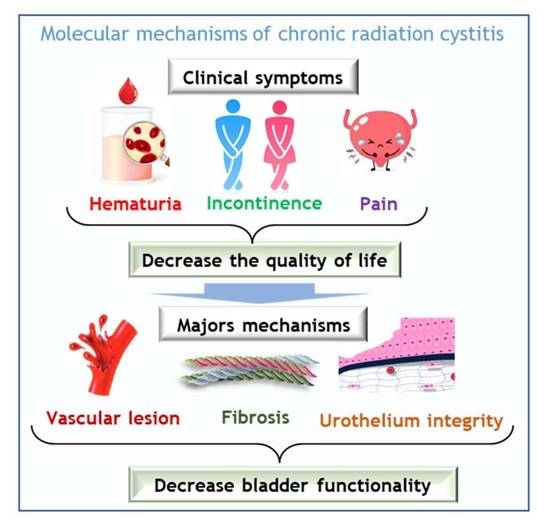
Understanding Molecular Mechanisms and Identifying Key Processes in Chronic Radiation Cystitis
Abstract
1. Introduction
2. Radiosensitivity of the Bladder
- The exposed bladder volume and surface area—if involved, the trigone is more problematic than the bladder dome.
- The dose rate—a rate below 0.8 Gy/h reduces the risk of cystitis and the dose of the daily fraction of irradiation. Doses higher than 2 Gy per fraction increase the risk of delayed sequelae.
3. Incidence of CRC
4. Pathophysiology of CRC in Patients
5. Grading CRC
6. Clinical Management of Radiation Cystitis
7. Mechanisms of CRC: Lesson from Preclinical Studies
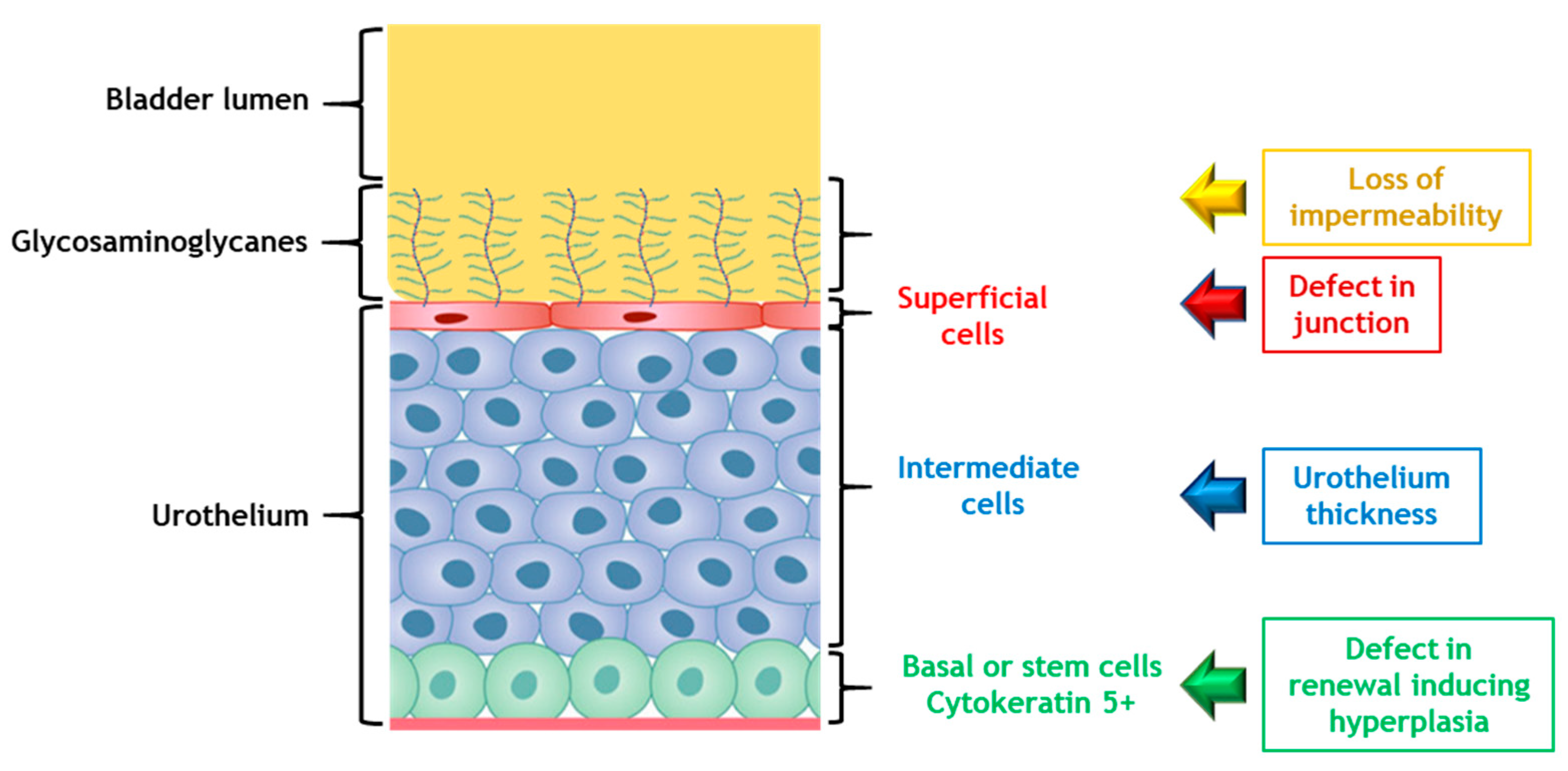
7.1. Urothelium: Unknown Actor but May Be the Key Element in Inflammation: A Paradigm Shift?
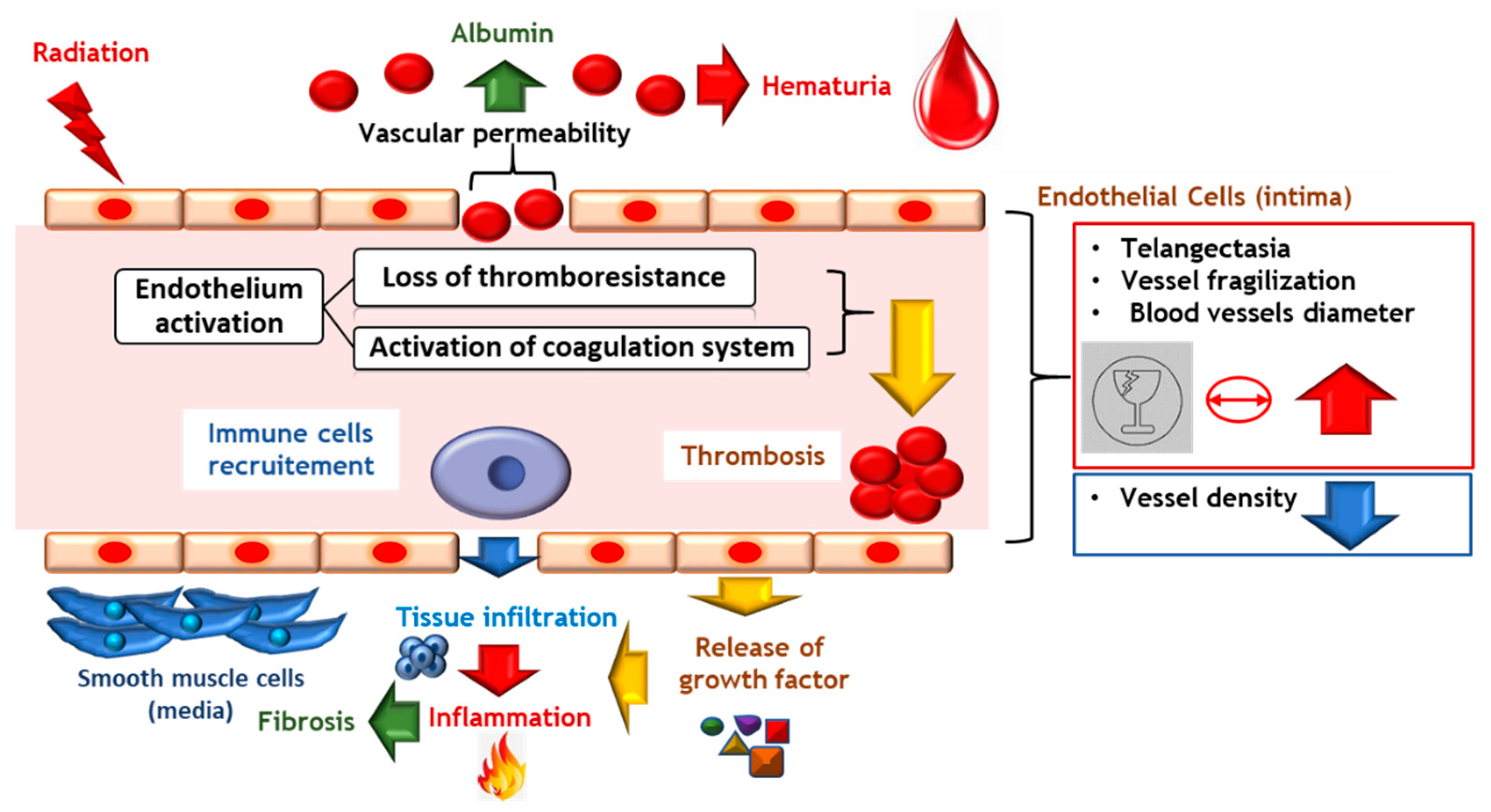
7.2. Vascular Lesions Are the Checkpoint in Chronicity Development
7.2.1. Vascular Damage Implicated in Late Radiation Injury
7.2.2. Vascular Lesions: Key Factor in the Onset of CRC
7.3. Inflammation Is a Major Tissue Response to Irradiation, Inducing Chronic Tissue Damage
Are Neuro-Inflammation and Lack of Neurogenic Control the Cause of Spasms and Pain?
