Effect of In Vitro Gastrointestinal Digestion on Amino Acids, Polyphenols and Antioxidant Capacity of Tamarillo Yoghurts
Abstract
:1. Introduction
2. Results and Discussion
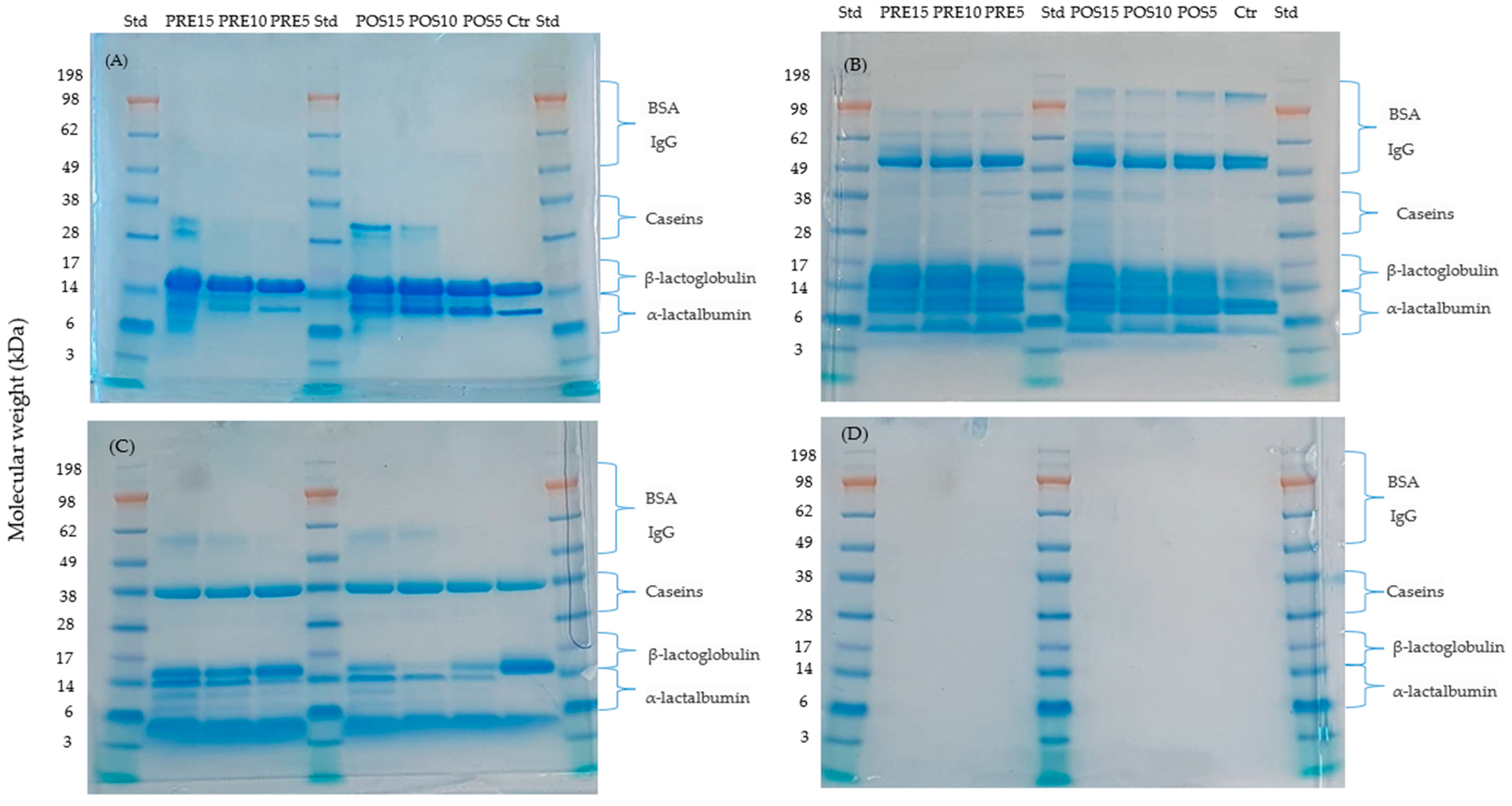
2.1. Protein Fractions by Mass
2.2. Effect of In Vitro Digestion on Free Amino Acid Profiles of Tamarillo Yoghurts
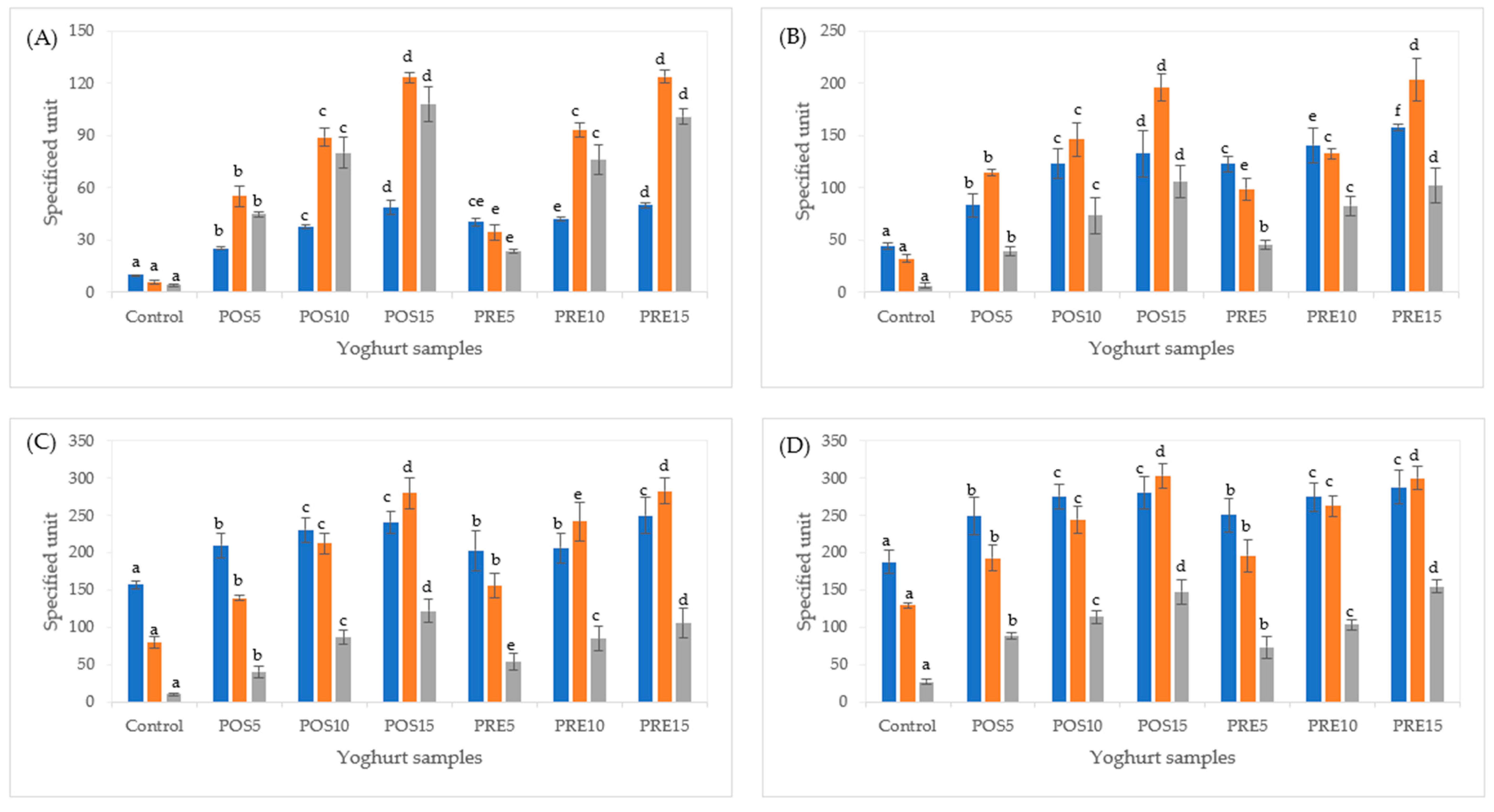
2.3. Effect of In Vitro Digestion on Total Phenolic Content and Antioxidant Activity of Tamarillo Yoghurts
2.4. Effect of In Vitro Digestion on the Polyphenol Profile of Tamarillo Yoghurts
3. Materials and Methods
3.1. Materials
3.2. Yoghurt Preparation
3.3. In Vitro Digestion of Yoghurt
3.4. Analysis of Soluble Proteins Using Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
3.5. Determination and Quantification of Free Amino Acids (FAAs)
3.6. Determination and Quantification of Phenolics and Anthocyanins
3.7. Total Phenolic Content (TPC) and Antioxidant Activity of Yoghurt
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.L. Whole fruits and fruit fiber emerging health effects. Nutrients 2018, 10, 1833. [Google Scholar] [CrossRef] [Green Version]
- Diep, T.T.; Pook, C.; Yoo, M.J.Y. Physicochemical properties and proximate composition of tamarillo (Solanum betaceum Cav.) fruits from New Zealand. J. Food Compost. Anal. 2020, 92, 103563. [Google Scholar] [CrossRef]
- Diep, T.; Pook, C.; Yoo, M. Phenolic and Anthocyanin Compounds and Antioxidant Activity of Tamarillo (Solanum betaceum Cav.). Antioxidants 2020, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Kou, M.C.; Yen, J.H.; Hong, J.T.; Wang, C.L.; Lin, C.W.; Wu, M.J. Cyphomandra betacea Sendt. phenolics protect LDL from oxidation and PC12 cells from oxidative stress. LWT Food Sci. Technol. 2009, 42, 458–463. [Google Scholar] [CrossRef]
- Abdul Kadir, N.A.; Rahmat, A.; Jaafar, H.Z. Protective Effects of Tamarillo (Cyphomandra betacea) Extract against High Fat Diet Induced Obesity in Sprague-Dawley Rats. J. Obes. 2015, 2015, 846041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutalib, M.A.; Ali, F.; Othman, F.; Ramasamy, R.; Rahmat, A. Phenolics profile and anti-proliferative activity of Cyphomandra betacea fruit in breast and liver cancer cells. SpringerPlus 2016, 5, 2105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutalib, M.A.; Rahmat, A.; Ali, F.; Othman, F.; Ramasamy, R. Nutritional Compositions and Antiproliferative Activities of Different Solvent Fractions from Ethanol Extract of Cyphomandra betacea (Tamarillo) Fruit. Malays. J. Med. Sci. 2017, 24, 19–32. [Google Scholar] [CrossRef]
