Gata3 Silencing Is Involved in Neuronal Differentiation and Its Abnormal Expression Impedes Neural Activity in Adult Retinal Neurocytes
Abstract
:1. Introduction
2. Results
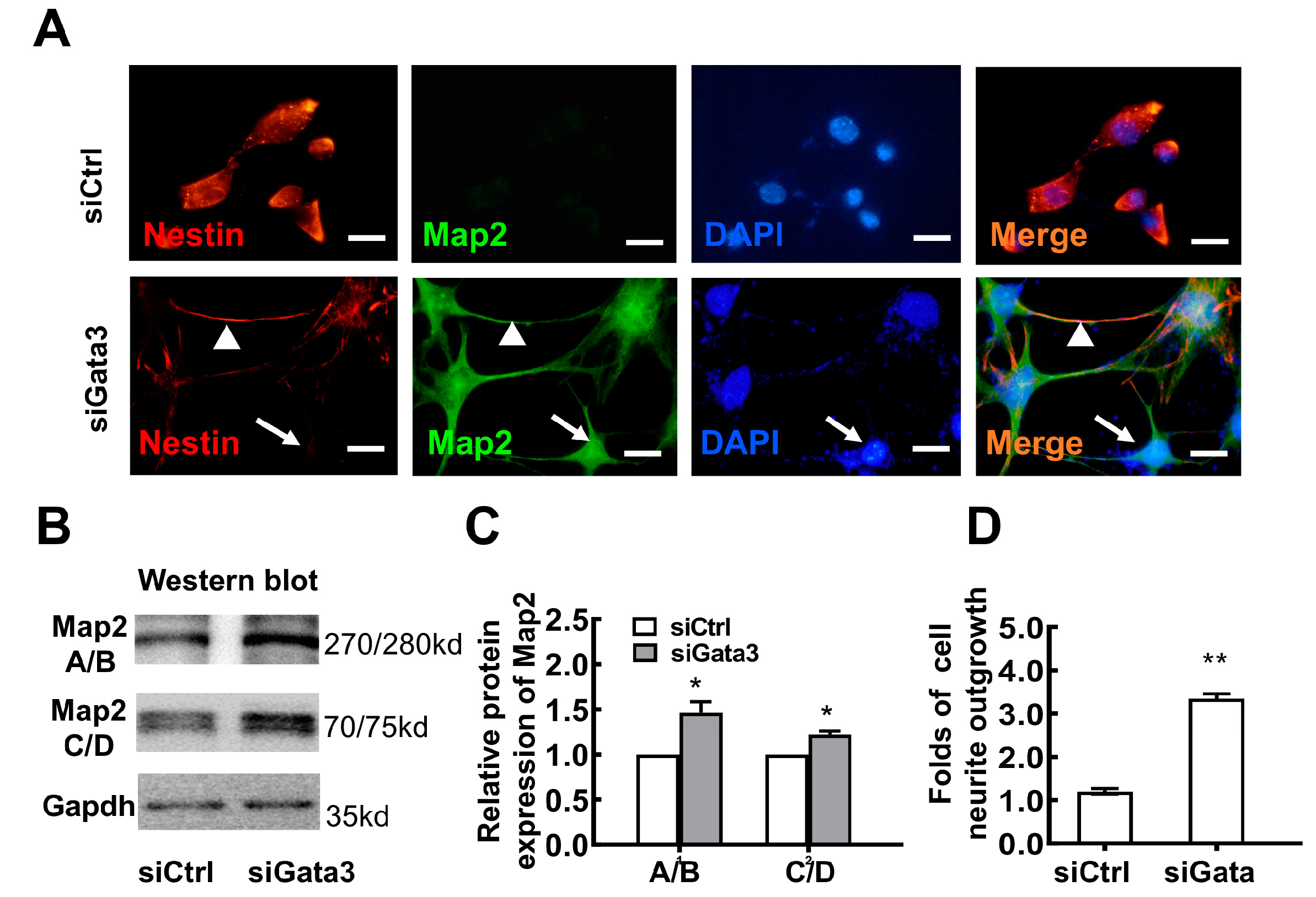
2.1. Gata3 Silencing Induces Differentiation and Reduces the Viability of 661W Cells
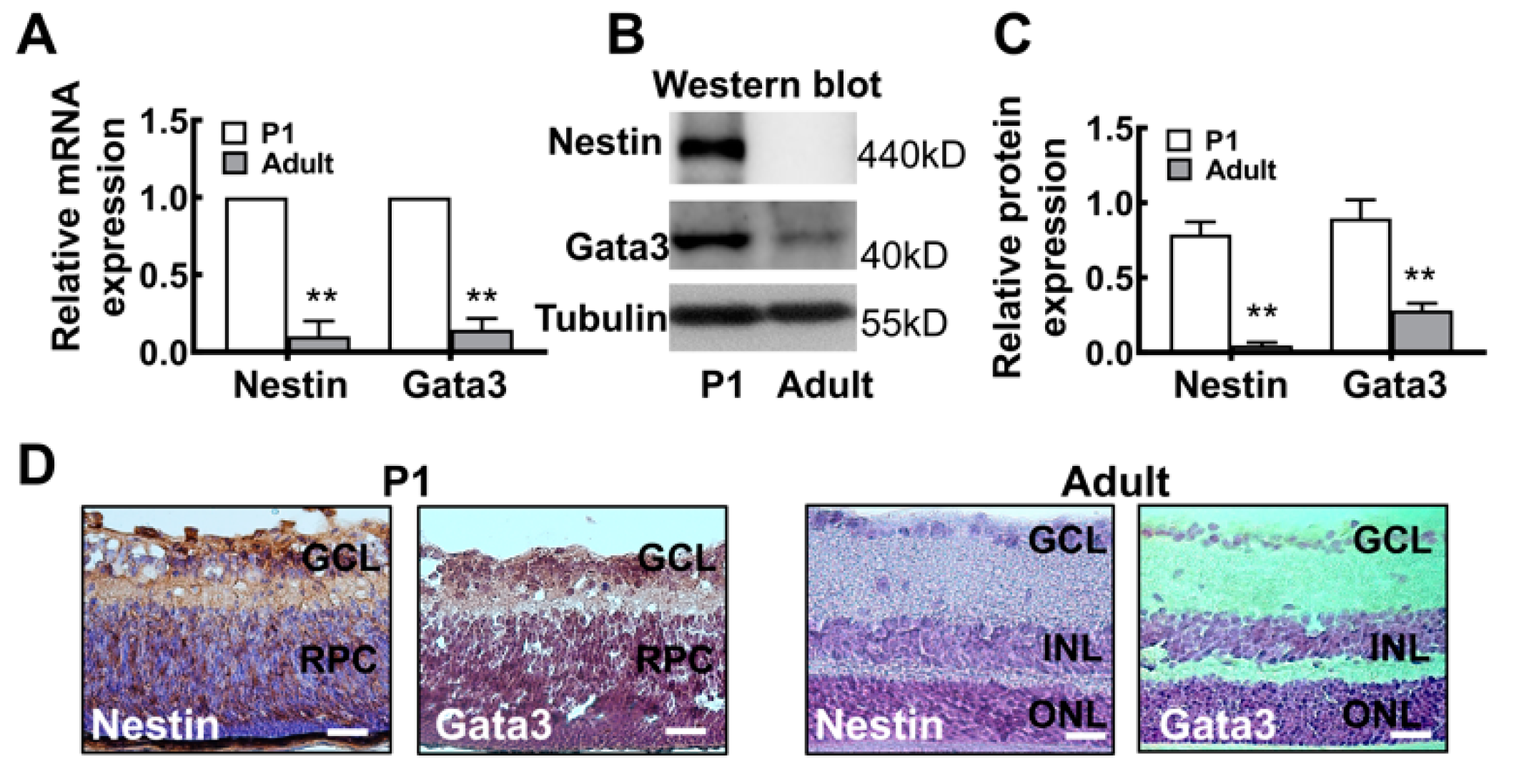
2.2. The Gata3 Expression Profile in the Developing Mouse Retina Is Generally Similar to That in Precursor-Like Cells
2.3. Exogenous Gata3 Inhibits Synaptophysin (Syn) Expression and Neurite Outgrowth and Reduces the Viability of Primary Retinal Neurocytes in Vitro
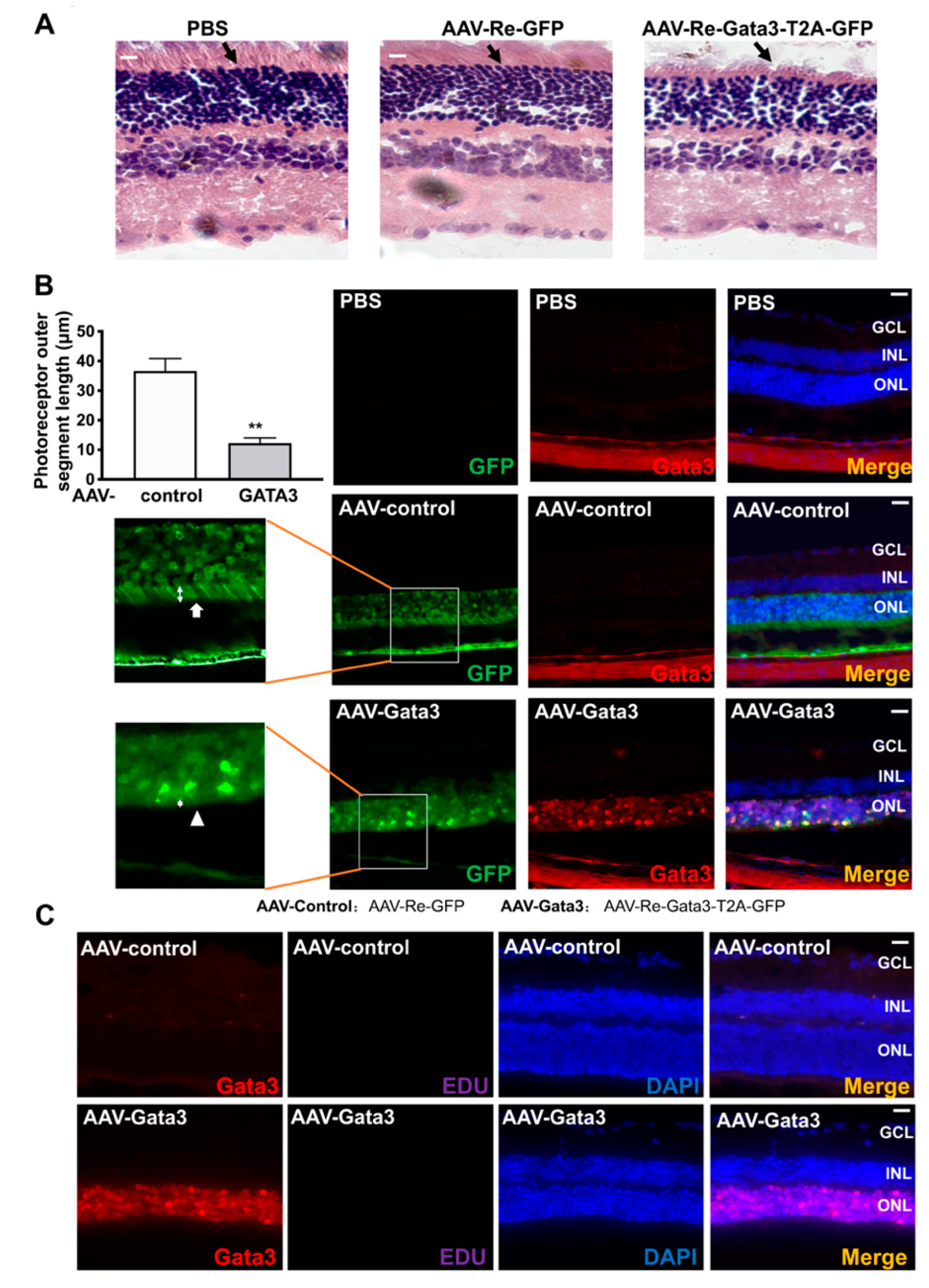
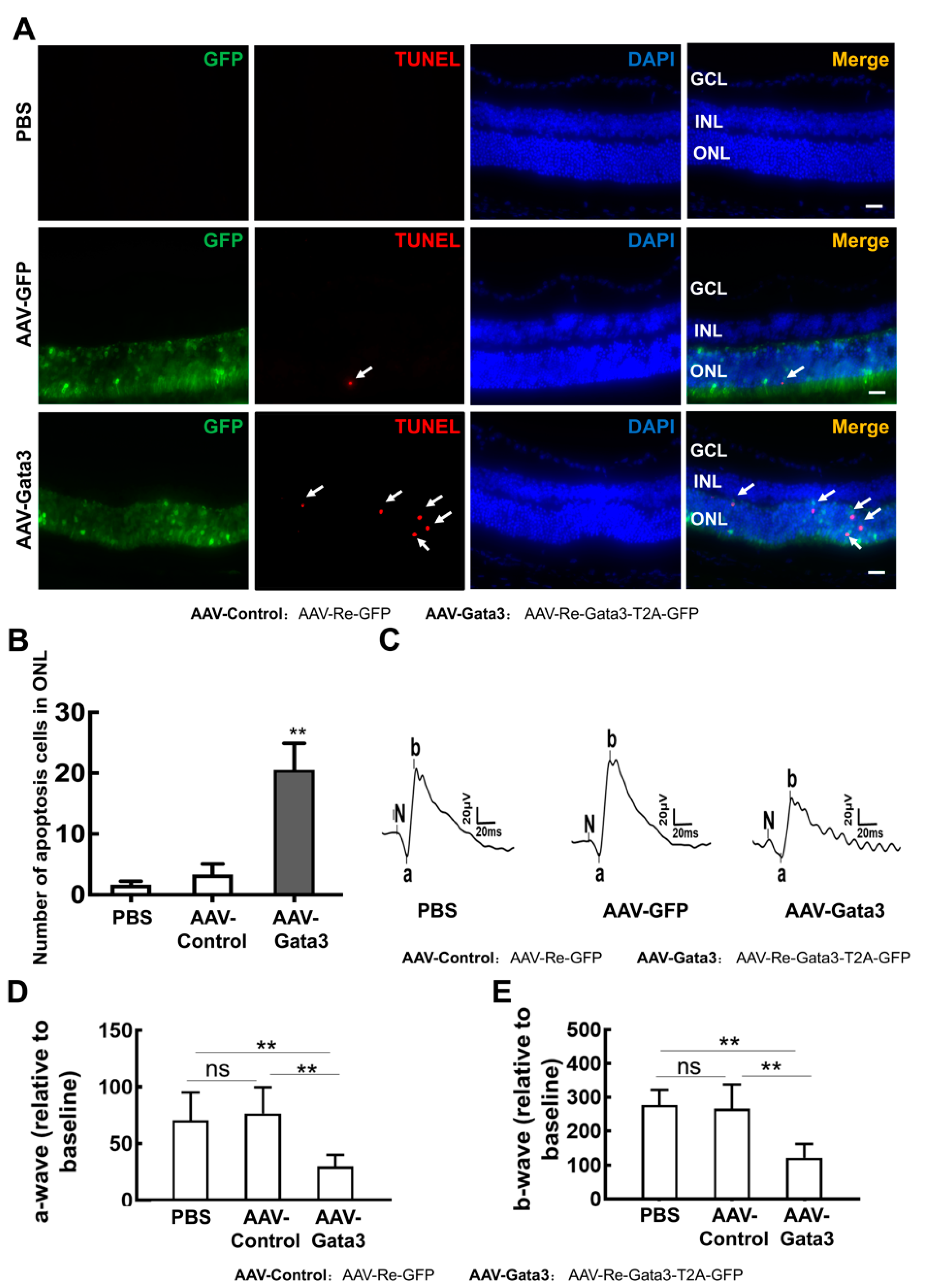
2.4. Gata3 Overexpression Impairs Mouse Retinal Function in Vivo
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chou, J.; Provot, S.; Werb, Z. GATA3 in development and cancer differentiation: Cells GATA have it! J. Cell. Physiol. 2010, 222, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Di, L.J. Wnt/beta-Catenin Mediates AICAR Effect to Increase GATA3 Expression and Inhibit Adipogenesis. J. Biol. Chem. 2015, 290, 19458–19468. [Google Scholar] [CrossRef] [Green Version]
- Van Esch, H.; Groenen, P.; Nesbit, M.A.; Schuffenhauer, S.; Lichtner, P.; Vanderlinden, G.; Harding, B.; Beetz, R.; Bilous, R.W.; Holdaway, I.; et al. GATA3 haplo-insufficiency causes human HDR syndrome. Nature 2000, 406, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Y.; Li, Z.Y.; Zou, H.L.; Hu, Z.L.; Song, N.N.; Zheng, M.H.; Su, C.J.; Ding, Y.Q. Expression of the transcription factor GATA3 in the postnatal mouse central nervous system. Neurosci. Res. 2008, 61, 420–428. [Google Scholar] [CrossRef]
