Investigation of Metabolome Underlying the Biological Mechanisms of Acute Heat Stressed Granulosa Cells
Abstract
:1. Introduction
2. Results
2.1. Influence of Heat Stress on the Physical Parameters of Bovine Granulosa Cells
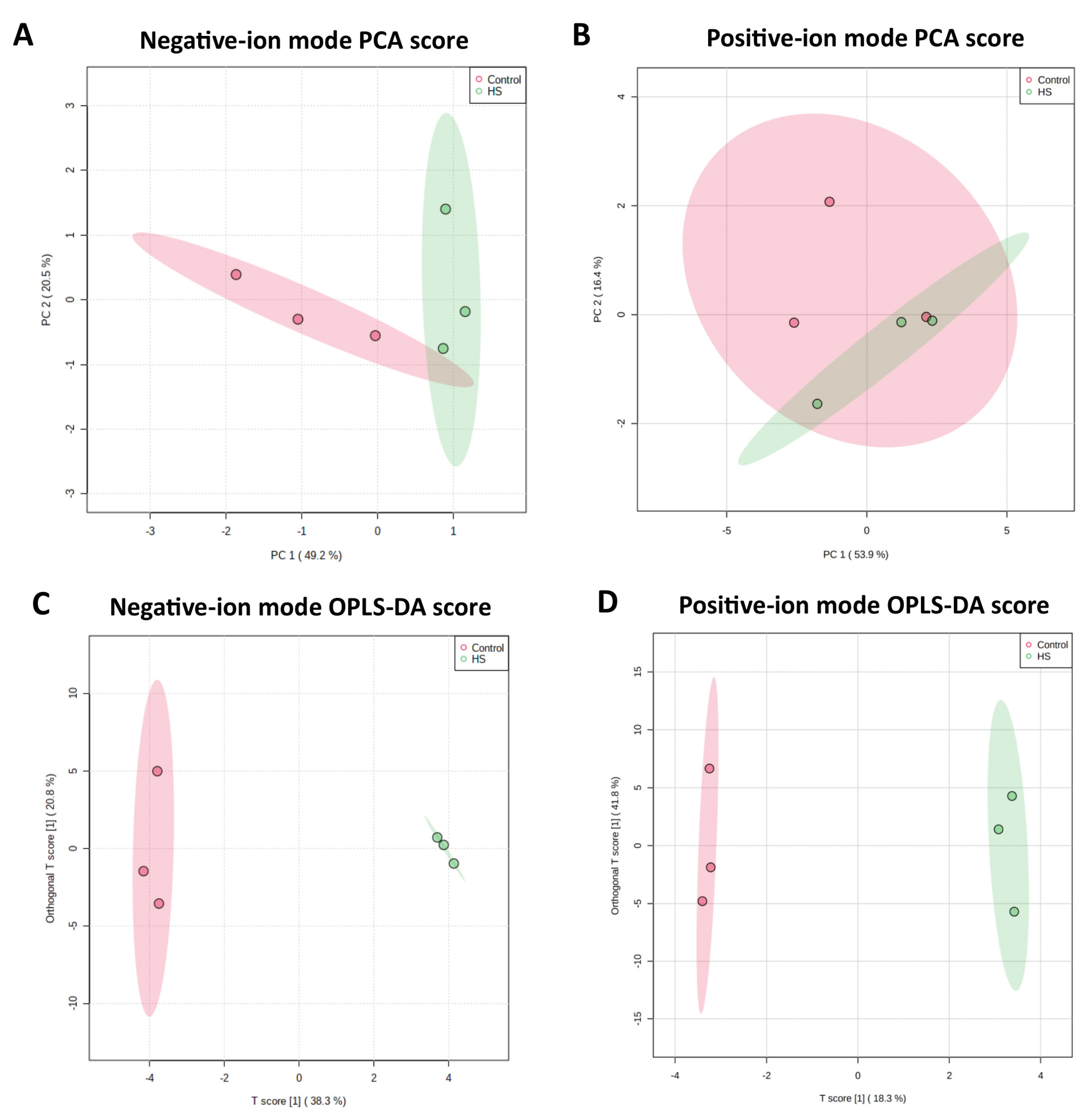
2.2. Metabolome Profile in the Culture Medium of Bovine Granulosa Cells
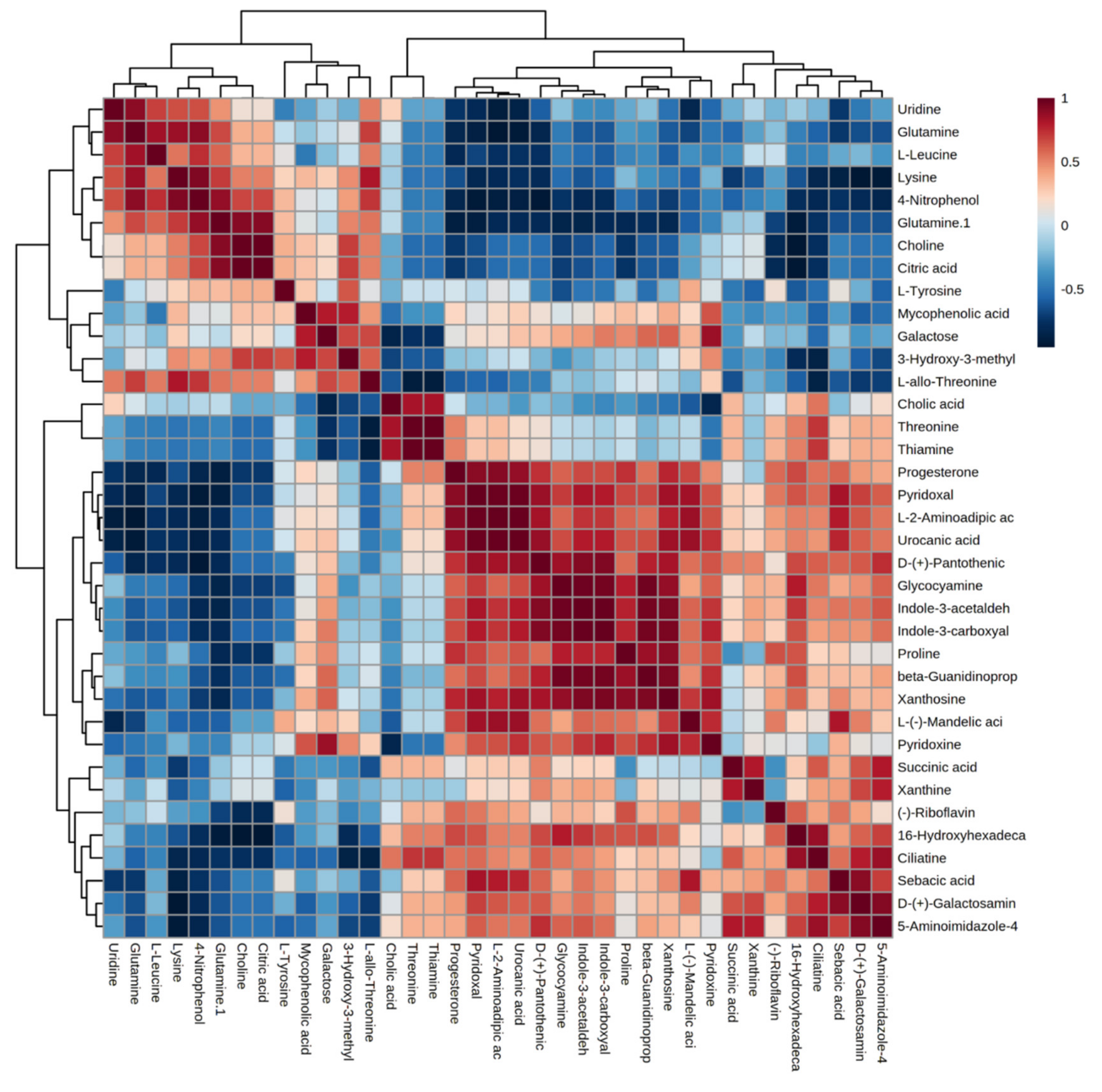
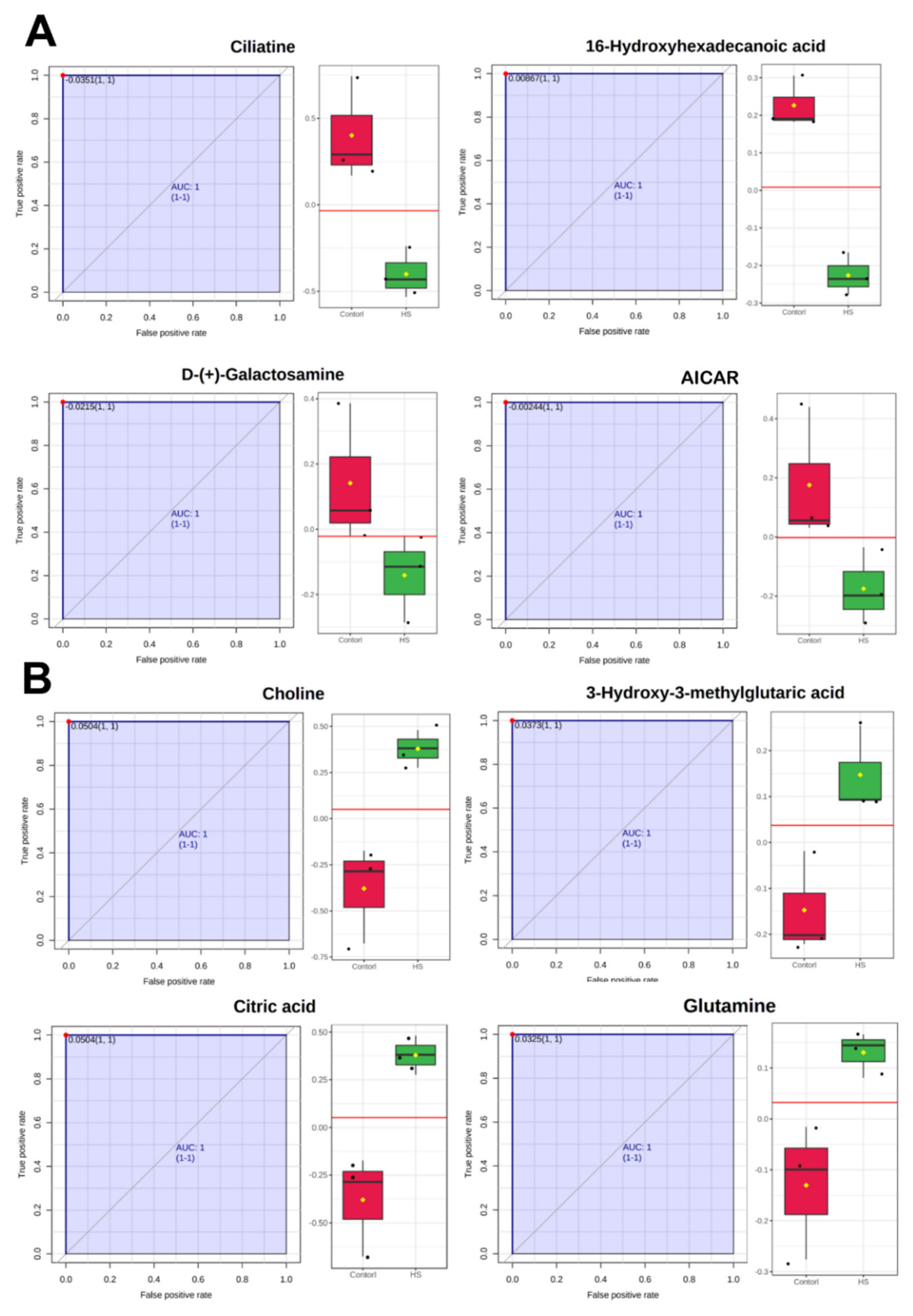
2.3. Differential Metabolites between Control and Heat Stress Groups
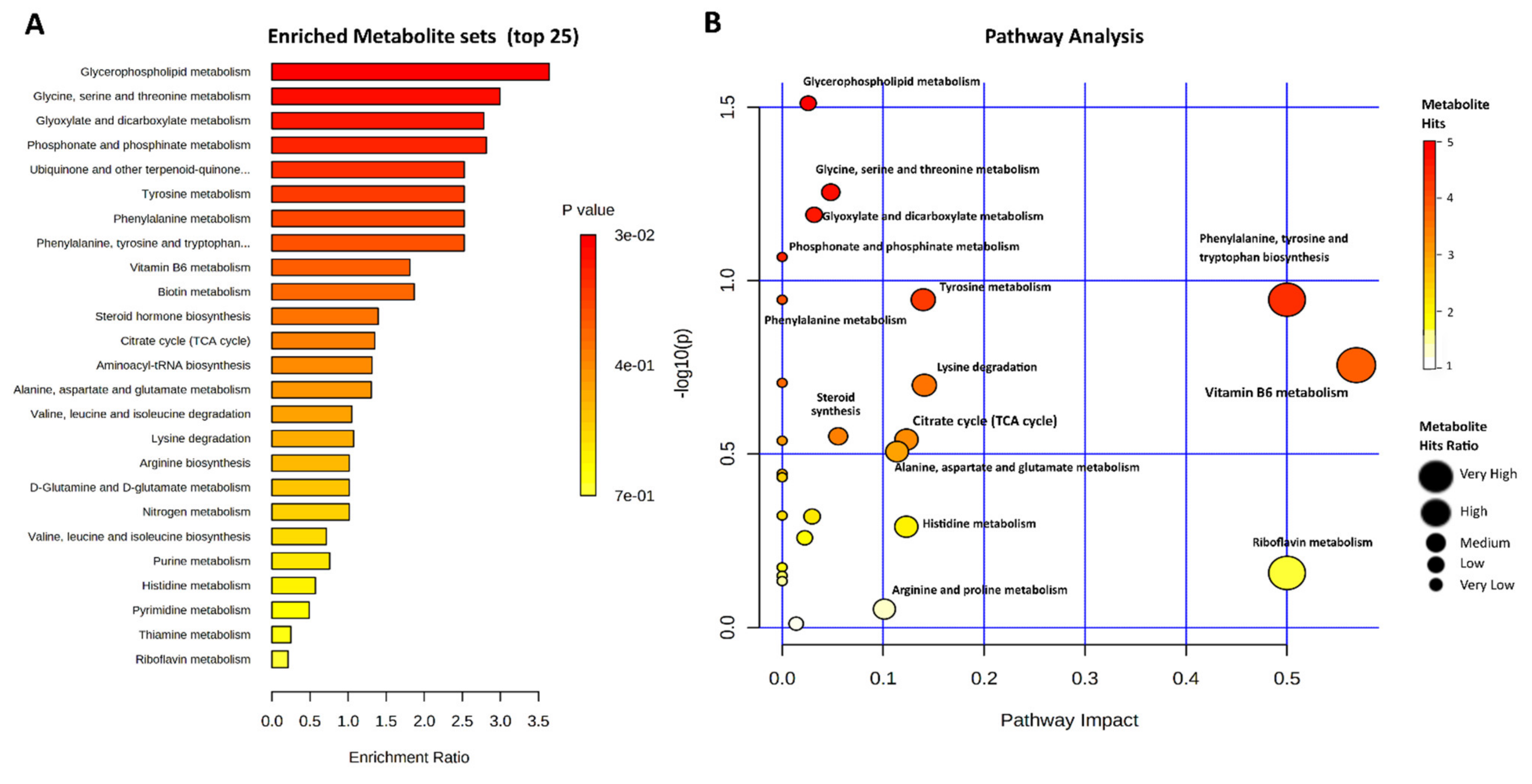
2.4. Metabolic Pathways Involved in the Heat Stress of Bovine Granulosa Cells
3. Discussion
4. Methods
4.1. Granulosa Cell Culture and Heat Treatment
4.2. Physical Parameters of Acute Heat-Stressed Granulosa Cells
4.3. Statistical Analysis of Physiological Parameters
4.4. Samples Preparation for LC–MS/MS
4.5. LC–MS/MS Analysis and Pre-Processing of Peaks
4.6. Metabolome Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherwood, S.C.; Huber, M. An adaptability limit to climate change due to heat stress. Proc. Natl. Acad. Sci. USA 2010, 107, 9552–9555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovats, R.S.; Hajat, S. Heat Stress and Public Health: A Critical Review. Proc. Annu. Rev. Public Health 2008, 29, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Sailo, L.; Verma, N.; Bharti, P.; Saikia, J.; Imtiwati; Kumar, R. Impact of heat stress on health and performance of dairy animals: A review. Vet. World 2016, 9, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Collier, R.J.; Collier, J.L. Environmental Physiology of Livestock; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Ray, D.; Correa-Calderon, A.; Armstrong, D.; Enns, M.; DeNise, S.; Howison, C. Thermoregulatory responses of Holstein and Brown Swiss Heat-Stressed dairy cows to two different cooling systems. Int. J. Biometeorol. 2004, 48, 142–148. [Google Scholar] [CrossRef]
- Asseng, S.; Spänkuch, D.; Hernandez-Ochoa, I.M.; Laporta, J. The upper temperature thresholds of life. Lancet Planet. Health 2021, 5, e378–e385. [Google Scholar] [CrossRef]
- Sammad, A.; Luo, H.; Qiu, W.; Galindez, J.M.; Wang, Y.; Guo, G.; Huang, X.; Wang, Y. Automated monitoring of seasonal and diurnal variation of rumination behaviour: Insights into thermotolerance management of Holstein cows. Biosyst. Eng. 2021. [Google Scholar] [CrossRef]
- Hansen, P.J. Embryonic mortality in cattle from the embryo’s perspective. J. Anim. Sci. 2002, 80, E33–E44. [Google Scholar] [CrossRef]
- Sammad, A.; Umer, S.; Shi, R.; Zhu, H.; Zhao, X.; Wang, Y. Dairy cow reproduction under the influence of heat stress. J. Anim. Physiol. Anim. Nutr. 2020, 104, 978–986. [Google Scholar] [CrossRef]
- Destaw, W.M.; Kefyalew, A.W. Evaluation of the reproductive performance of Holstein Friesian dairy cows in Alage ATVET college, Ethiopia. Int. J. Livest. Prod. 2018, 9, 131–139. [Google Scholar] [CrossRef]
- Jahromi, B.N.; Mosallanezhad, Z.; Matloob, N.; Davari, M.; Ghobadifar, M.A. The potential role of granulosa cells in the maturation rate of immature human oocytes and embryo development: A co-culture study. Clin. Exp. Reprod. Med. 2015, 42, 111–117. [Google Scholar] [CrossRef]