7.4. Fibrosis: Key Point in Maintaining the CRC
7.5. The Impaired Muscle Induces Contractility Deficit, and Increases Frequency of Miction as Well as Decreasing the Volume of Miction
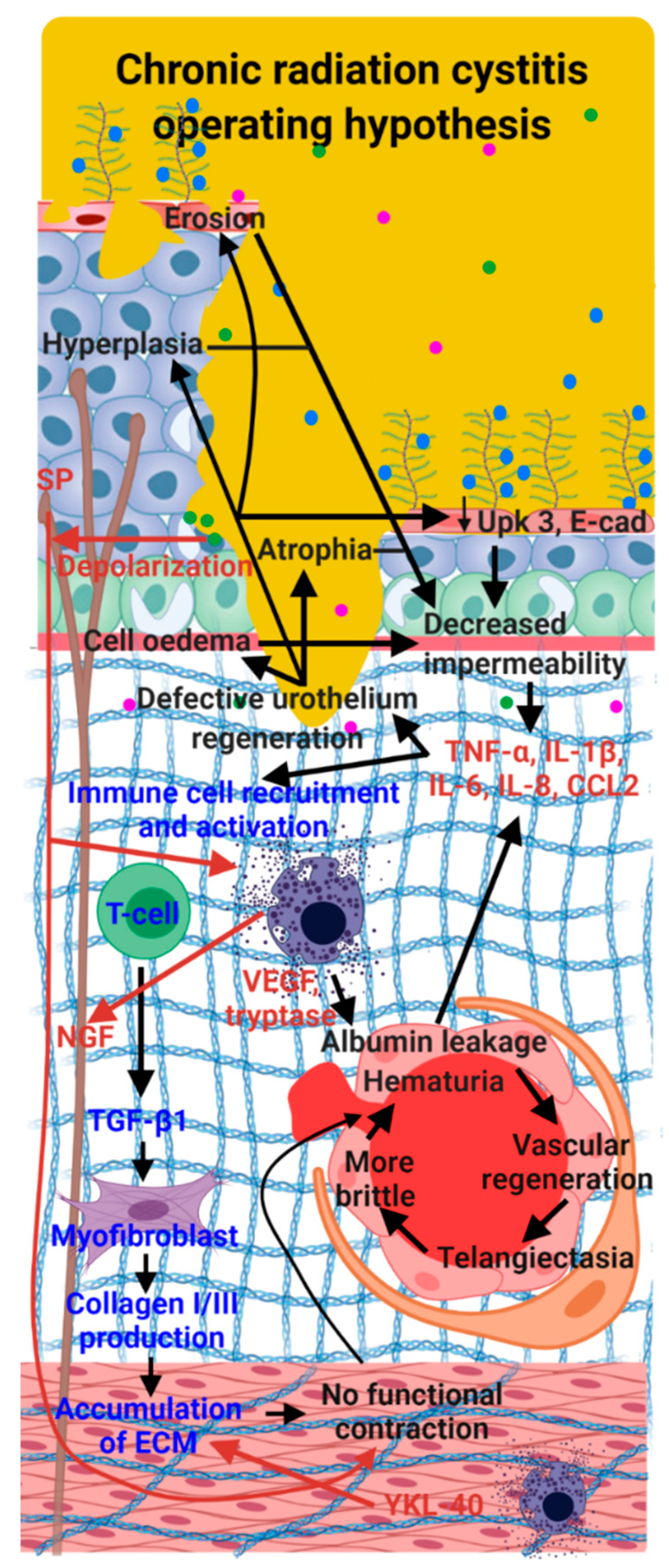
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The Global Cancer Observatory: International Agency for Research on Cancer: World Health Organization 2020 (Globocan). Number of New Cases in 2020 bs, All Ages and Number of Deaths in 2020, Both Sexes, All Ages. Available online: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf (accessed on 25 December 2021).
- INCA. Institut National du Cancer: Les Cancers en France en 2018—L’essentiel des Faits et Chiffres (éDition 2019). France: Institut National du Cancer; Février 2019. Available online: https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Les-cancers-en-France-en-2018-L-essentiel-des-faits-et-chiffres-edition-2019 (accessed on 25 December 2021).
- Andreyev, H.J.; Wotherspoon, A.; Denham, J.W.; Hauer-Jensen, M. “Pelvic radiation disease”: New understanding and new solutions for a new disease in the era of cancer survivorship. Scand. J. Gastroenterol. 2011, 46, 389–397. [Google Scholar] [CrossRef]
- Trotman, J.; Nivison-Smith, I.; Dodds, A. Haemorrhagic cystitis: Incidence and risk factors in a transplant population using hyperhydration. Bone Marrow Transplant. 1999, 23, 797–801. [Google Scholar] [CrossRef][Green Version]
- Cesaro, S.; Brugiolo, A.; Faraci, M.; Uderzo, C.; Rondelli, R.; Favre, C.; Zecca, M.; Garetto, G.; Dini, G.; Pillon, M.; et al. Incidence and treatment of hemorrhagic cystitis in children given hematopoietic stem cell transplantation: A survey from the Italian association of pediatric hematology oncology-bone marrow transplantation group. Bone Marrow Transplant. 2003, 32, 925–931. [Google Scholar] [CrossRef]
- Rigaud, J.; Hetet, J.F.; Bouchot, O. Management of radiation cystitis. Prog. Urol. J. Assoc. Fr. Urol. Soc. Fr. Urol. 2004, 14, 568–572. [Google Scholar]
- Smit, S.G.; Heyns, C.F. Management of radiation cystitis. Nat. Rev. Urol. 2010, 7, 206–214. [Google Scholar] [CrossRef]
- Helissey, C.; Cavallero, S.; Brossard, C.; Dusaud, M.; Chargari, C.; Francois, S. Chronic Inflammation and Radiation-Induced Cystitis: Molecular Background and Therapeutic Perspectives. Cells 2020, 10, 21. [Google Scholar] [CrossRef]
- Duverge, L.; Castelli, J.; Lizee, T.; de Crevoisier, R.; Azria, D. Doses to organs at risk for conformational and stereotactic radiotherapy: Bladder. Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2017, 21, 597–603. [Google Scholar] [CrossRef]
- SFRO. Guide des Procedures de Radiotherapie Externe 2007. France: Société Française de Radiothérapie Oncologique. 2007. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2008-08/guide_de_rth_des_tumeurs_v7_complet.pdf (accessed on 25 December 2021).
