A Comparative Genomic and Safety Assessment of Six Lactiplantibacillus plantarum subsp. argentoratensis Strains Isolated from Spontaneously Fermented Greek Wheat Sourdoughs for Potential Biotechnological Application
Abstract
1. Introduction
2. Results and Discussion
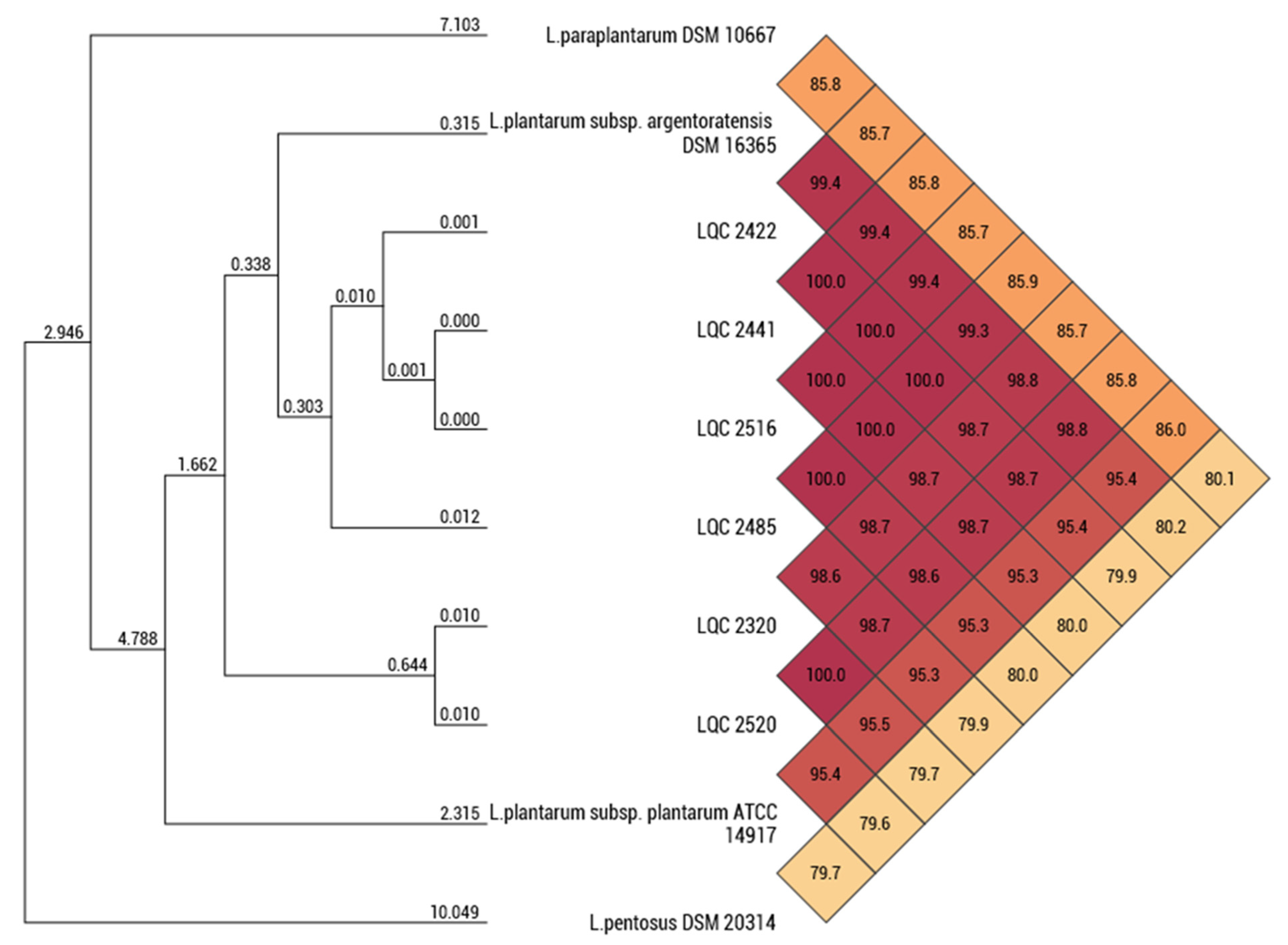
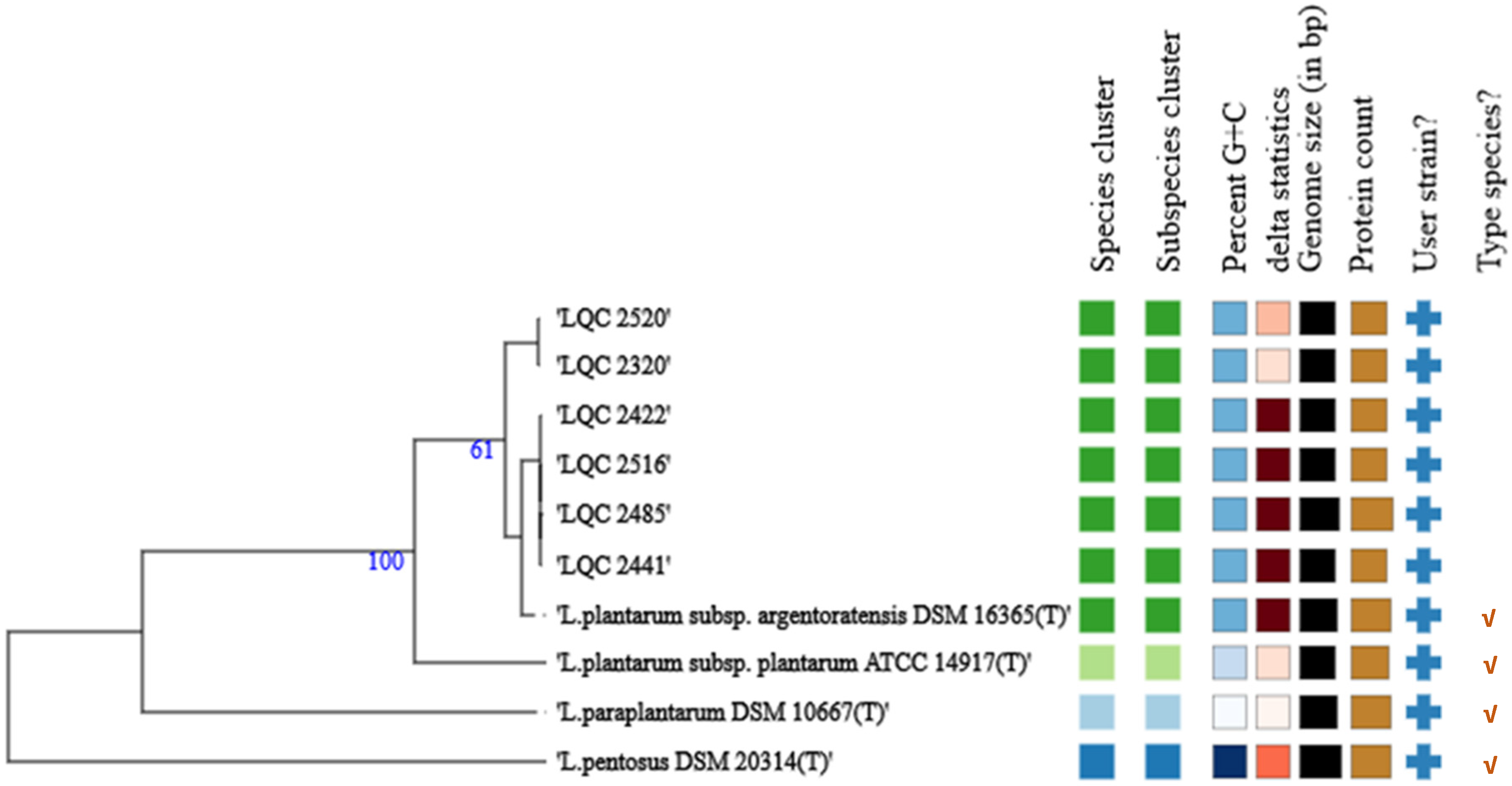
2.1. Strain Relatedness
2.2. Phenotypic Characterization and Pan-Genome Analysis
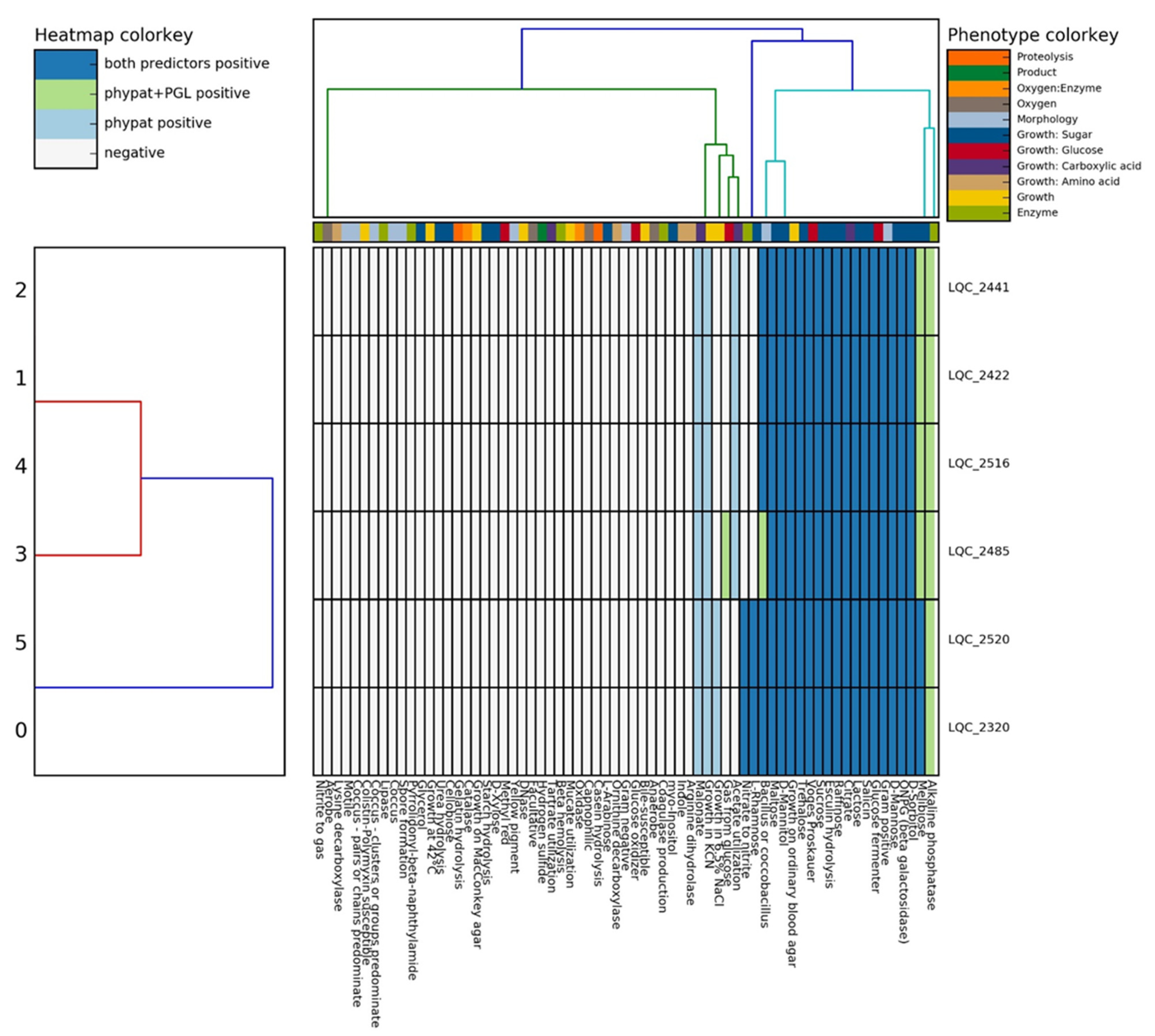
2.2.1. Phenotypic Properties
2.2.2. Pan-Genome Analysis and Annotation
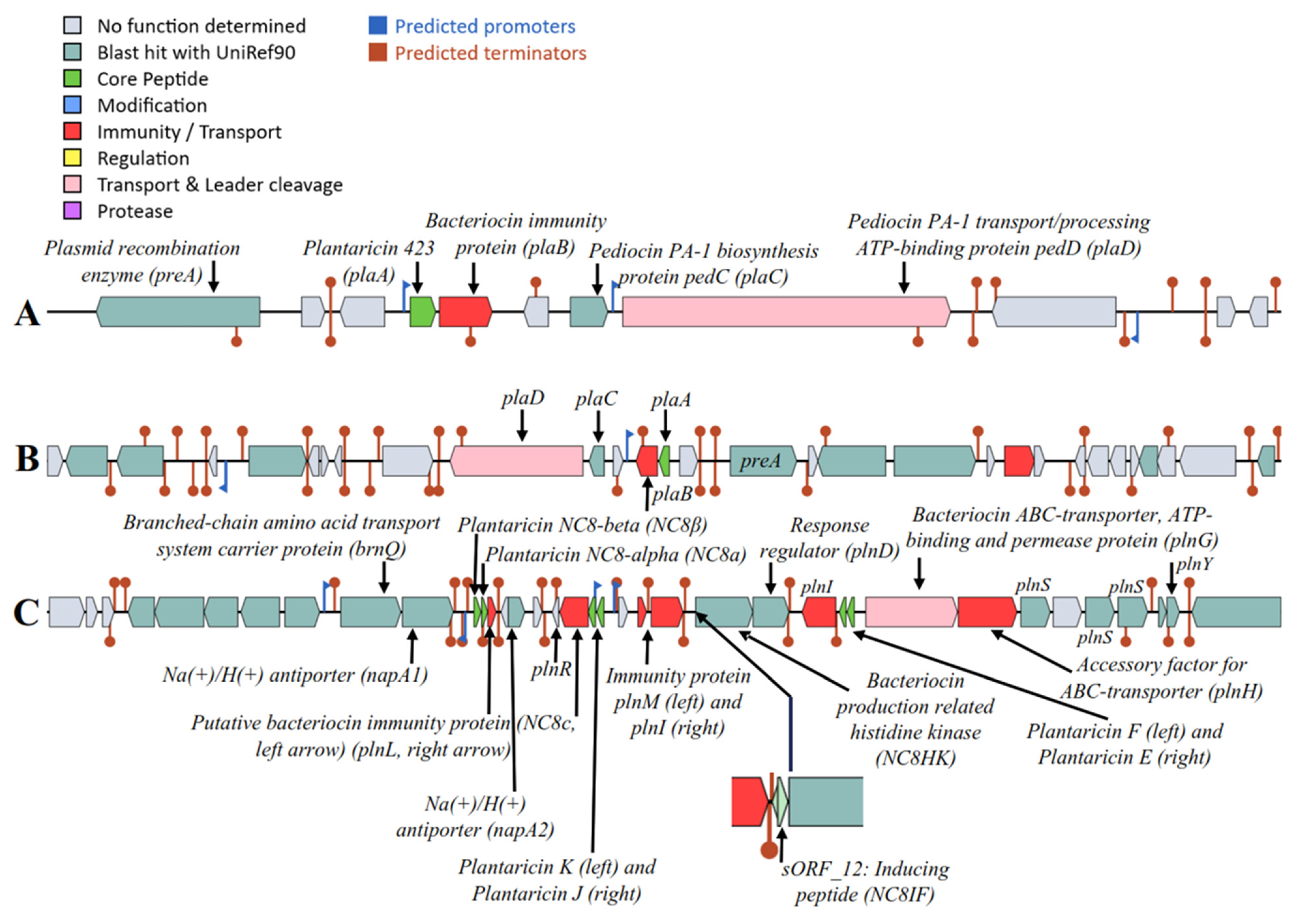
2.3. Safety Assessment
3. Materials and Methods
3.1. Microbial Strains
3.2. Strains Relatedness
3.3. Phenotypic Characterization and Pan-Genome Analysis
3.4. Genomic Aspects Related to Food Safety
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA. Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA–Opinion of the Scientific Committee. EFSA J. 2007, 5, 587. [Google Scholar] [CrossRef]
- Leuschner, R.G.; Robinson, T.P.; Hugas, M.; Cocconcelli, P.S.; Richard-Forget, F.; Klein, G.; Licht, T.R.; Nguyen-The, C.; Querol, A.; Richardson, M. Qualified presumption of safety (QPS): A generic risk assessment approach for biological agents notified to the European Food Safety Authority (EFSA). Trends Food Sci. Technol. 2010, 21, 425–435. [Google Scholar] [CrossRef]
- Cannon, J.P.; Lee, T.A.; Bolanos, J.T.; Danziger, L.H. Pathogenic relevance of Lactobacillus: A retrospective review of over 200 cases. Eur. J. Clin. Microbiol. Infect. Dis. 2005, 24, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kayser, F.H. Safety aspects of enterococci from the medical point of view. Int. J. Food Microbiol. 2003, 88, 255–262. [Google Scholar] [CrossRef]
- Salminen, M.K.; Rautelin, H.; Tynkkynen, S.; Poussa, T.; Saxelin, M.; Valtonen, V.; Järvinen, A. Lactobacillus bacteremia, species identification, and antimicrobial susceptibility of 85 blood isolates. Clin. Infect. Dis. 2006, 42, e35–e44. [Google Scholar] [CrossRef]
- Chokesajjawatee, N.; Santiyanont, P.; Chantarasakha, K.; Kocharin, K.; Thammarongtham, C.; Lertampaiporn, S.; Vorapreeda, T.; Srisuk, T.; Wongsurawat, T.; Jenjaroenpun, P.; et al. Safety assessment of Nham starter culture Lactobacillus plantarum BCC9546 via whole-genome analysis. Sci. Rep. 2020, 10, 10241. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, F.B.; Ripari, V.; Waszczynskyj, N.; Spier, M.R. Overview of sourdough technology: From production to marketing. Food Bioprocess Technol. 2018, 11, 242–270. [Google Scholar] [CrossRef]
