The Roles of Two CNG Channels in the Regulation of Ascidian Sperm Chemotaxis
Abstract
:1. Introduction
2. Results
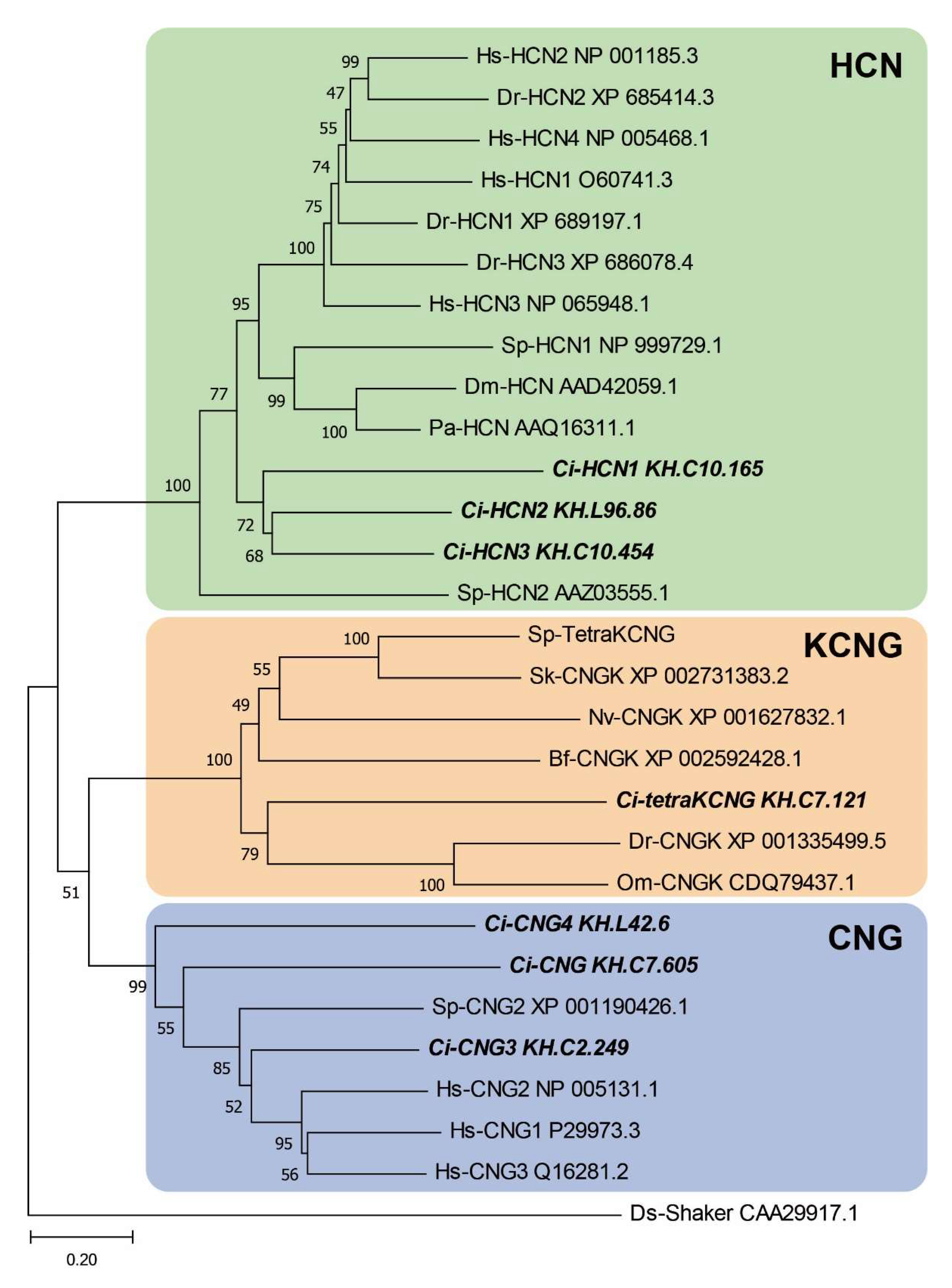
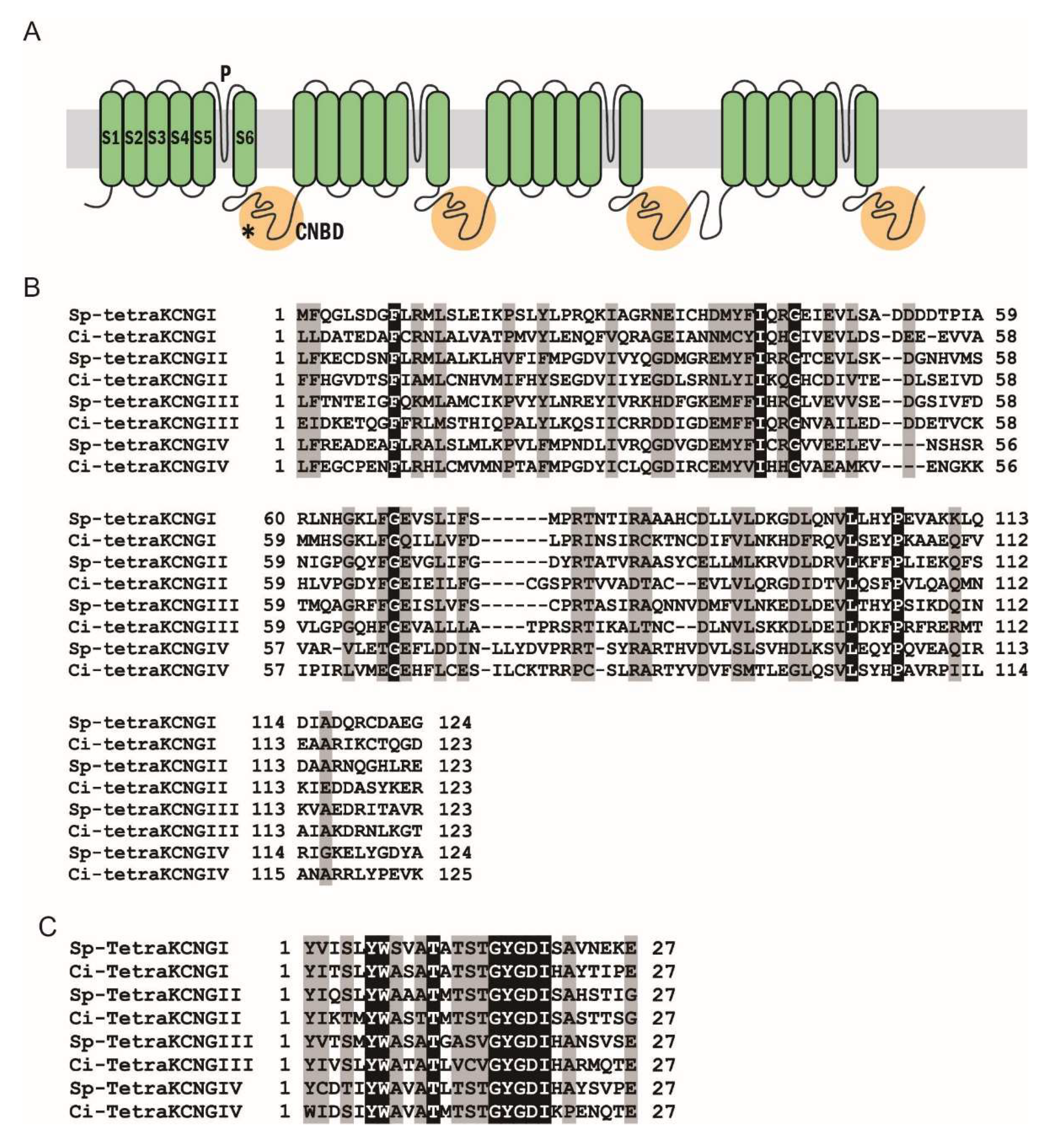
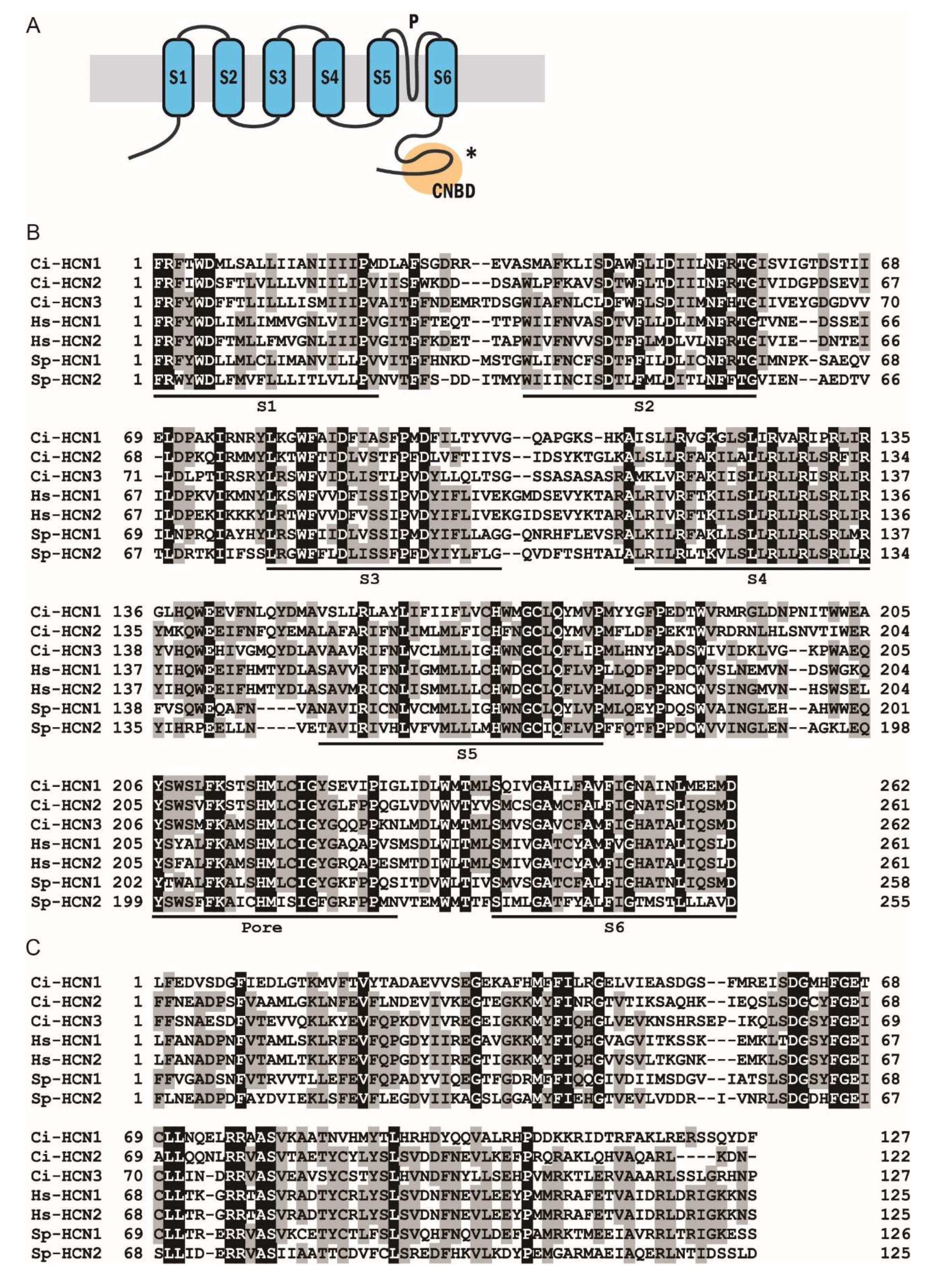
2.1. Phylogenetic and Sequence Analysis
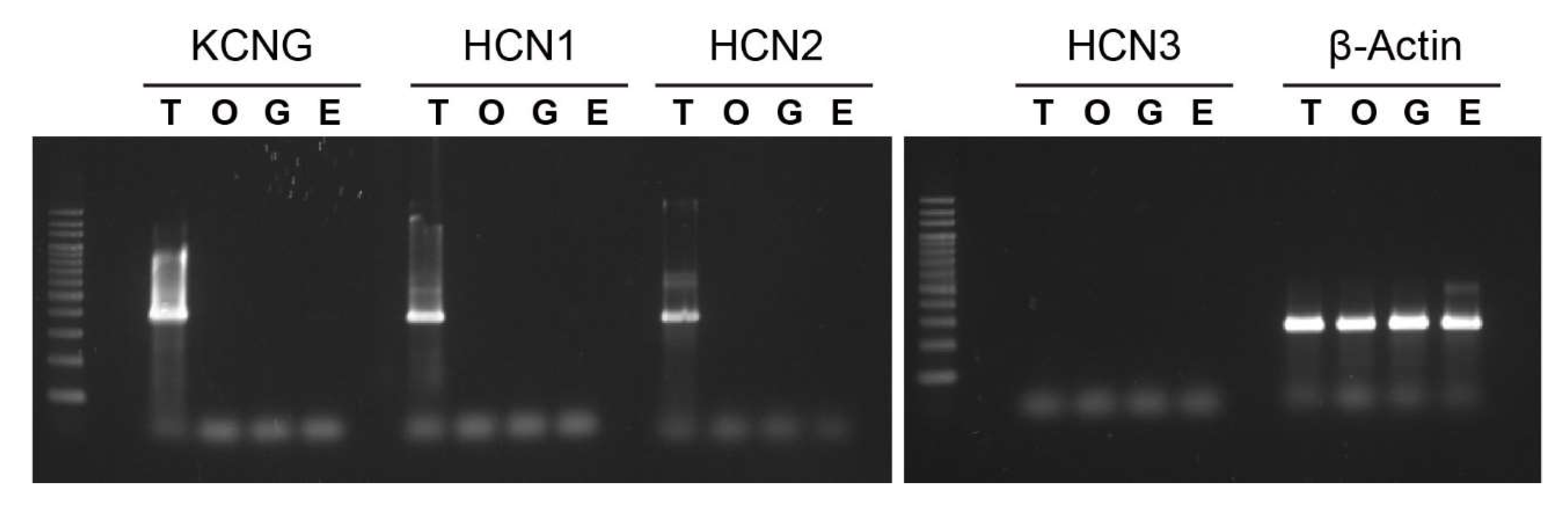
2.2. Expression of Tetra-KCNG and HCN Genes in Ciona Testis
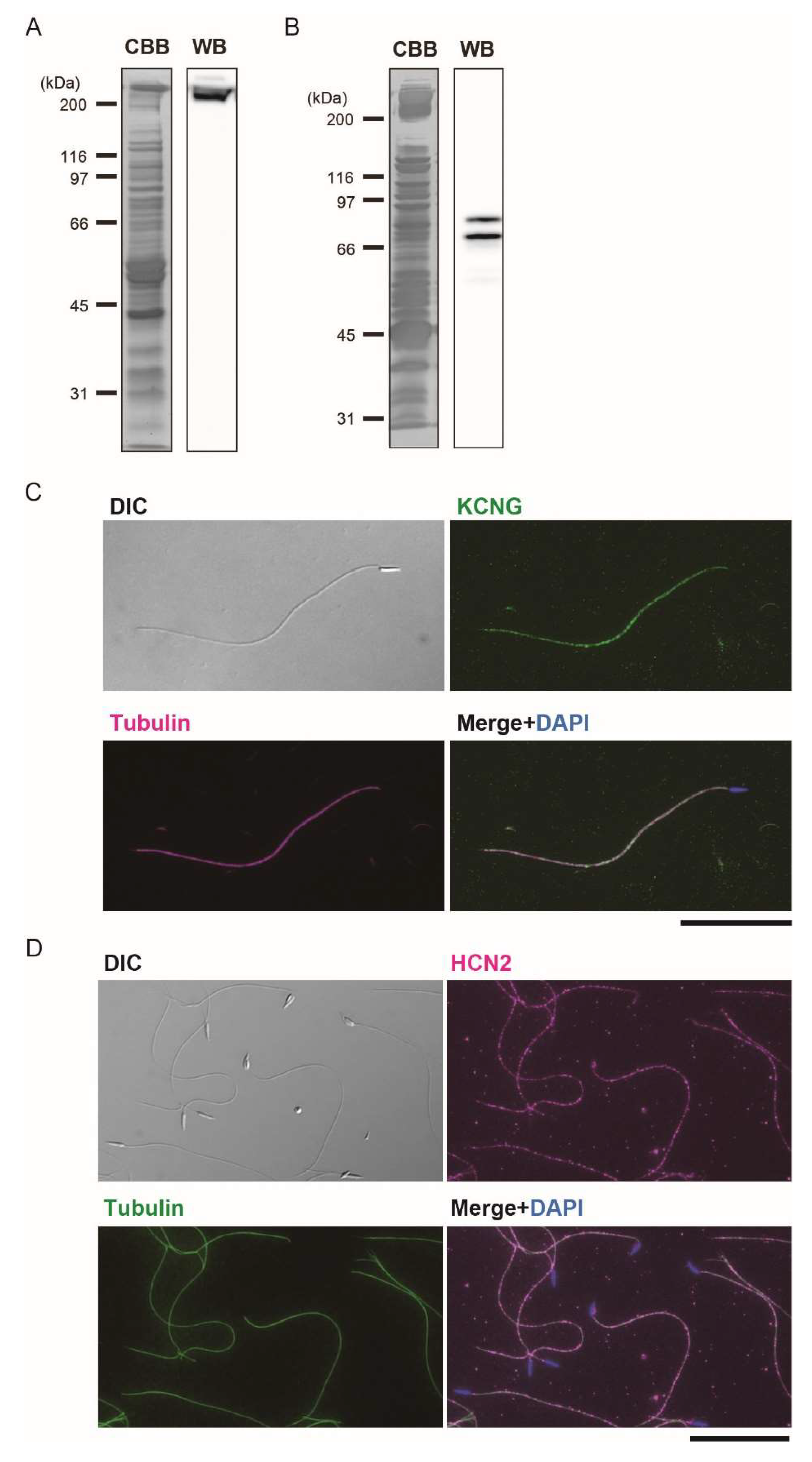
2.3. Immunoblotting and Immunofluorescent Microscopy
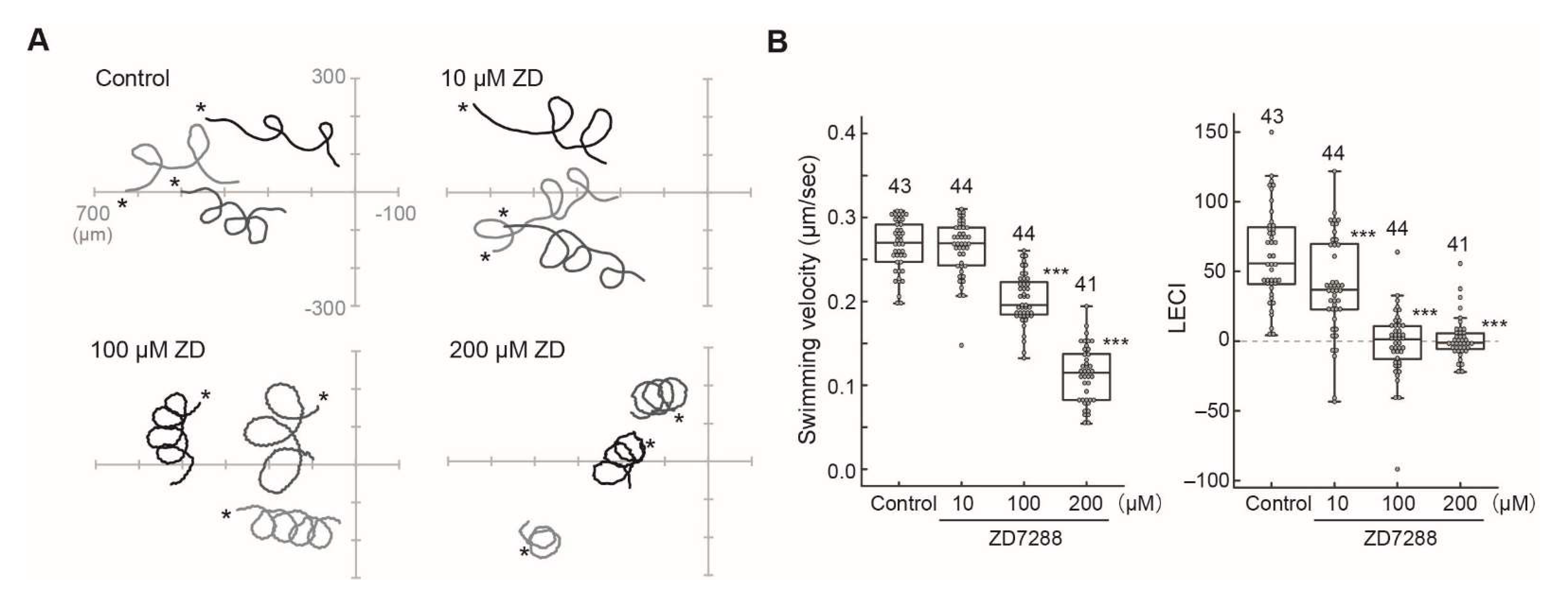
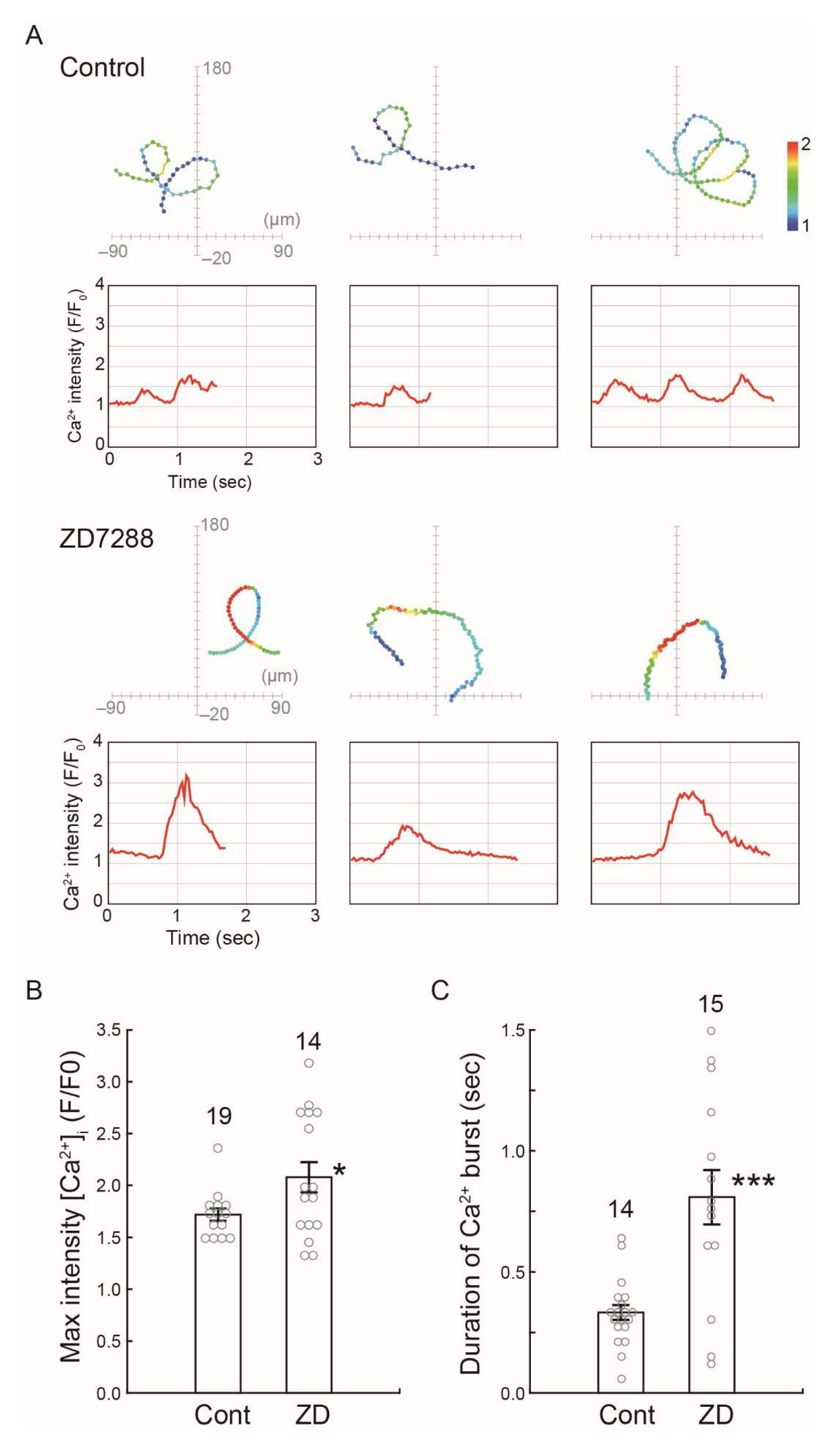
2.4. Effects of HCN Inhibitors on the Chemotaxis of Ciona Sperm
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chemical and Solutions
4.3. Sequence Analysis
4.4. Reverse-Transcriptase (RT) PCR
4.5. Preparation of Antibodies and Immunoblotting
4.6. Immunofluorescence Microscopy
4.7. Assessment of Sperm Chemotaxis
4.8. Imaging Analysis of Intracellular Ca2+
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inaba, K. Molecular Architecture of the Sperm Flagella: Molecules for Motility and Signaling. Zool. Sci. 2003, 20, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Wachten, D.; Jikeli, J.F.; Kaupp, U.B. Sperm