- Devisetty, K.; Mell, L.K.; Salama, J.K.; Schomas, D.A.; Miller, R.C.; Jani, A.B.; Roeske, J.C.; Aydogan, B.; Chmura, S.J. A multi-institutional acute gastrointestinal toxicity analysis of anal cancer patients treated with concurrent intensity-modulated radiation therapy (IMRT) and chemotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2009, 93, 298–301. [Google Scholar] [CrossRef]
- Vieillot, S.; Fenoglietto, P.; Lemanski, C.; Moscardo, C.L.; Gourgou, S.; Dubois, J.B.; Ailleres, N.; Azria, D. IMRT for locally advanced anal cancer: Clinical experience of the Montpellier Cancer Center. Radiat. Oncol. 2012, 7, 45. [Google Scholar] [CrossRef]
- Kachnic, L.A.; Pugh, S.L.; Tai, P.; Smith, M.; Gore, E.; Shah, A.B.; Martin, A.G.; Kim, H.E.; Nabid, A.; Lawton, C.A. RTOG 0518: Randomized phase III trial to evaluate zoledronic acid for prevention of osteoporosis and associated fractures in prostate cancer patients. Prostate Cancer Prostatic Dis. 2013, 16, 382–386. [Google Scholar] [CrossRef]
- Upadhyay, R.; Carthikeyan, S.M.; Pathy, S. Radiation cystitis: A late effect of radiotherapy in a patient with cervical rhabdomyosarcoma. J. Cancer Res. Ther. 2021, 17, 580–583. [Google Scholar] [CrossRef]
- Zwaans, B.M.M.; Wegner, K.A.; Bartolone, S.N.; Vezina, C.M.; Chancellor, M.B.; Lamb, L.E. Radiation cystitis modeling: A comparative study of bladder fibrosis radio-sensitivity in C57BL/6, C3H, and BALB/c mice. Physiol. Rep. 2020, 8, e14377. [Google Scholar] [CrossRef]
- Stewart, F.A. Mechanism of bladder damage and repair after treatment with radiation and cytostatic drugs. Br. J. Cancer. Suppl. 1986, 7, 280–291. [Google Scholar]
- El-Benhawy, S.A.; Sadek, N.A.; Kamel, M.M.; Sharaf, A.M.; Abderhman, I.G.; Morsi, M.I.; Abobakr, A. Study the relationship of endothelial damage/dysfunction due to occupational exposure to low dose ionizing radiation versus high dose exposure during radiotherapy. Cancer Treat. Res. Commun. 2020, 25, 100215. [Google Scholar] [CrossRef]
- Parsons, C.L.; Stein, P.C.; Bidair, M.; Lebow, D. Abnormal sensitivity to intravesical potassium in interstitial cystitis and radiation cystitis. Neurourol. Urodyn. 1994, 13, 515–520. [Google Scholar] [CrossRef]
- Marks, L.B.; Carroll, P.R.; Dugan, T.C.; Anscher, M.S. The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1257–1280. [Google Scholar] [CrossRef]
- Zwaans, B.M.; Krueger, S.; Bartolone, S.N.; Chancellor, M.B.; Marples, B.; Lamb, L.E. Modeling of chronic radiation-induced cystitis in mice. Adv. Radiat. Oncol. 2016, 1, 333–343. [Google Scholar] [CrossRef][Green Version]
- Akiyama, Y.; Luo, Y.; Hanno, P.M.; Maeda, D.; Homma, Y. Interstitial cystitis/bladder pain syndrome: The evolving landscape, animal models and future perspectives. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2020, 27, 491–503. [Google Scholar] [CrossRef]
- Vanneste, B.G.L.; Van Limbergen, E.J.; Marcelissen, T.A.; van Roermund, J.G.H.; Lutgens, L.C.; Arnoldussen, C.; Lambin, P.; Oelke, M. Development of a Management Algorithm for Acute and Chronic Radiation Urethritis and Cystitis. Urol. Int. 2022, 106, 63–74. [Google Scholar] [CrossRef]
- Fajardo, L.F.; Berthrong, M. Radiation injury in surgical pathology. Part I. Am. J. Surg. Pathol. 1978, 2, 159–199. [Google Scholar] [CrossRef]
- Gowing, N.F. Congenital fibro-elastosis of the endocardium. J. Pathol. Bacteriol. 1953, 65, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.; Randhawa, V.; Maughan, R. The RBE for mouse bladders after irradiation with 1 to 8 fractions of d(4)+ Be neutrons. Br. J. Radiol. 1986, 59, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Pavy, J.J.; Denekamp, J.; Letschert, J.; Littbrand, B.; Mornex, F.; Bernier, J.; Gonzales-Gonzales, D.; Horiot, J.C.; Bolla, M.; Bartelink, H.; et al. Late effects toxicity scoring: The SOMA scale. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1995, 35, 11–15. [Google Scholar] [CrossRef]
- Droller, M.J.; Saral, R.; Santos, G. Prevention of cyclophosphamide-induced hemorrhagic cystitis. Urology 1982, 20, 256–258. [Google Scholar] [CrossRef]
- Brugieres, L.; Hartmann, O.; Travagli, J.P.; Benhamou, E.; Pico, J.L.; Valteau, D.; Kalifa, C.; Patte, C.; Flamant, F.; Lemerle, J. Hemorrhagic cystitis following high-dose chemotherapy and bone marrow transplantation in children with malignancies: Incidence, clinical course, and outcome. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1989, 7, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Bearman, S.I.; Appelbaum, F.R.; Buckner, C.D.; Petersen, F.B.; Fisher, L.D.; Clift, R.A.; Thomas, E.D. Regimen-related toxicity in patients undergoing bone marrow transplantation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1988, 6, 1562–1568. [Google Scholar] [CrossRef]
- Arthur, R.R.; Shah, K.V.; Baust, S.J.; Santos, G.W.; Saral, R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N. Engl. J. Med. 1986, 315, 230–234. [Google Scholar] [CrossRef]
- Tucker, S.L.; Dong, L.; Bosch, W.R.; Michalski, J.; Winter, K.; Mohan, R.; Purdy, J.A.; Kuban, D.; Lee, A.K.; Cheung, M.R.; et al. Late rectal toxicity on RTOG 94-06: Analysis using a mixture Lyman model. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1253–1260. [Google Scholar] [CrossRef]
- LENT SOMA scales for all anatomic sites. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1049–1091. [CrossRef]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. United States of America: National Cancer Institute, 27 November 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 25 December 2021).
