Type I Interferons in Systemic Lupus Erythematosus: A Journey from Bench to Bedside
Abstract
1. Introduction
2. Interferon Pathways Leading to SLE
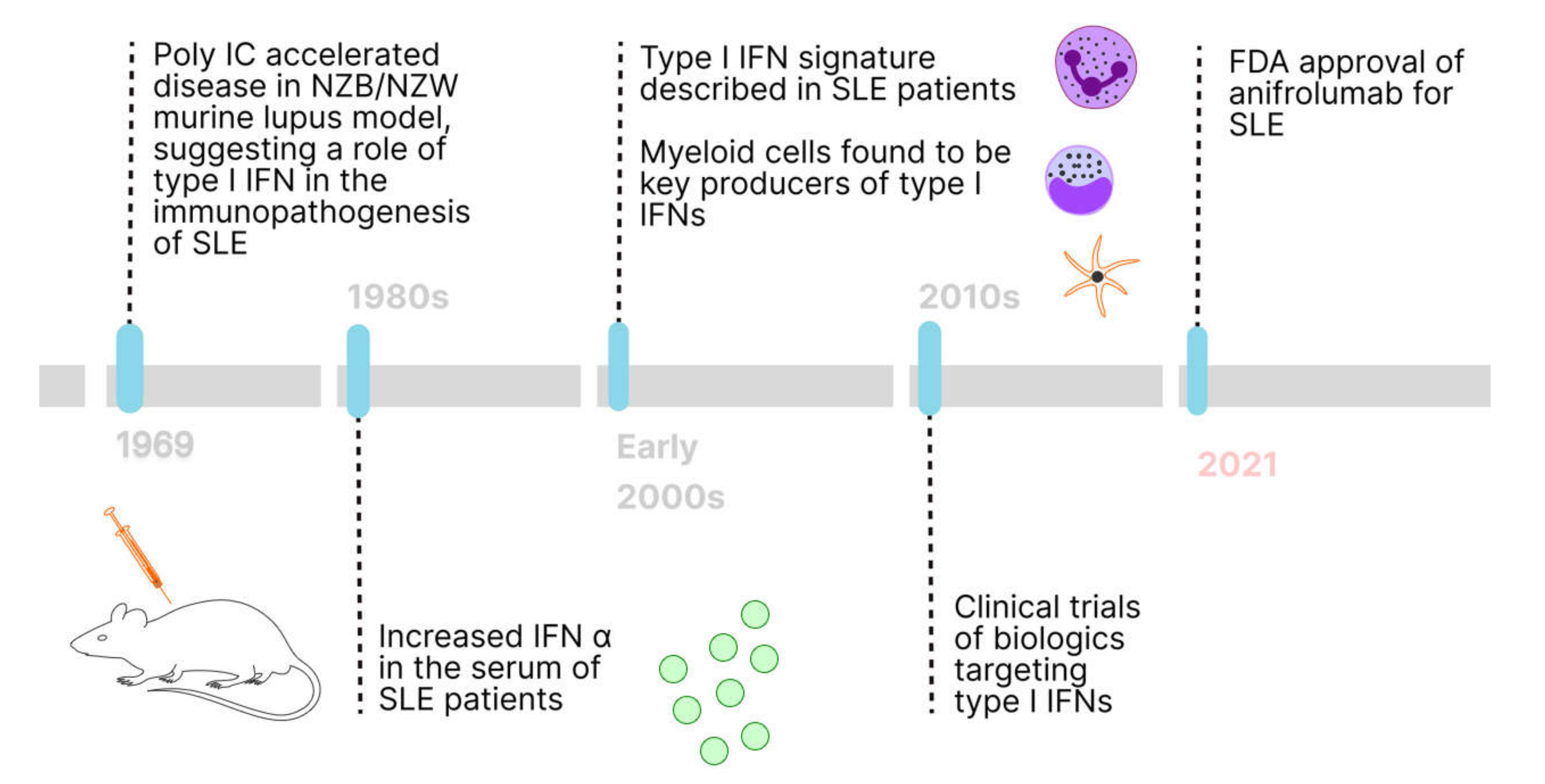
2.1. History of Type I IFNs in SLE
2.2. Contribution of Type II and III IFNs to SLE Immunopathogenesis
2.3. Physiological Role of Type I IFNs in Viral Infections
2.4. Interferon System and Disease Manifestations in SLE
3. Biologics Targeting Type I Interferons in SLE
3.1. Rontalizumab
3.2. Sifalimumab
3.3. Anifrolumab
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kaul, A.; Gordon, C.; Crow, M.K.; Touma, Z.; Urowitz, M.B.; van Vollenhoven, R.; Ruiz-Irastorza, G.; Hughes, G. Systemic lupus erythematosus. Nat. Rev. Dis. Primers 2016, 2, 16039. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.; Cheung, M.; Chiew, H.J.; Liu, Y.; Ho, R.C.-M. Global Trend of Survival and Damage of Systemic Lupus Erythematosus: Meta-Analysis and Meta-Regression of Observational Studies from the 1950s to 2000s. Semin. Arthritis Rheum. 2012, 41, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Margery-Muir, A.A.; Bundell, C.; Nelson, D.; Groth, D.M.; Wetherall, J.D. Gender balance in patients with systemic lupus erythematosus. Autoimmun. Rev. 2017, 16, 258–268. [Google Scholar] [CrossRef]
- Lewis, M.J.; Jawad, A.S. The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology 2016, 56, i67–i77. [Google Scholar] [CrossRef] [PubMed]
- Ugarte-Gil, M.F.; Mak, A.; Leong, J.; Dharmadhikari, B.; Kow, N.Y.; Reátegui-Sokolova, C.; Elera-Fitzcarrald, C.; Aranow, C.; Arnaud, L.; Askanase, A.D.; et al. Impact of glucocorticoids on the incidence of lupus-related major organ damage: A systematic literature review and meta-regression analysis of longitudinal observational studies. Lupus Sci. Med. 2021, 8, e000590. [Google Scholar] [CrossRef]
- Levy, R.A.; Gonzalez-Rivera, T.; Khamashta, M.; Fox, N.L.; Jones-Leone, A.; Rubin, B.; Burriss, S.W.; Gairy, K.; van Maurik, A.; Roth, D.A. 10 Years of belimumab experience: What have we learnt? Lupus 2021, 30, 1705–1721. [Google Scholar] [CrossRef]
- Rovin, B.H.; Teng, Y.K.O.; Ginzler, E.M.; Arriens, C.; Caster, D.J.; Romero-Diaz, J.; Gibson, K.; Kaplan, J.; Lisk, L.; Navarra, S.; et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2070–2080. [Google Scholar] [CrossRef]
- Morand, E.F.; Furie, R.; Tanaka, Y.; Bruce, I.N.; Askanase, A.D.; Richez, C.; Bae, S.-C.; Brohawn, P.Z.; Pineda, L.; Berglind, A.; et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N. Engl. J. Med. 2020, 382, 211–221. [Google Scholar] [CrossRef]
- Mode, D.; Stockholm, L.C. AstraZeneca: Saphnelo (anifrolumab) approved in the US for moderate to severe systemic lupus erythematosus. Available online: https://mfn.se/cis/a/astrazeneca/astrazeneca-saphnelo-anifrolumab-approved-in-the-us-for-moderate-to-severe-systemic-lupus-erythematosus-26759120 (accessed on 28 January 2022).
- Burki, T.K. FDA approval for anifrolumab in patients with lupus. Lancet Rheumatol. 2021, 3, e689. [Google Scholar] [CrossRef]
- Navarra, S.V.; Guzmán, R.M.; Gallacher, A.E.; Hall, S.; Levy, R.A.; Jimenez, R.E.; Li, E.K.-M.; Thomas, M.; Kim, H.-Y.; León, M.G.; et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: A randomised, placebo-controlled, phase 3 trial. Lancet 2011, 377, 721–731. [Google Scholar] [CrossRef]
- Furie, R.; Petri, M.; Zamani, O.; Cervera, R.; Wallace, D.J.; Tegzová, D.; Sanchez-Guerrero, J.; Schwarting, A.; Merrill, J.T.; Chatham, W.W. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 3918–3930. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Rovin, B.H.; Houssiau, F.; Malvar, A.; Teng, Y.O.; Contreras, G.; Amoura, Z.; Yu, X.; Mok, C.-C.; Santiago, M.B.; et al. Two-Year, Randomized, Controlled Trial of Belimumab in Lupus Nephritis. N. Engl. J. Med. 2020, 383, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Riggs, J.M.; Hanna, R.N.; Rajan, B.; Zerrouki, K.; Karnell, J.L.; Sagar, D.; Vainshtein, I.; Farmer, E.; Rosenthal, K.; Morehouse, C.; et al. Characterisation of anifrolumab, a fully human anti-interferon receptor antagonist antibody for the treatment of systemic lupus erythematosus. Lupus Sci. Med. 2018, 5, e000261. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.W.H.; Ng, C.H.; Tay, S.H. Biologics targeting type I interferons in SLE: A meta-analysis and systematic review of randomised controlled trials. Lupus 2020, 29, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Caielli, S.; Cardenas, J.; de Jesus, A.A.; Baisch, J.; Walters, L.; Blanck, J.P.; Balasubramanian, P.; Stagnar, C.; Ohouo, M.; Hong, S.; et al. Erythroid mitochondrial retention triggers myeloid-dependent type I interferon in human SLE. Cell 2021, 184, 4464–4479.e19. [Google Scholar] [CrossRef]
