Combined Impact of Magnetic Force and Spaceflight Conditions on Escherichia coli Physiology
Abstract
1. Introduction
2. Results
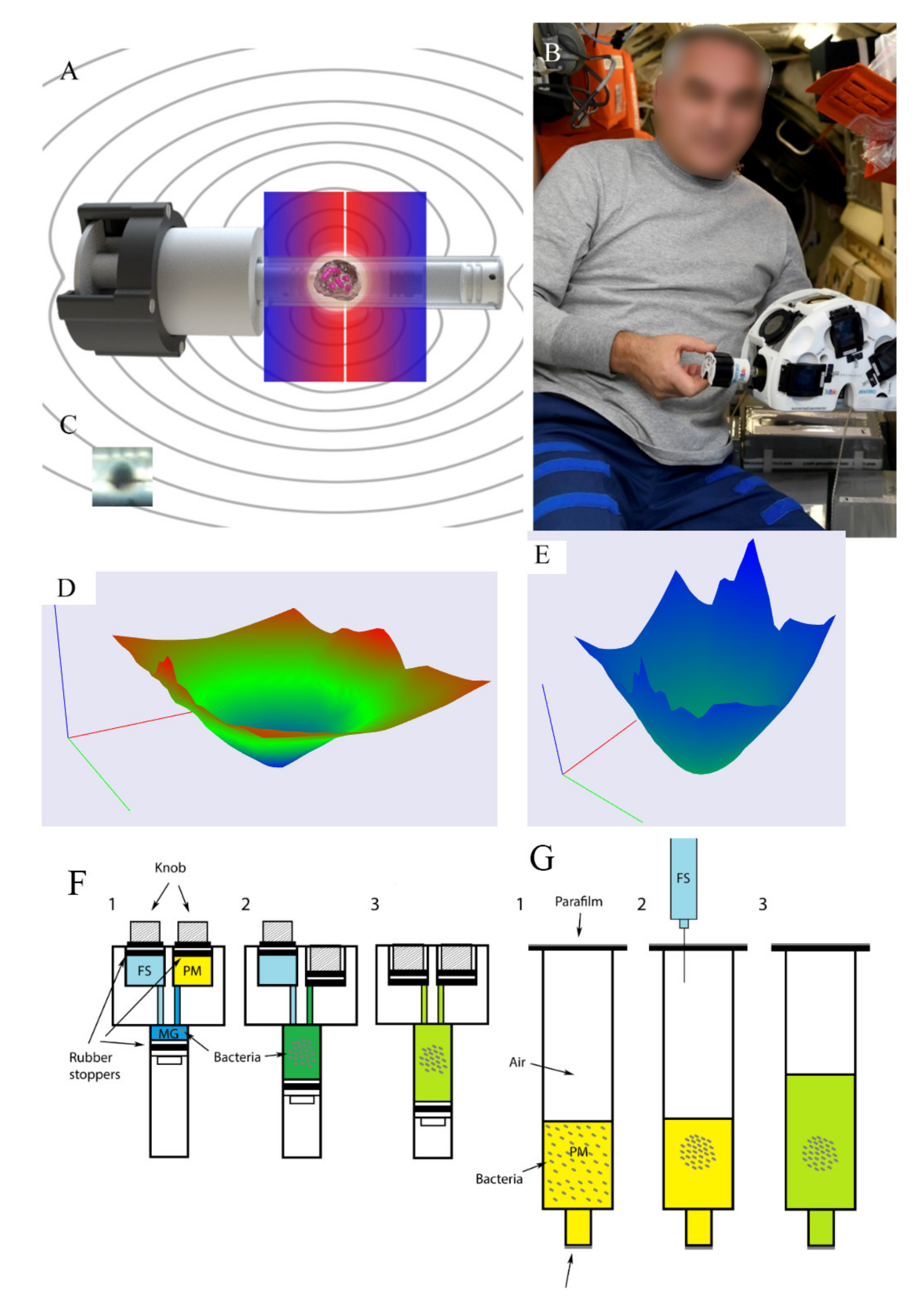
2.1. Experimental Design
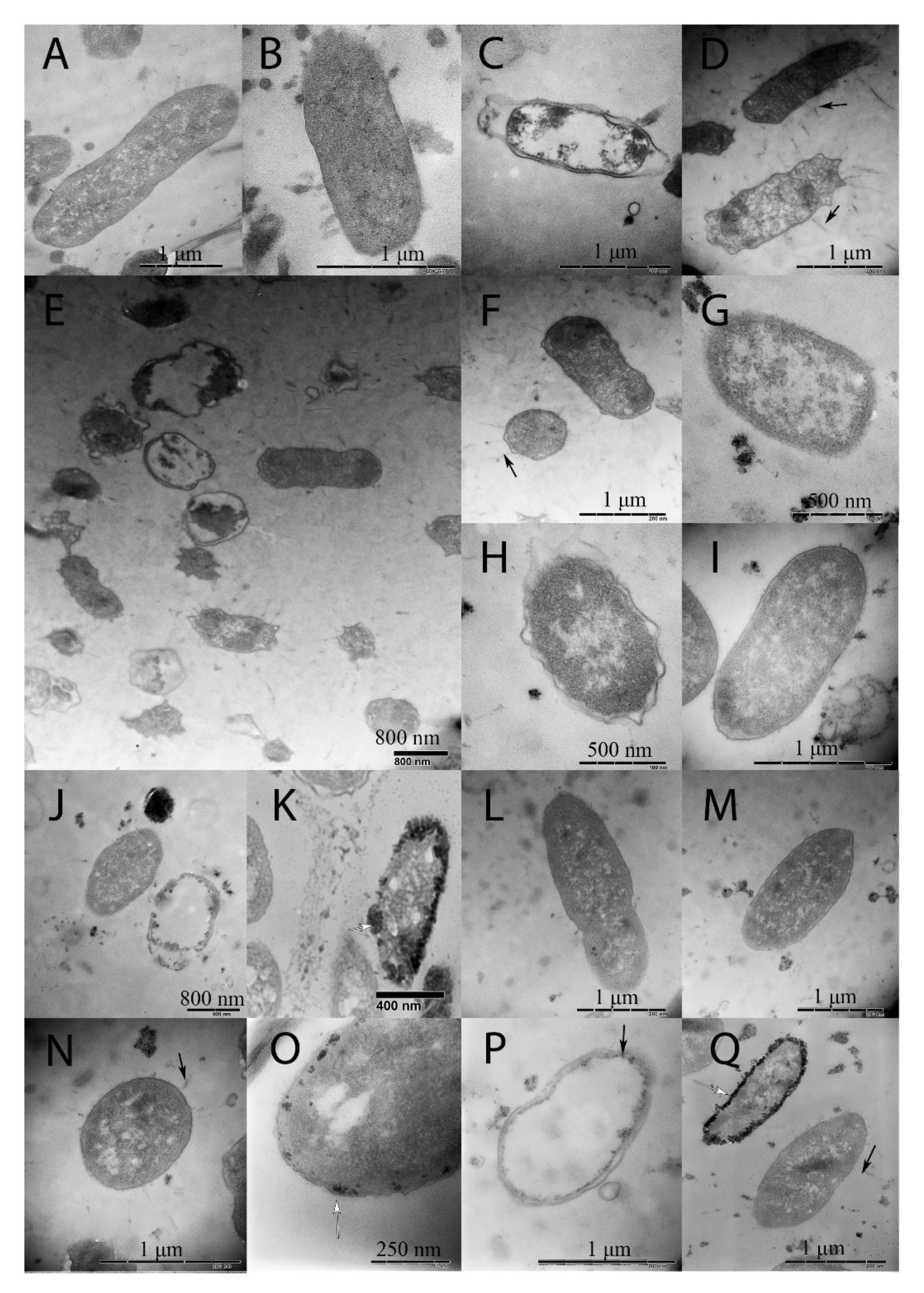
2.2. Changes in Morphology Caused by Spaceflight and a Magnetic Force
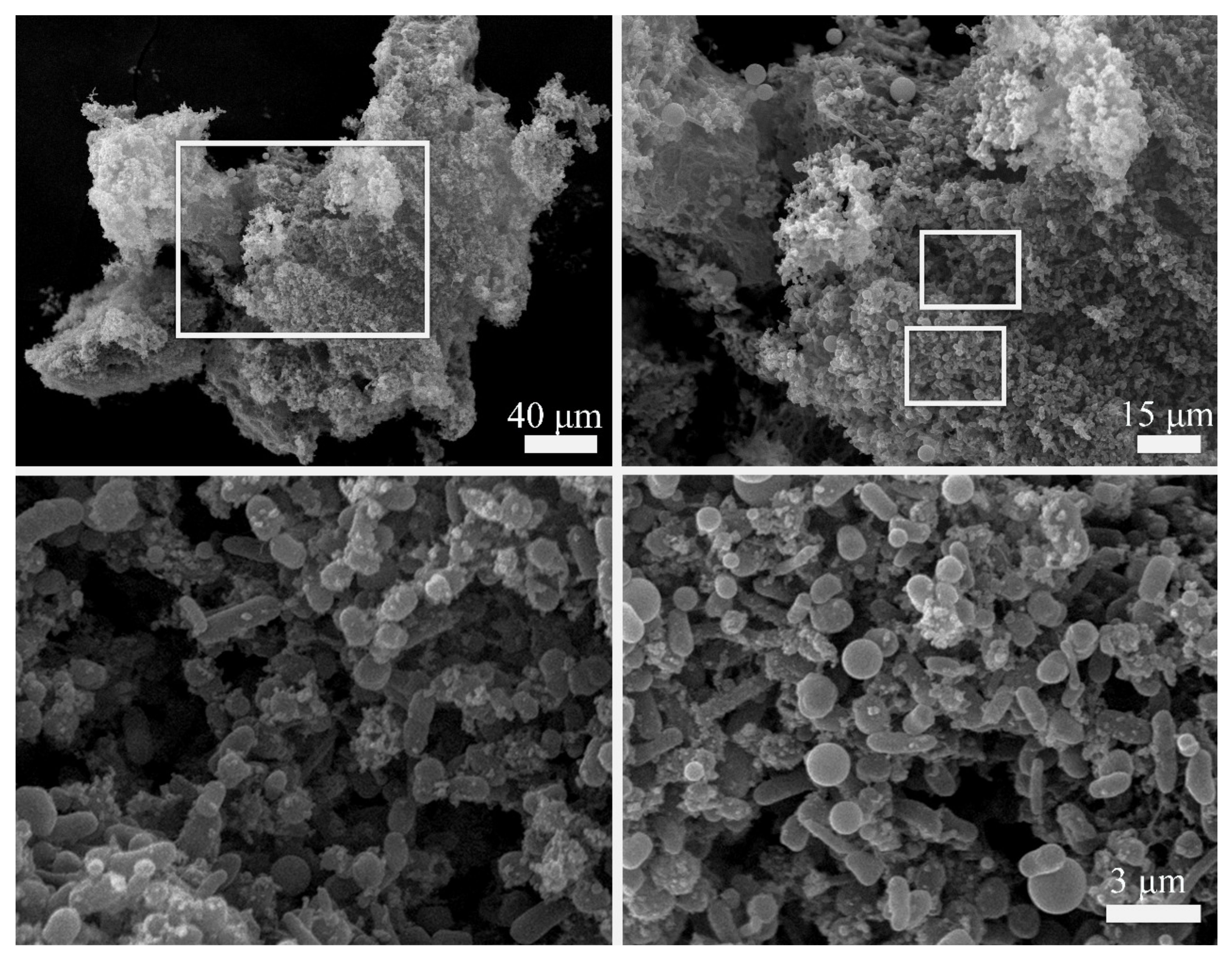
2.3. Aggregates Formed by Cells and Non-Cellular Matrix under SF + MF Conditions
2.4. Proteomic Studies
2.4.1. SF vs. Ground Control
2.4.2. SF + MF vs. Ground Control and SF
2.4.3. MF vs. Ground Control
2.4.4. SF vs. MF
3. Discussion
3.1. Reduced Bacterial Growth
3.2. Role of Cobalamin Outer Membrane Transporter BtuB for Prolonged Survival under SF, SF + MF, and Ground MF Conditions
3.3. Changes in Bacterial Sizes
3.4. Bacterial Autoaggregation
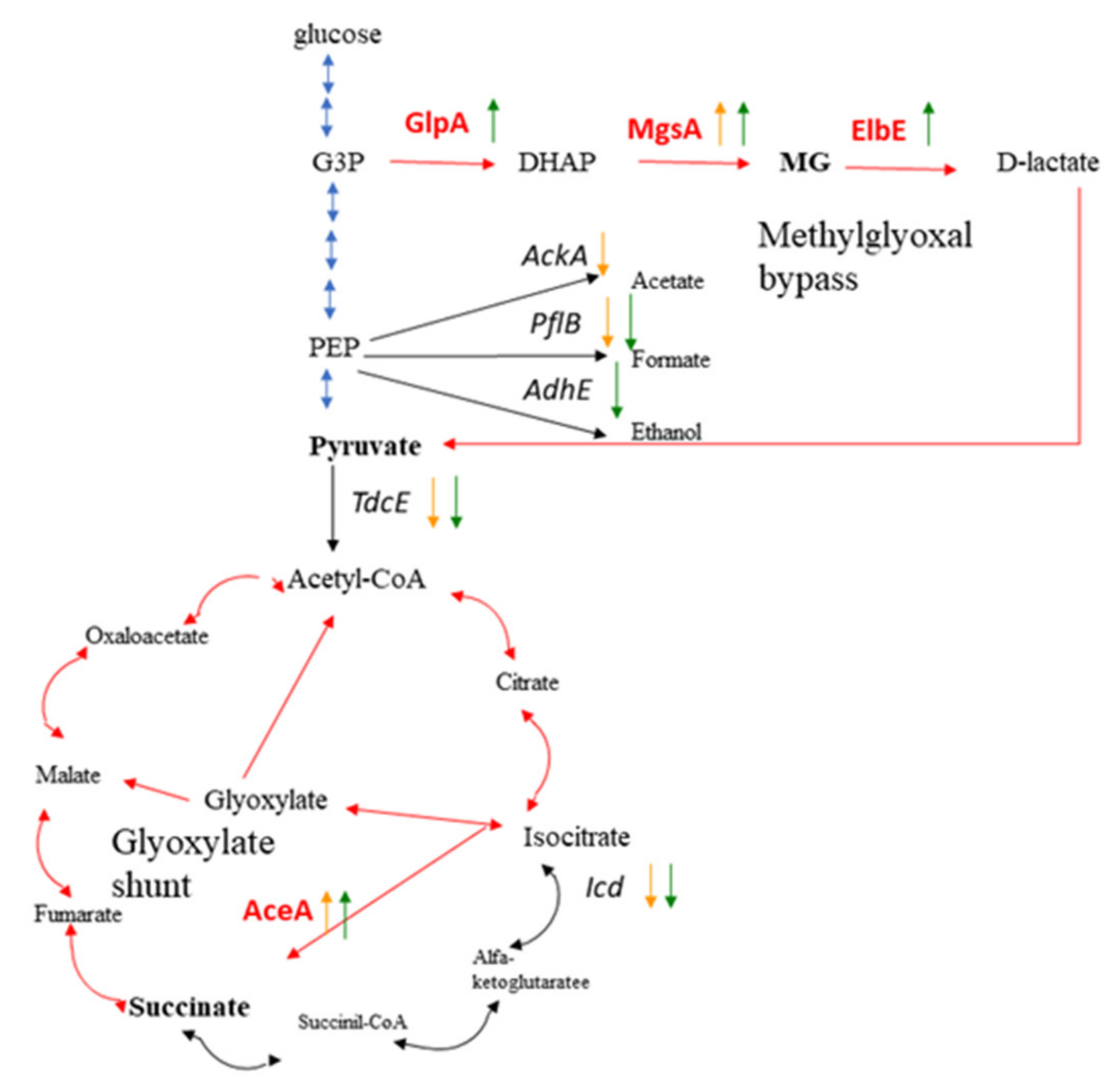
3.5. Role of Low Nutrient and Oxygen Availability and Carbohydrate Metabolism under Spaceflight Conditions
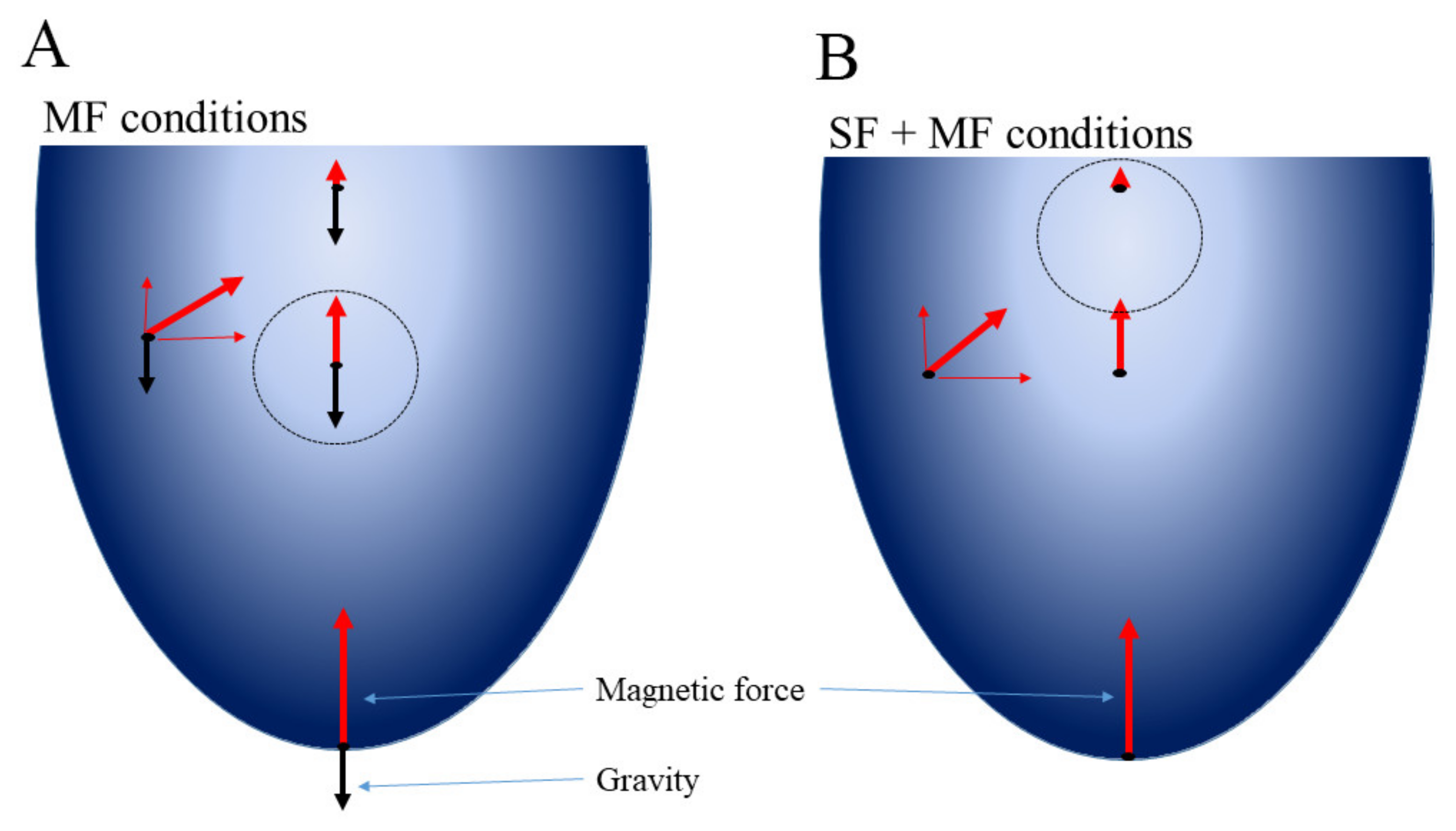
3.6. Effect of the Magnetic Force on Cell Response on Oxygen and Nutrient Limitations
3.7. Outer Membrane Vesicles, Heat-Shock Protein Downregulation, and Misfolded Protein Accumulation Stress Response
3.8. Role of the Autotransporter Ag43
4. Materials and Methods
4.1. Bacterial Strain
4.2. Spaceflight Experimental Settings
4.3. Ground Control Experimental Settings
4.4. Sample Preparation for Proteomic Analysis
4.5. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Based Proteomic Analysis
4.6. Transmission Electron Microscopy
4.7. Scanning Electron Microscopy
4.8. The Magnetic Experimental Setup and Calculation of Magnetic Force Affecting Bacteria
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dickson, K.J. Summary of biological spaceflight experiments with cells. ASGSB Bull. 1991, 4, 151–260. [Google Scholar]
- Nickerson, C.A.; Ott, C.M.; Wilson, J.W.; Ramamurthy, R.; Pierson, D.L. Microbial Responses to Microgravity and Other Low-Shear Environments. Microbiol. Mol. Biol. Rev. 2004, 68, 345–361. [Google Scholar] [CrossRef]
- Horneck, G.; Klaus, D.M.; Mancinelli, R.L. Space Microbiology. Microbiol. Mol. Biol. Rev. 2010, 74, 121–156. [Google Scholar] [CrossRef]