- Diaz, F.J.; Wigglesworth, K.; Eppig, J.J. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J. Cell Sci. 2007, 120, 1330–1340. [Google Scholar] [CrossRef] [Green Version]
- Alemu, T.W.; Pandey, H.O.; Salilew Wondim, D.; Gebremedhn, S.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Oxidative and endoplasmic reticulum stress defense mechanisms of bovine granulosa cells exposed to heat stress. Theriogenology 2018, 110, 130–141. [Google Scholar] [CrossRef]
- Khan, A.; Dou, J.; Wang, Y.; Jiang, X.; Khan, M.Z.; Luo, H.; Usman, T.; Zhu, H. Evaluation of heat stress effects on cellular and transcriptional adaptation of bovine granulosa cells. J. Anim. Sci. Biotechnol. 2020, 11, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.L.; Liu, D.; Ding, G.R.; Liao, P.F.; Zhang, J.W. Hypoxia-inducible factor-1α and Wnt/β-catenin signaling pathways promote the invasion of hypoxic gastric cancer cells. Mol. Med. Rep. 2015, 12, 3365–3373. [Google Scholar] [CrossRef] [Green Version]
- Roth, Z. Heat stress reduces maturation and developmental capacity in bovine oocytes. Reprod. Fertil. Dev. 2021, 66–75. [Google Scholar] [CrossRef]
- Roth, Z.; Meiden, R.; Braw-Tal, R.; Wolfenson, D. Immediate and delayed effects of heat stress on follicular development and its association with plasma FSH and inhibin concentration in cows. J. Reprod. Fertil. 2000, 120, 83–90. [Google Scholar] [CrossRef]
- Gaskins, A.J.; Mínguez-Alarcón, L.; VoPham, T.; Hart, J.E.; Chavarro, J.E.; Schwartz, J.; Souter, I.; Laden, F. Impact of ambient temperature on ovarian reserve. Fertil. Steril. 2021, 116, 1225–1235. [Google Scholar] [CrossRef]
- Fan, Y.; Chang, Y.; Wei, L.; Chen, J.; Li, J.; Goldsmith, S.; Silber, S.; Liang, X. Apoptosis of mural granulosa cells is increased in women with diminished ovarian reserve. J. Assist. Reprod. Genet. 2019, 36, 1225–1235. [Google Scholar] [CrossRef] [Green Version]
- Wolfenson, D.; Roth, Z. Impact of heat stress on cow reproduction and fertility. Anim. Front. 2019, 9, 32–38. [Google Scholar] [CrossRef]
- Tseng, J.K.; Chen, C.H.; Chou, P.C.; Yeh, S.P.; Ju, J.C. Influences of follicular size on parthenogenetic activation and in vitro heat shock on the cytoskeleton in cattle oocytes. Reprod. Domest. Anim. 2004, 39, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Shehab-El-Deen, M.A.M.M.; Leroy, J.L.M.R.; Fadel, M.S.; Saleh, S.Y.A.; Maes, D.; Van Soom, A. Biochemical changes in the follicular fluid of the dominant follicle of high producing dairy cows exposed to heat stress early post-partum. Anim. Reprod. Sci. 2010, 117, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Bridges, P.J.; Brusie, M.A.; Fortune, J.E. Elevated temperature (heat stress) in vitro reduces androstenedione and estradiol and increases progesterone secretion by follicular cells from bovine dominant follicles. Domest. Anim. Endocrinol. 2005, 29, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Baumgard, L.H.; Wheelock, J.B.; Sanders, S.R.; Moore, C.E.; Green, H.B.; Waldron, M.R.; Rhoads, R.P. Postabsorptive carbohydrate adaptations to heat stress and monensin supplementation in lactating Holstein cows1. J. Dairy Sci. 2011, 94, 5620–5633. [Google Scholar] [CrossRef]