- Asnagli, H.; Afkarian, M.; Murphy, K.M. Cutting edge: Identification of an alternative GATA-3 promoter directing tissue-specific gene expression in mouse and human. J. Immunol. 2002, 168, 4268–4271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, K.M.; Leonard, M.W.; Roth, M.E.; Lieuw, K.H.; Kioussis, D.; Grosveld, F.; Engel, J.D. Embryonic expression and cloning of the murine GATA-3 gene. Development 1994, 120, 2673–2686. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, J.; Thiesson, D.; Fujiwara, Y.; Tsai, F.Y.; Orkin, S.H. Expression and genetic interaction of transcription factors GATA-2 and GATA-3 during development of the mouse central nervous system. Dev. Biol. 1999, 210, 305–321. [Google Scholar] [CrossRef] [Green Version]
- London, A.; Benhar, I.; Schwartz, M. The retina as a window to the brain-from eye research to CNS disorders. Nat. Rev. Neurol. 2013, 9, 44–53. [Google Scholar] [CrossRef]
- Maeda, A.; Moriguchi, T.; Hamada, M.; Kusakabe, M.; Fujioka, Y.; Nakano, T.; Yoh, K.; Lim, K.C.; Engel, J.D.; Takahashi, S. Transcription factor GATA-3 is essential for lens development. Dev. Dyn. 2009, 238, 2280–2291. [Google Scholar] [CrossRef] [Green Version]
- Martynova, E.; Bouchard, M.; Musil, L.S.; Cvekl, A. Identification of Novel Gata3 Distal Enhancers Active in Mouse Embryonic Lens. Dev. Dyn. 2018, 247, 1186–1198. [Google Scholar] [CrossRef] [Green Version]
- Celikkaya, H.; Cosacak, M.I.; Papadimitriou, C.; Popova, S.; Bhattarai, P.; Biswas, S.N.; Siddiqui, T.; Wistorf, S.; Nevado-Alcalde, I.; Naumann, L.; et al. GATA3 Promotes the Neural Progenitor State but Not Neurogenesis in 3D Traumatic Injury Model of Primary Human Cortical Astrocytes. Front. Cell. Neurosci. 