- Furie, R.A.; Morand, E.F.; Bruce, I.N.; Manzi, S.; Kalunian, K.C.; Vital, E.M.; Ford, T.L.; Gupta, R.; Hiepe, F.; Santiago, M.; et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): A randomised, controlled, phase 3 trial. Lancet Rheumatol. 2019, 1, e208–e219. [Google Scholar] [CrossRef]
- Kalunian, K.C.; Merrill, J.T.; Maciuca, R.; McBride, J.M.; Townsend, M.; Wei, X.; Davis, J.C.; Kennedy, W.P. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE). Ann. Rheum. Dis. 2015, 75, 196–202. [Google Scholar] [CrossRef]
- Houssiau, F.A.; Thanou, A.; Mazur, M.; Ramiterre, E.; Mora, D.A.G.; Misterska-Skora, M.; Perich-Campos, R.A.; Smakotina, S.A.; Cruz, S.C.; Louzir, B. IFN-α kinoid in systemic lupus erythematosus: Results from a phase IIb, randomised, placebo-controlled study. Ann. Rheum. Dis. 2020, 79, 347–355. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves AstraZeneca’s anifrolumab for lupus. Nat. Rev. Drug Discov. 2021, 20, 658. [Google Scholar] [CrossRef]
- Hargraves, M.M.; Richmond, H.; Morton, R. Presentation of two bone marrow elements: The ‘tart’ cell and the, ‘L.E.’ cell. Proc. Staff. Meet. Mayo Clin. 1948, 23, 25–28. [Google Scholar]
- Isaacs, A.; Lindenmann, J. Virus interference. I. The interferon. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1957, 147, 258–267. [Google Scholar]
- Bezalel, S.; Guri, K.M.; Elbirt, D.; Asher, I.; Sthoeger, Z.M. Type I interferon signature in systemic lupus erythematosus. Isr. Med. Assoc. J. 2014, 16, 246–249. [Google Scholar] [PubMed]
- Crispín, J.C.; Kyttaris, V.C.; Terhorst, C.; Tsokos, G.C. T cells as therapeutic targets in SLE. Nat. Rev. Rheumatol. 2010, 6, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Dörner, T.; Jacobi, A.M.; Lee, J.; Lipsky, P.E. Abnormalities of B cell subsets in patients with systemic lupus erythematosus. J. Immunol. Methods 2011, 363, 187–197. [Google Scholar] [CrossRef]
- Crow, M.K.; Olferiev, M.; Kirou, K.A. Type I interferons in autoimmune disease. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 369–393. [Google Scholar] [CrossRef]
- Pisetsky, D.S.; Lipsky, P.E. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat. Rev. Rheumatol. 2020, 16, 565–579. [Google Scholar] [CrossRef]
- Kirou, K.A.; Lee, C.; George, S.; Louca, K.; Peterson, M.G.E.; Crow, M.K. Activation of the interferon-α pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Care Res. 2005, 52, 1491–1503. [Google Scholar] [CrossRef]
- Hubbard, E.L.; Pisetsky, D.S.; Lipsky, P.E. Anti-RNP antibodies are associated with the interferon gene signature but not decreased complement levels in SLE. Ann. Rheum. Dis. 2022. [Google Scholar] [CrossRef]
- Steinberg, A.D.; Baron, S.; Talal, N. The Pathogenesis of Autoimmunity in New Zealand Mice, I. Induction of Antinucleic Acid Antibodies by Polyinosinic·Polycytidylic Acid. Proc. Natl. Acad. Sci. USA 1969, 63, 1102–1107. [Google Scholar] [CrossRef]
- Hooks, J.J.; Moutsopoulos, H.M.; Geis, S.A.; Stahl, N.I.; Decker, J.L.; Notkins, A.L. Immune Interferon in the Circulation of Patients with Autoimmune Disease. N. Engl. J. Med. 1979, 301, 5–8. [Google Scholar] [CrossRef]
- Preble, O.T.; Black, R.J.; Friedman, R.M.; Klippel, J.H.; Vilček, J. Systemic Lupus Erythematosus: Presence in Human Serum of an Unusual Acid-Labile Leukocyte Interferon. Science 1982, 216, 429–431. [Google Scholar] [CrossRef]