- Syrokou, M.K.; Themeli, C.; Paramithiotis, S.; Mataragas, M.; Bosnea, L.; Argyri, A.A.; Chorianopoulos, N.G.; Skandamis, P.N.; Drosinos, E.H. Microbial ecology of Greek wheat sourdoughs identified by a culture-dependent and a culture-independent approach. Foods 2020, 9, 1603. [Google Scholar] [CrossRef]
- Syrokou, M.K.; Paramithiotis, S.; Skandamis, P.N.; Drosinos, E.H.; Bosnea, L.; Mataragas, M. High-quality draft genome sequence data of six Lactiplantibacillus plantarum subsp. argentoratensis strains isolated from various Greek wheat sourdoughs. Data Br. 2021, 37, 107172. [Google Scholar]
- EFSA. Guidance on the characterization of microorganisms used as feed additives or as production microorganisms. EFSA J. 2018, 16, e05206. [Google Scholar]
- Sun, Z.; Harris, H.M.B.; McCann, A.; Guo, C.; Argimón, S.; Zhang, W.; Yang, X.; Jeffrey, I.B.; Cooney, J.C.; Kagawa, T.F.; et al. Expanding the biotechnology potential of lactobacilli through comparative genomics of 213 strains and associated genera. Nat. Comm. 2015, 6, 8322. [Google Scholar] [CrossRef] [PubMed]
- Carpi, F.M.; Coman, M.M.; Silvi, S.; Picciolini, M.; Verdenelli, M.C.; Napolioni, V. Comprehensive pan- genome analysis of Lactiplantibacillus plantarum complete genomes. J. Appl. Microbiol. 2022, 132, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Jin, G.-D.; Park, J.; You, I.; Kim, E.B. Pan-genomics of Lactobacillus plantarum revealed group- specific genomic profiles without habitat association. J. Microbiol. Biotechnol. 2018, 28, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Inglin, R.C.; Meile, L.; Stevens, M.J.A. Clustering of pan- and core- genome of Lactobacillus provides novel evolutionary insights for differentiation. BMC Genom. 2018, 19, 284. [Google Scholar] [CrossRef]
- Evanovich, E.; de Souza Mendonça Mattos, P.J.; Guerreiro, J.F. Comparative genomic analysis of Lactobacillus plantarum: An overview. Int. J. Genom. 2019, 2019, 4973214. [Google Scholar]
- Rodrigo-Torres, L.; Yépez Aznar, A.R.; Arahal, D.R. Genomic insights into five strains of Lactobacillus plantarum with biotechnological potential isolated from chicha, a traditional maize-based fermented beverage from northwestern Argentina. Front. Microbiol. 2019, 10, 2232. [Google Scholar] [CrossRef]