- Shin, E.K.; Jeong, J.K.; Choi, I.S.; Kang, H.G.; Hur, T.Y.; Jung, Y.H.; Kim, I.H. Relationships among ketosis, serum metabolites, body condition, and reproductive outcomes in dairy cows. Theriogenology 2015, 84, 252–260. [Google Scholar] [CrossRef]
- Leroy, J.L.M.R.; Vanholder, T.; Mateusen, B.; Christophe, A.; Opsomer, G.; de Kruif, A.; Genicot, G.; Van Soom, A. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction 2005, 130, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Bromfield, J.J.; Sheldon, I.M. Lipopolysaccharide initiates inflammation in bovine granulosa cells via the TLR4 pathway and perturbs oocyte meiotic progression in vitro. Endocrinology 2011, 152, 5029–5040. [Google Scholar] [CrossRef] [Green Version]
- Jakobson, C.M.; Jarosz, D.F. Metabolites control stress granule disassembly. Nat. Cell Biol. 2021, 23, 1053–1055. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Elsheikh, N.A.H.; Li, C.; Yang, F.; Wang, G.; Li, L. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging 2019, 11, 5535–5547. [Google Scholar] [CrossRef]
- Fu, Y.; He, C.-J.; Ji, P.-Y.; Zhuo, Z.-Y.; Tian, X.-Z.; Wang, F.; Tan, D.-X.; Liu, G.-S. Effects of Melatonin on the Proliferation and Apoptosis of Sheep Granulosa Cells under Thermal Stress. Int. J. Mol. Sci. 2014, 15, 21090–21104. [Google Scholar] [CrossRef] [Green Version]
- Saadeldin, I.M.; Swelum, A.A.A.; Elsafadi, M.; Mahmood, A.; Osama, A.; Shikshaky, H.; Alfayez, M.; Alowaimer, A.N.; Magdeldin, S. Thermotolerance and plasticity of camel somatic cells exposed to acute and chronic heat stress. J. Adv. Res. 2020, 22, 105–118. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Swelum, A.A.A.; Elsafadi, M.; Mahmood, A.; Alfayez, M.; Alowaimer, A.N. Differences between the tolerance of camel oocytes and cumulus cells to acute and chronic hyperthermia. J. Therm. Biol. 2018, 74, 47–54. [Google Scholar] [CrossRef]
- King, A.; Selak, M.A.; Gottlieb, E. Succinate dehydrogenase and fumarate hydratase: Linking mitochondrial dysfunction and cancer. Oncogene 2006, 25, 4675–4682. [Google Scholar] [CrossRef] [Green Version]
- Idle, J.R.; Gonzalez, F.J. Metabolomics. Cell Metab. 2007, 6, 348–351. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Li, L.; Xiao, C.; Sun, Y.; Wang, G.L. Heat stress impairs mice granulosa cell function by diminishing steroids production and inducing apoptosis. Mol. Cell. Biochem. 2016, 412, 81–90. [Google Scholar] [CrossRef]
- Mahadevan, S.; Shah, S.L.; Marrie, T.J.; Slupsky, C.M. Analysis of metabolomic data using support vector machines. Anal. Chem. 2008, 80, 7562–7570. [Google Scholar] [CrossRef]
- Sammad, A.; Wang, Y.J.; Umer, S.; Lirong, H.; Khan, I.; Khan, A.; Ahmad, B.; Wang, Y. Nutritional Physiology and Biochemistry of Dairy Cattle under the Influence of Heat Stress: Consequences and Opportunities. Animals 2020, 10, 793. [Google Scholar] [CrossRef]
- Collier, R.J.; Baumgard, L.H.; Zimbelman, R.B.; Xiao, Y. Heat stress: Physiology of acclimation and adaptation. Anim. Front. 2019, 9, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, S.; Summers, C.M.; Pearce, S.C.; Gabler, N.K.; Valentine, R.J.; Baumgard, L.H.; Rhoads, R.P.; Selsby, J.T. Short-term heat stress altered metabolism and insulin signaling in skeletal muscle. J. Anim. Sci. 2018, 96, 154–167. [Google Scholar] [CrossRef] [Green Version]
