019, 31, 282–293. [Google Scholar] [CrossRef]
- Dunning, K.R.; Robker, R.L. Promoting lipid utilization with l-carnitine to improve oocyte quality. Anim. Reprod. Sci. 2012, 134, 69–75. [Google Scholar] [CrossRef]
- Ishikawa, H.; Takaki, A.; Tsuzaki, R. l-carnitine prevents progression of non-alcoholic steatohepatitis in a mouse model with upregulation of mitochondrial pathway. PLoS ONE 2014, 9, e100627. [Google Scholar] [CrossRef]
- Hanai, T.; Shiraki, M.; Imai, K.; Suetugu, A.; Takai, K.; Shimizu, M. Usefulness of Carnitine Supplementation for the Complications of Liver Cirrhosis. Nutrients 2020, 12, 1915. [Google Scholar] [CrossRef]
- Li, N.; Zhao, H. Role of carnitine in non-alcoholic fatty liver disease and other related diseases: An update. Front. Med. 2021, 8, 689042. [Google Scholar] [CrossRef]
- Borum, P.R. Carnitine. Annu. Rev. Nutr. 1983, 3, 233–259. [Google Scholar] [CrossRef]
- Galluzzi, L.; Keep, O.; Vander Heiden, M.G.; Kroemer, G. Metabolic targets for cancer therapy. Nat. Rev. Drug. Discov. 2013, 2, 829–846. [Google Scholar] [CrossRef]
- Savic, D.; Hodson, L.; Neubauer, S.; Pavlides, M. The importance of the fatty acid transporter l-carnitine in non-alcoholic fatty liver disease (NAFLD). Nutrients 2020, 12, 2178. [Google Scholar] [CrossRef] [PubMed]
- Kerner, J.; Yohannes, E.; Lee, K. Acetyl-l-carnitine increases mitochondrial protein acetylation in the aged rat heart. Mech. Ageing Dev. 2015, 145, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Amiri, R.; Tabandeh, M.R.; Hosseini, S.A. Novel cardioprotective effect of l-Carnitine on obese diabetic mice: Regulation of Chemerin and CMKLRI expression in heart and adipose tissues. Arq. Bras. Cardiol. 2021, 117, 15–725. [Google Scholar] [CrossRef]