- Schmid, M.; Frei, D.; Patrignani, A.; Schlapbach, R.; Frey, J.E.; Remus-Emsermann, M.N.P.; Ahrens, C.H. Pushing the limits of de novo genome assembly for complex prokaryotic genomes harboring very long, near identical repeats. Nucleic Acids Res. 2018, 46, 8953–8965. [Google Scholar] [CrossRef]
- Thakur, M.; Deshpande, H.W.; Bhate, M.A. Isolation and identification of lactic acid bacteria and their exploration in non-dairy probiotic drink. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1023–1030. [Google Scholar]
- Sawitzki, M.C.; Fiorentini, A.M.; Cunha Junior, A.; Bertol, T.M.; Sant’anna, E.S. Lactobacillus plantarum AJ2 isolated from naturally fermented sausage and its effects on the technological properties of Milano-type salami. Food Sci Technol. 2008, 28, 709–717. [Google Scholar] [CrossRef]
- Weimann, A.; Mooren, K.; Frank, J.; Pope, P.B.; Bremges, A.; McHardy, A.C. From genomes to phenotypes: Traitar, the microbial trait analyzer. mSystems 2016, 1, e00101–e00116. [Google Scholar] [CrossRef]
- Dorđević, T.M.; Siler-Marinković, S.S.; Durović-Pejčev, R.D.; Dimitrijević-Branković, S.I.; Gajić Umiljendić, J.S. Dissipation of pirimiphos-methyl during wheat fermentation by Lactobacillus plantarum. Lett. Appl. Microbiol. 2013, 57, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Xu, D.; Liu, J.-Q.; Zhao, X.-H. Enhanced degradation of five organophosphorus pesticides in skimmed milk by lactic acid bacteria and its potential relationship with phosphatase production. Food Chem. 2014, 164, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Nguyen, T.H.; Nguyen, T.T.; Maischberger, T.; Haltrich, D. β-galactosidase from Lactobacillus plantarum WCFS1: Biochemical characterization and formation of prebiotic galacto-oligosaccharides. Carbohydr. Res. 2010, 345, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Kittibunchakul, S.; Pham, M.-L.; Tran, A.-M.; Nguyen, T.-H. β-galactosidase from Lactobacillus helveticus DSM 20075: Biochemical characterization and recombinant expression for applications in dairy industry. Int. J. Mol. Sci. 2019, 20, 947. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef]
- Tettelin, H.; Riley, D.; Cattuto, C.; Medina, D. Comparative genomics: The bacterial pan-genome. Curr. Opin. Microbiol. 2008, 11, 472–477. [Google Scholar] [CrossRef]
- Russell, R.R.; Aduse-Opoku, J.; Sutcliffe, I.C.; Tao, L.; Ferretti, J.J. A binding protein-dependent transport system in Streptococcus mutans responsible for multiple sugar metabolism. J. Biol. Chem. 1992, 267, 4631–4637. [Google Scholar] [CrossRef]