- Henningsohn, L.; Wijkstrom, H.; Dickman, P.W.; Bergmark, K.; Steineck, G. Distressful symptoms after radical radiotherapy for urinary bladder cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2002, 62, 215–225. [Google Scholar] [CrossRef]
- Soytas, M.; Kactan, C.; Guven, S. Recurrent bladder cystitis: Who takes the role? World J. Urol. 2020, 38, 2755–2760. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, C.; Hakimi, Z.; Zur, R.; Siddiqui, E.; Maman, K.; Aballea, S.; Nazir, J.; Chapple, C. Efficacy and Tolerability of Mirabegron Compared with Antimuscarinic Monotherapy or Combination Therapies for Overactive Bladder: A Systematic Review and Network Meta-analysis. Eur. Urol. 2018, 74, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Villeirs, L.; Tailly, T.; Ost, P.; Waterloos, M.; Decaestecker, K.; Fonteyne, V.; Van Praet, C.; Lumen, N. Hyperbaric oxygen therapy for radiation cystitis after pelvic radiotherapy: Systematic review of the recent literature. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2020, 27, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Browne, C.; Davis, N.F.; Mac Craith, E.; Lennon, G.M.; Mulvin, D.W.; Quinlan, D.M.; Mc Vey, G.P.; Galvin, D.J. A Narrative Review on the Pathophysiology and Management for Radiation Cystitis. Adv. Urol. 2015, 2015, 346812. [Google Scholar] [CrossRef] [PubMed]
- Dautruche, A.; Delouya, G. A contemporary review about the management of radiation-induced hemorrhagic cystitis. Curr. Opin. Supportive Palliat. Care 2018, 12, 344–350. [Google Scholar] [CrossRef]
- Pascoe, C.; Duncan, C.; Lamb, B.W.; Davis, N.F.; Lynch, T.H.; Murphy, D.G.; Lawrentschuk, N. Current management of radiation cystitis: A review and practical guide to clinical management. BJU Int. 2019, 123, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Sanguedolce, F.; Sancho Pardo, G.; Mercade Sanchez, A.; Balana Lucena, J.; Pisano, F.; Cortez, J.C.; Territo, A.; Huguet Perez, J.; Gaya Sopena, J.; Esquina Lopez, C.; et al. Radiation-induced haemorrhagic cystitis after prostate cancer radiotherapy: Factors associated to hospitalization and treatment strategies. Prostate Int. 2021, 9, 48–53. [Google Scholar] [CrossRef]
- Haldar, S.; Dru, C.; Bhowmick, N.A. Mechanisms of hemorrhagic cystitis. Am. J. Clin. Exp. Urol. 2014, 2, 199–208. [Google Scholar]
- Choong, S.K.; Walkden, M.; Kirby, R. The management of intractable haematuria. BJU Int. 2000, 86, 951–959. [Google Scholar] [CrossRef]
- Seear, M.D.; Dimmick, J.E.; Rogers, P.C. Acute aluminum toxicity after continuous intravesical alum irrigation for hemorrhagic cystitis. Urology 1990, 36, 353–354. [Google Scholar] [CrossRef]
- Koc, S.; Hagglund, H.; Ireton, R.C.; Perez-Simon, J.A.; Collins, S.J.; Appelbaum, F.R. Successful treatment of severe hemorrhagic cystitis with cystectomy following matched donor allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2000, 26, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Ajith Kumar, S.; Prasanth, P.; Tripathi, K.; Ghosh, P. Hyperbaric oxygen—A new horizon in treating cyclophosphamide-induced hemorrhagic cystitis. Indian J. Urol. IJU J. Urol. Soc. India 2011, 27, 272–273. [Google Scholar] [CrossRef]
- Pasquier, D.; Hoelscher, T.; Schmutz, J.; Dische, S.; Mathieu, D.; Baumann, M.; Lartigau, E. Hyperbaric oxygen therapy in the treatment of radio-induced lesions in normal tissues: A literature review. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2004, 72, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Neheman, A.; Nativ, O.; Moskovitz, B.; Melamed, Y.; Stein, A. Hyperbaric oxygen therapy for radiation-induced haemorrhagic cystitis. BJU Int. 2005, 96, 107–109. [Google Scholar] [CrossRef]
- Heath, J.A.; Mishra, S.; Mitchell, S.; Waters, K.D.; Tiedemann, K. Estrogen as treatment of hemorrhagic cystitis in children and adolescents undergoing bone marrow transplantation. Bone Marrow Transplant. 2006, 37, 523–526. [Google Scholar] [CrossRef]
- Demesmay, K.; Tissot, E.; Bulabois, C.E.; Bertrand, M.A.; Racadot, E.; Woronoff-Lemsi, M.C.; Cahn, J.Y.; Deconinck, E. Factor XIII replacement in stem-cell transplant recipients with severe hemorrhagic cystitis: A report of four cases. Transplantation 2002, 74, 1190–1192. [Google Scholar] [CrossRef]
- Pihusch, M.; Bacigalupo, A.; Szer, J.; von Depka Prondzinski, M.; Gaspar-Blaudschun, B.; Hyveled, L.; Brenner, B. Recombinant activated factor VII in treatment of bleeding complications following hematopoietic stem cell transplantation. J. Thromb. Haemost. 2005, 3, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Ippoliti, C.; Przepiorka, D.; Mehra, R.; Neumann, J.; Wood, J.; Claxton, D.; Gajewski, J.; Khouri, I.; van Besien, K.; Andersson, B.; et al. Intravesicular carboprost for the treatment of hemorrhagic cystitis after marrow transplantation. Urology 1995, 46, 811–815. [Google Scholar] [CrossRef]
- Fanourgiakis, P.; Georgala, A.; Vekemans, M.; Triffet, A.; De Bruyn, J.M.; Duchateau, V.; Martiat, P.; De Clercq, E.; Snoeck, R.; Wollants, E.; et al. Intravesical instillation of cidofovir in the treatment of hemorrhagic cystitis caused by adenovirus type 11 in a bone marrow transplant recipient. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 40, 199–201. [Google Scholar] [CrossRef]
- Bridges, B.; Donegan, S.; Badros, A. Cidofovir bladder instillation for the treatment of BK hemorrhagic cystitis after allogeneic stem cell transplantation. Am. J. Hematol. 2006, 81, 535–537. [Google Scholar] [CrossRef]
- Linder, B.J.; Tarrell, R.F.; Boorjian, S.A. Cystectomy for refractory hemorrhagic cystitis: Contemporary etiology, presentation and outcomes. J. Urol. 2014, 192, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, J.; Slade, A.; McFarland, M.; Keihani, S.; Hotaling, J.N.; Myers, J.B. Scoping Review and Meta-analysis of Hyperbaric Oxygen Therapy for Radiation-Induced Hemorrhagic Cystitis. Curr. Urol. Rep. 2018, 19, 38. [Google Scholar] [CrossRef] [PubMed]
- Stewart, F.A.; Michael, B.D.; Denekamp, J. Late radiation damage in the mouse bladder as measured by increased urination frequency. Radiat. Res. 1978, 75, 649–659. [Google Scholar] [CrossRef]
