Criticality of Surface Characteristics of Intravenous Iron–Carbohydrate Nanoparticle Complexes: Implications for Pharmacokinetics and Pharmacodynamics
Abstract
:1. Introduction
2. Different Carbohydrate Types Are Used as the Ligands of the Polynuclear Iron Cores
2.1. The Correct Conformation of the Iron Carbohydrate Complex Is Required to Produce a Safe Iron–Carbohydrate Complex
2.2. The Carbohydrate Ligands of the Iron–Carbohydrate Preparations Are Complex and Structurally Heterogeneous
2.3. The Bond Interactions between the Polynuclear Iron Core and Carbohydrate Ligands Differ among the Iron–Carbohydrate Preparations
3. The IV Iron–Carbohydrate Complexes Have Significantly Different Physicochemical Properties
4. The IV Iron–Carbohydrate Complexes Have Significantly Different Pharmacological Effects in the Body, Indicating the Importance of Surface Characteristics
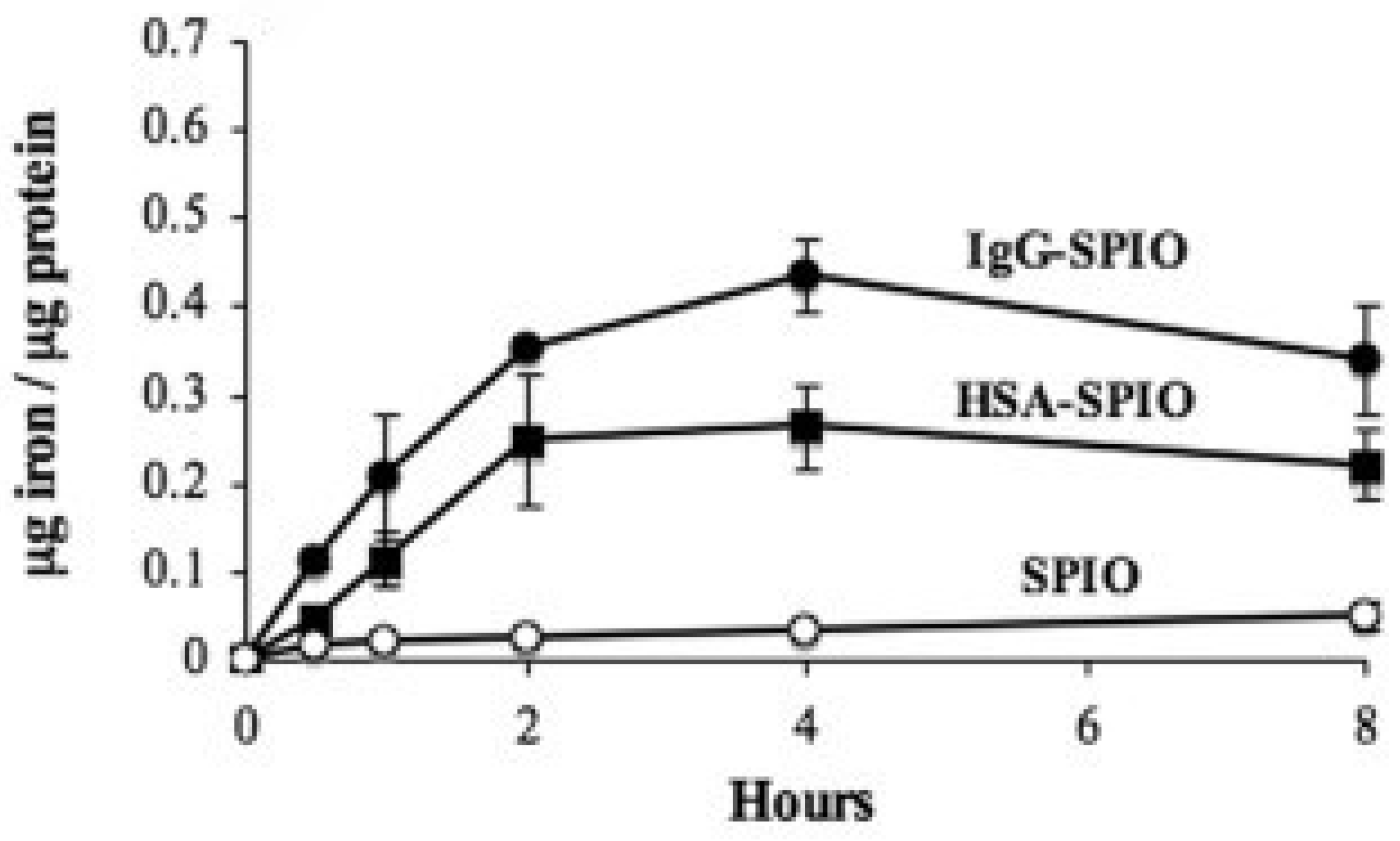
4.1. The Pharmacokinetics of Iron–Carbohydrate Complexes Are Different and the Rate and Extent of Iron Exposure Is Inextricably Linked to the Ligand
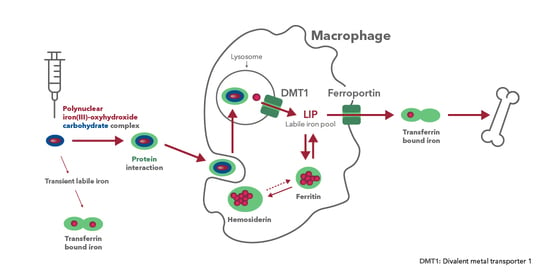
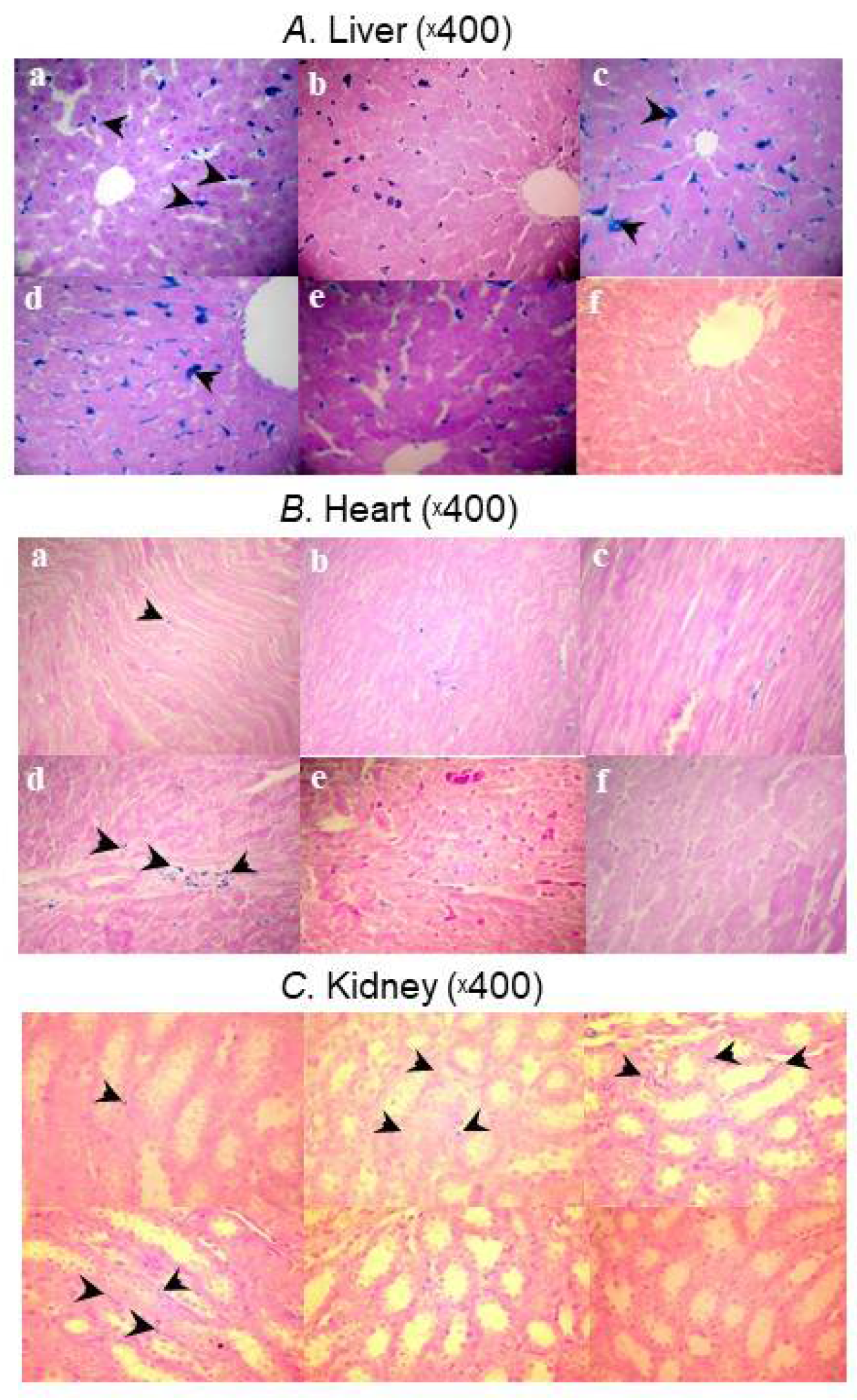
4.2. Biodistribution Profiles in Key Pharmacologic Target Tissues Differs Widely between Iron–Carbohydrate Complexes
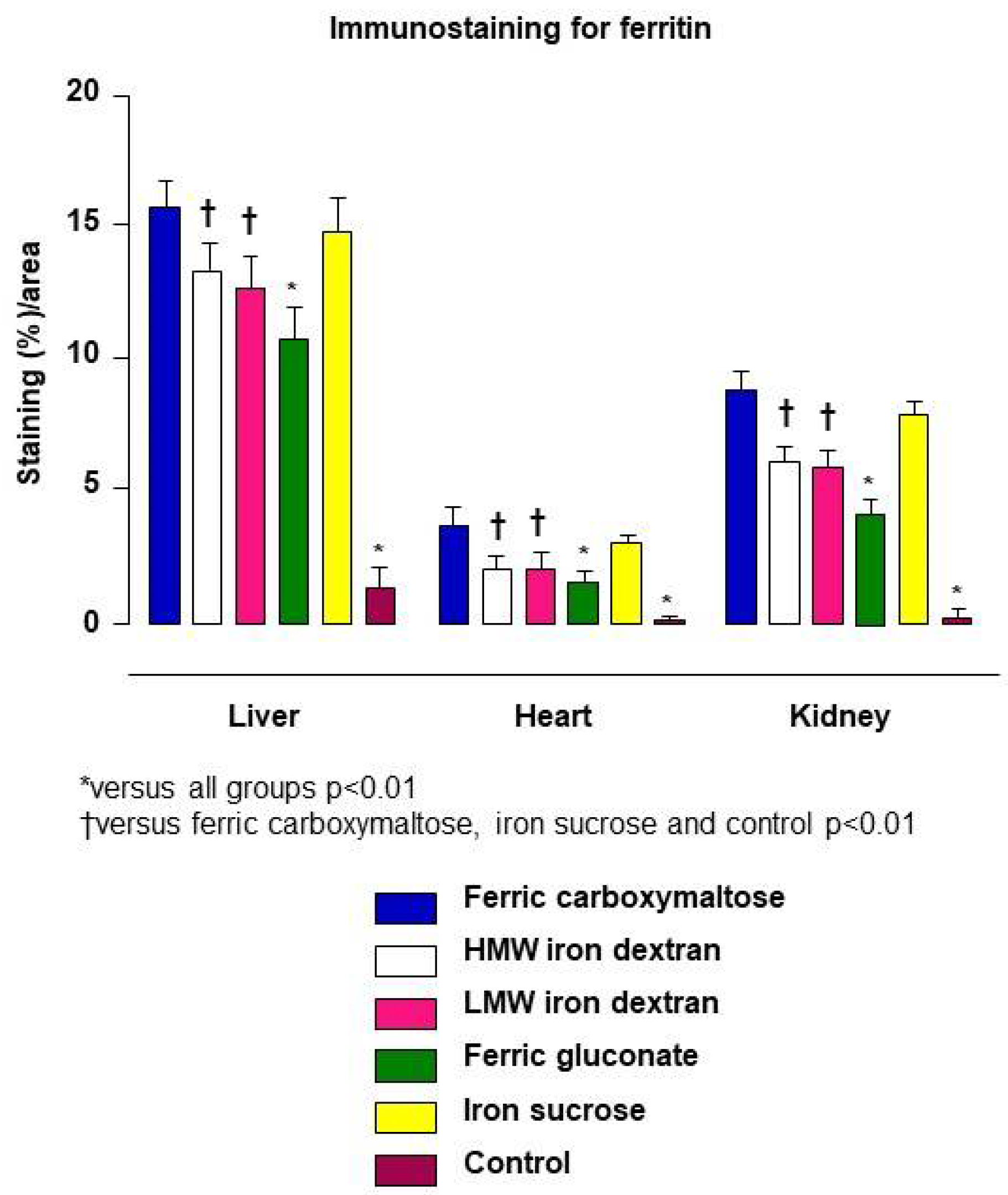
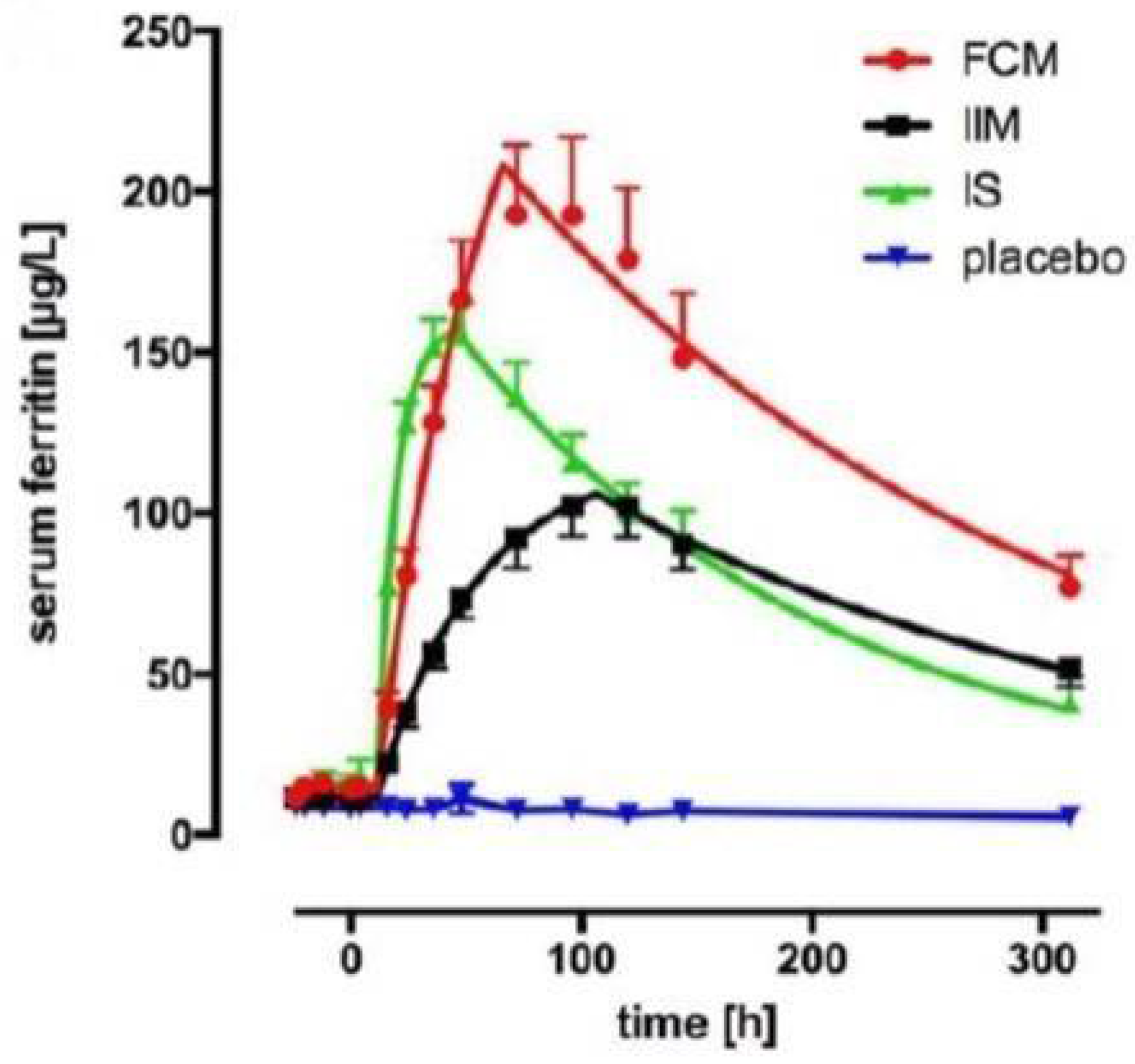
4.3. The Difference in Pharmacologic Activity between the Different IV Iron–Carbohydrate Complexes Is Further Illustrated by the Pharmacodynamic Marker Ferritin
