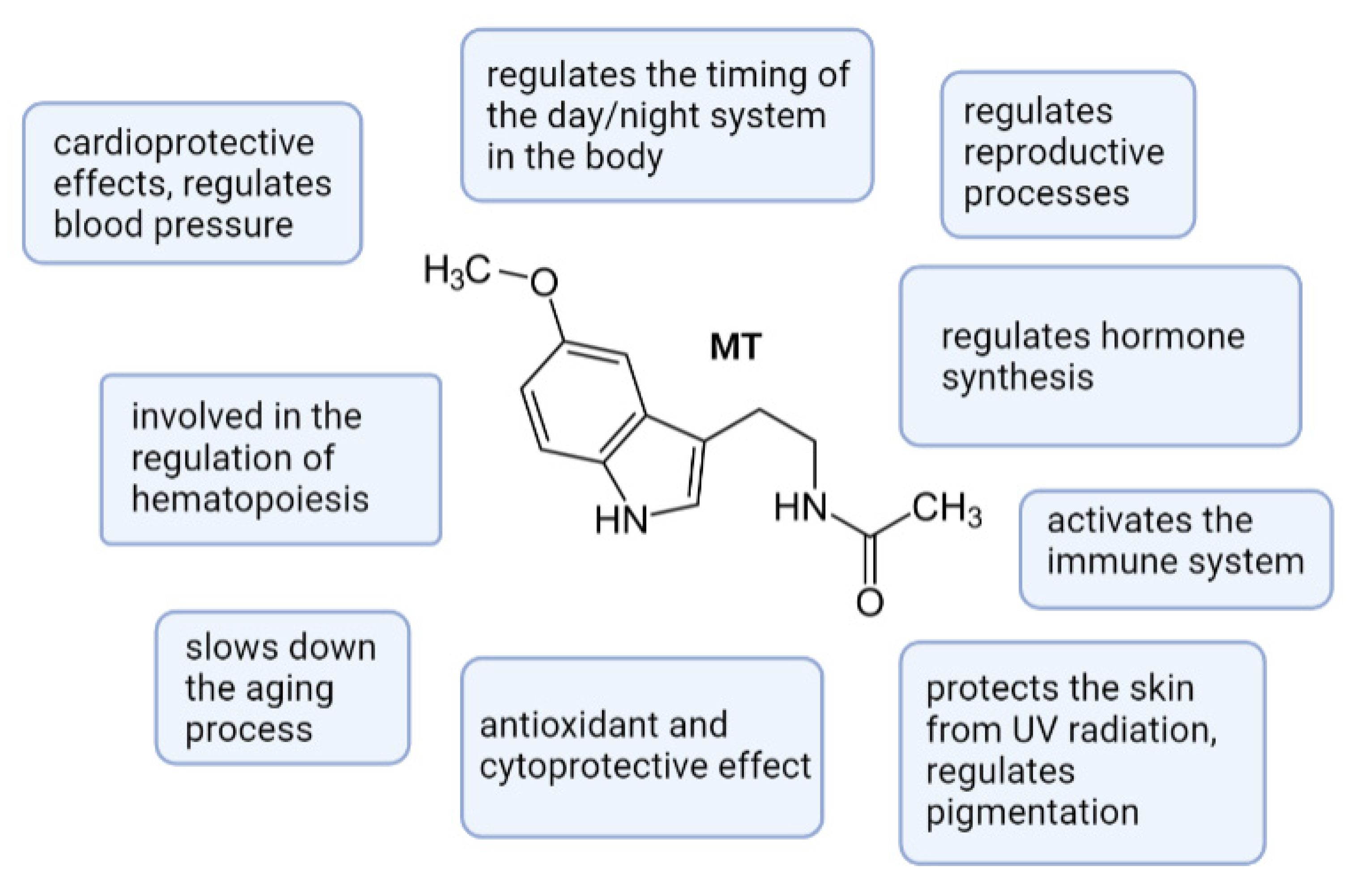
Melatonin as the Cornerstone of Neuroimmunoendocrinology
Abstract
:1. Introduction
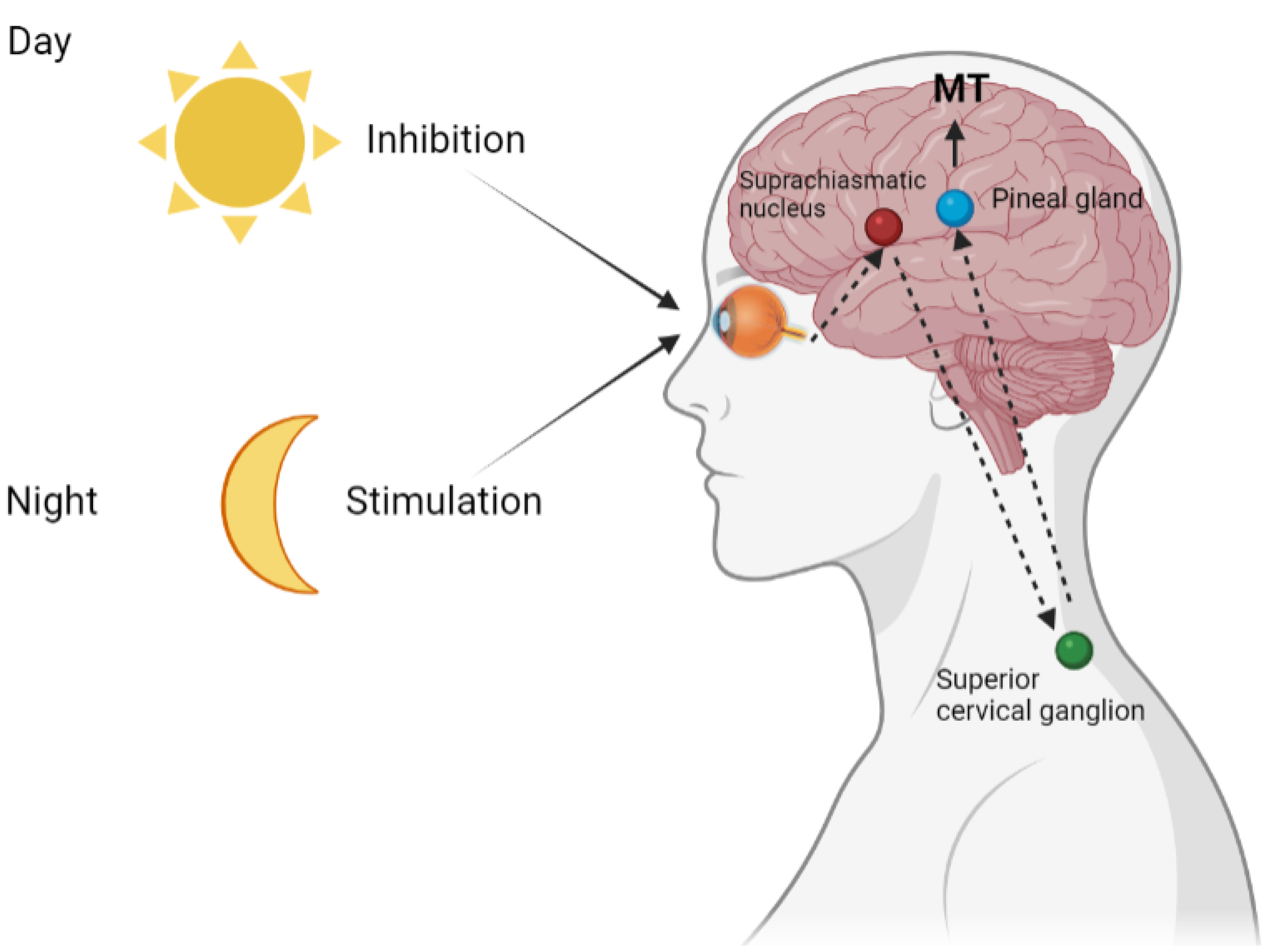
2. Pineal Melatonin
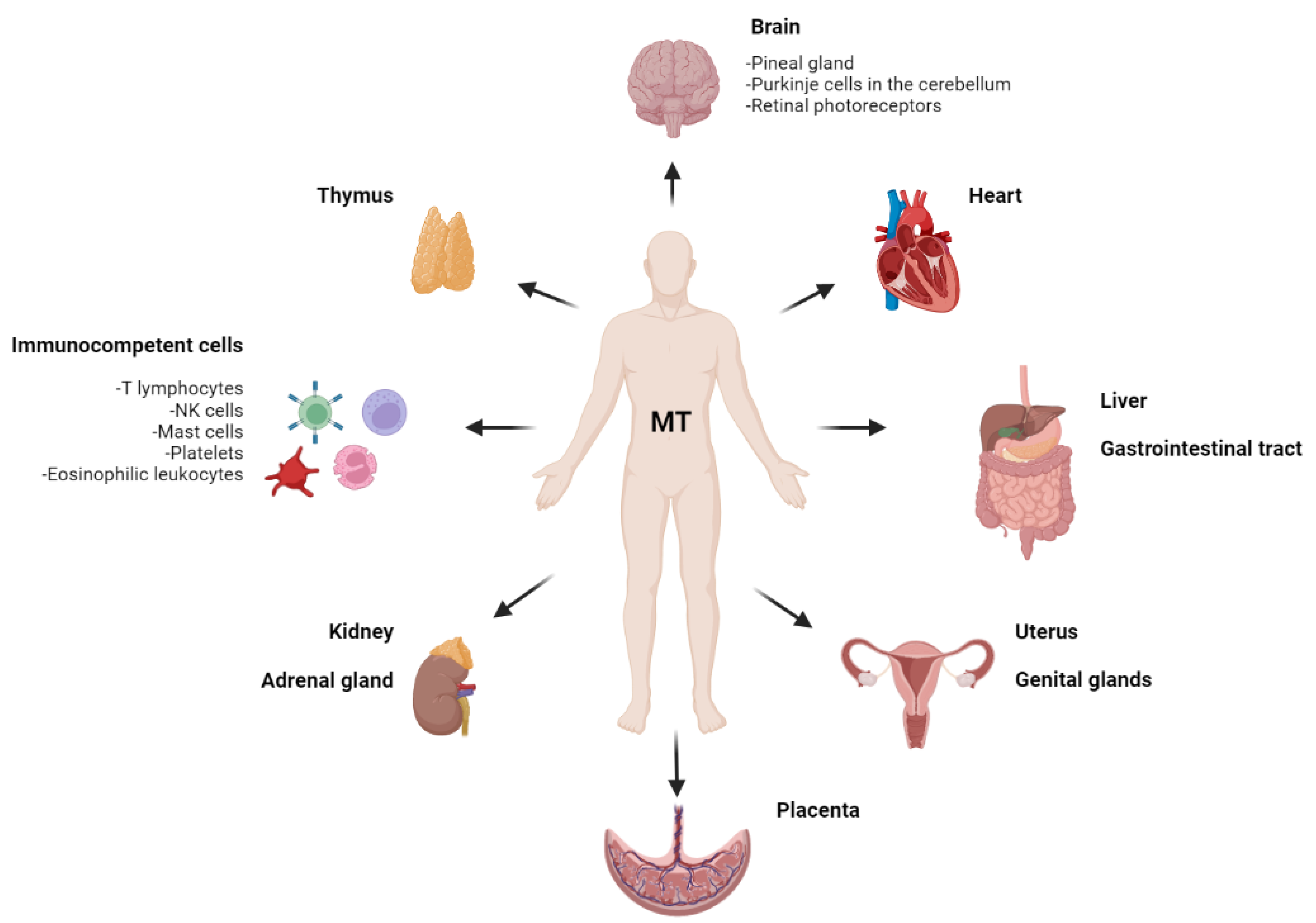
3. Extrapineal Melatonin
3.1. Melatonin in the Nervous System
3.2. Melatonin in the Immune System
3.3. Melatonin in the Gastrointestinal Tract
3.4. Melatonin in Other Visceral Organs
4. Melatonin and Mitochondria
5. Melatonin as a Neuroimmunoendocrine Marker and Molecular Target for Socially Significant Diseases
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlazzo, N.; Andolina, G.; Cannata, A.; Costanzo, M.G.; Rizzo, V.; Currò, M.; Ientile, R.; Caccamo, D. Is Melatonin the Cornucopia of the 21st Century? Antioxidants 2020, 9, 1088. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a Potent and Inducible Endogenous Antioxidant: Synthesis and Metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [Green Version]
- Pandi–Perumal, S.R.; Srinivasan, V.; Maestroni, G.J.M.; Cardinali, D.P.; Poeggedoler, B.; Hardeland, R. Melatonin Nature’s most versatile biological signal? FEBS J. 2006, 273, 2813–2838. [Google Scholar]
- Chitimus, D.M.; Popescu, M.R.; Voiculescu, S.E.; Panaitescu, A.M.; Pavel, B.; Zagrean, L.; Zagrean, A.-M. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules 2020, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G. The timing clock work of life. J. Biol. Regul. Homeost. Agents 2011, 25, 137–143. [Google Scholar] [PubMed]
- Kvetnoy, I.M.; Sinitskaya, N.S.; Kvetnaya, T.V. Extrapineal Melatonin Location and Role in Pathological Processes. In Melatonin: Biological Basis of its Function in Health and Disease; Pandi-Peruumal, S.R., Cardinali, D.P., Eds.; CRC Press: Boca Raton, FL, USA, 2005; Chapter 13; pp. 148–161. [Google Scholar]
- Kvetnoy, I.M. Extrapineal Melatonin: Location and role within diffuse neuroendocrine system. Histochem. J. 1999, 31, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Amaral, F.G. Melatonin as a hormone: New physiological and clinical insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venegas, C.; García, J.A.; Escames, G.; Ortiz, F.; López, A.; Doerrier, C.; García-Corzo, L.; López, L.C.; Reiter, R.J.; Acuña-Castroviejo, D. Extrapineal Melatonin: Analysis of its subcellular distribution and daily fluctuations. J. Pineal Res. 2012, 52, 217–227. [Google Scholar] [CrossRef]
- Acuna-Castroviejo, D.; Escames, G.; Venegas, C.; Diaz-Casado, M.E.; Lima-Cabello, E.; Lopez, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal Melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Peruumal, S.R.; Cardinali, D.P. Melatonin: Biological Basis of its Function in Health and Disease; CRC Press: Boca Raton, FL, USA, 2005; 283p. [Google Scholar]
- Barinaga, M. How the brain’s clock gets daily enlightenment. Science 2002, 295, 955–957. [Google Scholar] [CrossRef]
- Kvetnoy, I.; Sandvik, A.K.; Waldum, H.L.J. The diffuse neuroendocrine system and extrapineal Melatonin. Mol. Endocrinol. 1997, 18, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Idle, J.R.; Krausz, K.W.; Gonzalez, F.J. Metabolism of Melatonin by human cytochromes p 450. Drag Metab. Dispos. 2005, 33, 489–494. [Google Scholar] [CrossRef]
- Bubenik, G.A. Thirty-four years since the discovery of gastrointestinal Melatonin. J. Physiol. Pharm. 2008, 59, 33–51. [Google Scholar]
- Reppert, S.M.; Godson, C.; Mahle, C.D.; Weaver, D.R.; Slaugenhaupt, S.; Gusella, J.F. Molecular characterization of a second Melatonin receptor expressed in human retina and brain: The Mel1b Melatonin receptor. Proc. Natl. Acad. Sci. USA 1995, 92, 8734–8738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narváez-Rojas, A.R.; Moscote-Salazar, L.R.; Dolachee, A.A.; Alrawi, M.A.; Neamah, A.M.; AlBanaa, S.A. Physiology of the Pineal Gland. In Pineal Neurosurgery; Springer: Berlin/Heidelberg, Germany, 2020; pp. 21–29. [Google Scholar]
- Amini, H.; Rezabakhsh, A.; Heidarzadeh, M.; Hassanpour, M.; Hashemzadeh, S.; Ghaderi, S.; Sokullu, E.; Rahbarghazi, R.; Reiter, R.J. An Examination of the Putative Role of Melatonin in Exosome Biogenesis. Front. Cell Dev. Biol. 2021, 9, 686551. [Google Scholar] [CrossRef]
- Slominski, R.M.; Reiter, R.J.; Schlabritz-Loutsevitch, N.; Ostrom, R.S.; Slominski, A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell Endocrinol. 2012, 351, 152–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D. Update on Melatonin receptors: IUPHAR Review 20. Br. J. Pharm. 2016, 173, 2702–2725. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007, 8, 34–42. [Google Scholar] [CrossRef] [PubMed]
- McCord, C.P.; Allen, F.P. Evidences associating pineal function with alterations in pigmentation. J. Exp. Zool. 1917, 23, 207–243. [Google Scholar] [CrossRef] [Green Version]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Bartsch, C.; Bartsch, H.; Seebald, E.; Küpper, H.; Mecke, D. Modulation of pineal activity during the 23rd sunspot cycle: Melatonin rise during the ascending phase of the cycle is accompanied by an increase of the sympathetic tone. Indian J. Exp. Biol. 2014, 52, 438–447. [Google Scholar]