- Chapinal, N.; LeBlanc, S.J.; Carson, M.E.; Leslie, K.E.; Godden, S.; Capel, M.; Santos, J.E.P.; Overton, M.W.; Duffield, T.F. Herd-level association of serum metabolites in the transition period with disease, milk production, and early lactation reproductive performance. J. Dairy Sci. 2012, 95, 5676–5682. [Google Scholar] [CrossRef]
- Sammad, A.; Khan, M.Z.; Abbas, Z.; Hu, L.; Ullah, Q.; Wang, Y.; Zhu, H.; Wang, Y. Major Nutritional Metabolic Alterations Influencing the Reproductive System of Postpartum Dairy Cows. Metabolites 2022, 12, 60. [Google Scholar] [CrossRef]
- Dobson, H.; Smith, R.; Royal, M.; Knight, C.; Sheldon, I. The high-producing dairy cow and its reproductive performance. Reprod. Domest. Anim. 2007, 42 (Suppl. S2), 17–23. [Google Scholar] [CrossRef] [Green Version]
- Vergara, C.F.; Döpfer, D.; Cook, N.B.; Nordlund, K.V.; McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R. Risk factors for postpartum problems in dairy cows: Explanatory and predictive modeling. J. Dairy Sci. 2014, 97, 4127–4140. [Google Scholar] [CrossRef] [Green Version]
- Miqueo, E.; Chiarle, A.; Giuliodori, M.J.; Relling, A.E. Association between prepartum metabolic status and resumption of postpartum ovulation in dairy cows. Domest. Anim. Endocrinol. 2019, 69, 62–67. [Google Scholar] [CrossRef]
- Al-Katanani, Y.M.; Webb, D.W.; Hansen, P.J. Factors affecting seasonal variation in 90-day nonreturn rate to first service in lactating Holstein cows in a hot climate. J. Dairy Sci. 1999, 82, 2611–2616. [Google Scholar] [CrossRef]
- Siatka, K.; Sawa, A.; Piwczyński, D.; Bogucki, M.; Krężel-Czopek, S. Factors affecting first insemination success in Polish Holstein-Fresian cows. Anim. Sci. Pap. Rep. 2018, 36, 275–285. [Google Scholar]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Arif, M.; Taha, A.E.; Noreldin, A.E. Stress biomarkers and proteomics alteration to thermal stress in ruminants: A review. J. Therm. Biol. 2019, 79, 120–134. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Kikusato, M.; Sudo, S.; Amo, T.; Toyomizu, M. Time course of ROS production in skeletal muscle mitochondria from chronic heat-exposed broiler chicken. Comp. Biochem. Physiol.—A Mol. Integr. Physiol. 2010, 157, 266–271. [Google Scholar] [CrossRef]
- Gu, Z.T.; Li, L.; Wu, F.; Zhao, P.; Yang, H.; Liu, Y.S.; Geng, Y.; Zhao, M.; Su, L. Heat stress induced apoptosis is triggered by transcription-independent p53, Ca2+ dyshomeostasis and the subsequent Bax mitochondrial translocation. Sci. Rep. 2015, 5, 11497. [Google Scholar] [CrossRef] [Green Version]
- Hou, C.H.; Lin, F.L.; Hou, S.M.; Liu, J.F. Hyperthermia induces apoptosis through endoplasmic reticulum and reactive oxygen species in human Osteosarcoma cells. Int. J. Mol. Sci. 2014, 15, 17380–17395. [Google Scholar] [CrossRef] [Green Version]