- Ordonez, R.M.; Ordonez, A.A.L.; Sayago, J.E.; Moreno, M.I.N.; Isla, M.I. Antimicrobial activity of glycosidase inhibitory protein isolated from Cyphomandra betacea Sendt. fruit. Peptides 2006, 27, 1187–1191. [Google Scholar] [CrossRef]
- Castro-Vargas, H.I.; Benelli, P.; Ferreira, S.R.S.; Parada-Alfonso, F. Supercritical fluid extracts from tamarillo (Solanum betaceum Sendtn) epicarp and its application as protectors against lipid oxidation of cooked beef meat. J. Supercrit. Fluids 2013, 76, 17–23. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.; Pintado, M. Stability of polyphenols and carotenoids in strawberry and peach yoghurt throughout in vitro gastrointestinal digestion. Food Funct. 2015, 6, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Aryana, K.J.; Olson, D.W. A 100-Year Review: Yogurt and other cultured dairy products. J. Dairy Sci. 2017, 100, 9987–10013. [Google Scholar] [CrossRef] [Green Version]
- Helal, A.; Tagliazucchi, D. Impact of in-vitro gastro-pancreatic digestion on polyphenols and cinnamaldehyde bioaccessibility and antioxidant activity in stirred cinnamon-fortified yogurt. LWT 2018, 89, 164–170. [Google Scholar] [CrossRef]
- Anuyahong, T.; Chusak, C.; Adisakwattana, S. Incorporation of anthocyanin-rich riceberry rice in yogurts: Effect on physicochemical properties, antioxidant activity and in vitro gastrointestinal digestion. LWT 2020, 129, 109571. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Helal, A.; Verzelloni, E.; Conte, A. The type and concentration of milk increase the in vitro bioaccessibility of coffee chlorogenic acids. J. Agric. Food Chem. 2012, 60, 11056–11064. [Google Scholar] [CrossRef]
- Li, Z.; Scott, K.; Otter, D.; Zhou, P.; Hemar, Y. Effect of temperature and pH on the properties of skim milk gels made from a tamarillo (Cyphomandra betacea) coagulant and rennet. J. Dairy Sci. 2018, 101, 4869–4878. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.; Alexandre, E.M.; Coelho, M.; Lopes, C.; Almeida, D.P.; Pintado, M. Incorporation of strawberries preparation in yoghurt: Impact on phytochemicals and milk proteins. Food Chem. 2015, 171, 370–378. [Google Scholar] [CrossRef]