- Boden, M.J.; Varcoe, T.J.; Kennaway, D.J. Circadian regulation of reproduction: From gamete to offspring. Prog. Biophys. Mol. Biol. 2013, 113, 387–397. [Google Scholar] [CrossRef] [Green Version]
- Zisapel, N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharm. 2018, 175, 3190–3199. [Google Scholar] [CrossRef]
- Markus, R.P.; Fernandes, P.A.; Kinker, G.S.; da Silveira Cruz-Machado, S.; Marçola, M. Immune-pineal axis—Acute inflammatory responses coordinate Melatonin synthesis by pinealocytes and phagocytes. Br. J. Pharm. 2018, 175, 3239–3250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shavali, S.S.; Haldar, C. Effects of continuous light, continuous darkness and pinealectomy on pineal-thyroid-gonadal axis of the female Indian palm squirrel, Funambulus pennanti. J. Neural Transm. 1998, 105, 407–413. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Shen, Y.; Liu, Q.; Zhao, Z.; Sharma, R.; Reiter, R.J. Melatonin Synthesis and Function: Evolutionary History in Animals and Plants. Front. Endocrinol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Kvetnoy, I.M. Neuroimmunoendocrinology: Where is the field for study? Neuro Endocrinol. Lett. 2002, 23, 119–120. [Google Scholar] [PubMed]
- Hardeland, R. Melatonin—More than Just a Pineal Hormone. Biomed. J. Sci. Technol. Res. 2017, 1, 994–997. [Google Scholar] [CrossRef] [Green Version]
- Steful, J.; Hortner, M.; Ghosh, M.; Schauenstein, K.; Rinner, I.; Wolfler, A.; Liebmann, P.M. Gene expression of the key enzymes of Melatonin synthesis in extrapineal tissues of the rat. J. Pineal Res. 2001, 30, 243–247. [Google Scholar] [CrossRef]
- Jimenez-Jorge, S.; Guerrero, J.M.; Jimenez-Caliani, A.J.; Naranjo, M.C.; Lardone, P.J.; Carrillo-Vico, A.; Osuna, C.; Molinero, P. Evidence for Melatonin synthesis in the rat brain during development. J. Pineal Res. 2007, 42, 240–246. [Google Scholar] [CrossRef]
- Cardinali, D.P. Melatonin: A mammalian pineal hormone. Endocrin. Rev. 1981, 2, 327–346. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Tan, D.X.; Reiter, R.J. Kynuramines, metabolites of Melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. J. Pineal Res. 2009, 47, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhuang, J.; Zhu, H.Y.; Shen, Y.X.; Tan, Z.L.; Zhou, J.N. Cultured rat cortical astrocytes synthesize Melatonin: Absence of a diurnal rhythm. J. Pineal Res. 2007, 43, 232–238. [Google Scholar] [CrossRef] [PubMed]
- ZhuaUzng, T.; Qu, T.; Sugaya, K.; Manev, H. Neuronal expression of arylalkylamine N-acetyltransferase (AANAT) mRNA in the rat brain. Neurosci. Res. 2002, 42, 309–316. [Google Scholar] [CrossRef]
- Uz, T.; Ahmed, R.; Akhisaroglu, M.; Kurtuncu, M.; Imbesi, M.; Dirim, A.A.; Manev, H. Effect of fluoxetine and cocaine on the expression of clock genes in the mouse hippocampus and striatum. Neuroscience 2005, 134, 1309–1316. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin metabolism in the central nervous system. Curr. Neuropharmacol. 2010, 8, 168–171. [Google Scholar] [CrossRef] [Green Version]
- Sallanon, M.; Claustrat, B.; Touret, M. Presence of Melatonin in various cat brainstem nuclei determined by radioimmunoassay. Acta Endocrinol. 1982, 101, 161–165. [Google Scholar] [CrossRef]
- Seifman, M.A.; Adamides, A.A.; Nguyen, P.N.; Vallance, S.A.; Cooper, D.J.; Kossmann, T.; Morganti-Kossmann, M.C. Endogenous Melatonin increases in cerebrospinal fluid of patients aftersevere traumatic brain injury and correlates with oxidative stress and metabolic disarray. J. Cereb. Blood Flow. Metab. 2008, 28, 684–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babaee, A.; Eftekhar Vaghefi, S.H.; Dehghani Soltani, S.; Asadi Shekaari, M.; Shahrokhi, N.; Basiri, M. Hippocampal Astrocyte Response to Melatonin Following Neural Damage Induction in Rats. Basic Clin. Neurosci. 2021, 12, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, T.; Jin, X.; Wang, G.; Zhao, F.; Jin, Y. Roles ofCrosstalk between Astrocytes and Microglia in Triggering Neuroinflammation and Brain Edema Formation in 1,2-DichloroethaneIntoxicated Mice. Cells 2021, 10, 2647. [Google Scholar] [CrossRef]
- Rehman, S.U.; Ikram, M.; Ullah, N.; Alam, S.I.; Park, H.Y.; Badshah, H.; Choe, K.; Kim, M.O. Neurological Enhancement Effects of Melatonin against Brain Injury-Induced Oxidative Stress, Neuroinflammation, and Neurodegeneration via AMPK/CREB Signaling. Cells 2019, 8, 760. [Google Scholar] [CrossRef] [Green Version]
- Carloni, S.; Perrone, S.; Buonocore, G.; Longini, M.; Proietti, F.; Balduini, W. Melatonin protects from the long-term consequences of a neonatal hypoxic-ischemic brain injury in rats. J. Pineal Res. 2008, 44, 157–164. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B. Melatonin and synthetic melatonergic agonists: Actions and metabolism in the central nervous system. Cent. Nerv. Syst. Agents. Med. Chem. 2012, 12, 189–216. [Google Scholar] [CrossRef] [PubMed]