4.4. The Surface Characteristics Confer the Relative Immunogenicity Risk of Iron–Carbohydrate Complexes
5. The Entire Iron–Carbohydrate Complex Defines Properties Relevant to Furnish Pharmacological Activity
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macdougall, I.C.; Comin-Colet, J.; Breymann, C.; Spahn, D.R.; Koutroubakis, I.E. Iron Sucrose: A Wealth of Experience in Treating Iron Deficiency. Adv. Ther. 2020, 37, 1960–2002. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.; Breymann, C.; Brookes, M.J.; Lindgren, S.; Macdougall, I.C.; Mcmahon, L.P.; Munro, M.G.; Nemeth, E.; Rosano, G.M.C.; Schiefke, I.; et al. Questions and answers on iron deficiency treatment selection and the use of intravenous iron in routine clinical practice. Ann. Med. 2021, 53, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Gafter-Gvili, A.; Macdougall, I.C. Intravenous iron: A framework for changing the management of iron deficiency. Lancet Haematol. 2020, 7, e342–e350. [Google Scholar] [CrossRef]
- Heath, C.W.; Strauss, M.B.; Castle, W.B. Quantitative Aspects of Iron Deficiency in Hypochromic Anemia: (The Parenteral Administration of Iron). J. Clin. Investig. 1932, 11, 1293–1312. [Google Scholar] [CrossRef]
- Goetsch, A.T.; Moore, C.V.; Minnich, V. Observations on the effect of massive doses of iron given intravenously to patients with hypochromic anemia. Blood 1946, 1, 129–142. [Google Scholar] [CrossRef]
- Knutson, M.D. Non-transferrin-bound iron transporters. Free Radic. Biol. Med. 2019, 133, 101–111. [Google Scholar] [CrossRef]