- Wang, X.; Ye, A.; Lin, Q.; Han, J.; Singh, H. Gastric digestion of milk protein ingredients: Study using an in vitro dynamic model. J. Dairy Sci. 2018, 101, 6842–6852. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, L.; Gauthier, S.F.; Britten, M.; Turgeon, S.L. In vitro gastrointestinal digestion of liquid and semi-liquid dairy matrixes. LWT Food Sci. Technol. 2014, 57, 99–105. [Google Scholar] [CrossRef]
- Diep, T.T.; Yoo, M.J.Y.; Rush, E. Effect of Tamarillo Fortification and Fermentation Process on Physicochemical Properties and Nutrient and Volatiles Content of Yoghurt. Foods 2022, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Lorieau, L.; Halabi, A.; Ligneul, A.; Hazart, E.; Dupont, D.; Floury, J. Impact of the dairy product structure and protein nature on the proteolysis and amino acid bioaccessiblity during in vitro digestion. Food Hydrocoll. 2018, 82, 399–411. [Google Scholar] [CrossRef]
- Mandalari, G.; Adel-Patient, K.; Barkholt, V.; Baro, C.; Bennett, L.; Bublin, M.; Gaier, S.; Graser, G.; Ladics, G.; Mierzejewska, D. In vitro digestibility of β-casein and β-lactoglobulin under simulated human gastric and duodenal conditions: A multi-laboratory evaluation. Regul. Toxicol. Pharmacol. 2009, 55, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.; Portmann, R.; Dubois, S.; Recio, I.; Egger, L. Protein digestion of different protein sources using the INFOGEST static digestion model. Food Res. Int. 2020, 130, 108996. [Google Scholar] [CrossRef] [PubMed]
- Ye, A.; Cui, J.; Carpenter, E.; Prosser, C.; Singh, H. Dynamic in vitro gastric digestion of infant formulae made with goat milk and cow milk: Influence of protein composition. Int. Dairy J. 2019, 97, 76–85. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant proteins: Assessing their nutritional quality and effects on health and physical function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef]
- Wolfe, R.R.; Rutherfurd, S.M.; Kim, I.-Y.; Moughan, P.J. Protein quality as determined by the Digestible Indispensable Amino Acid Score: Evaluation of factors underlying the calculation. Nutr. Rev. 2016, 74, 584–599. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.; Sakr, S.; El-Dieb, S.; Elkashef, H. Developing functional yogurt rich in bioactive peptides and gamma-aminobutyric acid related to cardiovascular health. LWT 2018, 98, 390–397. [Google Scholar] [CrossRef]