- McLaughlin, R.E.; Ferretti, J.J. The multiple-sugar metabolism (msm) gene cluster of Streptococcus mutans is transcribed as a single operon. FEMS Microbiol. Lett. 1996, 140, 261–264. [Google Scholar] [CrossRef]
- Fedtke, I.; Kamps, A.; Krismer, B.; Götz, F. The nitrate reductase and nitrite reductase operons and the narT gene of Staphylococcus carnosus are positively controlled by the novel two-component system NreBC. J. Bacteriol. 2002, 184, 6624–6634. [Google Scholar] [CrossRef]
- Schönert, S.; Seitz, S.; Krafft, H.; Feuerbaum, E.-A.; Andernach, I.; Witz, G.; Dahl, M.K. Maltose and maltodextrin utilization by Bacillus subtilis. J. Bacteriol. 2006, 188, 3911–3922. [Google Scholar] [CrossRef]
- Pelletier, G.; Frenette, M.; Vadeboncoeur, C. Transport of mannose by an inducible phosphoenolpyruvate: Fructose phosphotransferase system in Streptococcus salivarius. Microbiology 1994, 140, 2433–2438. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Chun, B.H.; Kim, K.H.; Jeon, H.H.; Lee, S.H.; Jeon, C.O. Pan-genomic and transcriptomic analyses of Leuconostoc mesenteroides provide insights into its genomic and metabolic features and roles in kimchi fermentation. Sci. Rep. 2017, 7, 11504. [Google Scholar] [CrossRef] [PubMed]
- Carrizo, S.L.; Montes de Oca, C.E.; Laiño, J.E.; Suarez, N.E.; Vignolo, G.; LeBlanc, J.L.; Rollán, G. Ancestral Andean grain quinoa as source of lactic acid bacteria capable to degrade phytate and produce B-group vitamins. Food Res. Int. 2016, 89, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, X.; Gu, Q.; Lou, X.-Y.; Zhang, X.-M.; Song, D.-F.; Zhng, C. Comparative genomic analysis of Lactobacillus plantarum ZJ316 reveals its genetic adaptation and potential probiotic profiles. J. Zhejiang Univ. Sci. B 2016, 17, 569–579. [Google Scholar] [CrossRef]
- Goel, A.; Halami, P.M.; Tamang, J.P. Genome analysis of Lactobacillus plantarum isolated from some Indian fermented foods for bacteriocin production and probiotic marker genes. Front. Microbiol. 2020, 11, 40. [Google Scholar] [CrossRef]
- Begley, M.; Hill, C.; Gahan, C.G.M. Bile salt hydrolase activity in probiotics. Appl. Environ. Microbiol. 2006, 72, 1729–1738. [Google Scholar] [CrossRef]
- Benkerroum, N. Biogenic amines in dairy products: Origin, incidence, and control means. Compr. Rev. Food Sci. Food Saf. 2016, 15, 801–826. [Google Scholar] [CrossRef]