Sensory Signaling. Cold Spring Harb. Perspect. Biol. 2017, 9, a028225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darszon, A.; Nishigaki, T.; Beltran, C.; Trevino, C.L. Calcium Channels in the Development, Maturation, and Function of Spermatozoa. Physiol. Rev. 2011, 91, 1305–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishigaki, T.; José, O.; Gonzalez-Cota, A.L.; Romero, F.; Trevino, C.L.; Darszon, A. Intracellular pH in sperm physiology. Biochem. Biophys. Res. Commun. 2014, 450, 1149–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lishko, P.V.; Kirichok, Y.; Ren, D.; Navarro, B.; Chung, J.-J.; Clapham, D.E. The Control of Male Fertility by Spermatozoan Ion Channels. Annu. Rev. Physiol. 2012, 74, 453–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hozumi, A.; Padma, P.; Toda, T.; Ide, H.; Inaba, K. Molecular characterization of axonemal proteins and signaling molecules responsible for chemoattractant-induced sperm activation inCiona intestinalis. Cell Motil. Cytoskelet. 2008, 65, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Shiba, K.; Inaba, K. Distinct Roles of Soluble and Transmembrane Adenylyl Cyclases in the Regulation of Flagellar Motility in Ciona Sperm. Int. J. Mol. Sci. 2014, 15, 13192–13208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, M.; Murata, M.; Inaba, K.; Morisawa, M. A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proc. Natl. Acad. Sci. USA 2002, 99, 14831–14836. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Shiba, K.; Sakamoto, A.; Ikenaga, J.; Matsunaga, S.; Inaba, K.; Yoshida, M. Ca2+ efflux via plasma membrane Ca2+-ATPase mediates chemotaxis in ascidian sperm. Sci. Rep. 2018, 8, 16622. [Google Scholar] [CrossRef]
- Gauss, R.; Seifert, R.; Kaupp, U.B. Molecular identification of a hyperpolarization-activated channel in sea urchin sperm. Nature 1998, 393, 583–587. [Google Scholar] [CrossRef]
- Galindo, B.E.; Neill, A.T.; Vacquier, V.D. A new hyperpolarization-activated, cyclic nucleotide-gated channel from sea urchin sperm flagella. Biochem. Biophys. Res. Commun. 2005, 334, 96–101. [Google Scholar] [CrossRef]
- Galindo, B.E.; de la Vega-Beltrán, J.L.; Labarca, P.; Vacquier, V.D.; Darszon, A. Sp-tetraKCNG: A novel cyclic nucleotide gated K+ channel. Biochem. Biophys. Res. Commun. 2007, 354, 668–675. [Google Scholar] [CrossRef]