2019, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Kizil, C.; Kyritsis, N.; Dudczig, S.; Kroehne, V.; Freudenreich, D.; Kaslin, J.; Brand, M. Regenerative neurogenesis from neural progenitor cells requires injury-induced expression of Gata3. Dev. Cell 2012, 23, 1230–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, D.L.; Cepko, C.L. A common progenitor for neurons and glia persists in rat retina late in development. Nature 1987, 328, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Palmer, T.D.; Takahashi, J.; Gage, F.H. Widespread integration and survival of adult-derived neural progenitor cells in the developing optic retina. Mol. Cell. Neurosci. 1998, 12, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, H.S.; Shin, J.M.; Chun, M.H.; Oh, S.J. Nestin expressing progenitor cells during establishment of the neural retina and its vasculature. Anat. Cell Biol. 2012, 45, 38–46. [Google Scholar] [CrossRef]
- Pandolfi, P.P.; Roth, M.E.; Karis, A.; Leonard, M.W.; Dzierzak, E.; Grosveld, F.G.; Engel, J.D.; Lindenbaum, M.H. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat. Genet. 1995, 11, 40–44. [Google Scholar] [CrossRef]
- Chen, L.; Chen, B.; Leng, W.; Lui, X.; Wu, Q.; Ouyang, X.; Liang, Z. Identification of a novel de novo GATA3 mutation in a patient with HDR syndrome. J. Int. Med. Res. 2015, 43, 718–724. [Google Scholar] [CrossRef]
- Kyritsis, N.; Kizil, C.; Zocher, S.; Kroehne, V.; Kaslin, J.; Freudenreich, D.; Iltzsche, A.; Brand, M. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 2012, 338, 1353–1356. [Google Scholar] [CrossRef]