- Virmani, M.A.; Biselli, R.; Spadoni, A. Protective actions of l-carnitine and acetyl-l-carnitine on the neurotoxicity evoked by mitochondrial uncoupling or inhibitors. Pharmacol. Res. 1995, 32, 383–389. [Google Scholar] [CrossRef]
- Rai, S.N.; Singh, C.; Singh, A.; Singh, M.P.; Singh, B.K. Mitochondrial dysfunction: A potential therapeutic target to treat Alzheimer's disease. Mol. Neurobiol. 2020, 57, 3075–3088. [Google Scholar] [CrossRef]
- Virmani, M.A.; Caso, V.; Spadoni, A.; Rossi, S.; Russo, F.; Gaetani, F. The action of acetyl-l-carnitine on the neurotoxicity evoked by amyloid fragments and peroxide on primary rat cortical neurones. Ann. N. Y. Acad. Sci. 2001, 939, 162–178. [Google Scholar] [CrossRef]
- Mota, S.I.; Pita, I.; Águas, R. Mechanistic perspectives on differential mitochondrial-based neuroprotective effects of several carnitine forms in Alzheimer's disease in vitro model. Arch. Toxicol. 2021, 95, 2769–2784. [Google Scholar] [CrossRef]
- Cuevas, E.; Lantz, S.M.; Tobón-Velasco, J.C. On the in vivo early toxic properties of A-beta 25-35 peptide in the rat hippocampus: Involvement of the Receptor-for-Advanced Glycation-End-Products and changes in gene expression. Neurotoxicol. Teratol. 2011, 33, 288–296. [Google Scholar] [CrossRef]
- Abdul, H.M.; Calabrese, V.; Calvani, M.; Butterfield, D.A. Acetyl-l-carnitine-induced up-regulation of heat shock proteins protects cortical neurons against amyloid-beta peptide 1-42-mediated oxidative stress and neurotoxicity: Implications for Alzheimer's disease. J. Neurosci. Res. 2006, 84, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Dhitavat, S.; Ortiz, D.; Shea, T.B.; Rivera, E.R. Acetyl-l-carnitine protects against amyloid-beta neurotoxicity: Roles of oxidative buffering and ATP levels. Neurochem. Res. 2002, 27, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Virmani, A.; Gaetani, F.; Binienda, Z. Effects of metabolic modifiers such as