- Barbé, F.; Ménard, O.; Le Gouar, Y.; Buffière, C.; Famelart, M.-H.; Laroche, B.; Le Feunteun, S.; Rémond, D.; Dupont, D. Acid and rennet gels exhibit strong differences in the kinetics of milk protein digestion and amino acid bioavailability. Food Chem. 2014, 143, 1–8. [Google Scholar] [CrossRef]
- Zhang, R.; Yoo, M.J.; Realini, C.E.; Staincliffe, M.; Farouk, M.M. In-Bag Dry-vs. Wet-Aged Lamb: Quality, Consumer Acceptability, Oxidative Stability and In Vitro Digestibility. Foods 2021, 10, 41. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Zhou, J.; Wadhwa, S.S. Drinking yoghurts with berry polyphenols added before and after fermentation. Food Control 2013, 32, 450–460. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S.; Bhatti, M.S. Optimization of extraction conditions and enhancement of phenolic content and antioxidant activity of pearl millet fermented with Aspergillus awamori MTCC-548. Resour. Effic. Technol. 2016, 2, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Salar, R.K.; Certik, M.; Brezova, V. Modulation of phenolic content and antioxidant activity of maize by solid state fermentation with Thamnidium elegans CCF 1456. Biotechnol. Bioprocess Eng. 2012, 17, 109–116. [Google Scholar] [CrossRef]
- Adebo, O.A.; Gabriela Medina-Meza, I. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: A mini review. Molecules 2020, 25, 927. [Google Scholar] [CrossRef] [Green Version]
- Simonetti, A.; Perna, A.; Grassi, G.; Gambacorta, E. In vitro phenols bioaccessibility and antioxidant activity of goat milk yogurt fortified with Rhus coriaria leaf powder. J. Food Sci. 2021, 86, 1400–1409. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Everett, D.W. Antioxidant activity and recovery of green tea catechins in full-fat cheese following gastrointestinal simulated digestion. J. Food Compost. Anal. 2016, 48, 13–24. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Zhou, J.; Wadhwa, S.S. Effects of adding apple polyphenols before and after fermentation on the properties of drinking yoghurt. Food Bioprocess Technol. 2012, 5, 2674–2686. [Google Scholar] [CrossRef]