- Bönigk, W.; Loogen, A.; Seifert, R.; Kashikar, N.; Klemm, C.; Krause, E.; Hagen, V.; Kremmer, E.; Strünker, T.; Kaupp, U.B. An Atypical CNG Channel Activated by a Single cGMP Molecule Controls Sperm Chemotaxis. Sci. Signal. 2009, 2, ra68. [Google Scholar] [CrossRef]
- Strünker, T.; Weyand, I.; Bönigk, W.; Van, Q.; Loogen, A.; Brown, J.E.; Kashikar, N.; Hagen, V.; Krause, E.; Kaupp, U.B. A K+-selective cGMP-gated ion channel controls chemosensation of sperm. Nat. Cell Biol. 2006, 8, 1149–1154. [Google Scholar] [CrossRef]
- Inaba, K.; Padma, P.; Satouh, Y.; Shin-I, T.; Kohara, Y.; Satoh, N.; Satou, Y. EST analysis of gene expression in testis of the ascidianCiona intestinalis. Mol. Reprod. Dev. 2002, 62, 431–445. [Google Scholar] [CrossRef]
- Fechner, S.; Alvarez, L.; Bönigk, W.; Müller, A.; Berger, T.K.; Pascal, R.; Troetschel, C.; Poetsch, A.; Stölting, G.; Siegfried, K.; et al. A K+-selective CNG channel orchestrates Ca2+ signalling in zebrafish sperm. eLife 2015, 4, e07624. [Google Scholar] [CrossRef]