- Bennett, L.; Palucka, A.K.; Arce, E.; Cantrell, V.; Borvak, J.; Banchereau, J.; Pascual, V. Interferon and Granulopoiesis Signatures in Systemic Lupus Erythematosus Blood. J. Exp. Med. 2003, 197, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Baechler, E.C.; Batliwalla, F.M.; Karypis, G.; Gaffney, P.; Ortmann, W.A.; Espe, K.J.; Shark, K.B.; Grande, W.J.; Hughes, K.M.; Kapur, V.; et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA 2003, 100, 2610–2615. [Google Scholar] [CrossRef] [PubMed]
- Han, G.-M.; Chen, S.-L.; Shen, N.; Ye, S.; Bao, C.-D.; Gu, Y.-Y. Analysis of gene expression profiles in human systemic lupus erythematosus using oligonucleotide microarray. Genes Immun. 2003, 4, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Crow, M.K.; Wohlgemuth, J. Microarray analysis of gene expression in lupus. Arthritis Res. Ther. 2003, 5, 279–287. [Google Scholar] [CrossRef][Green Version]
- Haynes, W.A.; Haddon, D.J.; Diep, V.K.; Khatri, A.; Bongen, E.; Yiu, G.; Balboni, I.; Bolen, C.R.; Mao, R.; Utz, P.J.; et al. Integrated, multicohort analysis reveals unified signature of systemic lupus erythematosus. JCI Insight 2020, 5, e122312. [Google Scholar] [CrossRef]
- Banchereau, J.; Pascual, V. Type I Interferon in Systemic Lupus Erythematosus and Other Autoimmune Diseases. Immunity 2006, 25, 383–392. [Google Scholar] [CrossRef]
- Kim, H.; Sanchez, G.A.M.; Goldbach-Mansky, R. Insights from Mendelian Interferonopathies: Comparison of CANDLE, SAVI with AGS, Monogenic Lupus. Klin. Wochenschr. 2016, 94, 1111–1127. [Google Scholar] [CrossRef]
- Crouse, J.; Kalinke, U.; Oxenius, A. Regulation of antiviral T cell responses by type I interferons. Nat. Rev. Immunol. 2015, 15, 231–242. [Google Scholar] [CrossRef]
- Barrat, F.J.; Crow, M.K.; Ivashkiv, L.B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 2019, 20, 1574–1583. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Auto-antibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]
- Rönnblom, L.; Leonard, D. Interferon pathway in SLE: One key to unlocking the mystery of the disease. Lupus Sci. Med. 2019, 6, e000270. [Google Scholar] [CrossRef] [PubMed]
- Niewold, T.B.; Hua, J.; Lehman, T.J.A.; Harley, J.B.; Crow, M.K. High serum IFN-α activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007, 8, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Ghodke-Puranik, Y.; Niewold, T.B. Genetics of the type I interferon pathway in systemic lupus erythematosus. Int. J. Clin. Rheumatol. 2013, 8, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Nehar-Belaid, D.; Hong, S.; Marches, R.; Chen, G.; Bolisetty, M.; Baisch, J.; Walters, L.; Punaro, M.; Rossi, R.J.; Chung, C.-H.; et al. Mapping systemic lupus erythematosus heterogeneity at the single-cell level. Nat. Immunol. 2020, 21, 1094–1106. [Google Scholar] [CrossRef]
- Chaussabel, D.; Quinn, C.; Shen, J.; Patel, P.; Glaser, C.; Baldwin, N.; Stichweh, D.; Blankenship, D.; Li, L.; Munagala, I.; et al. A Modular Analysis Framework for Blood Genomics Studies: Application to Systemic Lupus Erythematosus. Immunity 2008, 29, 150–164. [Google Scholar] [CrossRef]
- Chiche, L.; Jourde-Chiche, N.; Whalen, E.; Presnell, S.; Gersuk, V.; Dang, K.; Anguiano, E.; Quinn, C.; Burtey, S.; Berland, Y. Modular transcriptional repertoire analyses of adults with systemic lupus erythematosus reveal distinct type I and type II interferon signatures. Arthritis Rheumatol. 2014, 66, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Zickert, A.; Oke, V.; Parodis, I.; Svenungsson, E.; Sundström, Y.; Gunnarsson, I. Interferon (IFN)-λ is a potential mediator in lupus nephritis. Lupus Sci. Med. 2016, 3, e000170. [Google Scholar] [CrossRef]
- Oke, V.; Gunnarsson, I.; Dorschner, J.; Eketjäll, S.; Zickert, A.; Niewold, T.B.; Svenungsson, E. High levels of circulating interferons type I, type II and type III associate with distinct clinical features of active systemic lupus erythematosus. Arthritis Res. Ther. 2019, 21, 107. [Google Scholar] [CrossRef]
- Rodero, M.; Decalf, J.; Bondet, V.; Hunt, D.; Rice, G.I.; Werneke, S.; McGlasson, S.L.; Alyanakian, M.-A.; Bader-Meunier, B.; Barnerias, C.; et al. Detection of interferon alpha protein reveals differential levels and cellular sources in disease. J. Exp. Med. 2017, 214, 1547–1555. [Google Scholar] [CrossRef]