carnitines, coenzyme Q10, and PUFAs against different forms of neurotoxic insults: Metabolic inhibitors, MPTP, and methamphetamine. Ann. N. Y. Acad. Sci. 2005, 1053, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Magi, S.; Preziuso, A.; Piccirillo, S. The neuroprotective effect of l-carnitine against glyceraldehyde-induced metabolic impairment: Possible implications in Alzheimer's disease. Cells 2021, 10, 2109. [Google Scholar] [CrossRef] [PubMed]
- Knottnerus, S.J.G.; Bleeker, J.C.; Wüst, R.C.I.; Ferdinandusse, S.; IJlst, L.; Wijburg, F.A.; Wanders, R.J.A.; Visser, G.; Houtkooper, R.H. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev. Endocr. Metab. Disord. 2018, 19, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Steiber, A.; Kerner, J.; Hoppel, C.L. Carnitine: A nutritional, biosynthetic, and functional perspective. Mol. Aspects Med. 2004, 25, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Sommerfeld, E.; Penn, D.; Wolf, H. Carnitine deficiency in premature infants receiving total parenteral nutrition: Effect of l-carnitine supplementation. J. Pediatr. 1983, 102, 931–935. [Google Scholar] [CrossRef]
- Agarwal, A.; Sengupta, P.; Durairajanayagam, D. Role of l-carnitine in female infertility. Reprod. Biol. Endocrinol. 2018, 16, 5. [Google Scholar] [CrossRef]
- Kitano, Y.; Hashimoto, S.; Matsumoto, H. Oral administration of l-carnitine improves the clinical outcome of fertility in patients with IVF treatment. Gynecol. Endocrinol. 2018, 34, 684–688. [Google Scholar] [CrossRef]
- Cavallini, G.; Ferraretti, A.P.; Gianaroli, L.; Biagiotti, G.; Vitali, G. Cinnoxicam and l-carnitine/acetyl-l-carnitine treatment for idiopathic and varicocele-associated oligoasthenospermia. J. Androl. 2004, 25, 761–772. [Google Scholar] [CrossRef]