- Pinato, L.; da Silveira Cruz Machado, S.; Franco, D.G.; Campos, L.M.; Cecon, E. Selective protection of the cerebellum against intracerebroventricular LPS is mediated by local Melatonin synthesis. Brain Struct. Funct. 2015, 220, 827–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.J.; Meng, F.T.; Wang, L.L.; Zhang, L.F.; Cheng, X.P.; Zhou, J.N. Apolipoprotein E influences Melatonin biosynthesis by regulating NAT and MAOA expression in C6 cells. J. Pineal Res. 2012, 52, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Alonso, A.; Ramirez-Rodriguez, G.; Benitez-King, G. Melatonin increases dendritogenesis in the hilus of hippocampal organotypic cultures. J. Pineal Res. 2012, 52, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Rubio, G.; Ortiz-Lopez, L.; Benitez-King, G. Melatonin modulates cytoskeletal organization in the rat brain hippocampus. Neurosci. Lett. 2012, 511, 47–51. [Google Scholar] [CrossRef]
- Chern, C.M.; Liao, J.F.; Wang, Y.H.; Shen, Y.C. Melatonin ameliorates neural function by promoting endogenous neurogenesis through the Melatonin2 Melatonin receptor in ischemic-stroke mice. Free Radic. Biol. Med. 2012, 52, 1634–1647. [Google Scholar] [CrossRef]
- Tosini, G.; Baba, K.; Hwang, C.K.; Iuvone, P.M. Melatonin: An underappreciated player in retinal physiology and pathophysiology. Exp. Eye Res. 2012, 103, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Zmijewski, M.A.; Sweatman, T.W.; Slominski, A.T. The Melatonin-producing system is fully functional in retinal pigment epithelium (ARPE-19). Mol. Cell. Endocrinol. 2009, 307, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Wiechmann, A.F.; Summers, J.A. Circadian rhythms in the eye: The physiological significance of Melatonin receptors in ocular tissues. Prog. Retin. Eye Res. 2008, 27, 137–160. [Google Scholar] [CrossRef]
- Marchiafava, P.L.; Longoni, B. Melatonin as an antioxidant in retinal photoreceptors. J. Pineal Res. 1999, 26, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.T.; Takahashi, N.; Abe, M.; Shimizu, K. Expression and cellular localization of Melatonin-synthesizing enzymes in the rat lens. J. Pineal Res. 2007, 42, 92–96. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A review of the multiple actions of Melatonin on the immune system. Endocrine 2005, 27, 189–200. [Google Scholar] [CrossRef]
- Maldonado, M.D.; Mora-Santos, M.; Naji, L.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Calvo, J.R. Evidence of Melatonin synthesis and release by mast cells. Possible modulatory role on inflammation. Pharmacol. Res. 2010, 62, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Naranjoa, M.C.; Guerreroa, J.M.; Rubioa, A.; Lardonea, P.J.; Carrillo-Vicoa, A.; Carrascosa-Salmorala, M.P.; Jimnez-Jorgea, S.; Arellanob, M.V.; Leal-Novalb, S.R.; Lealc, M.; et al. Melatonin biosynthesis in the thymus of humans and rats. Cell. Mol. Life Sci. 2007, 64, 781–790. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Santarelli, L.; Costarelli, L.; Cipriano, C.; Muti, E.; Giacconi, R.; Malavolta, M. Plasticity of neuroendocrine-thymus interactions during ontogeny and ageing: Role of zinc and arginine. Ageing Res. Rev. 2006, 5, 281–309. [Google Scholar] [CrossRef]
- Molinero, P.; Soutto, M.; Benot, S.; Hmadcha, A.; Guerrero, J.M. Melatonin is responsible for the nocturnal increase observed in serum and thymus of thymosin alpha1 and thymulin concentrations: Observations in rats and humans. J. Neuroimmunol. 2000, 103, 180–188. [Google Scholar] [CrossRef]
- Sainz, R.M.; Mayo, J.C.; Uria, H.; Kotler, M.; Antolin, I.; Rodriguez, C.; Menendez-Pelaez, A. The pineal neurohormone Melatonin prevents in vivo and in vitro apoptosis in thymocytes. J. Pineal Res. 1995, 19, 178–188. [Google Scholar] [CrossRef]
- Jimenez-Jorge, S.; Jimenez-Caliani, A.J.; Guerrero, J.M.; Naranjo, M.C.; Lardone, P.J.; Carrillo-Vico, A.; Osuna, C.; Molinero, P. Melatonin synthesis and Melatonin-membrane receptor (Melatonin1) expression during rat thymus development: Role of the pineal gland. J. Pineal Res. 2005, 39, 77–83. [Google Scholar] [CrossRef]
- Kvetnoy, I.M.; Polyakova, V.O.; Trofimov, A.V.; Yuzhakov, V.V.; Yarilin, A.A.; Kurilets, E.S.; Mikhina, L.N.; Sharova, N.I.; Nikonova, M.F. Hormonal function and proliferative activity of thymic cells in humans: Immunocytochemical correlations. Neuroendocrinol. Lett. 2003, 24, 263–268. [Google Scholar] [PubMed]
- Yarilin, A.A.; Belyakov, I.M. Cytokines in the thymus: Production and biological effects. Curr. Med. Chem. 2004, 11, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Sainz, R.M.; Mayo, J.C.; Kotler, M.; Uria, H.; Antolin, I.; Rodriguez, C. Melatonin decreases mRNA for histone of young rats. Life Sci. 1998, 63, 1109–1117. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Calvo, J.R.; Abreu, P.; Lardone, P.J.; Garcia-Maurino, S.; Reite, R.J.; Guerrero, J.M. Evidence of Melatonin synthesis by human lymphocytes and its physiological significance: Possible role as intracrine, autocrine, and/or paracrine substance. FASEB J. 2004, 18, 537–539. [Google Scholar] [CrossRef]
- NaveenKumar, S.K.; Hemshekhar, M.; Jagadish, S.; Manikanta, K.; Vishalakshi, G.J.; Kemparaju, K.; Kesturu, S.; Girish, K.S. Melatonin restores neutrophil functions and prevents apoptosis amid dysfunctional glutathione redox system. J. Pineal Res. 2020, 69, e12676. [Google Scholar] [CrossRef] [PubMed]