- Hasni, I.; Bourassa, P.; Hamdani, S.; Samson, G.; Carpentier, R.; Tajmir-Riahi, H.-A. Interaction of milk α-and β-caseins with tea polyphenols. Food Chem. 2011, 126, 630–639. [Google Scholar] [CrossRef]
- Sirk, T.W.; Brown, E.F.; Friedman, M.; Sum, A.K. Molecular binding of catechins to biomembranes: Relationship to biological activity. J. Agric. Food Chem. 2009, 57, 6720–6728. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Soto, M.-J.; Tomás-Barberán, F.-A.; García-Conesa, M.-T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- McDougall, G.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red wine–their stability under simulated gastrointestinal digestion. Phytochemistry 2005, 66, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J. Agric. Food Chem. 2005, 53, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Durmus, N.; Capanoglu, E.; Kilic-Akyilmaz, M. Activity and bioaccessibility of antioxidants in yoghurt enriched with black mulberry as affected by fermentation and stage of fruit addition. Int. Dairy J. 2021, 117, 105018. [Google Scholar] [CrossRef]
- Dupas, C.; Marsset Baglieri, A.; Ordonaud, C.; Tomé, D.; Maillard, M.N. Chlorogenic acid is poorly absorbed, independently of the food matrix: A Caco-2 cells and rat chronic absorption study. Mol. Nutr. Food Res. 2006, 50, 1053–1060. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Kristo, E.; LaPointe, G. Adding apple pomace as a functional ingredient in stirred-type yogurt and yogurt drinks. Food Hydrocoll. 2020, 100, 105453. [Google Scholar] [CrossRef]
- Sivakumaran, S.; Huffman, L.; Sivakumaran, S. The Concise New Zealand Food Composition Tables 2016, 12th ed.; The New Zealand Institute for Plant & Food Research Limited and Ministry of Health: Palmerston North, New Zealand, 2017. [Google Scholar]
- Zhang, R.; Yoo, M.J.; Gathercole, J.; Reis, M.G.; Farouk, M.M. Effect of animal age on the nutritional and physicochemical qualities of ovine bresaola. Food Chem. 2018, 254, 317–325. [Google Scholar] [CrossRef]
- Liu, D. Effect of Fuzhuan brick-tea addition on the quality and antioxidant activity of skimmed set-type yoghurt. Int. J. Dairy Technol. 2018, 71, 22–33. [Google Scholar] [CrossRef]
| Yoghurt Samples | Undigested | After Intestinal Digestion | ||||
|---|---|---|---|---|---|---|
| TEFAAs | TNEFAAs | TFAAs | TEFAAs | TNEFAAs | TFAAs | |