- Liu, C.-J.; Wang, R.; Gong, F.-M.; Liu, X.-F.; Zheng, H.-J.; Luo, Y.-Y.; Li, X.-R. Complete genome sequences and comparative genome analysis of Lactobacillus plantarum strain 5-2 isolated from fermented soybean. Genomics 2015, 106, 404–411. [Google Scholar] [CrossRef]
- Westra, E.R.; Semenova, E.; Datsenko, K.A.; Jackson, R.N.; Wiedenheft, B.; Severinov, K.; Brouns, S.J.J. Type I-E CRISPR-cas systems discriminate target from non-target DNA through base pairing-independent PAM recognition. PLoS Genet. 2013, 9, e1003742. [Google Scholar] [CrossRef]
- Marraffini, L.A.; Sontheimer, E.J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 2008, 322, 1843–1845. [Google Scholar] [CrossRef] [PubMed]
- Couvin, D.; Bernheim, A.; Toffano-Nioche, C.; Touchon, M.; Michalik, J.; Néron, B.; Rocha, E.P.C.; Vergnaud, G.; Gautheret, D.; Pourcel, C. CRISPRCasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for Cas proteins. Nucleic Acids Res. 2018, 46, 246–251. [Google Scholar] [CrossRef]
- Van Reenen, C.A.; Chikindas, M.L.; Van Zyl, W.H.; Dicks, L.M.T. Characterization and heterologous expression of a class IIa bacteriocin, plantaricin 423 from Lactobacillus plantarum 423, in Saccharomyces cerevisiae. Int. J. Food Microbiol. 2003, 81, 29–40. [Google Scholar] [CrossRef]
- Van Reenen, C.A.; Van Zyl, W.H.; Dicks, L.M.T. Expression of the immunity protein of plantaricin 423, produced by Lactobacillus plantarum 423, and analysis of the plasmid encoding the bacteriocin. Appl. Environ. Microbiol. 2006, 72, 7644–7651. [Google Scholar] [CrossRef] [PubMed]
- Diep, D.B.; Straume, D.; Kjos, M.; Torres, C.; Nes, I.F. An overview of the mosaic bacteriocin pln loci from Lactobacillus plantarum. Peptides 2009, 30, 1562–1574. [Google Scholar] [CrossRef]
- Maldonado, A.; Ruiz-Barba, J.L.; Jimenez-Diaz, R. Purification and genetic characterization of plantaricin NC8, a novel coculture-inducible two-peptide bacteriocin from Lactobacillus plantarum NC8. Appl. Environ. Microbiol. 2003, 69, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, A.; Ruiz-Barba, J.L.; Jimenez-Diaz, R. Production of plantaricin NC8 by Lactobacillus plantarum NC8 is induced in the presence of different types of Gram-positive bacteria. Arch. Microbiol. 2004, 181, 8–16. [Google Scholar] [CrossRef]
- Chun, J.; Rainey, F.A. Integrating genomics into the taxonomy and systematics of the Bacteria and Archaea. Int. J. Syst. Evol. Microbiol. 2014, 64, 316–324. [Google Scholar] [CrossRef]