- Tsarovina, K.; Reiff, T.; Stubbusch, J.; Kurek, D.; Grosveld, F.G.; Parlato, R.; Schutz, G.; Rohrer, H. The Gata3 transcription factor is required for the survival of embryonic and adult sympathetic neurons. J. Neurosci. 2010, 30, 10833–10843. [Google Scholar] [CrossRef]
- Lawoko-Kerali, G.; Rivolta, M.N.; Lawlor, P.; Cacciabue-Rivolta, D.I.; Langton-Hewer, C.; van Doorninck, J.H.; Holley, M.C. GATA3 and NeuroD distinguish auditory and vestibular neurons during development of the mammalian inner ear. Mech. Dev. 2004, 121, 287–299. [Google Scholar] [CrossRef]
- Nishimura, K.; Noda, T.; Dabdoub, A. Dynamic Expression of Sox2, Gata3, and Prox1 during Primary Auditory Neuron Development in the Mammalian Cochlea. PLoS ONE 2017, 12, e0170568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Han, D. GATA3 modulates neuronal survival through regulating TRPM2 in Parkinson’s disease. Int. J. Clin. Exp. Med. 2017, 10, 15178–15186. [Google Scholar]
- Tan, E.; Ding, X.Q.; Saadi, A.; Agarwal, N.; Naash, M.I.; Al-Ubaidi, M.R. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Investig. Ophthalmol. Vis. Sci 2004, 45, 764–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayyad, Z.; Sirohi, K.; Radha, V.; Swarup, G. 661W is a retinal ganglion precursor-like cell line in which glaucoma-associated optineurin mutants induce cell death selectively. Sci. Rep. 2017, 7, 16855. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.; Diaz-Nido, J.; Avila, J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog. Neurobiol. 2000, 61, 133–168. [Google Scholar] [CrossRef]