- Markus, R.P.; Ferreira, Z.S. The Immune-Pineal Axis: The Role of Pineal and Extra-Pineal Melatonin in Modulating Inflammation. Adv. Neuroimmune Biol. 2011, 1, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [Green Version]
- Martins, E., Jr.; Ferreira, A.C.; Skorupa, A.L.; Afeche, S.C.; Cipolla-Neto, J.; Costa Rosa, L.F. Tryptophan consumption and indoleamines production by peritoneal cavity macrophages. J. Leukoc Bio.l 2004, 75, 1116. [Google Scholar] [CrossRef] [Green Version]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; González-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Bernatoniene, J. Molecular Mechanisms of Melatonin-Mediated Cell Protection and Signaling in Health and Disease. Pharmaceutics 2021, 13, 129. [Google Scholar] [CrossRef]
- Maestroni, G.J.M.; Conti, A. Melatonin in Relation to the Immune System. In Melatonin; CRC Press: Boca Raton, FL, USA, 2020; pp. 289–309. [Google Scholar]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem. Cell Res. 2020, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furusawa, Y.; Yamamoto, T.; Hattori, A.; Suzuki, N.; Hirayama, J.; Sekiguchi, T.; Tabuchi, Y. De novo transcriptome analysis and gene expression profiling of fish scales isolated from Carassius auratus during space flight: Impact of melatonin on gene expression in response to space radiation. Mol. Med. Rep. 2020, 22, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Raikhlin, N.T.; Kvetnoy, I.M.; Tolkachev, V.N. Melatonin may be synthesized in enterochromaffine cells. Nature 1975, 255, 344–345. [Google Scholar] [CrossRef] [PubMed]
- Bubenik, G.A. Localization and Physiological Significance of Gastrointestinal Melatonin. In Melatonin in Health Promotion; Watson, R., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 21–39. [Google Scholar]
- Chen, C.Q.; Fichna, J.; Bashashati, M.; Li, Y.Y.; Storr, M. Distribution, function and physiological role of Melatonin in the lower gut. World J. Gastroenterol. 2011, 34, 3888–3898. [Google Scholar] [CrossRef]
- Huether, G. The contribution of extrapineal sites of Melatonin synthesis to circulating Melatonin levels in higher vertebrates. Experientia 1993, 49, 665–670. [Google Scholar] [CrossRef]
- Suofu, Y.; Li, W.; Jean-Alphonse, F.G.; Jia, J.; Khattar, N.K.; Li, J.; Baranov, S.V.; Leronni, D.; Mihalik, A.C.; He, Y.; et al. Dual role of mitochondria in producing Melatonin and driving GPCR signaling to block cytochrome c release. Proc. Natl. Acad. Sci. USA 2017, 114, 7997–8006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khizhkin, E.A.; Ilyukha, V.A.; Vinogradova, I.A.; Anisimov, V.N. Absence of photoperiodism and digestive enzymes in rats: The role of the age and endogenous Melatonin level. Adv. Gerontol. 2019, 32, 347–351. [Google Scholar] [CrossRef]
- Kvetnoy, I.M.; Ingel, I.E.; Kvetnaya, T.V.; Malinovskaya, N.K.; Rapoport, S.I.; Raikhlin, N.T.; Trofimov, A.V.; Yuzhakov, V.V. Gastrointestinal Melatonin: Cellular identification and biological role. Neuroendocrinol. Lett. 2002, 23, 121–132. [Google Scholar]
- Wang, R.X.; Liu, H.; Xu, L.; Zhang, H.; Zhou, R.X. Involvement of nuclear receptor RZR/RORγ in Melatonin-induced HIF-1α inactivation in SGC-7901 human gastric cancer cells. Oncol. Rep. 2015, 34, 2541–2546. [Google Scholar] [CrossRef] [Green Version]
- Ahluwalia, A.; Brzozowska, I.M.; Hoa, N.; Jones, M.K.; Tarnawski, A.S. Melatonin signaling in mitochondria extends beyond neurons and neuroprotection: Implications for angiogenesis and cardio/gastroprotection. Proc. Natl. Acad. Sci. USA 2018, 115, 1942–1943. [Google Scholar] [CrossRef] [Green Version]
- Mayo, J.C.; Aguado, A.; Cernuda-Cernuda, R.; Álvarez-Artime, A.; Cepas, V.; Quirós-González, I.; Hevia, D.; Sáinz, R.M. Melatonin Uptake by Cells: An Answer to Its Relationship with Glucose? Molecules 2018, 23, 1999. [Google Scholar] [CrossRef] [Green Version]
- Pal, P.K.; Bhattacharjee, B.; Chattopadhyay, A.; Bandyopadhyay, D. Pleiotropic roles of Melatonin against oxidative stress mediated tissue injury in the gastrointestinal tract: An overview. Melatonin Res. 2019, 2, 158–184. [Google Scholar] [CrossRef]
- Bubenik, G.A. Gastrointestinal Melatonin: Localization, function, and clinical relevance. Dig. Dis. Sci. 2002, 47, 2336–2348. [Google Scholar] [CrossRef]
- Pal, P.K.; Sarkar, S.; Chattopadhyay, A.; Tanc, D.-X.; Bandyopadhyay, D. Enterochromaffin cells as the source of Melatonin: Key findings and functional relevance in mammals. Melatonin Res. 2019, 2, 61–82. [Google Scholar] [CrossRef]
- Yasmin, F.; Sutradhar, S.; Das, P.; Mukherjee, S. Gut Melatonin: A potent candidate in the diversified journey of Melatonin research. Gen. Comp. Endocrinol. 2021, 303, 113693. [Google Scholar] [CrossRef]
- Sjoblom, M.; Flemstrom, G. Melatonin in the duodenal lumen is a potent stimulant of mucosal bicarbonate secretion. J. Pineal Res. 2003, 34, 288–293. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Noguera-Navarro, M.T.; Reiter, R.J.; Escames, G. Melatonin actions in the heart: More than a hormone. Melatonin Res. 2018, 1, 21–26. [Google Scholar] [CrossRef]