- Mataragas, M. Investigation of genomic characteristics and carbohydrates’ metabolic activity of Lactococcus lactis subsp. lactis during ripening of a Swiss-type cheese. Food Microbiol. 2020, 87, 103392. [Google Scholar]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.-W.; De Meyer, S.; et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef]
- Lee, I.; Chalita, M.; Ha, S.M.; Na, S.I.; Yoon, S.H.; Chun, J. ContEst16S: An algorithm that identifies contaminated prokaryotic genomes using 16S RNA gene sequences. Int. J. Syst. Evol. 2017, 67, 2053–2057. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Kim, Y.O.; Park, S.C.; Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2015, 66, 1100–1103. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.M.; Gupta, V.K.; Sutra, C. BPGA-an ultra-fast pan-genome analysis pipeline. Sci. Rep. 2016, 6, 24373. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Davis, J.J.; Wattam, A.R.; Aziz, R.K.; Brettin, T.; Butler, R.; Butler, R.M.; Chlenski, P.; Conrad, N.; Dickerman, A.; Dietrich, E.M.; et al. The PATRIC Bioinformatics Resource Center: Expanding data and analysis capabilities. Nucleic Acids Res. 2020, 48, D606–D612. [Google Scholar] [CrossRef]
- Aramaki, T.; Blanc-Mathieu, R.; Endo, H.; Ohkubo, K.; Kanehisa, M.; Goto, S.; Ogata, H. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics 2020, 36, 2251–2252. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Forslund, K.; Coelho, L.P.; Szklarczyk, D.; Jensen, L.J.; von Mering, C.; Bork, P. Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Mol. Biol. Evol. 2017, 34, 2115–2122. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2021, 42, D490–D495. [Google Scholar] [CrossRef]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.; Larsen, V.M.; Aarestrup, F.M.; Lund, O. PathogenFinder–distinguishing friend from foe using bacterial whole genome sequence data. PLoS ONE 2013, 8, e77302. [Google Scholar] [CrossRef]
- Van Heel, A.J.; De Jong, A.; Montalbán-López, M.; Kok, J.; Kuipers, O.P. BAGEL3: Automated identification of genes encoding bacteriocins and (non-) bactericidal post-translationally modified peptides. Nucleic Acids Res. 2013, 41, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Carattoli, A.; Zankari, E.; Garcia-Fernandez, A.; Larsen, M.V.; Lund, O.; Villa, L.; Aarestrup, F.M.; Hasman, H. In Silico detection and typing of plasmids using PlasmidFinder and Plasmid Multilocus Sequence Typing. Antimicrob. Agents Chemother. 