| Control | 1.56 ± 0.31 a | 5.42 ± 0.81 a | 6.98 ± 1.11 a | 722 ± 129 a | 599 ± 123 a | 1321 ± 251 a |
| POS5 | 12.3 ± 0.48 b | 238 ± 18.2 b | 251 ± 18.7 b | 778 ± 43.6 b | 918 ± 50.7 b | 1695 ± 94.4 b |
| POS10 | 20.0 ± 0.58 c | 404 ± 4.37 c | 424 ± 4.95 c | 651 ± 91.6 c | 1046 ± 128 c | 1697 ± 220 b |
| POS15 | 35.2 ± 1.76 d | 576 ± 44.6 d | 611 ± 46.4 d | 691 ± 87.7 ac | 1437 ± 169 d | 2129 ± 256 c |
| PRE5 | 77.4 ± 2.43 e | 250 ± 8.01 e | 327 ± 10.4 e | 1009 ± 190 d | 1282 ± 226 e | 2290 ± 416 c |
| PRE10 | 105 ± 5.75 f | 433 ± 15.5 f | 538 ± 21.2 f | 886 ± 146 e | 1550± 255 d | 2436 ± 401 d |
| PRE15 | 112 ± 9.96 g | 538 ± 31.9 d | 650 ± 42.0 d | 905 ± 128 de | 1819 ± 233 f | 2724 ± 361 e |
| Yoghurt Samples | Undigested | After Intestinal Digestion | ||||
|---|---|---|---|---|---|---|
| L-Aspartic Acid | L-Glutamic Acid | GABA | L-Aspartic Acid | L-Glutamic Acid | GABA | |
| Control | 0.16 ± 0.01 a | 0.81 ± 0.04 a | 0.03 ± 0.00 a | 34.0 ± 6.57 a | 75.3 ± 13.3 a | 0.49 ± 0.40 a |
| POS5 | 20.1 ± 1.51 b | 171 ± 13.5 b | 29.2 ± 2.05 b | 59.8 ± 1.90 b | 310 ± 11.1 b | 39.0 ± 3.19 b |
| POS10 | 37.3 ± 0.41 c | 285 ± 2.65 c | 54.8 ± 0.39 c | 68.5 ± 7.15 c | 452 ± 33.6 c | 61.6 ± 3.21 c |
| POS15 | 50.0 ± 2.37 d | 409 ± 37.6 d | 71.5 ± 2.82 d | 93.3 ± 9.95 d | 806 ± 91.03 d | 73.8 ± 8.32 d |
| PRE5 | 19.5 ± 0.43 b | 160 ± 5.49 e | 26.2 ± 0.80 b | 78.6 ± 16.0 e | 470 ± 69.1 c | 34.3 ± 3.36 b |
| PRE10 | 37.9 ± 1.49 c | 280 ± 7.88 c | 48.6 ± 2.52 e | 97.8 ± 18.7 d | 750 ± 119 d | 66.1 ± 10.17 c |
| PRE15 | 47.97 ± 1.92 e | 358 ± 20.6 f | 61.5 ± 2.96 f | 117 ± 19.3 f | 1027 ± 123 e | 90.6 ± 13.9 e |
| Polyphenols/Phases | Before Digestion | |||||
|---|---|---|---|---|---|---|
| POS5 | POS10 | POS15 | PRE5 | PRE10 | PRE15 | |
| Phenolics | ||||||
| Gallic Acid | 0.044 ± 0.000 a | 0.045 ± 0.000 a | 0.049 ± 0.001 b | 0.044 ± 0.000 a | 0.048 ± 0.001 b | 0.049 ± 0.001 b |
| Catechin | 0.119 ± 0.085 a | 0.165 ± 0.070 b | 0.219 ± 0.104 c | 0.035 ± 0.014 d | 0.113 ± 0.097 a | 0.208 ± 0.142 c |
| Caffeic acid | 0.021 ± 0.001 a | 0.031 ± 0.003 b | 0.050 ± 0.005 c | 0.024 ± 0.002 a | 0.059 ± 0.014 d | 0.060 ± 0.006 d |
| Chlorogenic acid | 4.387 ± 0.274 a | 7.005 ± 1.290 b | 10.31 ± 0.458 c | 4.072 ± 0.031 a | 6.052 ± 0.379 b | 9.502 ± 0.393 c |