- Zea, L.; Larsen, M.; Estante, F.; Qvortrup, K.; Moeller, R.; de Oliveira, S.D.; Stodieck, L.; Klaus, D. Phenotypic changes exhibited by E. coli cultured in space. Front. Microbiol. 2017, 8, 1598. [Google Scholar] [CrossRef]
- Zea, L.; Prasad, N.; Levy, S.E.; Stodieck, L.; Jones, A.; Shrestha, S.; Klaus, D. A molecular genetic basis explaining altered bacterial behavior in space. PLoS ONE 2016, 11, e0164359. [Google Scholar] [CrossRef]
- Crabbé, A.; Schurr, M.J.; Monsieurs, P.; Morici, L.; Schurr, J.; Wilson, J.W.; Ott, C.M.; Tsaprailis, G.; Pierson, D.L.; Stefanyshyn-Piper, H.; et al. Transcriptional and proteomic responses of Pseudomonas aeruginosa PAO1 to spaceflight conditions involve Hfq regulation and reveal a role for oxygen. Appl. Environ. Microbiol. 2011, 77, 1221–1230. [Google Scholar] [CrossRef]
- Wilson, J.W.; Ott, C.M.; Höner Zu Bentrup, K.; Ramamurthy, R.; Quick, L.; Porwollik, S.; Cheng, P.; McClelland, M.; Tsaprailis, G.; Radabaugh, T.; et al. Space flight alters bacterial gene expression and virulence and reveals a role for global regulator Hfq. Proc. Natl. Acad. Sci. USA 2007, 104, 16299–16304. [Google Scholar] [CrossRef]
- Tixador, R.; Gasset, G.; Eche, B.; Moatti, N.; Lapchine, L.; Woldringh, C.; Toorop, P.; Moatti, J.P.; Delmotte, F.; Tap, G. Behavior of bacteria and antibiotics under space conditions. Aviat. Sp. Environ. Med. 1994, 65, 551–556. [Google Scholar]
- Li, J.; Liu, F.; Wang, Q.; Ge, P.; Woo, P.C.Y.; Yan, J.; Zhao, Y.; Gao, G.F.; Liu, C.H.; Liu, C. Genomic and transcriptomic analysis of NDM-1 Klebsiella pneumoniae in spaceflight reveal mechanisms underlying environmental adaptability. Sci. Rep. 2014, 4, 6216. [Google Scholar] [CrossRef]
- Tucker, D.L.; Ott, C.M.; Huff, S.; Fofanov, Y.; Pierson, D.L.; Willson, R.C.; Fox, G.E. Characterization of Escherichia coli MG1655 grown in a low-shear modeled microgravity environment. BMC Microbiol. 2007, 7. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Wong, M.; Lu, T.; Zhang, Y.; Wu, H. Interplay of space radiation and microgravity in DNA damage and DNA damage response. npj Microgravity 2017, 3, 14. [Google Scholar] [CrossRef]