2014, 58, 3895–3903. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.H.K.; Bortolaia, V.; Tansirichaiya, S.; Aarestrup, F.M.; Roberts, A.P.; Petersen, T.N. Detection of mobile genetic elements associated with antibiotic resistance in Salmonella enterica using a newly developed web tool: MobileElementFinder. J. Antimicrob. Chemother. 2021, 76, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Zankari, E.; Allesøe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J. Antimicrob. Chemother. 2017, 72, 2764–2768. [Google Scholar] [CrossRef]
- Joensen, K.G.; Scheutz, F.; Lund, O.; Hasman, H.; Kaas, R.S.; Nielsen, E.M.; Aarestrup, F.M. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Microbiol. 2014, 52, 1501–1510. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, 16–21. [Google Scholar] [CrossRef]



| a/a | Strains | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LQC 2422 | - | |||||||||
| 2 | LQC 2441 | 99.9 | - | ||||||||
| 3 | LQC 2516 | 100 | 100 | - | |||||||
| 4 | LQC 2485 | 99.5 | 99.5 | 99.5 | - | ||||||
| 5 | LQC 2320 | 89.2 | 89.2 | 89.2 | 88.9 | - | |||||
| 6 | LQC 2520 | 89.2 | 89.2 | 89.2 | 88.9 | 100 | - | ||||
| 7 | L. paraplantarum DSM 10667T | 31.1 | 31.2 | 31.2 | 31.5 | 31.1 | 31.2 | - | |||
| 8 | L. plantarum subsp. argentoratensis DSM 16365T | 95.1 | 95.1 | 95.2 | 94 | 89.9 | 90.5 | 31.5 | - | ||
| 9 | L. plantarum subsp. plantarum ATCC 14917T | 62.5 | 62.4 | 62.4 | 62.3 | 63.1 | 63 | 31.1 | 62.8 | - | |
| 10 | L. pentosus DSM 20314T | 24.4 | 24.4 | 24.4 | 24.5 | 24 | 24 | 24.4 | 24.9 | 23.8 | - |
| a/a | AMR Element | KEGG KO | Gene (EC No) | Description |
|---|---|---|---|---|
| 1 | Bacitracin | K06153 | uppP, EC 3.6.1.27 | Catalyzes the dephosphorylation of the undecaprenyl diphosphate (UPP). Confers resistance to bacitracin |
| ABC transporter, permease protein, probably the 2 or 3 component bacitracin resistance efflux pump, BcrAB or BcrABC | ||||
| Chloramphenicol acetyltransferase | ||||
| 2 | Bacitracin | K01992 | - | D-alanyl-D-alanine-carboxypeptidase |
| Catalyzes hydrolysis of the D-alanyl-D-alanine dipeptide | ||||
| Beta-lactamase enzyme family | ||||
| 3 | Phenicol | K19271 | catA, EC 2.3.1.28 | Mediates bacterial resistance to the antibiotics streptomycin and spectomycin |
| 4 | Vancomycin | K07260 | vanY, EC 3.4.17.14 | |
| 5 | Vancomycin | K08641 | ddpX, EC 3.4.13.22 | |
| 6 | Beta-Lactam | K17836 | bla1, bla2, EC 3.5.2.6 | |