- Funk, F.; Ryle, P.; Canclini, C.; Neiser, S.; Geisser, P. The new generation of intravenous iron: Chemistry, pharmacology, and toxicology of ferric carboxymaltose. Arzneimittelforschung 2010, 60, 345–353. [Google Scholar] [CrossRef]
- Theil, E.C.; Sayers, D.E.; Brown, M.A. Similarity of the structure of ferritin and iron. dextran (imferon) determined by extended X-ray absorption fine structure analysis. J. Biol. Chem. 1979, 254, 8132–8134. [Google Scholar] [CrossRef]
- Geisser, P.; Burckhardt, S. The Pharmacokinetics and Pharmacodynamics of Iron Preparations. Pharmaceutics 2011, 3, 12–33. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. IUPAC. Compendium of Chemical Terminology, (the “Gold Book”): Polymer Complexation, 2nd ed.; Chalk, S.J., Ed.; Blackwell Scientific Publications: Oxford, UK, 1997. [Google Scholar]
- Alphandéry, E. Iron oxide nanoparticles for therapeutic applications. Drug Discov. Today 2020, 25, 141–149. [Google Scholar] [CrossRef]
- Nikravesh, N.; Borchard, G.; Hofmann, H.; Philipp, E.; Flühmann, B.; Wick, P. Factors influencing safety and efficacy of intravenous iron-carbohydrate nanomedicines: From production to clinical practice. Nanomed. Nanotechnol. Biol. Med. 2020, 26, 102178. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Ballard, H. Clinical Use of Intravenous Iron: Administration, Efficacy, and Safety. Hematology 2010, 2010, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Nissim, J. Intravenous Administration of Iron. Lancet 1947, 250, 49–51. [Google Scholar] [CrossRef]