- Sommansson, A.; Nylander, O.; Sjoblom, M. Melatonin decreases duodenal epithelial paracellular permeability via a nicotinic receptor-dependent pathway in rats in vivo. J. Pineal Res. 2013, 54, 282–291. [Google Scholar] [CrossRef]
- Yin, J.; Li, Y.; Han, H.; Chen, S.; Gao, J.; Liu, G.; Wu, X.; Deng, J.; Yu, Q.; Huang, X.; et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in highfat dietfed mice. J. Pineal Res. 2018, 65. [Google Scholar] [CrossRef]
- Favero, G.; Franceschetti, L.; Bonomini, F.; Rodella, L.F.; Rezzani, R. Melatonin as an anti-inflammatory agent modulating inflammasome activation. Int. J. Endocrinol. 2017, 1835195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, J.; Li, Y.; Han, H.; Ma, J.; Liu, G.; Wu, H.; Huang, H.; Fang, R.; Baba, K.; Bin, P.; et al. Administration of exogenous Melatonin improves the diurnal rhythms of the gut microbiota in mice fed a high-fat diet. Systems 2020, 5, e00002-20. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhang, J.; Reiter, R.J.; Ma, X. Melatonin mediates mucosal immune cells, microbial metabolism, and rhythm crosstalk: A therapeutic target to reduce intestinal inflammation. Med. Res. Rev. 2020, 40, 606–632. [Google Scholar] [CrossRef] [PubMed]
- Gunawardene, A.R.; Corfe, B.M.; Staton, C.A. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int. J. Exp. Pathol. 2011, 92, 219–231. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; López, L.C.; Hitos, A.B.; León, J. Melatonin and nitric oxide: Two required antagonists for mitochondrial homeostasis. Endocrine 2005, 27, 159–168. [Google Scholar] [CrossRef]
- Sarkar, S.; Chattopadhyay, A.; Bandyopadhyay, D. Melatonin as a prospective metabolic regulator in pathologically altered cardiac energy homeostasis. Melatonin Res. 2021, 4, 316–335. [Google Scholar] [CrossRef]
- Segovia-Roldan, M.; Diez, E.R.; Pueyo, E. Melatonin to Rescue the Aged Heart: Antiarrhythmic and Antioxidant Benefits. Oxidative Med. Cell. Longev. 2021, 13, 1–18. [Google Scholar] [CrossRef]
- Ivanov, D.; Mironova, E.; Polyakova, V.; Evsyukova, I.; Osetrov, M.; Kvetnoy, I. Sudden infant death syndrome: Melatonin, serotonin, and CD34 factor as possible diagnostic markers and prophylactic targets. PLoS ONE 2021, 16, e0256197. [Google Scholar] [CrossRef]
- Slominski, A.; Fischer, T.W.; Zmijewski, M.A.; Wortsman, J.; Semak, I.; Zbytek, B.; Slominski, R.M.; Tobin, D.J. On the role of Melatonin in skin physiology and pathology. Endocrine 2005, 27, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Slominski, A.T.; Hardeland, R.; Zmijewski, M.A.; Slominski, R.M.; Reiter, R.J.; Paus, R. Melatonin: A Cutaneous Perspective on its Production, Metabolism, and Functions. J. Investig. Derm. 2018, 138. [Google Scholar] [CrossRef] [Green Version]
- Acuña-Castroviejo, D.; López, L.C.; Escames, G.; López, A.; García, J.A.; Reiter, R.J. Melatonin-mitochondria interplay in health and disease. Curr. Top. Med. Chem. 2011, 11, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagatam, Y.; Shimamuram, K.; et al. Oxidative stress impairs oocyte quality and Melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal Res. 2008, 44, 280–287. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Chuffa, L.G.; Lupi, L.A.; Cucielo, M.S.; Silveira, H.S.; Reiter, R.J.; Seiva, F.R.F. Melatonin Promotes Uterine and Placental Health: Potential Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlomagno, G.; Minini, M.; Tilotta, M.; Unfer, V. From Implantation to Birth: Insight into Molecular Melatonin Functions. Int. J. Mol. Sci. 2018, 19, 2802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olcese, J.M. Melatonin and Female Reproduction: An Expanding Universe. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef] [Green Version]
- Itoh, M.T.; Ishizuka, B.; Kuribayashi, Y.; Amemiya, A.; Sumi, Y. Melatonin, its precursors, and synthesizing enzyme activities in the human ovary. Mol. Hum. Reprod. 1999, 5, 402–408. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Wang, R.X.; Li, M.H.; Sun, T.C.; Zhou, Y.W.; Li, Y.Y.; Sun, L.H.; Zhang, B.L.; Lian, Z.X.; Xue, S.G.; et al. Melatonin Reduces Androgen Production and Upregulates Hem Oxygenase-1 Expression in Granulosa Cells from PCOS Patients with Hypoestrogenia and Hyperandrogenia. Oxid. Med. Cell. Longev. 2019, 2019, 8218650. [Google Scholar] [CrossRef]
- Tamura, H.; Nakamura, Y.; Terron, M.P.; Flores, L.J.; Manchester, L.S.; Tan, D.-X.; Sugino, N.; Reiter, R.J. Melatonin and pregnancy in the human. Reprod. Toxicol. 2008, 25, 291–303. [Google Scholar] [CrossRef]
- Lampiao, F.D.P.S. New developments of the effect of Melatonin on reproduction. World J. Obstet. Gynecol. 2013, 2, 8–15. [Google Scholar] [CrossRef]
- Yang, M.; Tao, J.; Wu, H.; Guan, S.; Liu, L.; Zhang, L.; Deng, S.; He, C.; Ji, P.; Liu, J.; et al. Aanat Knockdown and Melatonin Supplementation in Embryo Development: Involvement of Mitochondrial Function and DNA Methylation. Antioxid. Redox Signal 2019, 30, 2050–2065. [Google Scholar] [CrossRef]
- Cha, J.; Sun, X.; Dey, S.K. Mechanisms of implantation: Strategies for successful pregnancy. Nat. Med. 2012, 18, 1754–1767. [Google Scholar] [CrossRef]