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Xiong, Y.-M.; Tan, Y.-J.; Wang, L.; Li, R.; Zhang, Y.; Liu, X.-M.; Lin, X.-H.; Jin, L.; Hu, Y.-T.; et al. Melatonin rescues impaired penetration ability of human spermatozoa induced by mitochondrial dysfunction. Reproduction 2019, 158, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Feturi, F.G.; Weinstock, M.; Zhao, W.; Zhang, W.; Schnider, J.T.; Erbas, V.E.; Oksuz, S.; Plock, J.A.; Rohan, L.; Spiess, A.M.; et al. Mycophenolic Acid for Topical Immunosuppression in Vascularized Composite Allotransplantation: Optimizing Formulation and Preliminary Evaluation of Bioavailability and Pharmacokinetics. Front. Surg. 2018, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, J.; Ye, S.; Bureau, D.P.; Liu, H.; Yin, J.; Mou, Z.; Lin, H.; Hao, F. Global metabolic responses of the lenok (Brachymystax lenok) to thermal stress. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 29, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Di, H.S.; Guo, L.; Li, Z.H.; Wang, G.L. Hyperthermia causes bovine mammary epithelial cell death by a mitochondrial-induced pathway. J. Therm. Biol. 2008, 33, 37–47. [Google Scholar] [CrossRef]
- Jones, D.E.; Perez, L.; Ryan, R.O. 3-Methylglutaric acid in energy metabolism. Clin. Chim. Acta 2020, 502, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Akram, M. Citric Acid Cycle and Role of its Intermediates in Metabolism. Cell Biochem. Biophys. 2013, 68, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.E.; Youness, E.R.; Mohammed, N.A.; Morsy, S.M.Y.; Omara, E.A.; Sleem, A.A. Citric acid effects on brain and liver oxidative stress in lipopolysaccharide-treated mice. J. Med. Food 2014, 17, 588–598. [Google Scholar] [CrossRef] [Green Version]
- Elbaz, A.M.; Ibrahim, N.S.; Shehata, A.M.; Mohamed, N.G.; Abdel-Moneim, A.M.E. Impact of multi-strain probiotic, citric acid, garlic powder or their combinations on performance, ileal histomorphometry, microbial enumeration and humoral immunity of broiler chickens. Trop. Anim. Health Prod. 2021, 53, 1–10. [Google Scholar] [CrossRef]
- Wu, Y.H.; Hu, S.Q.; Liu, J.; Cao, H.C.; Xu, W.; Li, Y.J.; Li, L.J. Nature and mechanisms of hepatocyte apoptosis induced by D-galactosamine/lipopolysaccharide challenge in mice. Int. J. Mol. Med. 2014, 33, 1498–1506. [Google Scholar] [CrossRef] [Green Version]
- Morikawa, A.; Sugiyama, T.; Kato, Y.; Koide, N.; Jiang, G.Z.; Takahashi, K.; Tamada, Y.; Yokochi, T. Apoptotic cell death in the response of D-galactosamine-sensitized mice to lipopolysaccharide as an experimental endotoxic shock model. Infect. Immun. 1996, 64, 734–738. [Google Scholar] [CrossRef] [Green Version]
- Stachlewitz, R.F.; Seabra, V.; Bradford, B.; Bradham, C.A.; Rusyn, I.; Germolec, D.; Thurman, R.G. Glycine and uridine prevent d-galactosamine hepatotoxicity in the rat: Role of kupffer cells. Hepatology 1999, 29, 737–745. [Google Scholar] [CrossRef]
- Di Biase, S.; Ma, X.; Wang, X.; Yu, J.; Wang, Y.C.; Smith, D.J.; Zhou, Y.; Li, Z.; Kim, Y.J.; Clarke, N.; et al. Creatine uptake regulates CD8 T cell antitumor immunity. J. Exp. Med. 2019, 216, 2869–2882. [Google Scholar] [CrossRef] [Green Version]
- Aguer, C.; Gambarotta, D.; Mailloux, R.J.; Moffat, C.; Dent, R.; McPherson, R.; Harper, M.-E. Correction: Galactose Enhances Oxidative Metabolism and Reveals Mitochondrial Dysfunction in Human Primary Muscle Cells. PLoS ONE 2012, 7, e28536. [Google Scholar] [CrossRef]
- Dayal, S.; Kalra, K.D.; Sahu, P. Comparative study of efficacy and safety of 45% mandelic acid versus 30% salicylic acid peels in mild-to-moderate acne vulgaris. J. Cosmet. Dermatol. 2020, 19, 393–399. [Google Scholar] [CrossRef]