- Stewart, F.A.; Denekamp, J.; Hirst, D.G. Proliferation kinetics of the mouse bladder after irradiation. Cell Tissue Kinet. 1980, 13, 75–89. [Google Scholar] [CrossRef]
- Stewart, F.A.; Randhawa, V.S.; Michael, B.D.; Denekamp, J. Repair during fractionated irradiation of the mouse bladder. Br. J. Radiol. 1981, 54, 799–804. [Google Scholar] [CrossRef]
- Stewart, F.A.; Randhawa, V.S.; Michael, B.D. Multifraction irradiation of mouse bladders. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1984, 2, 131–140. [Google Scholar] [CrossRef]
- Stewart, F.A. The proliferative and functional response of mouse bladder to treatment with radiation and cyclophosphamide. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1985, 4, 353–362. [Google Scholar] [CrossRef]
- Stewart, F.A.; Lundbeck, F.; Oussoren, Y.; Luts, A. Acute and late radiation damage in mouse bladder: A comparison of urination frequency and cystometry. Int. J. Radiat. Oncol. Biol. Phys. 1991, 21, 1211–1219. [Google Scholar] [CrossRef]
- Kohler, M.; Eppenberger, H.M.; Cordt-Riehle, I.; Michel, C. Urination frequency and cystic pressure resistance after fractionated whole or partial irradiation of the rabbit urinary bladder. Acta Oncol. 1992, 31, 673–677. [Google Scholar] [CrossRef]
- Kohler, M.; Michel, C.; Zimmermann, A. Histological changes after fractionated whole or partial irradiation of the rabbit urinary bladder. Acta Oncol. 1995, 34, 199–204. [Google Scholar] [CrossRef]
- Post, J.G.; te Poele, J.A.; Oussoren, Y.G.; Stewart, F.A. Radiation tolerance of normal mouse bladders after intravesical chemotherapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1995, 34, 30–38. [Google Scholar] [CrossRef]
- Kraft, M.; Oussoren, Y.; Stewart, F.A.; Dorr, W.; Schultz-Hector, S. Radiation-induced changes in transforming growth factor beta and collagen expression in the murine bladder wall and its correlation with bladder function. Radiat. Res. 1996, 146, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Jaal, J.; Dorr, W. Radiation induced inflammatory changes in the mouse bladder: The role of cyclooxygenase-2. J. Urol. 2006, 175, 1529–1533. [Google Scholar] [CrossRef]
- Jaal, J.; Dorr, W. Radiation-induced damage to mouse urothelial barrier. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2006, 80, 250–256. [Google Scholar] [CrossRef]
- Jaal, J.; Dorr, W. Radiation induced late damage to the barrier function of small blood vessels in mouse bladder. J. Urol. 2006, 176, 2696–2700. [Google Scholar] [CrossRef] [PubMed]
- Soler, R.; Vianello, A.; Fullhase, C.; Wang, Z.; Atala, A.; Soker, S.; Yoo, J.J.; Koudywilliam, J. Vascular therapy for radiation cystitis. Neurourol. Urodyn. 2011, 30, 428–434. [Google Scholar] [CrossRef]
- Ikeda, Y.; Zabbarova, I.V.; Birder, L.A.; Wipf, P.; Getchell, S.E.; Tyagi, P.; Fry, C.H.; Drake, M.J.; Kanai, A.J. Relaxin-2 therapy reverses radiation-induced fibrosis and restores bladder function in mice. Neurourol. Urodyn. 2018, 37, 2441–2451. [Google Scholar] [CrossRef]
- Lane, Z.; Epstein, J.I. Pseudocarcinomatous epithelial hyperplasia in the bladder unassociated with prior irradiation or chemotherapy. Am. J. Surg. Pathol. 2008, 32, 92–97. [Google Scholar] [CrossRef]
- Kreft, M.E.; Jezernik, K.; Kreft, M.; Romih, R. Apical plasma membrane traffic in superficial cells of bladder urothelium. Ann. N. Y. Acad. Sci. 2009, 1152, 18–29. [Google Scholar] [CrossRef]
- Kreft, M.E.; Hudoklin, S.; Jezernik, K.; Romih, R. Formation and maintenance of blood-urine barrier in urothelium. Protoplasma 2010, 246, 3–14. [Google Scholar] [CrossRef]
- Varley, C.L.; Stahlschmidt, J.; Smith, B.; Stower, M.; Southgate, J. Activation of peroxisome proliferator-activated receptor-gamma reverses squamous metaplasia and induces transitional differentiation in normal human urothelial cells. Am. J. Pathol. 2004, 164, 1789–1798. [Google Scholar] [CrossRef]
- Varley, C.L.; Stahlschmidt, J.; Lee, W.C.; Holder, J.; Diggle, C.; Selby, P.J.; Trejdosiewicz, L.K.; Southgate, J. Role of PPARgamma and EGFR signalling in the urothelial terminal differentiation programme. J. Cell Sci. 2004, 117, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.T. Altered phenotype of cultured urothelial and other stratified epithelial cells: Implications for wound healing. Am. J. Physiol. Ren. Physiol. 2006, 291, F9–F21. [Google Scholar] [CrossRef] [PubMed]
- Marilyn, P. Law. Radiation-Induced Vascular Injury and Its Relation to Late Effects in Normal Tissues. Adv. Radiat. Biol. 1991, 9, 37–73. [Google Scholar] [CrossRef]
- Milliat, F.; Francois, A.; Tamarat, R.; Benderitter, M. Role of endothelium in radiation-induced normal tissue damages. Ann. Cardiol. Angeiol. 2008, 57, 139–148. [Google Scholar] [CrossRef]
- Milliat, F.; Francois, A.; Isoir, M.; Deutsch, E.; Tamarat, R.; Tarlet, G.; Atfi, A.; Validire, P.; Bourhis, J.; Sabourin, J.C.; et al. Influence of endothelial cells on vascular smooth muscle cells phenotype after irradiation: Implication in radiation-induced vascular damages. Am. J. Pathol. 2006, 169, 1484–1495. [Google Scholar] [CrossRef]
- Richter, K.K.; Fink, L.M.; Hughes, B.M.; Shmaysani, H.M.; Sung, C.C.; Hauer-Jensen, M. Differential effect of radiation on endothelial cell function in rectal cancer and normal rectum. Am. J. Surg. 1998, 176, 642–647. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, H.; Ou, X.; Fink, L.M.; Hauer-Jensen, M. Deficiency of microvascular thrombomodulin and up-regulation of protease-activated receptor-1 in irradiated rat intestine: Possible link between endothelial dysfunction and chronic radiation fibrosis. Am. J. Pathol. 2002, 160, 2063–2072. [Google Scholar] [CrossRef]
- Guipaud, O.; Jaillet, C.; Clement-Colmou, K.; Francois, A.; Supiot, S.; Milliat, F. The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. Br. J. Radiol. 2018, 91, 20170762. [Google Scholar] [CrossRef]
- Verheij, M.; Dewit, L.G.; van Mourik, J.A. The effect of ionizing radiation on endothelial tissue factor activity and its cellular localization. Thromb. Haemost. 1995, 73, 894–895. [Google Scholar] [CrossRef]