- Mongioi, L.; Calogero, A.E.; Vicari, E. The role of carnitine in male infertility. Andrology 2016, 4, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Melegh, B. Carnitine supplementation in the premature. Biol. Neonatol. 1990, 58 (Suppl. 1), 93–106. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.A.; Kardas, Z.; Kardas, F.; Gunes, T.; Kurtoglu, S. Effects of l-carnitine supplementation on respiratory distress syndrome development and prognosis in premature infants: A single blind randomized controlled trial. Exp. Ther. Med. 2016, 11, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Crill, C.M.; Christensen, M.L.; Storm, M.C.; Helms, R.A. Relative bioavailability of carnitine supplementation in premature neonates. JPEN J. Parenter. Enteral. Nutr. 2006, 30, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Abolfathi, M.; Mohd-Yusof, B.N.; Hanipah, Z.N.; Mohd Redzwan, S.; Yusof, L.M.; Khosroshahi, M.Z. The effects of carnitine supplementation on clinical characteristics of patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 48, 102273. [Google Scholar] [CrossRef]
- Kuwasawa-Iwasaki, M.; Io, H.; Muto, M. Effects of l-Carnitine Supplementation in Patients Receiving Hemodialysis or Peritoneal Dialysis. Nutrients 2020, 12, 3371. [Google Scholar] [CrossRef]
- Maruyama, T.; Maruyama, N.; Higuchi, T. Efficacy of l-carnitine supplementation for improving lean body mass and physical function in patients on hemodialysis: A randomized controlled trial. Eur. J. Clin. Nutr. 2019, 73, 293–301. [Google Scholar] [CrossRef]
- Guo, H.L.; Jing, X.; Sun, J.Y. Valproic Acid and the Liver Injury in Patients with Epilepsy: An Update. Curr. Pharm. Des. 2019, 25, 343–351. [Google Scholar] [CrossRef]
- Lheureux, P.E.; Hantson, P. Carnitine in the treatment of valproic acid-induced toxicity. Clin. Toxicol. 2009, 47, 101–111. [Google Scholar] [CrossRef]