- Nishigaki, T.; Zamudio, F.Z.; Possani, L.D.; Darszon, A. Time-Resolved Sperm Responses to an Egg Peptide Measured by Stopped-Flow Fluorometry. Biochem. Biophys. Res. Commun. 2001, 284, 531–535. [Google Scholar] [CrossRef]
- Kinoshita-Terauchi, N.; Shiba, K.; Terauchi, M.; Romero, F.; Ramírez-Gómez, H.V.; Yoshida, M.; Motomura, T.; Kawai, H.; Nishigaki, T. High potassium seawater inhibits ascidian sperm chemotaxis, but does not affect the male gamete chemotaxis of a brown alga. Zygote 2019, 27, 225–231. [Google Scholar] [CrossRef]
- Kaupp, U.B.; Solzin, J.; Hildebrand, E.; Brown, J.E.; Helbig, A.; Hagen, V.; Beyermann, M.; Pampaloni, F.; Weyand, I. The signal flow and motor response controling chemotaxis of sea urchin sperm. Nat. Cell Biol. 2003, 5, 109–117. [Google Scholar] [CrossRef]
- Seifert, R.; Flick, M.; Bönigk, W.; Alvarez, L.; Troetschel, C.; Poetsch, A.; Müller, A.; Goodwin, N.; Pelzer, P.; Kashikar, N.D.; et al. The C at S per channel controls chemosensation in sea urchin sperm. EMBO J. 2015, 34, 379–392. [Google Scholar] [CrossRef] [Green Version]
- Shiba, K.; Baba, S.A.; Inoue, T.; Yoshida, M. Ca2+ bursts occur around a local minimal concentration of attractant and trigger sperm chemotactic response. Proc. Natl. Acad. Sci. USA 2008, 105, 19312–19317. [Google Scholar] [CrossRef] [Green Version]