- Nissim, J.A.; Robson, J.M. Preparation and standardization of saccharated iron oxide for intra venous administration. Lancet 1949, 1, 686–689. [Google Scholar] [CrossRef]
- Müller, A. Classification and properties of iron preparations. Arzneimittelforschung 1974, 24, 880–883. [Google Scholar] [PubMed]
- Drugs@FDA. Venofer Prescribing Information. January 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021135Orig1s037lbl.pdf (accessed on 30 November 2021).
- Drugs@FDA. Ferrlecit Prescribing Information. December 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020955s019lbl.pdf (accessed on 30 November 2021).
- Drugs@FDA. INFeD Prescribing Information. April 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/017441s179lbl.pdf (accessed on 30 November 2021).
- CosmoFer Prescribing Information Germany. January 2020. Available online: https://www.pharmacosmos.de/media/1665/cosmofer_januar_2020.pdf (accessed on 30 November 2021).
- Monofer Public Assessment Report, Scientific Discussion, SE/H/734/01/DC. November 2009. Available online: https://docetp.mpa.se/LMF/Monofer%20solution%20for%20injection%20or%20infusion%20ENG%20PAR_09001be68045522a.pdf (accessed on 30 November 2021).
- Drugs@FDA. Monoferric Prescribing Information. September 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208171s001lbl.pdf (accessed on 30 November 2021).