| Epicatechin | 0.695 ± 0.198 a | 1.339 ± 0.391 b | 1.670 ± 0.470 c | 0.837 ± 0.083 d | 0.886 ± 0.147 d | 1.245 ± 0.185 b |
| p-Cumaric acid | 0.017 ± 0.000 a | 0.028 ± 0.001 b | 0.048 ± 0.002 c | 0.026 ± 0.000 b | 0.029 ± 0.001 b | 0.040 ± 0.003 c |
| Ferulic acid | 0.003 ± 0.000 a | 0.005 ± 0.001 b | 0.009 ± 0.002 c | 0.028 ± 0.001 d | 0.033 ± 0.001 e | 0.037 ± 0.004 e |
| Rutin | 0.011 ± 0.000 a | 0.023 ± 0.005 b | 0.035 ± 0.009 c | 0.011 ± 0.000 a | 0.022 ± 0.002 b | 0.033 ± 0.004 c |
| Ellagic Acid | 0.004 ± 0.000 a | 0.003 ± 0.001 a | 0.009 ± 0.001 b | 0.002 ± 0.000 c | 0.002 ± 0.000 c | 0.005 ± 0.000 a |
| Kaempferol-3-rutinoside | 2.702 ± 0.075 a | 4.208 ± 0.102 b | 8.218 ± 0.707 c | 2.206 ± 0.010 a | 4.290 ± 0.054 b | 6.022 ± 0.140 d |
| Isorhamnetin-3-rutinoside | 0.003 ± 0.000 a | 0.005 ± 0.001 b | 0.008 ± 0.001 c | 0.003 ± 0.001 a | 0.005 ± 0.001 b | 0.007 ± 0.001 c |
| Kaempferol | 0.010 ± 0.001 a | 0.011 ± 0.001 a | 0.016 ± 0.001 b | 0.026 ± 0.003 c | 0.028 ± 0.002 c | 0.028 ± 0.001 c |
| Anthocyanins | ||||||
| Delphinidin-3-rutinoside | 20.59 ± 1.648 a | 23.96 ± 5.714 b | 38.22 ± 2.137 c | 19.62 ± 0.149 a | 21.37 ± 1.807 ab | 31.66 ± 1.650 d |
| Cyanidin-3-rutinoside | 0.503 ± 0.066 a | 0.685 ± 0.122 b | 1.267 ± 0.111 c | 0.539 ± 0.025 a | 0.626 ± 0.080 b | 0.947 ± 0.096 d |
| Pelargonidin-3-rutinoside | 7.733 ± 0.494 a | 11.12 ± 1.484 b | 23.56 ± 1.063 c | 5.100 ± 0.218 d | 11.72 ± 0.702 b | 16.36 ± 0.685 e |
| Polyphenols/Phases | After Digestion | |||||
|---|---|---|---|---|---|---|
| POS5 | POS10 | POS15 | PRE5 | PRE10 | PRE15 | |
| Phenolics | ||||||
| Gallic Acid | 0.002 ± 0.001 a | 0.002 ± 0.001 a | 0.003 ± 0.002 b | 0.003 ± 0.001 b | 0.008 ± 0.004 c | 0.009 ± 0.002 c |
| Catechin | 0.014 ± 0.006 a | 0.033 ± 0.010 b | 0.036 ± 0.006 b | 0.005 ± 0.000 c | 0.022 ± 0.008 ad | 0.026 ± 0.002 d |
| Caffeic acid | 0.004 ± 0.001 a | 0.008 ± 0.003 b | 0.021 ± 0.012 c | 0.019 ± 0.003 c | 0.045 ± 0.015 d | 0.048 ± 0.013 d |
| Chlorogenic acid | 1.926 ± 0.034 a | 3.767 ± 0.198 b | 4.553 ± 0.260 c | 1.724 ± 0.045 d | 4.507 ± 0.264 c | 5.825 ± 0.207 e |
| Epicatechin | 0.679 ± 0.015 a | 0.803 ± 0.062 b | 0.871 ± 0.185 c | 0.793 ± 0.018 b | 0.834 ± 0.025 bc | 0.835 ± 0.088 c |
| p-Cumaric acid | 0.011 ± 0.006 a | 0.012 ± 0.006 b | 0.012 ± 0.003 a | 0.010 ± 0.005 a | 0.011 ± 0.005 a | 0.012 ± 0.008 b |