- Klaus, D.; Simske, S.; Todd, P.; Stodieck, L. Investigation of space flight effects on Escherichia coil and a proposed model of underlying physical mechanisms. Microbiology 1997, 143, 449–455. [Google Scholar] [CrossRef]
- Berry, M.V.; Geim, A.K. Of flying frogs and levitrons. Eur. J. Phys. 1997, 18, 307. [Google Scholar] [CrossRef]
- Durmus, N.G.; Tekin, H.C.; Guven, S.; Sridhar, K.; Yildiz, A.A.; Calibasi, G.; Ghiran, I.; Davis, R.W.; Steinmetz, L.M.; Demirci, U. Magnetic levitation of single cells. Proc. Natl. Acad. Sci. USA 2015, 112, E3661–E3668. [Google Scholar] [CrossRef]
- Parfenov, V.A.; Mironov, V.A.; Van Kampen, K.A.; Karalkin, P.A.; Koudan, E.V.; Pereira, F.D.A.S.; Petrov, S.V.; Nezhurina, E.K.; Petrov, O.F.; Myasnikov, M.I.; et al. Scaffold-free and label-free biofabrication technology using levitational assembly in a high magnetic field. Biofabrication 2020, 12, 045022. [Google Scholar] [CrossRef]
- Domnin, P.; Arkhipov, A.; Petrov, S.; Sysolyatina, E.; Parfenov, V.; Karalkin, P.; Mukhachev, A.; Gusarov, A.; Moisenovich, M.; Khesuani, Y.; et al. An in vitro model of nonattached biofilm-like bacterial aggregates based on magnetic levitation. Appl. Environ. Microbiol. 2020, 86, e01974-20. [Google Scholar] [CrossRef]
- Herranz, R.; Larkin, O.J.; Dijkstra, C.E.; Hill, R.J.A.; Anthony, P.; Davey, M.R.; Eaves, L.; van Loon, J.J.W.A.; Medina, F.J.; Marco, R. Microgravity simulation by diamagnetic levitation: Effects of a strong gradient magnetic field on the transcriptional profile of Drosophila melanogaster. BMC Genomics 2012, 13, 52. [Google Scholar] [CrossRef]
- Hemmersbach, R.; Simon, A.; Waßer, K.; Hauslage, J.; Christianen, P.C.M.; Albers, P.W.; Lebert, M.; Richter, P.; Alt, W.; Anken, R. Impact of a high magnetic field on the orientation of gravitactic unicellular organisms—A critical consideration about the application of magnetic fields to mimic functional weightlessness. Astrobiology 2014, 14, 205–215. [Google Scholar] [CrossRef]
- Henderson, I.R.; Meehan, M.; Owen, P. Antigen 43, a phase-variable bipartite outer membrane protein, determines colony morphology and autoaggregation in Escherichia coli K-12. FEMS Microbiol. Lett. 2006, 149, 115–120. [Google Scholar] [CrossRef]