- Monofer Prescribing Information Germany. July 2013. Available online: https://s3.eu-central-1.amazonaws.com/prod-cerebro-ifap/media_all/49872.pdf (accessed on 30 November 2021).
- Drugs@FDA. Injectafer Prescribing Information. November 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/203565Orig1s016lbledt.pdf (accessed on 30 November 2021).
- Neiser, S.; Rentsch, D.; Dippon, U.; Kappler, A.; Weidler, P.G.; Göttlicher, J.; Steininger, R.; Wilhelm, M.; Braitsch, M.; Funk, F.; et al. Physico-chemical properties of the new generation IV iron preparations ferumoxytol, iron isomaltoside 1000 and ferric carboxymaltose. BioMetals 2015, 28, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Drugs@FDA. Feraheme Prescribing Information. September 2020. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/022180s024lbl.pdf (accessed on 30 November 2021).
- Swissmedic. IKS Bulletin. 1949. Available online: https://wwwswissmedicch/swissmedic/de/home/ueber-uns/publikationen/swissmedic-journal/monthly-reports-iks-archivehtml (accessed on 6 December 2021).
- Drugs@FDA. Ferrlecit Pharmacology Review. Available online: https://wwwaccessdatafdagov/drugsatfda_docs/nda/99/20955_Ferrlecit_pharmrpdf (accessed on 6 December 2021).
- Pink Sheet. 1991. Available online: https://pinkpharmaintelligenceinformacom/PS019780/SCHEIN-LAUNCHING-INFED-INJECTABLE-IRON (accessed on 6 December 2021).
- Ferinject Public Assessment Report, Decentralised Procedure, UK/H/0894/001/DC. Available online: https://wwwgeneesmiddeleninformatiebanknl/Pars/h33865pdf (accessed on 6 December 2021).