| 7 | Aminoglycoside | K00984 | aadA, EC 2.7.7.47 |
| Strain | Total CRISPR Regions | CRISPR Regions with EL 1 or 2 1 | CRISPR Regions with EL 3 or 4 | cas Gene Clusters Nearby CRISPR Regions 2 | Type of cas Gene Cluster | Total cas Genes 3 |
|---|---|---|---|---|---|---|
| LQC 2320 | 4 | 2 of EL1 | 2 of EL4 | 2 | CAS-typeI and CAS-typeIE | 11 |
| LQC 2422 | 4 | 1 of EL1 | 1 of EL3 & 2 of EL4 | 2 | CAS-typeI and CAS-typeIE | 11 |
| LQC 2441 | 4 | 1 of EL1 | 1 of EL3 & 2 of EL4 | 2 | CAS-typeI and CAS-typeIE | 11 |
| LQC 2485 | 5 | 1 of EL1 | 4 of EL4 | 2 | CAS-typeI and CAS-typeIE | 11 |
| LQC 2516 | 4 | 1 of EL1 | 1 of EL3 & 2 of EL4 | 2 | CAS-typeI and CAS-typeIE | 11 |
| LQC 2520 | 4 | 2 of EL1 | 2 of EL4 | 2 | CAS-typeI and CAS-typeIE | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syrokou, M.K.; Paramithiotis, S.; Drosinos, E.H.; Bosnea, L.; Mataragas, M. A Comparative Genomic and Safety Assessment of Six Lactiplantibacillus plantarum subsp. argentoratensis Strains Isolated from Spontaneously Fermented Greek Wheat Sourdoughs for Potential Biotechnological Application. Int. J. Mol. Sci. 2022, 23, 2487. https://doi.org/10.3390/ijms23052487
Syrokou MK, Paramithiotis S, Drosinos EH, Bosnea L, Mataragas M. A Comparative Genomic and Safety Assessment of Six Lactiplantibacillus plantarum subsp. argentoratensis Strains Isolated from Spontaneously Fermented Greek Wheat Sourdoughs for Potential Biotechnological Application. International Journal of Molecular Sciences. 2022; 23(5):2487. https://doi.org/10.3390/ijms23052487
Chicago/Turabian StyleSyrokou, Maria K., Spiros Paramithiotis, Eleftherios H. Drosinos, Loulouda Bosnea, and Marios Mataragas. 2022. "A Comparative Genomic and Safety Assessment of Six Lactiplantibacillus plantarum subsp. argentoratensis Strains Isolated from Spontaneously Fermented Greek Wheat Sourdoughs for Potential Biotechnological Application" International Journal of Molecular Sciences 23, no. 5: 2487. https://doi.org/10.3390/ijms23052487
APA StyleSyrokou, M. K., Paramithiotis, S., Drosinos, E. H., Bosnea, L., & Mataragas, M. (2022). A Comparative Genomic and Safety Assessment of Six Lactiplantibacillus plantarum subsp. argentoratensis Strains Isolated from Spontaneously Fermented Greek Wheat Sourdoughs for Potential Biotechnological Application. International Journal of Molecular Sciences, 23(5), 2487. https://doi.org/10.3390/ijms23052487