- Denny, M.F.; Yalavarthi, S.; Zhao, W.; Thacker, S.G.; Anderson, M.; Sandy, A.R.; McCune, W.J.; Kaplan, M.J. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 2010, 184, 3284–3297. [Google Scholar] [CrossRef]
- Decker, P. Neutrophils and interferon-α-producing cells: Who produces interferon in lupus? Arthritis Res. Ther. 2011, 13, 118. [Google Scholar] [CrossRef]
- Sadler, A.J.; Williams, B.R.G. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 2008, 8, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Plisch, E.H.; Sullivan, J.; Thomas, C.; Czuprynski, C.J.; Williams, B.R.G.; Suresh, M. Role of PKR and Type I IFNs in Viral Control during Primary and Secondary Infection. PLOS Pathog. 2010, 6, e1000966. [Google Scholar] [CrossRef]
- Yan, N.; Chen, Z.J. Intrinsic antiviral immunity. Nat. Immunol. 2012, 13, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Tatouli, I.P.; Rosen, L.B.; Hasni, S.; Alevizos, I.; Manna, Z.G.; Rivera, J.; Jiang, C.; Siegel, R.M.; Holland, S.M.; et al. Distinct Functions of Autoantibodies Against Interferon in Systemic Lupus Erythematosus: A Comprehensive Analysis of Anticytokine Autoantibodies in Common Rheumatic Diseases. Arthritis Rheumatol. 2016, 68, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Braunstein, I.; Klein, R.S.; Okawa, J.; Werth, V.P. The interferon-regulated gene signature is elevated in subacute cutaneous lupus erythematosus and discoid lupus erythematosus and correlates with the cutaneous lupus area and severity index score. Br. J. Dermatol. 2012, 166, 971–975. [Google Scholar] [CrossRef]
- Petri, M.; Fu, W.; Ranger, A.; Allaire, N.; Cullen, P.; Magder, L.S.; Zhang, Y. Association between changes in gene signatures expression and disease activity among patients with systemic lupus erythematosus. BMC Med. Genom. 2019, 12, 4. [Google Scholar] [CrossRef]
- Berthier, C.C.; Tsoi, L.C.; Reed, T.J.; Stannard, J.N.; Myers, E.M.; Namas, R.; Xing, X.; Lazar, S.; Lowe, L.; Kretzler, M.; et al. Molecular Profiling of Cutaneous Lupus Lesions Identifies Subgroups Distinct from Clinical Phenotypes. J. Clin. Med. 2019, 8, 1244. [Google Scholar] [CrossRef]
- Casey, K.A.; Guo, X.; Smith, M.A.; Wang, S.; Sinibaldi, D.; Sanjuan, M.A.; Wang, L.; Illei, G.G.; White, W.I. Type I interferon receptor blockade with anifrolumab corrects innate and adaptive immune perturbations of SLE. Lupus Sci. Med. 2018, 5, e000286. [Google Scholar] [CrossRef]
- De Palma, G.; Castellano, G.; Del Prete, A.; Sozzani, S.; Fiore, N.; Loverre, A.; Parmentier, M.; Gesualdo, L.; Grandaliano, G.; Schena, F.P. The possible role of ChemR23/Chemerin axis in the recruitment of dendritic cells in lupus nephritis. Kidney Int. 2011, 79, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; Cafiero, C.; Divella, C.; Sallustio, F.; Gigante, M.; Pontrelli, P.; De Palma, G.; Rossini, M.; Grandaliano, G.; Gesualdo, L. Local synthesis of interferon-alpha in lupus nephritis is associated with type I interferons signature and LMP7 induction in renal tubular epithelial cells. Arthritis Res. Ther. 2015, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, E.; Yalavarthi, S.; Berthier, C.C.; Hodgin, J.B.; Khandpur, R.; Lin, A.M.; Rubin, C.J.; Zhao, W.; Olsen, S.H.; Klinker, M.; et al. Netting Neutrophils Induce Endothelial Damage, Infiltrate Tissues, and Expose Immunostimulatory Molecules in Systemic Lupus Erythematosus. J. Immunol. 2011, 187, 538–552. [Google Scholar] [CrossRef] [PubMed]
- Boedigheimer, M.J.; Martin, D.A.; Amoura, Z.; Sánchez-Guerrero, J.; Romero-Diaz, J.; Kivitz, A.; Aranow, C.; Chan, T.M.; Chong, Y.B.; Chiu, K.; et al. Safety, pharmacokinetics and pharmacodynamics of AMG 811, an anti-interferon-γ monoclonal antibody, in SLE subjects without or with lupus nephritis. Lupus Sci. Med. 2017, 4, e000226. [Google Scholar] [CrossRef] [PubMed]
- Der, E.; Ranabothu, S.; Suryawanshi, H.; Akat, K.M.; Clancy, R.; Morozov, P.; Kustagi, M.; Czuppa, M.; Izmirly, P.; Belmont, H.M.; et al. Single cell RNA sequencing to dissect the molecular heterogeneity in lupus nephritis. JCI Insight 2017, 2, e93009. [Google Scholar] [CrossRef]