- Tervo, D.G.; Hwang, B.Y.; Viswanathan, S.; Gaj, T.; Lavzin, M.; Ritola, K.D.; Lindo, S.; Michael, S.; Kuleshova, E.; Ojala, D.; et al. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 2016, 92, 372–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarsa, L.; Goda, Y. Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc. Natl. Acad. Sci. USA 2002, 99, 1012–1016. [Google Scholar] [CrossRef] [Green Version]
- Knaus, P.; Betz, H.; Rehm, H. Expression of synaptophysin during postnatal development of the mouse brain. J. Neurochem. 1986, 47, 1302–1304. [Google Scholar] [CrossRef]
- McMurray, C.T. To die or not to die: DNA repair in neurons. Mutat. Res. 2005, 577, 260–274. [Google Scholar] [CrossRef]
- Iacovoni, J.S.; Caron, P.; Lassadi, I.; Nicolas, E.; Massip, L.; Trouche, D.; Legube, G. High-resolution profiling of gammaH2AX around DNA double strand breaks in the mammalian genome. EMBO J. 2010, 29, 1446–1457. [Google Scholar] [CrossRef] [Green Version]
- Lobrich, M.; Shibata, A.; Beucher, A.; Fisher, A.; Ensminger, M.; Goodarzi, A.A.; Barton, O.; Jeggo, P.A. γH2AX foci analysis for monitoring DNA double-strand break repair: Strengths, limitations and optimization. Cell Cycle 2010, 9, 662–669. [Google Scholar] [CrossRef] [Green Version]
- Marz, M.; Chapouton, P.; Diotel, N.; Vaillant, C.; Hesl, B.; Takamiya, M.; Lam, C.S.; Kah, O.; Bally-Cuif, L.; Strahle, U. Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia 2010, 58, 870–888. [Google Scholar] [CrossRef] [PubMed]
- Kaslin, J.; Kroehne, V.; Benato, F.; Argenton, F.; Brand, M. Development and specification of cerebellar stem and progenitor cells in zebrafish: From embryo to adult. Neural Dev. 2013, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, E.J.; Hughes, E.H.; Carter, D.A.; Dick, A.D. Nestin positive cells in adult human retina and in epiretinal membranes. Br. J. Ophthalmol. 