- Iuchi, S.; Cole, S.T.; Lin, E.C.C. Multiple regulatory elements for the glpA operon encoding anaerobic glycerol-3-phosphate dehydrogenase and the glpD operon encoding aerobic glycerol-3-phosphate dehydrogenase in Escherichia coli: Further characterization of respiratory control. J. Bacteriol. 1990, 172, 179–184. [Google Scholar] [CrossRef]
- Nickerson, C.A.; Ott, C.M.; Wilson, J.W.; Ramamurthy, R.; LeBlanc, C.L.; Höner zu Bentrup, K.; Hammond, T.; Pierson, D.L. Low-shear modeled microgravity: A global environmental regulatory signal affecting bacterial gene expression, physiology, and pathogenesis. J. Microbiol. Methods 2003, 54, 1–11. [Google Scholar] [CrossRef]
- Yim, J.; Cho, S.W.; Kim, B.; Park, S.; Han, Y.H.; Seo, S.W. Transcriptional profiling of the probiotic Escherichia Coli nissle 1917 strain under simulated microgravity. Int. J. Mol. Sci. 2020, 21, 2666. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, J.A.; Abogunde, O.; Thomas, K.; Lawal, A.; Nguyen, Y.U.; Sodipe, A.; Jejelowo, O. Spaceflight and modeled microgravity effects on microbial growth and virulence. Appl. Microbiol. Biotechnol. 2010, 85, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Vukanti, R.; Model, M.A.; Leff, L.G. Effect of modeled reduced gravity conditions on bacterial morphology and physiology. BMC Microbiol. 2012, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Tixador, R.; Richoilley, G.; Gasset, G.; Planel, H.; Moatti, N.; Lapchine, L.; Enjalbert, L.; Raffin, J.; Bost, R.; Zaloguev, S.N.; et al. Preliminary results of cytos 2 experiment. Acta Astronaut. 1985, 12, 131–134. [Google Scholar] [CrossRef]
- Bouloc, P.; D’Ari, R. Escherichia coli metabolism in space. J. Gen. Microbiol. 1991, 137, 2839–2843. [Google Scholar] [CrossRef]
- Gasset, G.; Tixador, R.; Eche, B.; Lapchine, L.; Moatti, N.; Toorop, P.; Woldringh, C. Growth and division of Escherichia coli under microgravity conditions. Res. Microbiol. 1994, 145, 111–120. [Google Scholar] [CrossRef]
- Chakraborty, S.; Karmakar, K.; Chakravortty, D. Cells producing their own nemesis: Understanding methylglyoxal metabolism. IUBMB Life 2014, 66, 667–678. [Google Scholar] [CrossRef]
- Chang, G.W.; Chang, J.T. Evidence for the B12-dependent enzyme ethanolamine deaminase in Salmonella. Nature 1975, 254, 150–151. [Google Scholar] [CrossRef]