| Ferulic acid | 0.005 ± 0.002 a | 0.005 ± 0.001 a | 0.008 ± 0.001 b | 0.009 ± 0.003 b | 0.014 ± 0.003 c | 0.014 ± 0.004 c |
| Rutin | 0.006 ± 0.002 a | 0.015 ± 0.005 b | 0.018 ± 0.005 c | 0.005 ± 0.001 a | 0.019 ± 0.006 c | 0.020 ± 0.004 c |
| Ellagic acid | 0.065 ± 0.011 a | 0.073 ± 0.011 b | 0.076 ± 0.019 b | 0.059 ± 0.009 a | 0.064 ± 0.006 a | 0.092 ± 0.009 c |
| Kaempferol-3-rutinoside | 0.825 ± 0.092 a | 1.880 ± 0.227 b | 4.137 ± 0.380 c | 1.501 ± 0.249 b | 3.647 ± 0.525 d | 4.155 ± 0.272 c |
| Isorhamnetin-3-rutinoside | 0.005 ± 0.003 a | 0.006 ± 0.002 ab | 0.007 ± 0.003 b | 0.003 ± 0.001 a | 0.004 ± 0.001 a | 0.004 ± 0.001 a |
| Kaempferol | 0.018 ± 0.001 a | 0.018 ± 0.002 a | 0.023 ± 0.005 b | 0.018 ± 0.001 a | 0.022 ± 0.004 b | 0.024 ± 0.001 b |
| Anthocyanins | ||||||
| Delphinidin-3-rutinoside | 1.147 ± 0.010 a | 2.802 ± 0.064 b | 5.527 ± 0.004 c | 4.560 ± 0.008 d | 8.159 ± 0.090 e | 9.033 ± 0.016 f |
| Cyanidin-3-rutinoside | 0.639 ± 0.024 a | 0.641 ± 0.051 b | 0.672 ± 0.052 c | 0.653 ± 0.210 b | 0.667 ± 0.181 c | 0.687 ± 0.192 c |
| Pelargonidin-3-rutinoside | 1.601 ± 0.019 a | 2.072 ± 0.059 b | 4.147 ± 0.092 c | 1.740 ± 0.067 a | 2.557 ± 0.045 d | 2.617 ± 0.009 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diep, T.T.; Yoo, M.J.Y.; Rush, E. Effect of In Vitro Gastrointestinal Digestion on Amino Acids, Polyphenols and Antioxidant Capacity of Tamarillo Yoghurts. Int. J. Mol. Sci. 2022, 23, 2526. https://doi.org/10.3390/ijms23052526
Diep TT, Yoo MJY, Rush E. Effect of In Vitro Gastrointestinal Digestion on Amino Acids, Polyphenols and Antioxidant Capacity of Tamarillo Yoghurts. International Journal of Molecular Sciences. 2022; 23(5):2526. https://doi.org/10.3390/ijms23052526
Chicago/Turabian StyleDiep, Tung Thanh, Michelle Ji Yeon Yoo, and Elaine Rush. 2022. "Effect of In Vitro Gastrointestinal Digestion on Amino Acids, Polyphenols and Antioxidant Capacity of Tamarillo Yoghurts" International Journal of Molecular Sciences 23, no. 5: 2526. https://doi.org/10.3390/ijms23052526
APA StyleDiep, T. T., Yoo, M. J. Y., & Rush, E. (2022). Effect of In Vitro Gastrointestinal Digestion on Amino Acids, Polyphenols and Antioxidant Capacity of Tamarillo Yoghurts. International Journal of Molecular Sciences, 23(5), 2526. https://doi.org/10.3390/ijms23052526