- Darszon, A.; Guerrero, A.; Galindo, B.E.; Nishigaki, T.; Wood, C.D. Sperm-activating peptides in the regulation of ion fluxes, signal transduction and motility. Int. J. Dev. Biol. 2008, 52, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Kaupp, U.B.; Hildebrand, E.; Weyand, I. Sperm chemotaxis in marine invertebrates—Molecules and mechanisms. J. Cell. Physiol. 2006, 208, 487–494. [Google Scholar] [CrossRef]
- Bentley, J.K.; Shimomura, H.; Garbers, D.L. Retention of a functional resact receptor in isolated sperm plasma membranes. Cell 1986, 45, 281–288. [Google Scholar] [CrossRef]
- Nakachi, M.; Nakajima, A.; Nomura, M.; Yonezawa, K.; Ueno, K.; Endo, T.; Inaba, K. Proteomic profiling reveals compartment-specific, novel functions of ascidian sperm proteins. Mol. Reprod. Dev. 2011, 78, 529–549. [Google Scholar] [CrossRef]
- Nomura, M.; Inaba, K.; Morisawa, M. Cyclic AMP- and calmodulin-dependent phosphorylation of 21 and 26 kDa proteins in axoneme is a prerequisite for SAAF-induced motile activation in ascidian spermatozoa. Dev. Growth Differ. 2000, 42, 129–138. [Google Scholar] [CrossRef]