- Urbanowski, M.L.; Stauffer, L.T.; Plamann, L.S.; Stauffer, G.V. A new methionine locus, metR, that encodes a trans-acting protein required for activation of metE and metH in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 1987, 169, 1391–1397. [Google Scholar] [CrossRef]
- Kaval, K.G.; Garsin, D.A. Ethanolamine utilization in bacteria. MBio 2018, 9, e00066-18. [Google Scholar] [CrossRef] [PubMed]
- Crabbé, A.; De Boever, P.; Van Houdt, R.; Moors, H.; Mergeay, M.; Cornelis, P. Use of the rotating wall vessel technology to study the effect of shear stress on growth behaviour of Pseudomonas aeruginosa PA01. Environ. Microbiol. 2008, 10, 2098–2110. [Google Scholar] [CrossRef]
- Castro, S.L.; Nelman-Gonzalez, M.; Nickerson, C.A.; Ott, C.M. Induction of attachment-independent biofilm formation and repression of hfq expression by low-fluid-shear culture of Staphylococcus aureus. Appl. Environ. Microbiol. 2011, 77, 6368–6378. [Google Scholar] [CrossRef] [PubMed]
- Hoehler, T.M.; Jørgensen, B.B. Microbial life under extreme energy limitation. Nat. Rev. Microbiol. 2013, 11, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, R.S.; Cozzarelli, N.R.; Epstein, W. Accumulation of toxic concentrations of methylglyoxal by wild type Escherichia coli K12. J. Bacteriol. 1974, 119, 357–362. [Google Scholar] [CrossRef]
- Hopper, D.J.; Cooper, R.A. The regulation of Escherichia coli methylglyoxal synthase; a new control site in glycolysis? FEBS Lett. 1971, 13, 213–216. [Google Scholar] [CrossRef]
- Kim, I.; Kim, E.; Yoo, S.; Shin, D.; Min, B.; Song, J.; Park, C. Ribose utilization with an excess of mutarotase causes cell death due to accumulation of methylglyoxal. J. Bacteriol. 2004, 186, 7229–7235. [Google Scholar] [CrossRef]
- Ferguson, G.P.; Tötemeyer, S.; MacLean, M.J.; Booth, I.R. Methylglyoxal production in bacteria: Suicide or survival? Arch. Microbiol. 1998, 170, 209–218. [Google Scholar] [CrossRef]
- Maloy, S.R.; Bohlander, M.; Nunn, W.D. Elevated levels of glyoxylate shunt enzymes in Escherichia coli strains constitutive for fatty acid degradation. J. Bacteriol. 1980, 143, 720–725. [Google Scholar] [CrossRef]