- Ríos-Garcés, R.; Cervera, R. Targeting interferon I in SLE: A promising new perspective. Lancet Rheumatol. 2020, 2, e581–e582. [Google Scholar] [CrossRef]
- Bezalel, S.; Asher, I.; Elbirt, D.; Sthoeger, Z.M. Novel biological treatments for systemic lupus erythematosus: Current and future modalities. Isr. Med. Assoc. J. 2012, 14, 508. [Google Scholar]
- McBride, J.; Jiang, J.; Abbas, A.; Morimoto, A.; Li, J.; Maciuca, R.; Townsend, M.; Wallace, D.; Kennedy, W.; Drappa, J. Safety and pharmacodynamic results of rontalizumab in a phase I, placebo controlled, double blind, dose escalation study in systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 3666–3676. [Google Scholar] [CrossRef]
- Merrill, J.T.; Wallace, D.J.; Petri, M.; Kirou, K.A.; Yao, Y.; White, W.I.; Robbie, G.; Levin, R.; Berney, S.M.; Chindalore, V.; et al. Safety profile and clinical activity of sifalimumab, a fully human anti-interferon monoclonal antibody, in systemic lupus erythematosus: A phase I, multicentre, double-blind randomised study. Ann. Rheum. Dis. 2011, 70, 1905–1913. [Google Scholar] [CrossRef]
- Petri, M.; Wallace, D.J.; Spindler, A.; Chindalore, V.; Kalunian, K.C.; Mysler, E.; Neuwelt, C.M.; Robbie, G.; White, W.I.; Higgs, B.; et al. Sifalimumab, a Human Anti-Interferon-α Monoclonal Antibody, in Systemic Lupus Erythematosus: A Phase I Randomized, Controlled, Dose-Escalation Study. Arthritis Care Res. 2013, 65, 1011–1021. [Google Scholar] [CrossRef]
- Khamashta, M.; Merrill, J.T.; Werth, V.P.; Furie, R.; Kalunian, K.; Illei, G.G.; Drappa, J.; Wang, L.; Greth, W. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: A randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2016, 75, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Tummala, R.; Rouse, T.; Berglind, A.; Santiago, L. Safety, tolerability and pharmacokinetics of subcutaneous and intravenous anifrolumab in healthy volunteers. Lupus Sci. Med. 2018, 5, e000252. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Khamashta, M.; Merrill, J.T.; Werth, V.P.; Kalunian, K.; Brohawn, P.; Illei, G.G.; Drappa, J.; Wang, L.; Yoo, S. Anifrolumab, an anti-interferon-α receptor monoclonal antibody, in moderate-to-severe systemic lupus erythematosus. Arthritis Rheumatol. 2017, 69, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.; Rovin, B.; Mysler, E.F.; Furie, R.A.; Houssiau, F.A.; Trasieva, T.; Knagenhjelm, J.; Schwetje, E.; Chia, Y.L.; Tummala, R.; et al. Phase II randomised trial of type I interferon inhibitor anifrolumab in patients with active lupus nephritis. Ann. Rheum. Dis. 2022. [Google Scholar] [CrossRef]
- Hay, E.; Bacon, P.; Gordon, C.; Isenberg, D.; Maddison, P.; Snaith, M.; Symmons, D.; Viner, N.; Zoma, A. The BILAG index: A reliable and valid instrument for measuring clinical disease activity in systemic lupus erythematosus. QJM Int. J. Med. 1993, 86, 447–458. [Google Scholar]
- Chaichian, Y.; Wallace, D.J.; Weisman, M.H. A promising approach to targeting type 1 IFN in systemic lupus erythematosus. J. Clin. Investig. 2019, 129, 958–961. [Google Scholar] [CrossRef]
- Peng, L.; Oganesyan, V.; Wu, H.; Dall’Acqua, W.F.; Damschroder, M.M. In Molecular basis for antagonistic activity of anifrolumab, an anti-interferon-α receptor 1 antibody, MAbs, 2015. Taylor Fr. 2015, 7, 428–439. [Google Scholar]
- Fanouriakis, A.; Kostopoulou, M.; Cheema, K.; Anders, H.J.; Aringer, M.; Bajema, I.; Boletis, J.; Frangou, E.; Houssiau, F.A.; Hollis, J.; et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann. Rheum. Dis. 2020, 79, 713–723. [Google Scholar] [CrossRef]
- Morales, E.; Galindo, M.; Trujillo, H.; Praga, M. Update on Lupus Nephritis: Looking for a New Vision. Nephron Exp. Nephrol. 2020, 145, 1–13. [Google Scholar] [CrossRef]
- Wallace, D.J. Improved strategies for designing lupus trials with targeted therapies: Learning from 65 years of experience. Lupus 2016, 25, 1141–1149. [Google Scholar] [CrossRef]
- Wallace, D.J. Advances in drug therapy for systemic lupus erythematosus. BMC Med. 2010, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Tsang-A-Sjoe, M.W.P.; Bultink, I.E.M. New developments in systemic lupus erythematosus. Rheumatology 2021, 60, vi21–vi28. [Google Scholar] [CrossRef] [PubMed]