2003, 87, 1154–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, E.J.; Carter, D.A.; Ren, Y.; Hughes, E.H.; Rice, C.M.; Halfpenny, C.A.; Scolding, N.J.; Dick, A.D. Neural progenitor cells from postmortem adult human retina. Br. J. Ophthalmol. 2005, 89, 102–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, C.H.; Cho, H.; Kim, Y.K.; Park, T.K. Nestin Expression in the Adult Mouse Retina with Pharmaceutically Induced Retinal Degeneration. J. Korean Med. Sci. 2017, 32, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Valamanesh, F.; Monnin, J.; Morand-Villeneuve, N.; Michel, G.; Zaher, M.; Miloudi, S.; Chemouni, D.; Jeanny, J.C.; Versaux-Botteri, C. Nestin expression in the retina of rats with inherited retinal degeneration. Exp. Eye Res. 2013, 110, 26–34. [Google Scholar] [CrossRef]
- Yao, P.; Li, Y.L.; Chen, Y.; Shen, W.; Wu, K.Y.; Xu, W.H. Overexpression of long non-coding RNA Rian attenuates cell apoptosis from cerebral ischemia-reperfusion injury via Rian/miR-144-3p/GATA3 signaling. Gene 2020, 737, 144411. [Google Scholar] [CrossRef]
- Jing, H.; Liu, L.; Jia, Y.; Yao, H.; Ma, F. Overexpression of the long non-coding RNA Oprm1 alleviates apoptosis from cerebral ischemia-reperfusion injury through the Oprm1/miR-155/GATA3 axis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2431–2439. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, J.; Li, F.; Liu, X.; Liu, Z.; Lin, J.; Ge, Y.; Kaminski, J.M.; Summers, J.B.; Wang, Z.; Ge, J.; et al. Lithium chloride protects retinal neurocytes from nutrient deprivation by promoting DNA non-homologous end-joining. Biochem. Biophys. Res. Commun. 2009, 380, 650–654. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, N.; Tian, S.; Li, F.; Hu, H.; Chen, P.; Cai, X.; Xu, L.; Zhang, J.; Chen, Z.; et al. Lithium promotes DNA stability and survival of ischemic retinal neurocytes by upregulating DNA ligase IV. Cell Death Dis. 2016, 7, e2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Wu, Y.; Zhuang, J.; Liu, X.; Luo, Q.; Wang, Q.; Jiang, Z.; He, A.; Chen, S.; Chen, X.; et al. Gata3 Silencing Is Involved in Neuronal Differentiation and Its Abnormal Expression Impedes Neural Activity in Adult Retinal Neurocytes. Int. J. Mol. Sci. 2022, 23, 2495. https://doi.org/10.3390/ijms23052495
Chen P, Wu Y, Zhuang J, Liu X, Luo Q, Wang Q, Jiang Z, He A, Chen S, Chen X, et al. Gata3 Silencing Is Involved in Neuronal Differentiation and Its Abnormal Expression Impedes Neural Activity in Adult Retinal Neurocytes. International Journal of Molecular Sciences. 2022; 23(5):2495. https://doi.org/10.3390/ijms23052495
Chicago/Turabian StyleChen, Pei, Yihui Wu, Jiejie Zhuang, Xuan Liu, Qian Luo, Qiyun Wang, Zihua Jiang, Anqi He, Shuilian Chen, Xi Chen, and et al. 2022. "Gata3 Silencing Is Involved in Neuronal Differentiation and Its Abnormal Expression Impedes Neural Activity in Adult Retinal Neurocytes" International Journal of Molecular Sciences 23, no. 5: 2495. https://doi.org/10.3390/ijms23052495
APA StyleChen, P., Wu, Y., Zhuang, J., Liu, X., Luo, Q., Wang, Q., Jiang, Z., He, A., Chen, S., Chen, X., Qiu, J., Li, Y., Yang, Y., Yu, K., & Zhuang, J. (2022). Gata3 Silencing Is Involved in Neuronal Differentiation and Its Abnormal Expression Impedes Neural Activity in Adult Retinal Neurocytes. International Journal of Molecular Sciences, 23(5), 2495. https://doi.org/10.3390/ijms23052495