- Verheij, M.; Dewit, L.; van Mourik, J.A. Radiation-induced von Willebrand factor release. Blood 1997, 90, 2109–2110. [Google Scholar] [CrossRef]
- Rubin, D.B.; Drab, E.A.; Ts’ao, C.H.; Gardner, D.; Ward, W.F. Prostacyclin synthesis in irradiated endothelial cells cultured from bovine aorta. J. Appl. Physiol. 1985, 58, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhao, Y.; Li, P.; Bai, X.; Ruan, C. Thrombomodulin as a marker of radiation-induced endothelial cell injury. Radiat. Res. 1992, 131, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Kempuraj, D.; Sant, G.R. Mast cell involvement in interstitial cystitis: A review of human and experimental evidence. Urology 2001, 57, 47–55. [Google Scholar] [CrossRef]
- He, S.; Walls, A.F. Human mast cell tryptase: A stimulus of microvascular leakage and mast cell activation. Eur. J. Pharmacol. 1997, 328, 89–97. [Google Scholar] [CrossRef]
- Mühlstädt, S.; Mohammed, N.; Weigand, K.; Schumann, A.; Kawan, F.; Göllert, C.; Fornara, P. Radiogen bedingte Zystitis. Der Urol. 2017, 56, 301–305. [Google Scholar] [CrossRef]
- Kawamoto, K.; Noguchi, S.; Sakuramoto, T.; Shyuin, T.; Noguchi, K.; Kinoshita, Y.; Kubota, Y.; Hosaka, M. Clinical analysis of 46 cases of radiation cystitis. Hinyokika Kiyo. Acta Urol. Jpn. 1992, 38, 395–398. [Google Scholar]
- Gh, D.; Kong, D.; Gautrot, J.; Vootla, S.K. Fabrication and Characterization of Conductive Conjugated Polymer-Coated Antheraea mylitta Silk Fibroin Fibers for Biomedical Applications. Macromol. Biosci. 2017, 17, 1600443. [Google Scholar] [CrossRef]
- Yahyapour, R.; Motevaseli, E.; Rezaeyan, A.; Abdollahi, H.; Farhood, B.; Cheki, M.; Najafi, M.; Villa, V. Mechanisms of Radiation Bystander and Non-Targeted Effects: Implications to Radiation Carcinogenesis and Radiotherapy. Curr. Radiopharm. 2018, 11, 34–45. [Google Scholar] [CrossRef]
- Najafi, M.; Shirazi, A.; Motevaseli, E.; Rezaeyan, A.H.; Salajegheh, A.; Rezapoor, S. Melatonin as an anti-inflammatory agent in radiotherapy. Inflammopharmacology 2017, 25, 403–413. [Google Scholar] [CrossRef]
- Najafi, M.; Shirazi, A.; Motevaseli, E.; Geraily, G.; Norouzi, F.; Heidari, M.; Rezapoor, S. The melatonin immunomodulatory actions in radiotherapy. Biophys. Rev. 2017, 9, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Fardid, R.; Salajegheh, A.; Mosleh-Shirazi, M.A.; Sharifzadeh, S.; Okhovat, M.A.; Najafi, M.; Rezaeyan, A.; Abaszadeh, A. Melatonin Ameliorates the Production of COX-2, iNOS, and the Formation of 8-OHdG in Non-Targeted Lung Tissue after Pelvic Irradiation. Cell J. 2017, 19, 324–331. [Google Scholar] [CrossRef]
- Peeker, R.; Haghsheno, M.A.; Holmang, S.; Fall, M. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis: A prospective, randomized double-blind study. J. Urol. 2000, 164, 1912–1916. [Google Scholar] [CrossRef]
- Parsons, C.L.; Zupkas, P.; Parsons, J.K. Intravesical potassium sensitivity in patients with interstitial cystitis and urethral syndrome. Urology 2001, 57, 428–432. [Google Scholar] [CrossRef]
- Sant, G.R.; Kempuraj, D.; Marchand, J.E.; Theoharides, T.C. The mast cell in interstitial cystitis: Role in pathophysiology and pathogenesis. Urology 2007, 69, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Chen, K.H.; Sung, P.H.; Yang, C.C.; Cheng, B.C.; Chen, C.H.; Chang, C.L.; Sheu, J.J.; Lee, F.Y.; Shao, P.L.; et al. Extracorporeal shock wave markedly alleviates radiation-induced chronic cystitis in rat. Am. J. Transl. Res. 2018, 10, 1036–1052. [Google Scholar] [PubMed]
- Chen, Y.L.; Lin, Y.P.; Sun, C.K.; Huang, T.H.; Yip, H.K.; Chen, Y.T. Extracorporeal shockwave against inflammation mediated by GPR120 receptor in cyclophosphamide-induced rat cystitis model. Mol. Med. 2018, 24, 60. [Google Scholar] [CrossRef]
- Chen, Y.T.; Yang, C.C.; Sung, P.H.; Lin, K.C.; Chiang, J.Y.; Huang, C.R.; Huang, K.H.; Chuang, F.C.; Chu, Y.C.; Huang, E.Y.; et al. Long-term effect of extracorporeal shock wave therapy on attenuating radiation-induced chronic cystitis in rat. Am. J. Transl. Res. 2020, 12, 999–1015. [Google Scholar]
- Durand, C.; Pezet, S.; Eutamene, H.; Demarquay, C.; Mathieu, N.; Moussa, L.; Daudin, R.; Holler, V.; Sabourin, J.C.; Milliat, F.; et al. Persistent visceral allodynia in rats exposed to colorectal irradiation is reversed by mesenchymal stromal cell treatment. Pain 2015, 156, 1465–1476. [Google Scholar] [CrossRef]
- Giglio, D.; Podmolikova, L.; Tobin, G. Changes in the Neuronal Control of the Urinary Bladder in a Model of Radiation Cystitis. J. Pharmacol. Exp. Ther. 2018, 365, 327–335. [Google Scholar] [CrossRef]
- Koh, S.D.; Lee, H.; Ward, S.M.; Sanders, K.M. The Mystery of the Interstitial Cells in the Urinary Bladder. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 603–623. [Google Scholar] [CrossRef] [PubMed]
- Steineck, G.; Bull, C.; Kalm, M.; Sjoberg, F.; Alevronta, E.; Malipatlolla, D.K.; Bergmark, K.; Jeppsson, B.; Wilderang, U.; Bjork-Eriksson, T. Radiation physiology—Evidence for a higher biological effect of 24 Gy in four fractions as compared to three. Acta Oncol. 2017, 56, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
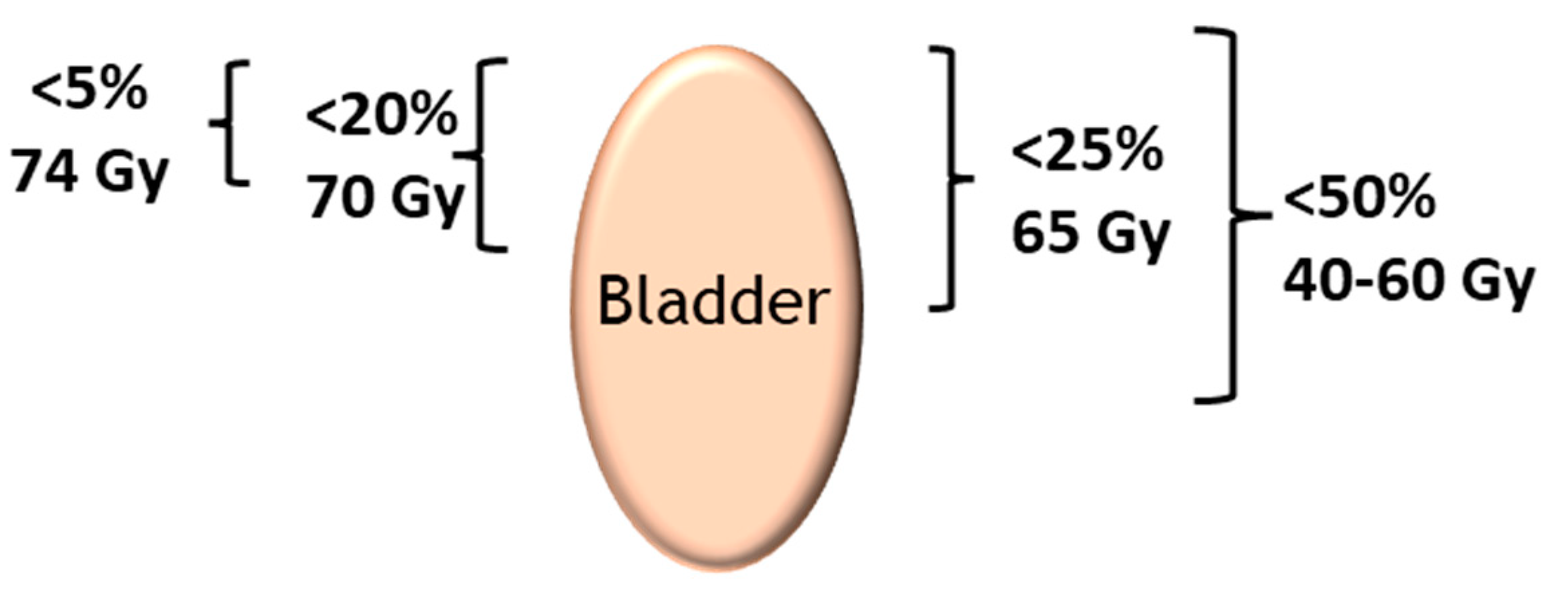
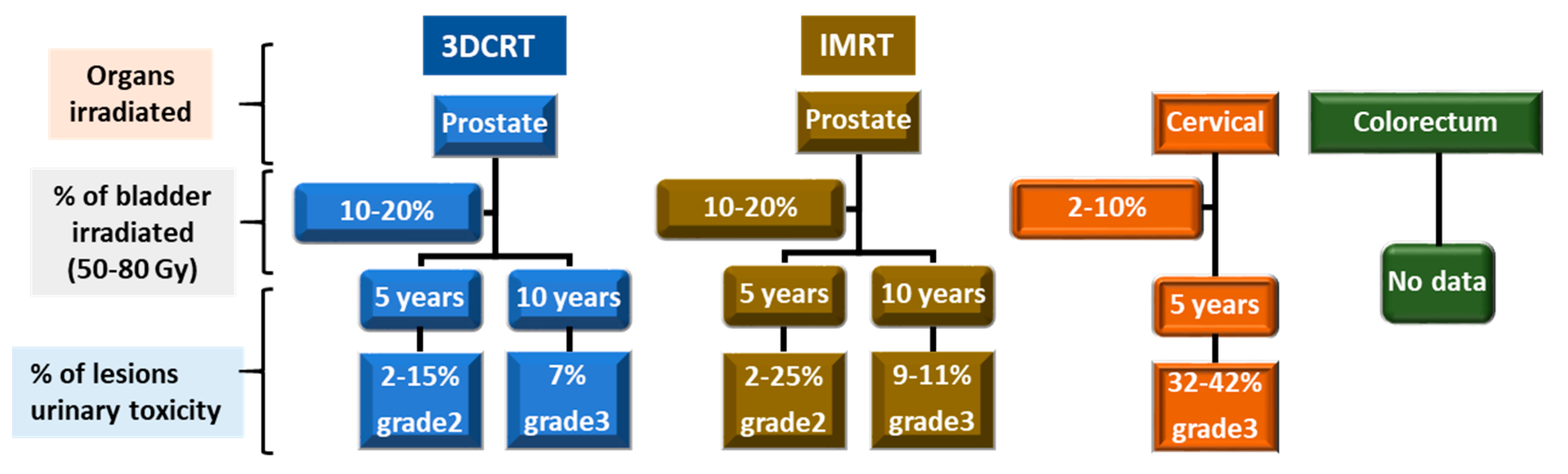
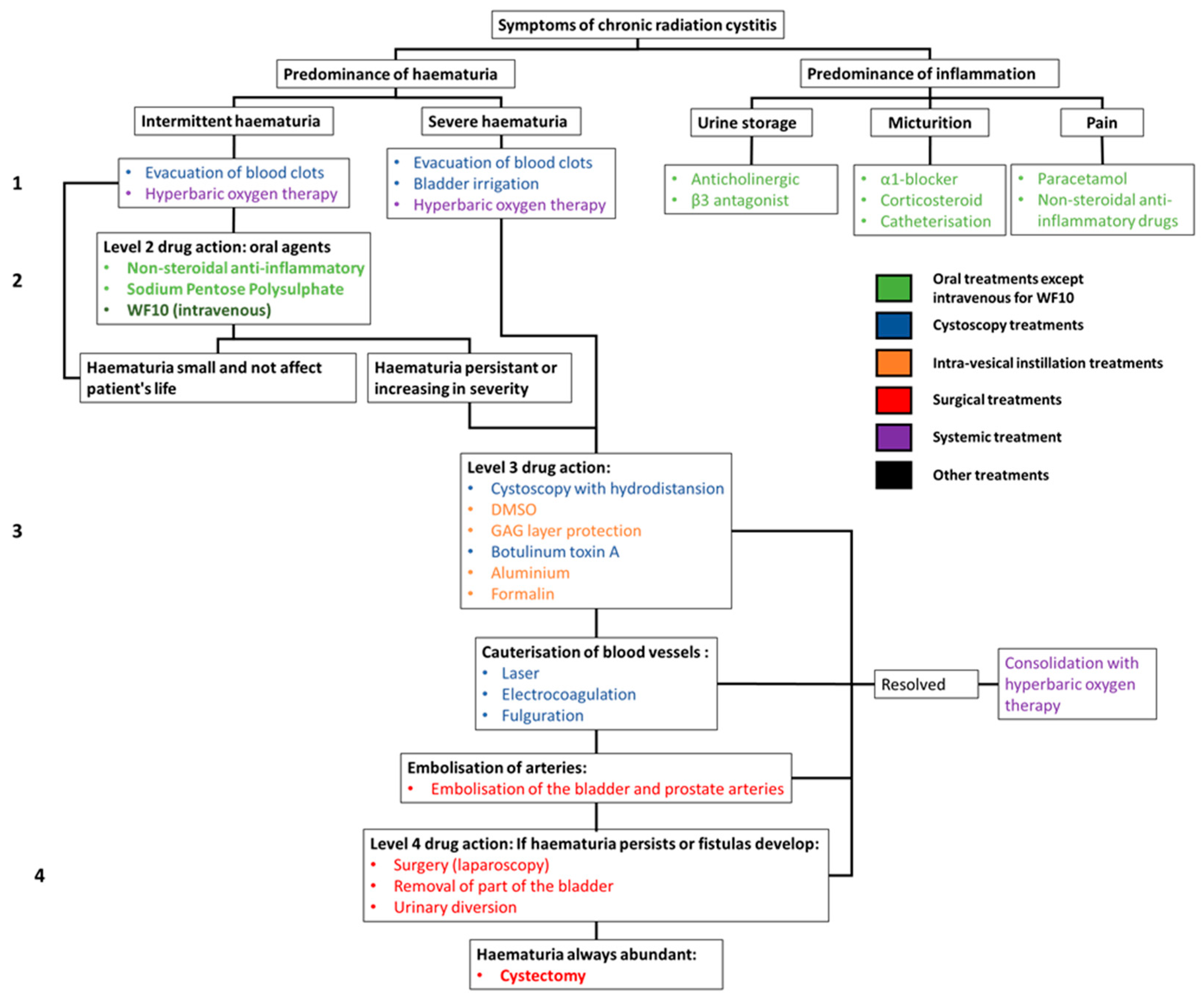


| Organ Treated by Radiotherapy | Organ at Risk |
|---|---|
| Rectum | Small intestine |
| Anal canal | Small intestine, bladder, vulva, labia majora |
| Cervix | Rectum, bladder, anal canal, small intestine, sigmoid colon, vagina |
| Endometrium | Rectum, bladder, anal canal, small intestine, sigmoid colon, vagina |
| Vulva | Rectum, bladder, urethra, anal canal, small intestine, sigmoid |
| Prostate | Rectum, bladder, anal canal |
| Bladder | Rectum, anal canal, small intestine, sigmoid colon |
| Testis | Spinal cord, kidney, stomach, small intestine, colon |
| Grade | CTCAE v5.0 [33] | RTOG/EORTC [26] | LENT-SOMA [32] |
|---|---|---|---|
| I | Minimal or microscopic bleeding intervention not indicated | Slight epithelial atrophy Minor telangiectasia (microscopic hematuria) | Hematuria occasional and microscopic Dysuria occasional Incontinence weekly episodes |