- Israelachvili, J.N. Intermolecular and Surface Forces, 3rd ed.; Academic Press: Burlington, MA, USA, 2011; 674p. [Google Scholar]
- Kästele, X.; Sturm, C.; Klüfers, P. 13C NMR spectroscopy as a tool for the in situ characterisation of iron-supplementing preparations. Eur. J. Pharm. Biopharm. 2014, 86, 469–477. [Google Scholar] [CrossRef]
- Kudasheva, D.S.; Lai, J.; Ulman, A.; Cowman, M.K. Structure of carbohydrate-bound polynuclear iron oxyhydroxide nanoparticles in parenteral formulations. J. Inorg. Biochem. 2004, 98, 1757–1769. [Google Scholar] [CrossRef]
- Shah, R.B.; Yang, Y.; Khan, M.A.; Raw, A.; Yu, L.X.; Faustino, P.J. Pharmaceutical characterization and thermodynamic stability assessment of a colloidal iron drug product: Iron sucrose. Int. J. Pharm. 2014, 464, 46–52. [Google Scholar] [CrossRef]
- Yang, Y.; Shah, R.B.; Faustino, P.J.; Raw, A.; Yu, L.X.; Khan, M.A. Thermodynamic stability assessment of a colloidal iron drug product: Sodium ferric gluconate. J. Pharm. Sci. 2010, 99, 142–153. [Google Scholar] [CrossRef]
- Di Francesco, T.; Sublet, E.; Borchard, G. Nanomedicines in clinical practice: Are colloidal iron sucrose ready-to-use intravenous solutions interchangeable? Eur. J. Pharm. Sci. 2019, 131, 69–74. [Google Scholar] [CrossRef]
- Philipp, E.; Braitsch, M.; Bichsel, T.; Mühlebach, S. Diluting ferric carboxymaltose in sodium chloride infusion solution (0.9% w/v) in polypropylene bottles and bags: Effects on chemical stability. Eur. J. Hosp. Pharm. 2016, 23, 22–27. [Google Scholar] [CrossRef]
- Monofer Prescribing Information Switzerland. May 2021. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/208171s000lbl.pdf (accessed on 30 November 2021).
- Jahn, M.R.; Andreasen, H.B.; Fütterer, S.; Nawroth, T.; Schünemann, V.; Kolb, U.; Hofmeister, W.; Muñoz, M.; Bock, K.; Meldal, M.; et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer®), a new intravenous iron preparation and its clinical implications. Eur. J. Pharm. Biopharm. 2011, 78, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Funk, F.; Hautot, D.; Büchi, R.; Christl, I.; Weidler, P.G. Physical and Chemical Characterization of Therapeutic Iron Containing Materials: A Study of Several Superparamagnetic Drug Formulations with the β-FeOOH or Ferrihydrite Structure. Hyperfine Interact. 2001, 136, 73–95. [Google Scholar] [CrossRef]
- Fütterer, S.; Andrusenko, I.; Kolb, U.; Hofmeister, W.; Langguth, P. Structural characterization of iron oxide/hydroxide nanoparticles in nine different parenteral drugs for the treatment of iron deficiency anaemia by electron diffraction (ED) and X-ray powder diffraction (XRPD). J. Pharm. Biomed. Anal. 2013, 86, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, V.S.; Rao, M.; Kausz, A.T.; Brenner, L.; Pereira, B.J.G.; Frigo, T.B.; Lewis, J.M. Physicochemical properties of ferumoxytol, a new intravenous iron preparation. Eur. J. Clin. Investig. 2009, 39, 489–496. [Google Scholar] [CrossRef]
- Garbowski, M.W.; Bansal, S.; Porter, J.B.; Mori, C.; Burckhardt, S.; Hider, R.C. Intravenous iron preparations transiently generate non-transferrin-bound iron from two proposed pathways. Haematologica 2021, 106, 2885. [Google Scholar] [CrossRef] [PubMed]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic Iron Oxide Nanoparticles—Current and Prospective Medical Applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef]
- Beduneau, A.; Ma, Z.; Grotepas, C.B.; Kabanov, A.; Rabinow, B.E.; Gong, N.; Mosley, R.L.; Dou, H.; Boska, M.D.; Gendelman, H.E. Facilitated Monocyte-Macrophage Uptake and Tissue Distribution of Superparmagnetic Iron-Oxide Nanoparticles. PLoS ONE 2009, 4, e4343. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
- Spicher, K.; Brendler-Schwaab, S.; Schlösser, C.; Catarinolo, M.; Fütterer, S.; Langguth, P.; Enzmann, H. Differences in tissue distribution of iron from various clinically used intravenous iron complexes in fetal avian heart and liver. Regul. Toxicol. Pharmacol. 2015, 73, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Toblli, J.E.; Cao, G.; Olivieri, L.; Angerosa, M. Comparison of the renal, cardiovascular and hepatic toxicity data of original intravenous iron compounds. Nephrol. Dial. Transplant. 2010, 25, 3631–3640. [Google Scholar] [CrossRef] [PubMed]