- Renilla, S.; Bernal, V.; Fuhrer, T.; Castaño-Cerezo, S.; Pastor, J.M.; Iborra, J.L.; Sauer, U.; Cánovas, M. Acetate scavenging activity in Escherichia coli: Interplay of acetyl-CoA synthetase and the PEP-glyoxylate cycle in chemostat cultures. Appl. Microbiol. Biotechnol. 2012, 93, 2109–2124. [Google Scholar] [CrossRef]
- Zhang, S.; Bryant, D.A. Biochemical validation of the glyoxylate cycle in the cyanobacterium Chlorogloeopsis fritschii strain PCC 9212. J. Biol. Chem. 2015, 290, 14019–14030. [Google Scholar] [CrossRef]
- Li, N.; Zhang, B.; Chen, T.; Wang, Z.; Tang, Y.J.; Zhao, X. Directed pathway evolution of the glyoxylate shunt in Escherichia coli for improved aerobic succinate production from glycerol. J. Ind. Microbiol. Biotechnol. 2013, 40, 1461–1475. [Google Scholar] [CrossRef]
- Beauprez, J.J.; De Mey, M.; Soetaert, W.K. Microbial succinic acid production: Natural versus metabolic engineered producers. Process. Biochem. 2010, 45, 1103–1114. [Google Scholar] [CrossRef]
- Kulp, A.; Kuehn, M.J. Biological Functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184. [Google Scholar] [CrossRef] [PubMed]
- Kjaergaard, K.; Schembri, M.A.; Hasman, H.; Klemm, P. Antigen 43 from Escherichia coli induces inter- and intraspecies cell aggregation and changes in colony morphology of Pseudomonas fluorescens. J. Bacteriol. 2000, 182, 4789–4796. [Google Scholar] [CrossRef][Green Version]
- Guerrero Montero, I.; Dolata, K.M.; Schlüter, R.; Malherbe, G.; Sievers, S.; Zühlke, D.; Sura, T.; Dave, E.; Riedel, K.; Robinson, C. Comparative proteome analysis in an Escherichia coli CyDisCo strain identifies stress responses related to protein production, oxidative stress and accumulation of misfolded protein. Microb. Cell Fact. 2019, 18, 19. [Google Scholar] [CrossRef]
- Belova, I.V.; Tochilina, A.G.; Soloveva, I.V.; Gorlova, I.S.; Efimov, E.I.; Zhirnov, V.A.; Ivanova, T.P. Phenotypic and genotypic characteristics of E. coli m-17 the probiotic strain. Modern Probl. Sci. Educ. 2017, 3, 26533. [Google Scholar] [CrossRef][Green Version]