- Wertheimer, E.; Krapf, D.; de la Vega-Beltran, J.L.; Sánchez-Cárdenas, C.; Navarrete, F.; Haddad, D.; Escoffier, J.; Salicioni, A.M.; Levin, L.R.; Buck, J.; et al. Compartmentalization of Distinct cAMP Signaling Pathways in Mammalian Sperm. J. Biol. Chem. 2013, 288, 35307–35320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speer, K.F.; Allen-Waller, L.; Novikov, D.R.; Barott, K.L. Molecular mechanisms of sperm motility are conserved in an early-branching metazoan. Proc. Natl. Acad. Sci. USA 2021, 118, e2109993118. [Google Scholar] [CrossRef] [PubMed]
- Shiba, K.; Márián, T.; Krasznai, Z.; Baba, S.A.; Morisawa, M.; Yoshida, M. Na+/Ca2+ exchanger modulates the flagellar wave pattern for the regulation of motility activation and chemotaxis in the ascidian spermatozoa. Cell Motil. Cytoskelet. 2006, 63, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Oishi, T.; Tsuchikawa, H.; Murata, M.; Yoshida, M.; Morisawa, M. Synthesis of endogenous sperm-activating and attracting factor isolated from ascidian Ciona intestinalis. Tetrahedron Lett. 2003, 44, 6387–6389. [Google Scholar] [CrossRef]