- Torti, F.M.; Torti, S.V. Regulation of ferritin genes and protein. Blood 2002, 99, 3505–3516. [Google Scholar] [CrossRef]
- Fischer, R.; Harmatz, P.R. Non-invasive assessment of tissue iron overload. Hematol. Am. Soc. Hematol. Educ. Program 2009, 2009, 215–221. [Google Scholar] [CrossRef]
- Wysowski, D.K.; Swartz, L.; Borders-Hemphill, B.V.; Goulding, M.R.; Dormitzer, C. Use of parenteral iron products and serious anaphylactic-type reactions. Am. J. Hematol. 2010, 85, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, H.; Kaluza, K.; Numan, S.; Goodnough, L.T. Frequency and Associated Costs of Anaphylaxis- and Hypersensitivity-Related Adverse Events for Intravenous Iron Products in the USA: An Analysis Using the US Food and Drug Administration Adverse Event Reporting System. Drug Saf. 2021, 44, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Koskenkorva-Frank, T.S.; Weiss, G.; Koppenol, W.; Burckhardt, S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic. Biol. Med. 2013, 65, 1174–1194. [Google Scholar] [CrossRef] [PubMed]
- Neiser, S.; Koskenkorva, T.S.; Schwarz, K.; Wilhelm, M.; Burckhardt, S. Assessment of Dextran Antigenicity of Intravenous Iron Preparations with Enzyme-Linked Immunosorbent Assay (ELISA). Int. J. Mol. Sci. 2016, 17, 1185. [Google Scholar] [CrossRef]
- Mahmoudi, M. The need for robust characterization of nanomaterials for nanomedicine applications. Nat. Commun. 2021, 12, 1–5. [Google Scholar] [CrossRef]
| Ligand(s) | Trade Names | International Nonproprietary Names and/or Common Names | Ligand Description |
|---|---|---|---|
| Sucrose | Venofer® | Iron sucrose Iron saccharate Saccharated iron oxide | Disaccharide |
| Fesin® | |||
| Ferrivenin® | |||
| Dextran (polyisomaltose) | Imferon® (HMWID) | Iron dextran | Polysaccharide, maltose units 1→6-linked |
| Dexferrum® (HMWID) | |||
| INFeD/Cosmofer® (LMWID) | |||
| Sorbitol and citric acid | Jectofer® | Iron sorbitex | Monosaccharide and carboxylic acid |
| Dextrin (polymaltose) | Amylofer® | Dextriferron Iron polymaltose | Polysaccharide, maltose units 1→4-linked |
| Gluconate and sucrose | Ferrlecit® | Sodium ferric gluconate | Carboxylic acid and disaccharide |
| Chondroitin sulfate | Blutal® | Iron chondroitin sulfate | Sulfated poly-glycosaminoglycan, alternating N-acetylgalactosamine and glucuronic acid |
| Carboxymaltose | Injectafer®/Ferinject® | Ferric carboxymaltose | Polysaccharide, maltose units 1→4-linked, oxidized |
| Polyglucose sorbitol carboxymethyl ether and mannitol (excipient) | Feraheme® | Ferumoxytol | Polysaccharide, maltose units 1→6-linked, hydrogenated and carboxymethylated, and monosaccharide |
| Isomaltoside 1000/derisomaltose, and citrate | Monoferric®/Monofer® | Iron isomaltoside 1000 Ferric derisomaltose | Oligosaccharide, maltose units 1→6-linked, hydrogenated, and carboxylic acid |
| Product (Year of First Market Entry) | Carbohydrates | Iron Content (mg Fe/mL) | Carbohydrate Content (mg/mL) | Carbohydrate Content (mg/mg Fe) | pH |
|---|---|---|---|---|---|
| Iron sucrose [17] (1949) [27] | Sucrose | 20 | 300 | 15 | 10.5–11.1 |
| Sodium ferric Gluconate [18] (1951) [28] | Sucrose Gluconate | 12.5 | 195 22 | 16 1.7 | 7.7–9.7 |
| Iron dextran [19,20] (1974) [29] | Dextran | 50 | 206 | 4.1 | 4.5–7.0 |
| Ferric derisomaltose [22] (2009) [21] | Derisomaltose Citrate | 100 | 230 10 | 2.3 0.1 | 5.0–7.0 |
| Ferric carboxymaltose [24] (2007) [30] | Carboxymaltose | 50 | 75 | 1.5 | 5.0–7.0 |
| Ferumoxytol [26] (2009) [26] | polyglucose sorbitol carboxymethylether Maltose (excipient) | 30 | 29 44 | 0.97 1.5 | 6–8 |
| Product | Carbohydrates | Van der Waals Forces | Non-ionic Hydrogen Bonds | Ionic Hydrogen Bonds | Coordinative Bonds |
|---|---|---|---|---|---|