- Ivanov, D.; Mazzoccoli, G.; Anderson, G.; Linkova, N.; Dyatlova, A.; Mironova, E.; Polyakova, V.; Kvetnoy, I.; Evsyukova, I.; Carbone, A. Melatonin, Its Beneficial Effects on Embryogenesis from Mitigating Oxidative Stress to Regulating Gene Expression. Int. J. Mol. Sci. 2021, 22, 5885. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.; Lacasse, A.A.; Lanoix, D.; Sagrillo-Fagundes, L.; Boulard, V.; Vaillancourt, C. Placental Melatonin system is present throughout pregnancy and regulates villous trophoblast differentiation. J. Pineal Res. 2015, 59, 38–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, S.; Nakazawa, K.; Sacai, J.; Kometani, K.; Iwashita, M.; Yoshimura, Y.; Maruyama, I. Melatonin as local regulator of human placental function. J. Pineal Res. 2005, 39, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Lanoix, D.; Beghdadi, H.; Lafond, J.; Vaillancourt, C. Human placental trophoblasts synthesize Melatonin and express its receptors. J. Pineal Res. 2008, 45, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Sagrillo-Fagundes, L.; Salustiano, E.M.A.; Ruano, R.; Markus, R.P.; Vaillancourt, C. Melatonin modulates autophagy and inflammation protecting human placental trophoblast from hypoxia/reoxygenation. J. Pieal Res. 2018, 65, 12520. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Tamura, H.; Kashida, S.; Nakayama, H.; Yamagata, Y.; Karube, A.; Sugino, N.; Kato, H. Changes of serum Melatonin level and its relationship to feto-placental unit during pregnancy. J. Pineal Res. 2001, 30, 29–33. [Google Scholar] [CrossRef]
- Okatani, Y.; Okamoto, K.; Hayashi, K.; Wakatsuki, A.; Tamura, S.; Sagara, Y. Materna-fetal transfer of Melatonin in pregnant women near term. J. Pineal Res. 1998, 125, 129–134. [Google Scholar] [CrossRef]
- Valenzuela, F.J.; Vera, J.; Venegas, C.; Pino, F.; Lagunas, C. Circadian System and Melatonin Hormone: Risk Factors for Complications during Pregnancy. Obstet. Gynecol. Int. 2015, 2015, 825802. [Google Scholar] [CrossRef]
- Ivanov, D.O.; Evsyukova, I.I.; Mazzoccoli, G.; Anderson, G.; Polyakova, V.O.; Kvetnoy, I.M.; Carbone, A.; Nasyrov, R.A. The Role of Prenatal Melatonin in the Regulation of Childhood Obesity. Biology 2020, 9, 72. [Google Scholar] [CrossRef] [Green Version]
- Ejaz, H.; Figaro, J.K.; Woolner, A.M.F.; Thottakam, B.M.V.; Galley, H.F. Maternal Serum Melatonin Increases During Pregnancy and Falls Immediately After Delivery Implicating the Placenta as a Major Source of Melatonin. Front. Endocrinol. 2021, 11, 1175. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Korkmaz, A.; Rosales-Corral, S.A. Melatonin and stabile circadian rhythms optimize maternal, placental and fetal physiology. Hum. Reprod. Update 2014, 20, 293–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okatani, Y.; Wakatsuki, A.; Shinohara, K.; Taniguchi, K.; Fukaya, T. Melatonin protects against oxidative mitochondrial damage induced in rat placenta by ischemia and reperfusion. J. Pineal Res. 2001, 31, 173–178. [Google Scholar] [CrossRef]
- Richter, H.J.; Hansell, J.A.; Raut, S.; Glussani, D.A. Melatonin improves placental efficiency and birth weight increases the placental expression of antioxidant enzymes in undernourished pregnancy. J. Pineal Res. 2009, 46, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, M.J.; Hu, B. Melatonin as a Mitochondria Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecule 2017, 23, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kivela, A. Serum Melatonin during human pregnancy. Acta Endocrinol. 1991, 124, 233–237. [Google Scholar] [CrossRef]
- Evsyukova, I.I. The Role of Melatonin in Prenatal Ontogenesis. J. Evol. Biochim. Physiol. 2021, 57, 33–43. [Google Scholar] [CrossRef]
- Thomas, J.E.; Purvis, C.C.; Drew, J.E.; Abramovich, D.R.; Williams, L.M. Melatonin receptors in human fetal brain: 2-[(125)]iodoMelatonin binding and Melatonin1 gene expression. J. Pineal Res. 2002, 33, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Lolova, I.S.; Davidoff, M.S.; Itzev, D.E. Histological and immunocytochemical data on the differentiation of intestinal endocrine cells in human fetus. Acta Physiol. Pharmacol. Bulg. 1998, 23, 61–71. [Google Scholar]
- Voiculescu, S.E.; Zygouropoulos, N.; Zahiu, C.D.; Zagrean, A.M. Role of Melatonin in embryo fetal development. J. Med. Life 2014, 7, 488–492. [Google Scholar]
- Weaver, D.R.; Rivkees, S.A.; Reppert, S.M. Localization and characterization of Melatonin receptors in rodent brain. J. Neurosci 1989, 9, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Ramracheva, R.D.; Muller, D.S.; Squires, P.E.; Brereton, H.; Sugden, D.; Huang, G.C.; Amiel, S.A.; Jones, P.M.; Persaud, S.J. Function and expression of Melatonin receptors on human pancreatic islets. J. Pineal Res. 2008, 44, 273–279. [Google Scholar] [CrossRef]
- Arendt, J. Melatonin and human rhythms. Chronobiol. Int. 2006, 23, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Seron-Ferre, M.; Torres-Farfan, C.; Valenzuela, F.J.; Castillo-Galan, S.; Rojas, A.; Mendez, N.; Reynolds, H.; Valenzuela, G.J.; Llanos, A.J. Deciphering the Function of the Blunt Circadian Rhythm of Melatonin in the Newborn Lamb: Impact on Adrenal and Heart. Endocrinology 2017, 158, 2895–2905. [Google Scholar] [CrossRef]