- Shimomura, Y.; Inahata, M.; Komori, M.; Kagawa, N. Reduction of Tryptophan Hydroxylase Expression in the Brain of Medaka Fish After Repeated Heat Stress. Zoöl. Sci. 2019, 36, 223–230. [Google Scholar] [CrossRef]
- Miura, H.; Ozaki, N.; Sawada, M.; Isobe, K.; Ohta, T.; Nagatsu, T. A link between stress and depression: Shifts in the balance between the kynurenine and serotonin pathways of tryptophan metabolism and the etiology and pathophysiology of depression. Stress 2009, 11, 198–209. [Google Scholar] [CrossRef]
- Katahira, R.; Ashihara, H. Profiles of purine biosynthesis, salvage and degradation in disks of potato (Solanum tuberosum L.) tubers. Planta 2006, 225, 115–126. [Google Scholar] [CrossRef]
- Battelli, M.G.; Polito, L.; Bortolotti, M.; Bolognesi, A. Xanthine oxidoreductase in cancer: More than a differentiation marker. Cancer Med. 2016, 5, 546–557. [Google Scholar] [CrossRef]
- McMichael, L.E.; Heath, H.; Johnson, C.M.; Fanter, R.; Alarcon, N.; Quintana-Diaz, A.; Pilolla, K.; Schaffner, A.; Jelalian, E.; Wing, R.R.; et al. Metabolites involved in purine degradation, insulin resistance, and fatty acid oxidation are associated with prediction of Gestational diabetes in plasma. Metabolomics 2021, 17, 105. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Kai, Y.; Kawano, Y.; Yamamoto, H.; Narahara, H. A possible role for AMP-activated protein kinase activated by metformin and AICAR in human granulosa cells. Reprod. Biol. Endocrinol. 2015, 13, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Kuang, M.; Wang, G.; Ali, I.; Tang, Y.; Yang, C.; Li, Y.; Li, L. Choline attenuates heat stress-induced oxidative injury and apoptosis in bovine mammary epithelial cells by modulating PERK/Nrf-2 signaling pathway. Mol. Immunol. 2021, 135, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Sreevidya, C.S.; Fukunaga, A.; Khaskhely, N.M.; Masaki, T.; Ono, R.; Nishigori, C.; Ullrich, S.E. Agents that Reverse UV-Induced Immune Suppression and Photocarcinogenesis Affect DNA Repair. J. Investig. Dermatol. 2010, 130, 1428–1437. [Google Scholar] [CrossRef] [Green Version]
- Korhonen, E.; Bisevac, J.; Hyttinen, J.M.T.; Piippo, N.; Hytti, M.; Kaarniranta, K.; Petrovski, G.; Kauppinen, A. UV-B-Induced Inflammasome Activation Can Be Prevented by Cis-Urocanic Acid in Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2020, 61, 7. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, S.; Reynolds, C.; Hollinger, K.; Pearce, S.C.; Gabler, N.K.; Baumgard, L.H.; Rhoads, R.P.; Selsby, J.T. Twelve hours of heat stress induces inflammatory signaling in porcine skeletal muscle. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2016, 310, R1288–R1296. [Google Scholar] [CrossRef] [Green Version]
- Lerner, A.A.C.; Salamone, D.F.; Chiappe, M.E.; Barañao, J.L. Comparative studies between freshly isolated and spontaneously immortalized bovine granulosa cells: Protein secretion, steroid metabolism, and responsiveness to growth factors. J. Cell. Physiol. 1995, 164, 348–351. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Basu, S.; Duren, W.; Evans, C.R.; Burant, C.F.; Michailidis, G.; Karnovsky, A. Sparse network modeling and metscape-based visualization methods for the analysis of large-scale metabolomics data. Bioinformatics 2017, 33, 1545–1553. [Google Scholar] [CrossRef]