- Sim, T.M.; Tarini, D.; Dheen, S.T.; Bay, B.H.; Srinivasan, D.K. Nanoparticle-Based Technology Approaches to the Management of Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 6070. [Google Scholar] [CrossRef] [PubMed]
- Chatenoud, L. Precision medicine for autoimmune disease. Nat. Biotechnol. 2016, 34, 930–932. [Google Scholar] [CrossRef]
- Thanou-Stavraki, A.; Sawalha, A.H. An update on belimumab for the treatment of lupus. Biol. Targets Ther. 2011, 5, 33. [Google Scholar]
- Banchereau, R.; Hong, S.; Cantarel, B.; Baldwin, N.; Baisch, J.; Edens, M.; Cepika, A.-M.; Acs, P.; Turner, J.; Anguiano, E. Personalized immunomonitoring uncovers molecular networks that stratify lupus patients. Cell 2016, 165, 551–565. [Google Scholar] [CrossRef]
- Zielinski, J.M.; Luke, J.J.; Guglietta, S.; Krieg, C. High Throughput Multi-Omics Approaches for Clinical Trial Evaluation and Drug Discovery. Front. Immunol. 2021, 12, 590742. [Google Scholar] [CrossRef]
| Study, n, SLE Population | Phase | Results | Effects on IFN Signature | Significant Adverse Events |
|---|---|---|---|---|
| McBride et al., 2012 [69], N = 60 (Placebo n = 12 vs. Rontalizumab n = 48). Subjects with stable, mildly active SLE as defined by SELENA-SLEDAI a score. ~50% of patients had baseline high interferon signature. | I | The pharmacokinetic properties of rontalizumab were as expected for an IgG1 monoclonal antibody and were found to be proportional to dose. | At baseline, patients were categorized by type I IFN signature high or low from expression of seven selected type I IFN inducible genes. IFN-regulated genes expression demonstrated a dose-dependent decline, which was evident in the majority of patients, regardless of high or low baseline IFN signature was sustained beyond 28 days after dosing. | An acceptable safety profile was demonstrated. The incidence of serious adverse events was comparable across cohorts. These serious adverse events were classified as unrelated to rontalizumab. |
| Kalunian et al., 2015 (ROSE) [18], N = 238 (Placebo n = 79 vs. Rontalizumab n = 159). Subjects with moderate to severe SLE as defined by BILAG b index. 75.6% of patients had baseline high interferon signature. | II | There was no significant treatment difference in BILAG c Index Response and SRI(4) c in rontalizumab and placebo groups. | Baseline IFN signature was stratified by gene expression of a 3-gene set of IFN-regulated genes. The patients with baseline low IFN signature appeared to be the most responsive to rontalizumab and had, on average, lower anti-dsDNA titres and less profound hypocomplementemia (C3, C4) compared with the patients with baseline high IFN signature. | The incidence of serious adverse effects was comparable between the placebo and rontalizumab. |
| Merrill et al., 2011 [70], N = 51 (Placebo n = 17 vs. Sifalimumab n = 34). Patients with mild to moderate SLE as defined by SLEDAI score and BILAG index. ~58% of patients had baseline high IFN signature. | I | Consistent trends of greater improvement in the sifalimumab group were found using different measures, although statistical significance was not reported. Sifalimumab subjects were less likely to exhibit a SLEDAI flare or a BILAG flare. | Baseline type I IFN high or low signature statuses were determined by expression levels of 21 type I IFN-inducible genes using RT-PCR. Sifalimumab was found to neutralize overexpression of type I IFN signature in a dose-dependent manner. | Adverse event rates were similar among groups and were mostly mild. No relationship was apparent between Sifalimumab dose and severity or frequency of adverse events. |