- Parfenov, V.A.; Koudan, E.V.; Bulanova, E.A.; Karalkin, P.A.; Das Pereira, F.; Norkin, N.E.; Knyazeva, A.D.; Gryadunova, A.A.; Petrov, O.F.; Vasiliev, M.M.; et al. Scaffold-free, label-free and nozzle-free biofabrication technology using magnetic levitational assembly. Biofabrication 2018, 10, 034104. [Google Scholar] [CrossRef] [PubMed]

| Conditions | Length 1 of Preserved Cells ± SD (μm) |
|---|---|
| Ground 24 h | 3.74 ± 0.31 |
| Ground 144 h | 3.27 ± 0.41 |
| SF | 3.0 ± 0.25 |
| SF + MF | 3.64 ± 0.42 |
| Ground MF | 1.92 ± 0.33 |
| Protein Name | Log2 Expression Ratio | Protein Activity | Protein Function | |||
|---|---|---|---|---|---|---|
| SF/144 h Control | SF + MF/144 h Control | ML/ 144 h Control | SF/ML | |||
| Genetic information processing | ||||||
| RS1 | 0.1 1 | n.d. 2 | 0.8 3 | −0.8 | Ribosomal protein S1 | Translation |
| RS6 | 0.7 | 1.2 | −0.4 | 1.2 | Ribosomal protein S6 | Translation |
| Pnp | 0.7 | 2.4 | −0.5 | 1.2 | Polynucleotide phosphorylase | tRNA processing |
| RpoB | −1.4 | −2.6 | −0.3 | −1.1 | Beta’-subunit RNA-polymerase | Transcription |
| Rho | 0.9 | 1.0 | −0.1 | 1.0 | Transcription terminator | Regulation of transcription |
| Stress response | ||||||
| KatG | 1.0 | 1.8 | 0.25 | 0.8 | Hydroperoxidase I | Oxidative stress response |
| WrbA | 1.5 | 2.0 | 1.0 | 0.5 | NADH:quinone oxidoreductase | Oxidative stress response |
| AhpC | 0.2 | 1.1 | −0.9 | 1.1 | Alkyl hydroperoxide reductase subunit C | Oxidative stress response |
| DnaK | −1.6 | −3.5 | 0.3 | −1.9 | Chaperone protein | Protein misfolding control |
| ClpB | −1.0 | −3.1 | 1.1 | −2.1 | Chaperone protein | Protein misfolding control |
| ClpXP | −0.9 | n.d. | 2.6 | −3.7 | Chaperone protease | Protein misfolding control |
| Carbohydrate metabolism | ||||||
| AceA | 2.1 | 3.3 | 1.3 | 1.2 | Isocitrate lyase | Glyoxylate shunt |
| Idh | −1.1 | −4.1 | 1.9 | −3.0 | Isocitrate dehydrogenase | TCA |
| TdcE | −1.8 | −3.8 | 0.2 | −2.0 | Pyruvate formate lyase 4 | Glycolysis |
| PflB | −1.6 | −2.5 | 0.1 | −1.6 | Pyruvate formate-lyase | Glycolysis |
| MgsA | 0.7 | 2.5 | −1.0 | 1.8 | Methylglyoxal synthase | Synthesis of methylglyoxal |
| ElbB | 1.3 | n.d. | 0.1 | 1.3 | Glyoxalase III | Methylglyoxal detoxification |
| AckA | −0.8 | n.d. | −1.2 | 0.5 | Acetate kinase | Acetate metabolism |