- Wu, Y.V.; Burnham, W.M.I. Progesterone, 5a-dihydropogesterone and allopregnanolone’s effects on seizures: A review of animal and clinical studies. Seizure 2018, 63, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Wu, S.; Yang, L.; Xue, R.; Li, H.; Wang, Y.; Zhao, H. Exogenous progesterone alleviates heat and high light stress-induced inactivation of photosystem II in wheat by enhancing antioxidant defense and D1 protein stability. Plant Growth Regul. 2014, 74, 311–318. [Google Scholar] [CrossRef]
- Ishihara, Y.; Takemoto, T.; Ishida, A.; Yamazaki, T. Protective actions of 17 β -Estradiol and progesterone on oxidative neuronal injury induced by organometallic compounds. Oxidative Med. Cell. Longev. 2015, 2015, 343706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Metabolites | Mode | VIP | FC | log2(FC) | Chemical Nature | KEGG |
|---|---|---|---|---|---|---|
| D-(+)-Galactosamine | POS | 2.105 | 0.863 | −0.213 | Amino acid derivative | C02262 |
| Mycophenolic acid | POS | 1.921 | 1.109 | 0.149 | Aromatic acid | C20380 |
| Choline | POS | 1.882 | 2.157 | 1.109 | Quaternary amine | C00114 |
| Glycocyamine | POS | 1.714 | 0.805 | −0.314 | Amino acid derivative | C00581 |
| 3-Hydroxy-3-methylglutaric acid | NEG | 1.701 | 1.134 | 0.182 | Dicarboxylic acid | C03761 |
| Indole-3-acetaldehyde | POS | 1.688 | 0.815 | −0.295 | Acetaldehyde | C00637 |
| 16-Hydroxyhexadecanoic acid | NEG | 1.679 | 0.767 | −0.383 | Fatty acid | C13949 |
| Ciliatine | NEG | 1.570 | 0.394 | −1.345 | Phosphonic acid | C03557 |
| Xanthine | NEG | 1.520 | 0.880 | −0.185 | Organic compound | C00385 |
| Uridine | NEG | 1.502 | 0.891 | −0.167 | Ribonucleoside | C00299 |
| Serotonin | POS | 1.474 | 0.855 | −0.227 | Amino acid | C00780 |
| Citric acid | NEG | 1.445 | 2.157 | 1.109 | Tricarboxylic acid | C00158 |
| Indole-3-carboxyaldehyde | POS | 1.440 | 0.834 | −0.263 | Organic acid | C08493 |
| Galactose | POS | 1.437 | 0.851 | −0.231 | Monosaccharide | C00029 |
| L-(−)-Mandelic acid | NEG | 1.431 | 0.931 | −0.103 | Organic acid | C01984 |
| L-Leucine | NEG | 1.410 | 1.039 | 0.056 | Amino acid | C00123 |
| Lysine | POS | 1.393 | 1.245 | 0.316 | Amino acid | C00047 |
| Pyridoxal | POS | 1.369 | 0.398 | −1.329 | Organic compound | C00250 |
| Succinic acid | NEG | 1.332 | 0.415 | −1.268 | Organic compound | C00042 |
| L-allo-Threonine | POS | 1.323 | 1.108 | 0.147 | Alpha-Amino acid | C05519 |
| Proline | POS | 1.319 | 0.848 | −0.238 | Alpha-Amino acid | C00148 |
| beta-Guanidinopropionic acid | POS | 1.316 | 0.864 | −0.210 | Alpha-Amino acid | C03065 |
| Progesterone | POS | 1.300 | 0.403 | −1.312 | Amines | C00410 |
| D-(+)-Pantothenic acid | NEG | 1.257 | 0.868 | −0.204 | Pantothenic acid | C00864 |
| (−)-Riboflavin | POS | 1.249 | 0.738 | −0.438 | Organic compound | C00255 |
| Sebacic acid | NEG | 1.233 | 0.944 | −0.083 | Dicarboxylic acid | C08277 |
| AICAR | NEG | 1.221 | 0.811 | −0.303 | Peptide | C04677 |
| Cholic acid | NEG | 1.189 | 0.794 | −0.332 | Bile acid | C00695 |
| 4-Nitrophenol | NEG | 1.154 | 1.137 | 0.185 | Phenol | C00870 |