| Petri et al., 2013 [71], N = 161 (Placebo n = 40 vs. sifalilumab n = 121). Most patients had moderate to severe SLE, as defined by SLEDAI score of ≥6. 75.2% of subjects had baseline high IFN signature. | I | Serum sifalimumab concentrations increased in a linear and dose-proportional manner. No statistically significant differences in clinical activity, as measured by SLEDAI and BILAG between sifalimumab and the placebo, were observed. However, when adjusted for excess burst steroids, SLEDAI change from baseline showed a positive trend over time. A trend toward normal complement C3 or C4 level at week 26 was seen in the sifalimumab groups compared with baseline. | At baseline, patients were categorized by type I IFN–inducible gene signature from a panel of 21 type I IFN–inducible genes. Dose-dependent neutralization of the type I IFN gene signature (21-gene panel) in the blood with sifalimumab treatment was observed in patients who had overexpression of the type I IFN signature at baseline. Patients with a baseline high IFN signature showed a greater mean reduction from baseline in SELENA–SLEDAI score in the combined sifalimumab group compared with the placebo group. Inhibition of the type I IFN by sifalilumab was found to be dose-dependent. | The frequencies of severe adverse effects were similar between the two treatment groups, with no apparent dose effects across the individual sifalimumab dose groups. Most adverse effects were mild or moderate and the most frequent treatment-related adverse effects were urinary tract infection, nausea and headache. Sifalilumab was generally well-tolerated. |
| Khamashta et al., 2016 [72], N = 431 (Placebo n = 108 vs. sifalilumab n = 323). Patients with moderate to severe SLE as defined by SLEDAI-2K d and BILAG scores. ~81% of subjects had baseline high IFN signature. | IIb | Compared with placebo, a greater percentage of patients who received sifalimumab met the primary end point of SRI (4). Improvements were consistent across various clinical end points, including global and organ-specific measures of disease activity. | Baseline IFN signature was measured based on expression of four type I IFN-regulated genes.Substantial improvements in SRI (4) and BICLA e were observed for baseline IFN-high patients vs. placebo. A meaningful statistical comparison between patients based on baseline IFN signature low vs. high was not possible due to the small number of patients with baseline low IFN gene signature. | Adverse events occurred with similar frequencies in the sifalimumab and placebo groups, except that herpes zoster infections were more frequent with sifalimumab treatment. The incidence of adverse effects was similar between sifalimumab and placebo groups. |
| Tummala et al., 2018 [73], N = 30. Anifrolumab in healthy subjects. | I | Anifrolumab reached maximum serum concentration after 4–7 days and were below the limit of detection by day 84 of administration. Subcutaneous administration of anifrolumab 300 mg and 600 mg exhibited dose-proportional pharmacokinetics. | - | Anifrolumab was generally safe and well-tolerated. |
| Furie et al., 2017 (MUSE) [74], N = 305 (Placebo n = 102 vs. anifrolumab n = 203). Patients with moderate to severe SLE, as determined by SLEDAI-2K and BILAG scores. ~75% of subjects had baseline high IFN signature. | IIb | A greater proportion of subjects treated with anifrolumab exhibited an SRI(4) response at week 24 than subjects who received placebo. Anifrolumab-treated patients had greater improvements in organ-specific and global outcomes compared to the placebo. | Baseline IFN signature was measured based on expression of four type I IFN-regulated genes. Greater efficacy of anifrolumab was found in subjects with a baseline high IFN signature as compared to the placebo. In the baseline high IFN signature subpopulation of patients, anifrolumab was found to be effective in supressing type I IFN gene expression. The median neutralization ratio was 89.7 and 91.7 for anifrolumab 300 mg and 600 mg, respectively. No neutralization was observed with the placebo.The efficacy of anifrolumab in subjects with baseline low IFN signature was similar to that of the placebo group. | Anifrolumab was well-tolerated, and incidence of adverse events was similar in anifrolumab and placebo groups. However, a dose-related increase in respiratory tract infections such as herpes zoster and influenza was observed. |