- Plioplys, A.V.; Plioplys, S. Serum levels of carnitine in chronic fatigue syndrome: Clinical correlates. Neuropsychobiology 1995, 32, 132–138. [Google Scholar] [CrossRef]
- Latham, L.E.; Wang, C.; Patterson, T.A.; Slikker, W.; Liu, F. Neuroprotective Effects of Carnitine and Its Potential Application to Ameliorate Neurotoxicity. Chem. Res. Toxicol. 2021, 34, 1208–1222. [Google Scholar] [CrossRef] [PubMed]
- Fathizadeh, H.; Milajerdi, A.; Reiner, Ž.; Kolahdooz, F.; Asemi, Z. The effects of l-carnitine supplementation on glycemic control: A systematic review and meta-analysis of randomized controlled trials. EXCLI J. 2019, 18, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Casariego, A.; Burgos-Peláez, R.; Martínez-Faedo, C. Metabolic effects of l-carnitine on type 2 diabetes mellitus: Systematic review and meta-analysis. Exp. Clin. Endocrinol. Diabetes 2013, 121, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Förster, L.; Indra, D.; Rosenberger, K.; Zver, L.; Hofbauer, R. l-carnitine exerts a nutrigenomic effect via direct modulation of nuclear receptor signaling in adipocytes, hepatocytes and SKMC, demonstrating its nutritional impact. Nutr. Res. 2021, 85, 84–98. [Google Scholar] [CrossRef]
- Zambrano, S.; Blanca, A.J.; Ruiz-Armenta, M.V. The renoprotective effect of l-carnitine in hypertensive rats is mediated by modulation of oxidative stress-related gene expression. Eur. J. Nutr. 2013, 52, 1649–1659. [Google Scholar] [CrossRef]
- Pagano, G.; Manfredi, C.; Pallardó, F.V.; Lyakhovich, A.; Tiano, L.; Trifuoggi, M. Potential roles of mitochondrial cofactors in the adjuvant mitigation of proinflammatory acute infections, as in the case of sepsis and COVID-19 pneumonia. Inflamm. Res. 2021, 70, 159–170. [Google Scholar] [CrossRef]
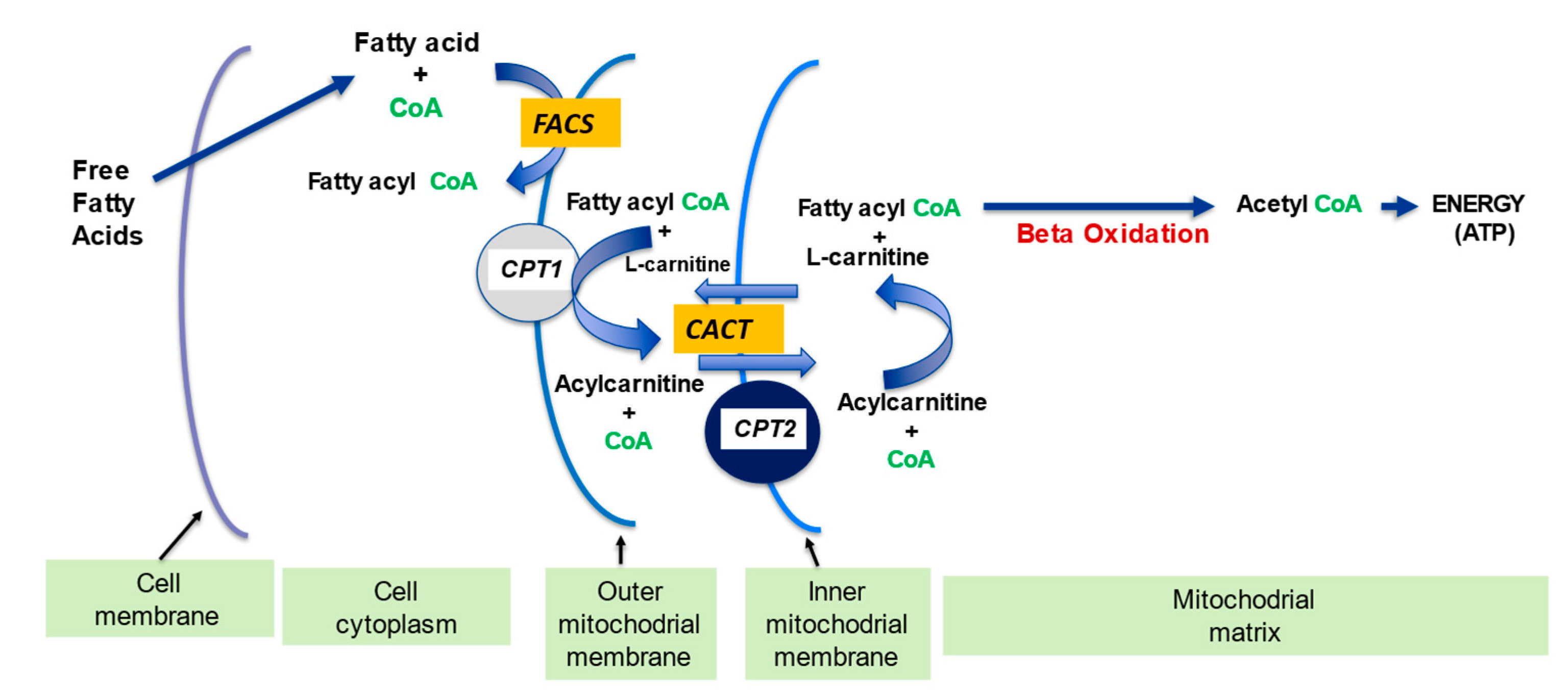
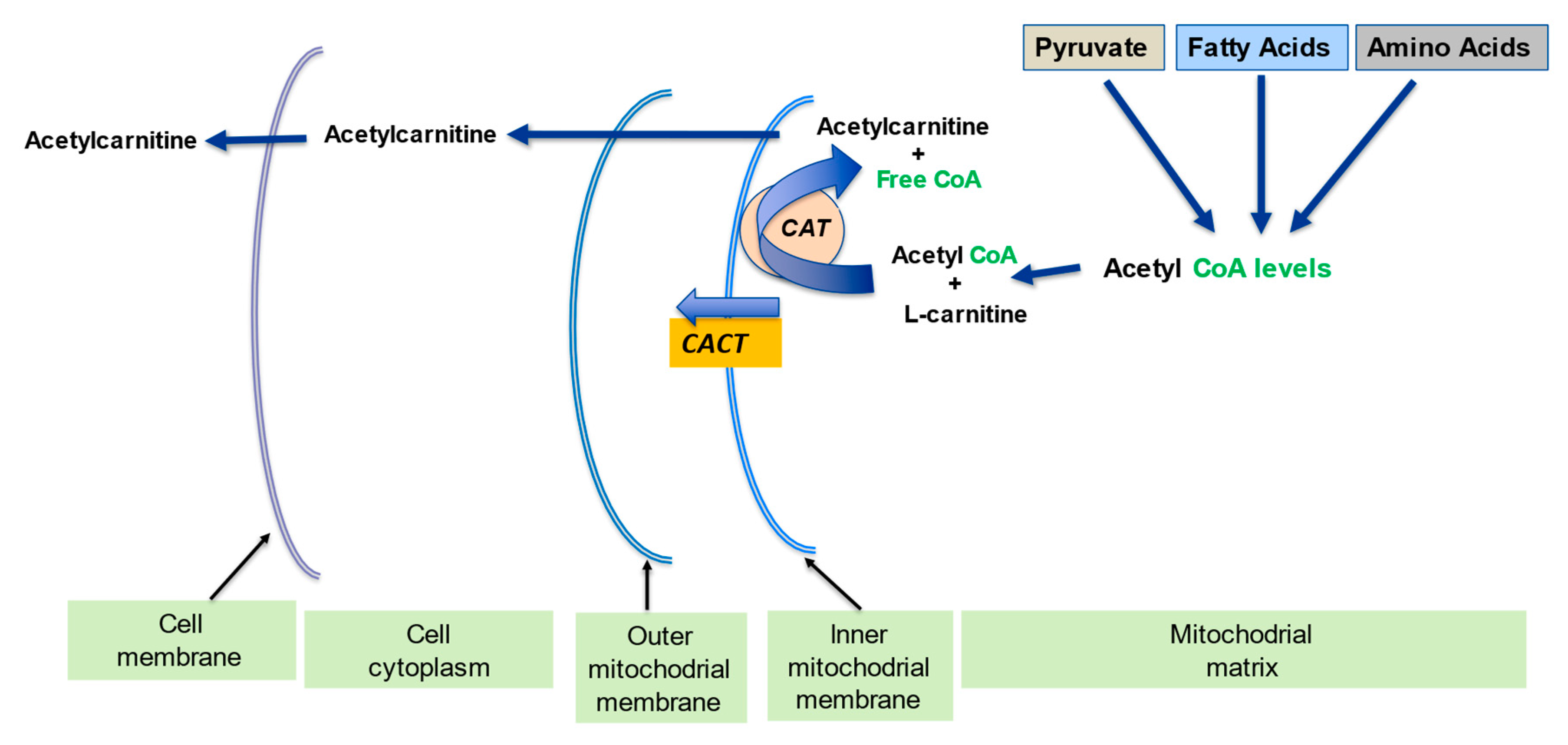