- Pontes, G.N.; Cardoso, E.C.; Carneiro-Sampaio, M.M.S.; Markus, R.P. Injury switches Melatonin production source from endocrine (pineal) to paracrine (phagocytes)—Melatonin in human colostrum and colostrum phagocytes. J. Pineal Res. 2006, 41, 136–141. [Google Scholar] [CrossRef]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [CrossRef]
- Akins, N.S.; Nielson, T.C.; Le, H.V. Inhibition of glycolysis and glutaminolysis: An emerging drug discovery approach to combat cancer. Curr. Top. Med. Chem. 2018, 201, 494–504. [Google Scholar] [CrossRef]
- Madeira, V.M.C. Overview of mitochondrial bioenergetics. Methods Mol. Biol. 2018, 1782, 1–6. [Google Scholar]
- Hickman, A.B.; Klein, D.C.; Dyda, F. Melatonin biosynthesis: The structure of serotonin N-acetyltransferase at 2.5 A resolution suggests a catalytic mechanism. Mol. Cell 1999, 3, 23–32. [Google Scholar] [CrossRef]
- Tatoyan, A.; Giulivi, C. Purification and characterization of a nitric-oxide synthase from rat liver mitochondria. J. Biol. Chem. 1998, 273, 11044–11048. [Google Scholar] [CrossRef] [Green Version]
- Jou, M.J.; Peng, T.I.; Reiter, R.J. Protective stabilization of mitochondrial permeability transition and mitochondrial oxidation during mitochondrial Ca2+ stress by Melatonin’s cascade metabolites C3-OHM and AFMK in RBA1 astrocytes. J. Pineal Res. 2019, 66, e12538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, X.; Wang, C.; Yu, Z.; Peng, Y.; Wang, S.; Feng, S.; Zhang, S.; Tian, X.; Sun, C.; Liu, K.; et al. Human transporters, PEPT1/2, facilitate Melatonin transportation into mitochondria of cancer cells: An implication of the therapeutic potential. J. Pineal Res 2017, 62, e12390. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sainz, R.M.; González-Menéndez, P.; Hevia, D.; Cernuda-Cernuda, R. Melatonin transport into mitochondria. Cell. Mol. Life. Sci 2017, 74, 3927–3940. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Mayo, J.C.; Sain, R.M.; Antoli, I.; Herrera, F.; Martin, V.; Rodriguez, C. Melatonin regulation of antioxidant enzyme gene expression. Cell. Mol. Life. Sci. 2002, 59, 1706–1713. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. New insights into the role of Melatonin in plants and animals. Chem. Biol. Interact 2019, 299, 163–167. [Google Scholar] [CrossRef]
- Zhao, Z.; Lu, C.; Li, T.; Wang, W.; Ye, W.; Zeng, R.; Ni, L.; Lai, Z.; Wang, X.; Liu, C. The Protective Effect of Melatonin on Brain Ischemia and Reperfusion in Rats and Humans: In Vivo Assessment and a Randomized Controlled Trial. J. Pineal Res. 2018, 65, e12521. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the free radical scavenging activities of Melatonin’s metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef]
- Ressmeyer, A.R.; Mayo, J.C.; Zelosko, V.; Sáinz, R.M.; Tan, D.X.; Poeggeler, B.; Antolín, I.; Zsizsik, B.K.; Reiter, R.J.; Hardeland, R. Antioxidant properties of the Melatonin metabolite N1-acetyl-5-methoxykynuramine (AMK): Scavenging of free radicals and prevention of protein destruction. Redox Rep. 2003, 8, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Acuna-Castroviejo, D.; Escames, G.; Rodriguez, M.I.; Lopez, L.C. Melatonin: Role in the mitochondrial function. Front. Bioschen 2007, 12, 947–963. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Pilar Terron, M.; Flores, L.J.; Koppisepi, S. Medical implications of Melatonin: Receptor mediated and receptor-independent actions. Adv. Med. Sci. 2007, 52, 11–28. [Google Scholar]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, A.; Ordóñez, R.; Reiter, R.J.; González-Gallego, J.; Mauriz, J.L. Melatonin and endoplasmic reticulum stress: Relation to autophagy and apoptosis. J. Pineal Res. 2015, 59, 292–307. [Google Scholar] [CrossRef]
- Mayo, J.C.; Sainz, R.M.; González Menéndez, P.; Cepas, V.; Tan, D.X.; Reiter, R.J. Melatonin and sirtuins: A “not-so unexpected” relationship. J. Pineal Res. 2017, 62, e12391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, F.; Sandhu, A.F.; Rungratanawanich, W.; Williams, G.E.; Akbar, M.; Zhou, S.; Song, B.J.; Wang, X. Melatonin and Autophagy in Aging-Related Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 7174. [Google Scholar] [CrossRef] [PubMed]
- Barja, G. Towards a unified mechanistic theory of aging. Exp. Gerontol. 2019, 124, 110627. [Google Scholar] [CrossRef]
- Reiter, R.J.; Sharma, R.; de Campos Zuccari, D.A.P.; de Almeida Chuffa, L.G.; Manucha, W.; Rodriguez, C. Melatonin synthesis in and uptake by mitochondria: Implications for diseased cells with dysfunctional mitochondria. Future Med. Chem 2021, 13, 335–339. [Google Scholar] [CrossRef]
- Ivanov, D.O.; Evsyukova, I.I.; Mironova, E.S.; Polyakova, V.O.; Kvetnoy, I.M.; Nasyrov, R.A. Maternal Melatonin Deficiency Leads to Endocrine Pathologies in Children in Early Ontogenesis. Int. J. Mol. Sci. 2021, 22, 2058. [Google Scholar] [CrossRef]