| Furie et al., 2019 (TULIP-1) [17], N = 457 (Placebo n = 184 vs. anifrolumab n = 273). Patients with moderate to severe SLE as determined by SLEDAI-2K score. ~82% of subjects had baseline high IFN signature. | III | The proportion of patients with an SRI(4) response was initially similar between anifrolumab and placebo. Following adjustment of medication rules, key analyses were reperformed and anifrolumab was found to improve organ-specific measures, BICLA response and sustained oral corticosteroid dose reduction. | Baseline IFN signature was measured based on expression of four type I IFN-regulated genes. In patients with high baseline IFN signature, a 21-gene assay assessing neutralization of type I IFN-induced transcripts found that anifrolumab 300mg caused early suppression (median percentage of baseline signature at week 12 was 12·6%), which was sustained through 52 weeks. No IFN gene signature suppression was observed with the placebo. | Anifrolumab was well-tolerated and had an acceptable safety profile. Incidence of adverse events was similar across groups. |
| Morand et al., 2019 (TULIP-2) [8], N = 362 (Placebo n = 182 vs. anifrolumab n = 180). Patients with moderate to severe, active SLE as determined by SLEDAI-2K score. ~83% of subjects had baseline high IFN signature. | III | Monthly administration of anifrolumab was found to result in a significantly higher proportion of patients with a BICLA response than the placebo. Anifrolumab treatment was also associated with reductions in oral glucocorticoid dose, severity of skin disease and counts of swollen and tender joints. | Baseline IFN signature was measured based on expression of four type I IFN-regulated genes. A 21-gene assay assessing neutralization of type I IFN-induced transcripts found that anifrolumab achieved neutralization of IFN signature early in treatment of patients with baseline high IFN signature. Among patients with baseline high IFN signature group, the percentage of patients treated with anifrolumab with a BICLA response at week 52 was significantly higher than those in the placebo group. | Anifrolumab was generally safe and well-tolerated. Incidence of herpes zoster was found to be increased in the anifrolumab group, as compared to the placebo group. |
| Jayne et al.,2022 (TULIP-LN) [75], N = 145, (Placebo n = 49 vs. anifrolumab n = 98). Patients with a biopsy-proven diagnosis of class III/IV ± V LN. ~94.8% of subjects had baseline high IFN signature. | II | The primary end point of a change in baseline 24-h UPCR for the combined anifrolumab compared to the placebo group at week 52 was not met. The intensified regimen of anifrolumab was associated with numerical improvements across various secodnary end points, such as percentage of subjects attaining aCRR and sustained glucocorticoid reduction. | Baseline type I IFN high or low signature statuses were determined by expression levels of 21 type I IFN-inducible genes using RT-PCR. In patients with high baseline IFN signature, the 21-gene assay found that anifrolumab displayed a dose-dependent neutralization of >80% of IFN signature. No IFN gene signature suppression was observed with the placebo. | Anifrolumab was safe and well-tolerated. The safety profile in LN patient was found to be similar to that in SLE patients without active renal disease. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, T.M.; Ong, S.J.; Mak, A.; Tay, S.H. Type I Interferons in Systemic Lupus Erythematosus: A Journey from Bench to Bedside. Int. J. Mol. Sci. 2022, 23, 2505. https://doi.org/10.3390/ijms23052505
Sim TM, Ong SJ, Mak A, Tay SH. Type I Interferons in Systemic Lupus Erythematosus: A Journey from Bench to Bedside. International Journal of Molecular Sciences. 2022; 23(5):2505. https://doi.org/10.3390/ijms23052505
Chicago/Turabian StyleSim, Tao Ming, Siying Jane Ong, Anselm Mak, and Sen Hee Tay. 2022. "Type I Interferons in Systemic Lupus Erythematosus: A Journey from Bench to Bedside" International Journal of Molecular Sciences 23, no. 5: 2505. https://doi.org/10.3390/ijms23052505
APA StyleSim, T. M., Ong, S. J., Mak, A., & Tay, S. H. (2022). Type I Interferons in Systemic Lupus Erythematosus: A Journey from Bench to Bedside. International Journal of Molecular Sciences, 23(5), 2505. https://doi.org/10.3390/ijms23052505