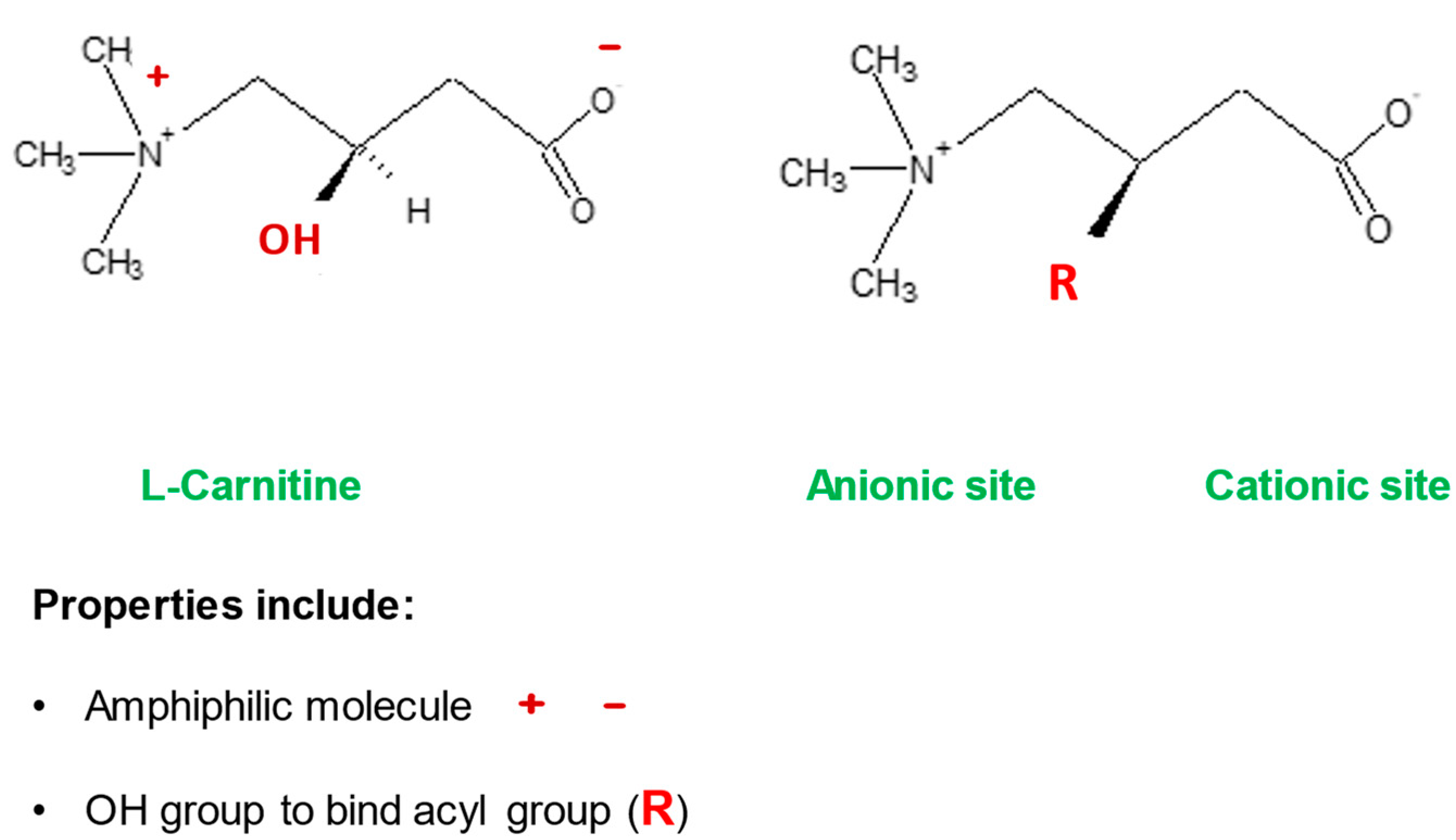
| l-Carnitine (LC) Function | Description | References |
|---|---|---|
| Fatty acid metabolism | LC transports LCFA across inner mitochondrial membrane for subsequent beta oxidation and ATP production | [4,5] |
| Regulation of mitochondrial acetyl-CoA/CoA ratio and acyl-CoA/CoA ratio | LC forms an effective transport system for acetyl or acyl groups out of the mitochondria to maintain free CoA levels important for glycolysis and other processes | [2,5] |
| Detoxification of potentially toxic metabolite | LC binds acyl residues and helps in their elimination thereby maintaining metabolic flexibility | [7] |
| Stabilization of cell membranes | LC helps stabilize cell membranes via its effects on acetylation of membrane phospholipids and surface membrane effects | [8,9] |
| Control of ketogenesis and gluconeogenesis | LC affects ketogenesis by transporting free fatty acids into mitochondria for subsequent use in the production of ketones in the mitochondria. | [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virmani, M.A.; Cirulli, M. The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. Int. J. Mol. Sci. 2022, 23, 2717. https://doi.org/10.3390/ijms23052717
Virmani MA, Cirulli M. The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. International Journal of Molecular Sciences. 2022; 23(5):2717. https://doi.org/10.3390/ijms23052717
Chicago/Turabian StyleVirmani, Mohamed Ashraf, and Maria Cirulli. 2022. "The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation" International Journal of Molecular Sciences 23, no. 5: 2717. https://doi.org/10.3390/ijms23052717
APA StyleVirmani, M. A., & Cirulli, M. (2022). The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. International Journal of Molecular Sciences, 23(5), 2717. https://doi.org/10.3390/ijms23052717