- Oishi, T.; Tsuchikawa, H.; Murata, M.; Yoshida, M.; Morisawa, M. Synthesis and identification of an endogenous sperm activating and attracting factor isolated from eggs of the ascidian Ciona intestinalis; an example of nanomolar-level structure elucidation of novel natural compound. Tetrahedron 2004, 60, 6971–6980. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiba, K.; Inaba, K. The Roles of Two CNG Channels in the Regulation of Ascidian Sperm Chemotaxis. Int. J. Mol. Sci. 2022, 23, 1648. https://doi.org/10.3390/ijms23031648
Shiba K, Inaba K. The Roles of Two CNG Channels in the Regulation of Ascidian Sperm Chemotaxis. International Journal of Molecular Sciences. 2022; 23(3):1648. https://doi.org/10.3390/ijms23031648
Chicago/Turabian StyleShiba, Kogiku, and Kazuo Inaba. 2022. "The Roles of Two CNG Channels in the Regulation of Ascidian Sperm Chemotaxis" International Journal of Molecular Sciences 23, no. 3: 1648. https://doi.org/10.3390/ijms23031648
APA StyleShiba, K., & Inaba, K. (2022). The Roles of Two CNG Channels in the Regulation of Ascidian Sperm Chemotaxis. International Journal of Molecular Sciences, 23(3), 1648. https://doi.org/10.3390/ijms23031648