| GlpA | 0.2 | 1.6 | −1.3 | −1.4 | Anaerobic Glyceraldehyde 3-phosphate dehydrogenase | Glycolysis/gluconeogenesis |
| Tkt1 | 0.1 | 1.6 | −1.3 | 1.4 | Transketolase | Pentose phosphate pathway |
| Surface structures | ||||||
| BtuB | 4.0 | 6.2 | 1.8 | 2.1 | Cobalamin/cobinamide outer membrane transporter | Vitamin B12 transport |
| Ag43 | 1.4 | 1.3 | 1.3 | 0.1 | Autotransporter adhesin Antigen 43 | Control of autoaggregation |
| FliC | 0.1 | n.d. | 3.8 | −3.8 | Flagella structural subunit | Motility |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domnin, P.A.; Parfenov, V.A.; Kononikhin, A.S.; Petrov, S.V.; Shevlyagina, N.V.; Arkhipova, A.Y.; Koudan, E.V.; Nezhurina, E.K.; Brzhozovskiy, A.G.; Bugrova, A.E.; et al. Combined Impact of Magnetic Force and Spaceflight Conditions on Escherichia coli Physiology. Int. J. Mol. Sci. 2022, 23, 1837. https://doi.org/10.3390/ijms23031837
Domnin PA, Parfenov VA, Kononikhin AS, Petrov SV, Shevlyagina NV, Arkhipova AY, Koudan EV, Nezhurina EK, Brzhozovskiy AG, Bugrova AE, et al. Combined Impact of Magnetic Force and Spaceflight Conditions on Escherichia coli Physiology. International Journal of Molecular Sciences. 2022; 23(3):1837. https://doi.org/10.3390/ijms23031837
Chicago/Turabian StyleDomnin, Pavel A., Vladislav A. Parfenov, Alexey S. Kononikhin, Stanislav V. Petrov, Nataliya V. Shevlyagina, Anastasia Yu. Arkhipova, Elizaveta V. Koudan, Elizaveta K. Nezhurina, Alexander G. Brzhozovskiy, Anna E. Bugrova, and et al. 2022. "Combined Impact of Magnetic Force and Spaceflight Conditions on Escherichia coli Physiology" International Journal of Molecular Sciences 23, no. 3: 1837. https://doi.org/10.3390/ijms23031837
APA StyleDomnin, P. A., Parfenov, V. A., Kononikhin, A. S., Petrov, S. V., Shevlyagina, N. V., Arkhipova, A. Y., Koudan, E. V., Nezhurina, E. K., Brzhozovskiy, A. G., Bugrova, A. E., Moysenovich, A. M., Levin, A. A., Karalkin, P. A., Pereira, F. D. A. S., Zhukhovitsky, V. G., Lobakova, E. S., Mironov, V. A., Nikolaev, E. N., Khesuani, Y. D., & Ermolaeva, S. A. (2022). Combined Impact of Magnetic Force and Spaceflight Conditions on Escherichia coli Physiology. International Journal of Molecular Sciences, 23(3), 1837. https://doi.org/10.3390/ijms23031837