- Opie, L.H.; Lecour, S. Melatonin Has Multiorgan Effects. Eur. Heart J. Cardiovasc. Pharm. 2016, 2, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.H.; Xu, J.H.; Ren, Q.C.; Duan, T.; Mo, F.; Zhang, W. Melatonin Promotes Secondary Hair Follicle Development of Early Postnatal Cashmere Goat and Improves Cashmere Quantity and Quality by Enhancing Antioxidant Capacity and Suppressing Apoptosis. J. Pineal Res. 2019, 67, e12569. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Zheng, X.; Du, X. Therapeutic Strategies of Melatonin in Cancer Patients: A Systematic Review and Meta-Analysis. Oncol. Targets 2018, 11, 7895–7908. [Google Scholar]
- Laudon, M.; Frydman-Marom, A. Therapeutic Eects of Melatonin Receptor Agonists on Sleep and Comorbid Disorders. Int. J. Mol. Sci. 2014, 15, 15924–15950. [Google Scholar] [CrossRef] [Green Version]
- Robertson, N.J.; Faulkner, S.; Fleiss, B.; Bainbridge, A.; Andorka, C.; Price, D.; Powell, E.; Lecky-Thompson, L.; Thei, L.; Chandrasekaran, M. Melatonin Augments Hypothermic Neuroprotection in a Perinatal Asphyxia Model. Brain 2013, 136, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Hardeland, R. Inflammaging, Metabolic Syndrome and Melatonin: A Call for Treatment Studies. Neuroendocrinology 2017, 104, 382–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez-Rodriguez, A.; Abreu-Gonzalez, P. Future Strategies for Acute Cardioprotection: “Melatonin as Promising Therapy”. Cardiovasc. Res. 2017, 113, 1418. [Google Scholar] [CrossRef]
- Zhou, H.; Ma, Q.; Zhu, P.; Ren, J.; Reiter, R.J.; Chen, Y. Protective Role of Melatonin in Cardiac Ischemia-Reperfusion Injury: From Pathogenesis to Targeted Therapy. J. Pineal Res. 2018, 64, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Zephy, D.; Ahmad, J. Type 2 Diabetes Mellitus: Role of Melatonin and Oxidative Stress. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 127–131. [Google Scholar] [CrossRef]
- Rehman, J.; Zhang, H.J.; Toth, P.T.; Zhang, Y.; Marsboom, G.; Hong, Z.; Salgia, R.; Husain, A.N.; Wietholt, C.; Archer, S.L. Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J. 2012, 26, 2175–2186. [Google Scholar] [CrossRef] [Green Version]
- Alghamdi, B.S. The Neuroprotective Role of Melatonin in Neurological Disorders. J. Neurosci. Res. 2018, 96, 1136–1149. [Google Scholar] [CrossRef]
- Carbone, A.; Linkova, N.; Polyakova, V.; Mironova, E.; Hashimova, U.; Gadzhiev, A.; Safikhanova, K.; Kvetnaia, T.; Krylova, J.; Tarquini, R.; et al. Melatonin and Sirtuins in Buccal Epithelium: Potential Biomarkers of Aging and Age-Related Pathologies. Int. J. Mol. Sci. 2020, 21, 8134. [Google Scholar] [CrossRef]
- Cardinali, D.P. Melatonin: Clinical Perspectives in Neurodegeneration. Front. Endocrinol. 2019, 10, 480. [Google Scholar] [CrossRef] [PubMed]
- Menegardo, C.S.; Friggi, F.A.; Scardini, J.B.; Rossi, T.S.; Vieira, T.D.S.; Tieppo, A.; Morelato, R.L. Sundown Syndrome in Patients with Alzheimer’s Disease Dementia. Dement. Neuropsychol. 2019, 13, 469–474. [Google Scholar] [CrossRef]
- Paul, R.; Phukan, B.C.; Justin Thenmozhi, A.; Manivasagam, T.; Bhattacharya, P.; Borah, A. Melatonin Protects against Behavioral Deficits, Dopamine Loss and Oxidative Stress in Homocysteine Model of Parkinson’s Disease. Life Sci. 2018, 192, 238–245. [Google Scholar] [CrossRef]
- Maestroni, G. Exogenous melatonin as potential adjuvant in anti-SarsCov2 vaccines. J. Neuroimmune Pharm. 2020, 15, 572–573. [Google Scholar] [CrossRef] [PubMed]
- Dowling, G.A.; Mastick, J.; Colling, E.; Carter, J.H.; Singer, C.M.; Amino, M.J. Melatonin for Sleep Disturbances in Parkinson’s Disease. Sleep Med. 2005, 6, 459–466. [Google Scholar] [CrossRef] [PubMed]

| Gene-Targets/Signaling Pathways | MT Effects | Physiological/Pathological Manifestations |
| PTEN/AKT [76] | Stimulating | Anti-inflammation |
| SIRT1 [77] | Stimulating | Anti-aging |
| TOLLR4 [77] | Stimulating | Anti-aging, immunomodulation |
| iNO [77] | Inhibitory | Anti-oxidative |
| NRF-2, CBR1, CLPP, SOD2 [78] | Inhibitory | Anti-oxidative |
| ANAPC4, HSPA4/Ubiquitination pathway [77] | Inhibitory | Neuroprotection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kvetnoy, I.; Ivanov, D.; Mironova, E.; Evsyukova, I.; Nasyrov, R.; Kvetnaia, T.; Polyakova, V. Melatonin as the Cornerstone of Neuroimmunoendocrinology. Int. J. Mol. Sci. 2022, 23, 1835. https://doi.org/10.3390/ijms23031835
Kvetnoy I, Ivanov D, Mironova E, Evsyukova I, Nasyrov R, Kvetnaia T, Polyakova V. Melatonin as the Cornerstone of Neuroimmunoendocrinology. International Journal of Molecular Sciences. 2022; 23(3):1835. https://doi.org/10.3390/ijms23031835
Chicago/Turabian StyleKvetnoy, Igor, Dmitry Ivanov, Ekaterina Mironova, Inna Evsyukova, Ruslan Nasyrov, Tatiana Kvetnaia, and Victoria Polyakova. 2022. "Melatonin as the Cornerstone of Neuroimmunoendocrinology" International Journal of Molecular Sciences 23, no. 3: 1835. https://doi.org/10.3390/ijms23031835
APA StyleKvetnoy, I., Ivanov, D., Mironova, E., Evsyukova, I., Nasyrov, R., Kvetnaia, T., & Polyakova, V. (2022). Melatonin as the Cornerstone of Neuroimmunoendocrinology. International Journal of Molecular Sciences, 23(3), 1835. https://doi.org/10.3390/ijms23031835