| II | Gross bleeding, medical intervention or urinary tract irrigation indicated | Moderate frequency Generalized telangiectasia Intermittent macroscopic hematuria | Hematuria intermittent, macroscopic and <10% decrease hemoglobin Dysuria intermittent Incontinence daily episodes |
| III | Transfusion, interventional radiology, endoscopic or operative intervention indicated, radiation therapy (i.e., hemostasis of bleeding site) | Severe frequency and dysuria Severe generalized telangiectasia (often with petechiae) Frequent hematuria Reduction in bladder capacity (<150 cc) | Hematuria persistent, macroscopic and 10–20% decrease hemoglobin Dysuria persistent and intense Incontinence daily episodes |
| IV | Life-threatening consequences, major urgent intervention indicated | Necrosis/Contracted bladder (capacity <100 cc) Severe hemorrhagic cystitis | Hematuria persistent, >20% decrease hemoglobin Dysuria refractory and excruciating Incontinence complete refractory |
| Model | Dose | Irradiation Source | Study Time | Main Characteristic Studied | Method Used | Main Results | Ref |
|---|---|---|---|---|---|---|---|
| CBA Female Mouse | Single dose from 10 to 40 Gy (5 Gy increments) | Linear accelerator (1.8 MeV, 75 pulses/sec). 112.5 Gy/min | Monthly (for 16 months) | Miction | Metabolic Cage | Increase in urinary frequency with the irradiation dose | [57] |
| CBA Female Mouse | Single dose from 10 to 40 Gy (5 Gy increments) | Linear accelerator (1.8 MeV, 75 pulses/sec). 112.5 Gy/min | 3, 7, 9, 12, 19 and 22 months | Urothelium regeneration Endothelium regeneration | Tritium marking Tritium marking | Increase in urothelium regeneration speed between 6 and 22 months inversely proportional to the dose Dose-dependent increase in endothelium regeneration rate between 6 and 22 months. | [58] |
| CBA Female Mouse | Single or fraction dose: 2 (24 h) and 5 fractions (4 days) Single dose: 15 to 32.5 Gy (2.5 Gy increments) Dose for 2 fractions: 22.5 to 37.5 Gy (5 Gy increments)Dose for 5 fractions: 30 to 55 Gy (5 Gy increments) | Linear accelerator (1.8 MeV, 75 pulses/sec). 112.5 Gy/min | 6 to 15 months (monthly) | Miction Bladder capacity | Metabolic Cage | Increase in urinary frequency with the irradiation dose, but decrease in urinary frequency with the number of fractions from 12 months onwards. Progressive reduction of bladder capacity proportional to dose and increase of capacity proportional to the number of fractionations starting at 12 months. | [59] |
| Female mouse CBA/Ht Gy f BSVS | Fractionation: 1, 5, 10 or 20 fractions (equal dose per fraction spread over 1 to 2 weeks (condition 20 fractions = 2 fractions per day separated by 5 h)) Irradiation dose for 1 fraction: 20, 25 and 30 Gy. For 5 fractions: 35, 45, 55 and 60 Gy. For 10 fractions: 45, 55, 65 and 75 Gy. For 20 fractions: 50 to 90 Gy (increment of 10 Gy). | Linear accelerator (1.8 MeV, 75 pulses/sec). 112.5 Gy/min | 9, 10, 11, 13, 14 months | Miction Bladder capacity | Metabolic cage | Increase in urinary frequency with the irradiation dose, but decrease in urinary frequency with the number of fractions from 10 months onwards Progressive reduction of bladder capacity proportional to dose and increase of capacity proportional to the number of fractionations starting at 10 months. | [60] |
| Female mouse CBA/Ht Gy f BSVS | Electron: 1 fraction: 17.5, 22.5, 25, 30 Gy 2 fractions: 22.5, 27.5, 32.5, 37.5 Gy; 4 fractions: 35, 45, 55 Gy 8 fractions: 35, 45, 55, 65 Gy Neutron: 1 fraction: 7, 8, 9, 10 Gy 2 fractions: 7.5, 9, 11, 12.5 Gy 4 fractions: 8, 10, 12, 14 Gy 8 fractions: 8, 10, 12, 14, 16 Gy | 1.8 MeV electron linear accelerator or 3 MeV neutron linear accelerator | 9 to 14 months (monthly) | Miction | Metabolic cage | Increase in urinary frequency with the irradiation dose but decrease in urinary frequency with the number of fractions from 9 months onwards Irradiation with electron is less damaging. | [25] |
| Female mouse CBA/Ht Gy BSVS | Single dose from 15 to 32.5 (2.5 Gy increments) | Linear accelerator (1.8 MeV, 75 pulses/sec). 112.5 Gy/min | 16 months (monthly) | Miction Urothelium regeneration Endothelium regeneration | Metabolic cage Tritium marking Tritium marking | Increase in urinary frequency from 7 months and proportional to the irradiation dose Limited urothelium regeneration in the first 3 months after irradiation. Limited regeneration of the endothelium in the first 3 months after irradiation. | [61] |
| Female mouse C3H/Hen Af-nu+ | Single dose of 10, 15, 20, 25, 27.5 and 30 Gy | X-rays 250 kV, filtered at 0.5 mm Cu, at 15 mA. At 2.1 Gy/min | Day 5, 12, 19 then every 4 to 6 weeks (over 53 weeks) | Urinary frequency Max. bladder volume Urothelium | Metabolic cage Cystoscopy Histology | Increase in urinary frequency as a function of time (acute phase in the 1st month) and proportional to the dose. Decrease in max. bladder volume as a function of time (acute phase in the 1st month) and proportional to the dose Epithelial denudation and focal hyperplasia, with fibrosis and ulceration. | [62] |
| New Zealand male rabbit | Fractionated doses over 5 consecutive days (once a day) for a total of 33, 36 and 39 Gy. Irradiation of the entire bladder or half of the bladder on the cranial or caudal side. | X-ray of 300 kV 0.8 Gy/min (Seifert isovolt 3002; filtration: 1.0 mm Al) | 100 weeks | Miction | Metabolic cage | Increase in frequency of urination decrease in max. volume for 100 weeks for dose 39 Gy or 36 Gy (total bladder irradiation), and from 20 weeks onwards for irradiation on half of the bladder (39 Gy at the cranial part of the bladder and 39 Gy or 36 Gy at the caudal part) | [63] |
| New Zealand male rabbit | Fractionated doses over 5 consecutive days (once a day) for a total of 33, 36 and 39 Gy. Irradiation of the entire bladder or half of the bladder on the cranial or caudal side. | X-ray of 300 kV 0.8 Gy/min (Seifert isovolt 3002; filtration: 1.0 mm Al) | 100 weeks | Tissue alteration | Histology | Dose-dependent urothelial hyperplasia or atrophy of the urothelium and more pronounced in the body of the bladder and trigone, submucosal and muscular tissues showed dose-dependent fibrosis and changes in the blood and lymphatic vessels, the body and trigone are more sensitive to radiation (more fibrosis, changes in the vessels) | [64] |