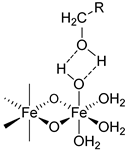 | 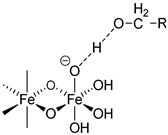 | 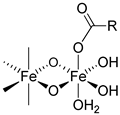 | |||
| Iron sucrose | Sucrose | √ | √ | √ | - |
| Sodium ferric gluconate | Sucrose Gluconate | √ | √ √ | √ - | - √ |
| Iron dextran | Dextran | √ | √ | - | - |
| Ferric derisomaltose | Derisomaltose Citrate | √ | √ √ | - . | - √ |
| Ferric carboxymaltose | Carboxymaltose | √ | √ | - | √ |
| Ferumoxytol | polyglucose sorbitol carboxymethylether Maltose (excipient) | √ | √ √ | - - | √ - |
| Product | Molecular Weight (kDa) | Particle Size (nm) [39] | Zeta Potential (mV) [39] | Crystalline Structure | Reduction Potential (mV) ° [25] | Reduction Kinetics k(Θ) × 103 (min−1) Θ = 0.1/0.5/0.8 [25] | Blocking Temperature (K) [25,40] |
|---|---|---|---|---|---|---|---|
| Iron sucrose | 34–60 [17,41] 42–44 [25] 252 [42] 140 [39] | 8.3 (PDI 0.192) | pH 7.43: −26.20 pH 11.03 *: −28.15 | 2-line ferrihydrite [39] Ferrihydrite and lepidocrocite [41] Akageneite [33] 2-line ferrihydrite-like [40] No clear identification [25] | −494 | 107/89/117 § | 55 |
| Sodium ferric gluconate | 289–440 [18] 37.5 [9] 200 [42] 164 [39] | 8.6 (PDI 0.244) | pH 7.4: −29.70 pH 8.36 *: −29.10 | 2-line ferrihydrite ([39] Ferrihydrite and lepidocrocite [41] Akaganeite [33] | nd | nd | nd |
| Iron dextran | 165 [20] 165 [39] | 12.2 (PDI 0.149) | pH 6.4 *: −15.30 pH 7.31: −17.25 | Akageneite [39,41] | nd | nd | nd |
| Ferric derisomaltose | 155 [22] 63–69 [25] 150 [39] | 9.9 (PDI 0.182) | pH 6.3 *: −22.0 pH 7.35: −21.05 | Akaganeite [25,39,41] | −338/−508 | 21/41/63 | 56 |
| Ferric carboxymaltose | ≈ 150 [24] 145–155 [25] 233 [39] | 23.1 (PDI 0.07) | pH 5.36: 3.68 pH 7.26: −8.52 | Akaganeite [25,39,41] | −333 | 18/35/55 | 114 |
| Ferumoxytol | 750 [26] 172–188 [25] 731 [42] 276 [39] | 23.6 (PDI 0.143) | pH 6.6: −43.20 pH 7.36: −30.55 | Magnetite/Maghemite [39] Magnetite [41] Maghemite [25] | −245/−768 | 36/67/98 | 73 |
| Parameter | Sodium Ferric Gluconate [9] | Iron Sucrose [9] | Ferric Carboxymaltose [9] | Ferumoxytol [9] | Ferric Derisomaltose [22] | Iron Dextran [19] |
|---|---|---|---|---|---|---|
| Dosage used for PK characteristics, mg Fe | 125 | 100 | 100/1000 | 316 | 1000 | NA |
| Terminal t1/2, h | 1.42 | 5.3 | 7.4/9.4 | 14.7 | 27 | 20 |
| Cmax, mg Fe/L | 20.6 | 35.5 | 37/331 | 130 | 408 | NA |
| AUC, mg Fe/L*h | 43.7 | 83.3 | 333/6277 | 2912 | 17730 | NA |
| AUC, standardized for a dose of 100 mg Fe, mg Fe/L*h | 35.0 | 83.3 | 333/627 | 922 | 1773 | NA |
| CL, L/h | 2.99 | 1.23 | 0.26/0.16 | 0.11 | NA | NA |
| IVIP-Fe * cmax (mmol/L) tmax (h) t1/2 (h) AUC0-inf (h*mmol/L) | 0.85 ± 0.17 0.35 (0.33–0.37) 3.43 ± 1.55 2.59 ± 0.5 | 1.16 ± 0.09 0.34 (0.33–0.7) 6.82 ± 1.93 12.39 ± 1.2 | 1.29 ± 0.1 0.67 (0.33–6) 20.3 ± 2.27 36.76 ± 4.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funk, F.; Flühmann, B.; Barton, A.E. Criticality of Surface Characteristics of Intravenous Iron–Carbohydrate Nanoparticle Complexes: Implications for Pharmacokinetics and Pharmacodynamics. Int. J. Mol. Sci. 2022, 23, 2140. https://doi.org/10.3390/ijms23042140
Funk F, Flühmann B, Barton AE. Criticality of Surface Characteristics of Intravenous Iron–Carbohydrate Nanoparticle Complexes: Implications for Pharmacokinetics and Pharmacodynamics. International Journal of Molecular Sciences. 2022; 23(4):2140. https://doi.org/10.3390/ijms23042140
Chicago/Turabian StyleFunk, Felix, Beat Flühmann, and Amy E. Barton. 2022. "Criticality of Surface Characteristics of Intravenous Iron–Carbohydrate Nanoparticle Complexes: Implications for Pharmacokinetics and Pharmacodynamics" International Journal of Molecular Sciences 23, no. 4: 2140. https://doi.org/10.3390/ijms23042140
APA StyleFunk, F., Flühmann, B., & Barton, A. E. (2022). Criticality of Surface Characteristics of Intravenous Iron–Carbohydrate Nanoparticle Complexes: Implications for Pharmacokinetics and Pharmacodynamics. International Journal of Molecular Sciences, 23(4), 2140. https://doi.org/10.3390/ijms23042140