| Glutamine | NEG | 1.132 | 1.131 | 0.178 | Amino acid | C00064 |
| L-Tyrosine | NEG | 1.125 | 1.159 | 0.213 | Amino acid | C00082 |
| L-2-Aminoadipic acid | POS | 1.120 | 0.804 | −0.314 | Alpha-Amino acid | C00956 |
| Xanthosine | NEG | 1.094 | 0.898 | −0.156 | Organic compound | C01762 |
| Threonine | NEG | 1.076 | 0.898 | −0.155 | Amino acid | C00188 |
| Pyridoxine | NEG | 1.054 | 1.042 | 0.059 | Organic compound | C00314 |
| Urocanic acid | POS | 1.037 | 0.666 | −0.586 | Amino acid derivative | C06559 |
| Thiamine | POS | 1.003 | 0.898 | −0.155 | Organic compound | C00068 |
| Metabolites | Degree | Betweenness | KEGG |
|---|---|---|---|
| Glutamine | 20 | 64.81 | C00064 |
| 4-Nitrophenol | 17 | 28.59 | C00870 |
| Urocanic acid | 16 | 19.06 | C00785 |
| L-2-Aminoadipic acid | 15 | 14.37 | C00956 |
| D-(+)-Pantothenic acid | 14 | 13.15 | C00864 |
| D-(+)-Galactosamine | 13 | 14.85 | C02262 |
| Uridine | 13 | 14.64 | C00299 |
| L-Leucine | 12 | 18.25 | C00123 |
| Xanthosine | 12 | 9.89 | C01762 |
| Lysine | 10 | 12.94 | C00047 |
| Succinic acid | 10 | 7.97 | C00042 |
| Indole-3-acetaldehyde | 9 | 2.36 | C00637 |
| Progesterone | 8 | 10.59 | C00410 |
| 16-Hydroxyhexadecanoic acid | 8 | 7.44 | C18218 |
| beta-Guanidinopropionic acid | 8 | 7.31 | C03065 |
| Xanthine | 7 | 15.23 | C00385 |
| Citric acid | 7 | 13.85 | C00158 |
| L-allo-Threonine | 7 | 12.1 | C05519 |
| Pyridoxine | 7 | 11.91 | C00314 |
| Sebacic acid | 7 | 10.68 | C08277 |
| (−)-Riboflavin | 7 | 6.85 | C00255 |
| Glycocyamine | 7 | 4.26 | C00581 |
| Thiamine | 6 | 5.95 | C00378 |
| Pyridoxal | 6 | 1.74 | C00250 |
| 3-Hydroxy-3-methylglutaric acid | 6 | 1.08 | C03761 |
| Proline | 5 | 8.89 | C00148 |
| Choline | 5 | 8.78 | C00114 |
| Threonine | 5 | 8.58 | C00188 |
| Ciliatine | 5 | 5.94 | C03557 |
| Galactose | 4 | 7.14 | C00984 |
| Mycophenolic acid | 4 | 5.81 | C20380 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sammad, A.; Hu, L.; Luo, H.; Abbas, Z.; Umer, S.; Zhao, S.; Xu, Q.; Khan, A.; Wang, Y.; Zhu, H.; et al. Investigation of Metabolome Underlying the Biological Mechanisms of Acute Heat Stressed Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 2146. https://doi.org/10.3390/ijms23042146
Sammad A, Hu L, Luo H, Abbas Z, Umer S, Zhao S, Xu Q, Khan A, Wang Y, Zhu H, et al. Investigation of Metabolome Underlying the Biological Mechanisms of Acute Heat Stressed Granulosa Cells. International Journal of Molecular Sciences. 2022; 23(4):2146. https://doi.org/10.3390/ijms23042146
Chicago/Turabian StyleSammad, Abdul, Lirong Hu, Hanpeng Luo, Zaheer Abbas, Saqib Umer, Shanjiang Zhao, Qing Xu, Adnan Khan, Yajing Wang, Huabin Zhu, and et al. 2022. "Investigation of Metabolome Underlying the Biological Mechanisms of Acute Heat Stressed Granulosa Cells" International Journal of Molecular Sciences 23, no. 4: 2146. https://doi.org/10.3390/ijms23042146
APA StyleSammad, A., Hu, L., Luo, H., Abbas, Z., Umer, S., Zhao, S., Xu, Q., Khan, A., Wang, Y., Zhu, H., & Wang, Y. (2022). Investigation of Metabolome Underlying the Biological Mechanisms of Acute Heat Stressed Granulosa Cells. International Journal of Molecular Sciences, 23(4), 2146. https://doi.org/10.3390/ijms23042146