| Female mouse C3H/Hen Af-nu+ | Single dose of 10, 15, 20, 22.5, and 25 Gy | X-ray 250 kV, filtered at 0.5 mm Cu, at 15 mA, 2.1 Gy/min | 1, 2 and 4 weeks after irradiation then monthly up to 61 weeks (irradiation in week 4 or 12) | Urinary frequency Volume per voiding Max. bladder volume | Absorbent paper (15 cm/h) Scanning tablet (Calcomp B.V., Amstelveen, The Netherlands) with special program Cystoscopy | Increased frequency in acute phase during the first 4 weeks, and late phase from 15 to 37 weeks (dose-dependent). Volume reduction per voiding between 52 and 56 weeks for 25 Gy. Decrease in total bladder volume as a function of the dose. | [65] |
| Female mouse C3H/Neu Female mouse C3H/Hen Af-nu+ | Single dose of 19 Gy Single dose of 25 Gy and 14 Gy. | Seifert Isovolt 320 300 kV, 5.3 Gy/min Seifert Isovolt 320 250 kV 2.1 Gy/min | 90 to 360 days | Bladder function Fibrosis Urothelium | Cystoscopy Immunohistochemistry Histology | Correlation between decrease in max. bladder volume and the expression of TGFβ and collagen, decrease in total bladder volume Increased tissue protein expression in TGFβ (in both strains) as a function of time and dose, increased collagen I and III from 180 days after irradiation, but decreased collagen I/III ratio in C3H/Hen Af-nu+ mice and increased in CH3/Neu mice Increased denudation or hyperplasia as a function of the dose, edema. | [66] |
| Female mouse C3H/Neu | Single dose of 20 Gy | Seifert Isovolt 320/20 X-ray machine (Seifert X-ray Corp., Fairview Village, PA) 200 kV, 0.85 Gy/min | 2, 4, 7, 10, 13, 16, 19, 22, 25, 28, 31 days (early), then on days 90, 120, 180, 240 and 360 (late) after irradiation. | Vascular | Immunohistochemistry | Increased COX-2 expression in the blood vessel wall during the precocious phase (4 to 16 days) but not in the late phase. | [67] |
| Female mouse C3H/Neu | Single dose of 20 Gy | Seifert Isovolt 320/20 X-ray machine (Seifert X-ray Corp., Fairview Village, PA) 200 kV, 0.85 Gy/min | 2, 4, 7, 10, 13, 16, 19, 22, 25, 28, 31 days (early), then on days 90, 120, 180, 240 and 360 (late) after irradiation. | Urothelium | Immunohistochemistry | Decrease in superficial cells in early phase (day 0 to 31) until the beginning of late phase (90 to 120 days), progressive loss of Uroplakine III expression at the cell surface correlated with the loss of superficial cells, but increase in cytoplasmic expression of Uroplakine III in superficial cells until the beginning of late phase (120 days) | [68] |
| Female mouse C3H/Neu | Single dose of 20 Gy | Seifert Isovolt 320/20 X-ray machine (Seifert X-ray Corp., Fairview Village, PA) 200 kV, 0.85 Gy/min | 90, 120, 180, 240 and 360 (late response phase) after irradiation | Fibrosis Vascular | Histology Immunohistology | Infiltration of albumin around small blood vessels in early and late phase Increase in collagen deposition in the bladder. | [69] |
| Lewis Rats female | Single dose of 20 Gy | Cesium irradiation (about 4 Gy/min) | 1.5 and 3 months | Fibrosis Vascular lesion | Histology Histology and transcriptomic | Increase in collagen at 3 months in muscle with fibrosis and overexpression of the TGF β gene at 3 months. Decrease in the number of blood vessels at 1.5 and 3 months, preferably in the dome, without increasing the expression of the VEGF gene. | [70] |
| Female mouse C3H/HeN | Single dose of 20 Gy | Small Animal Radiation Research Platform (SARRP), 2 Gy/min 220 kV and 13 mA | 19 weeks | Miction Fibrosis Urothelium Inflammation | Metabolic cage Histology, Immunohistology Histology | Increase in urinary frequency with decrease in volume per void. Increased fibrosis Decreased Upk III, E-cadherin and CD31 expression, hyperplasia. Increase of mast cells | [20] |
| Female mouse C57Bl/6 | Single dose of 10 Gy, external bladder | X-RAD 320 biological irradiator (Precision X-Ray, North Branford, CT), dose rate not given | 1, 2, 4, 6 and 9 and 12 weeks | Miction Bladder contraction Fibrosis | Whatman filter paper Electromyogram Histology | Increase in urinary frequency/incontinence 9 weeks after irradiation Decrease in contractility and compliance, increase in passive tension 9 weeks after irradiation. Increased collagen deposition (changes wall relaxation). | [71] |
| Female Mouse C57BL/6, C3H and BALB/c | Single dose of 40 Gy | Small Animal Radiation Research Platform (SARRP), 2 Gy/min 220 kV et 13 mA | 12 months | Miction Fibrosis Vascular | Metabolic Cage Histology, immunohistology Photos | Increased urinary frequency with decreased volume per void in C57BL/6 Increased fibrosis, collagen densification in C57BL/6 and BALB/c, increase in collagen I and III than in C57BL/6. Pale avascular lesions, hemorrhages and telangiectasias. | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brossard, C.; Lefranc, A.-C.; Simon, J.-M.; Benderitter, M.; Milliat, F.; Chapel, A. Understanding Molecular Mechanisms and Identifying Key Processes in Chronic Radiation Cystitis. Int. J. Mol. Sci. 2022, 23, 1836. https://doi.org/10.3390/ijms23031836
Brossard C, Lefranc A-C, Simon J-M, Benderitter M, Milliat F, Chapel A. Understanding Molecular Mechanisms and Identifying Key Processes in Chronic Radiation Cystitis. International Journal of Molecular Sciences. 2022; 23(3):1836. https://doi.org/10.3390/ijms23031836
Chicago/Turabian StyleBrossard, Clément, Anne-Charlotte Lefranc, Jean-Marc Simon, Marc Benderitter, Fabien Milliat, and Alain Chapel. 2022. "Understanding Molecular Mechanisms and Identifying Key Processes in Chronic Radiation Cystitis" International Journal of Molecular Sciences 23, no. 3: 1836. https://doi.org/10.3390/ijms23031836
APA StyleBrossard, C., Lefranc, A.-C., Simon, J.-M., Benderitter, M., Milliat, F., & Chapel, A. (2022). Understanding Molecular Mechanisms and Identifying Key Processes in Chronic Radiation Cystitis. International Journal of Molecular Sciences, 23(3), 1836. https://doi.org/10.3390/ijms23031836