Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine
Abstract
:1. Introduction
2. The Role of Essential Transition Metals in Living Systems
3. The Potential Antioxidant/Pro-Oxidant Effects of Ascorbic Acid
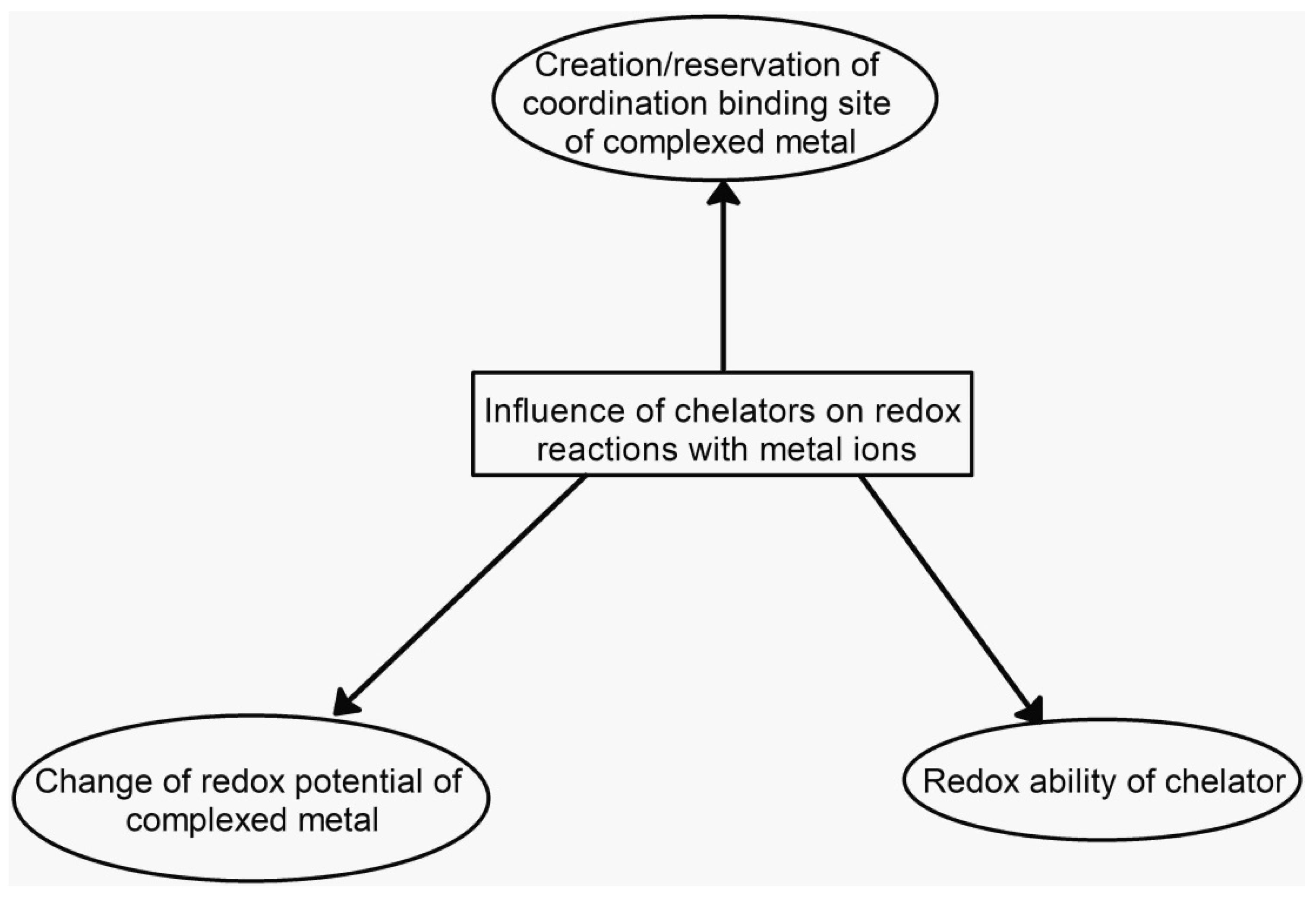
4. Redox Properties and Biological Implications of Chelating Drugs
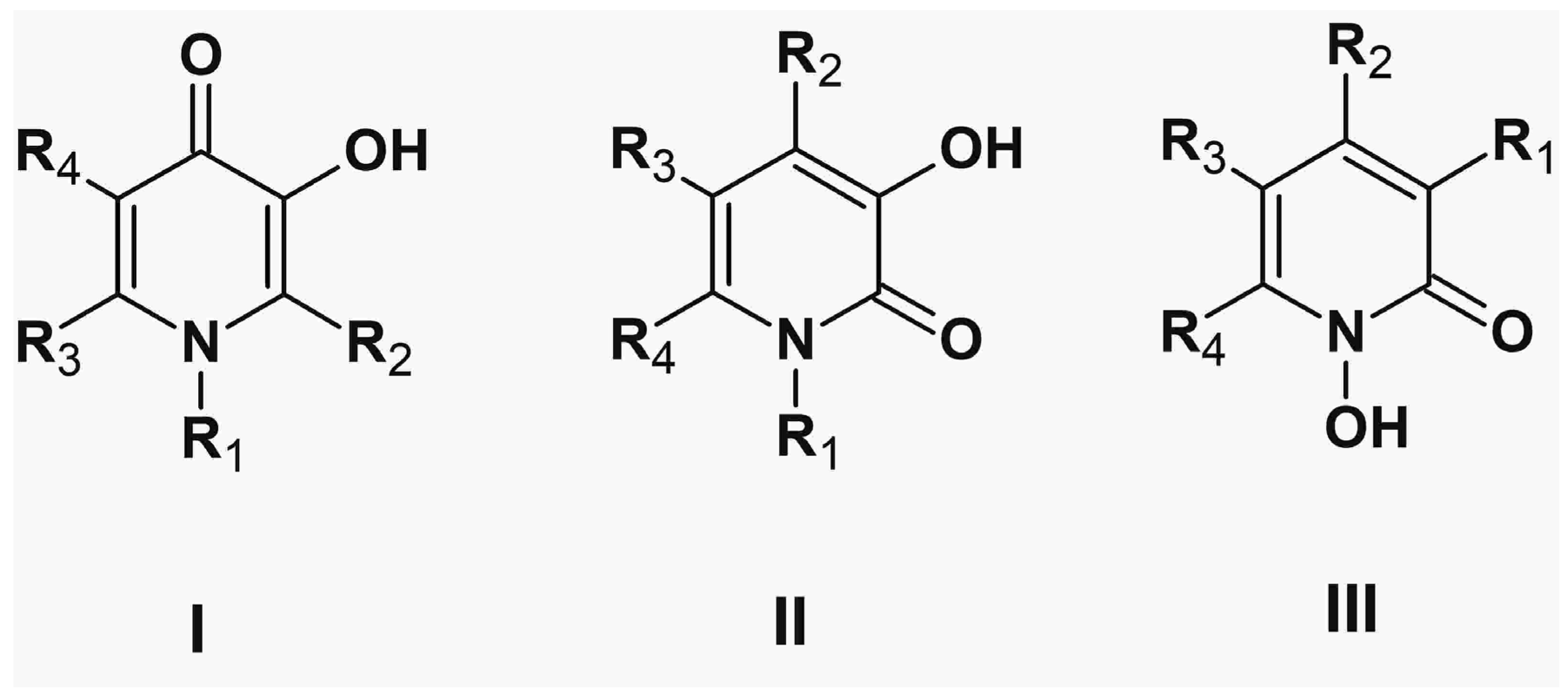
5. Alpha-Ketohydroxypyridine Chelators
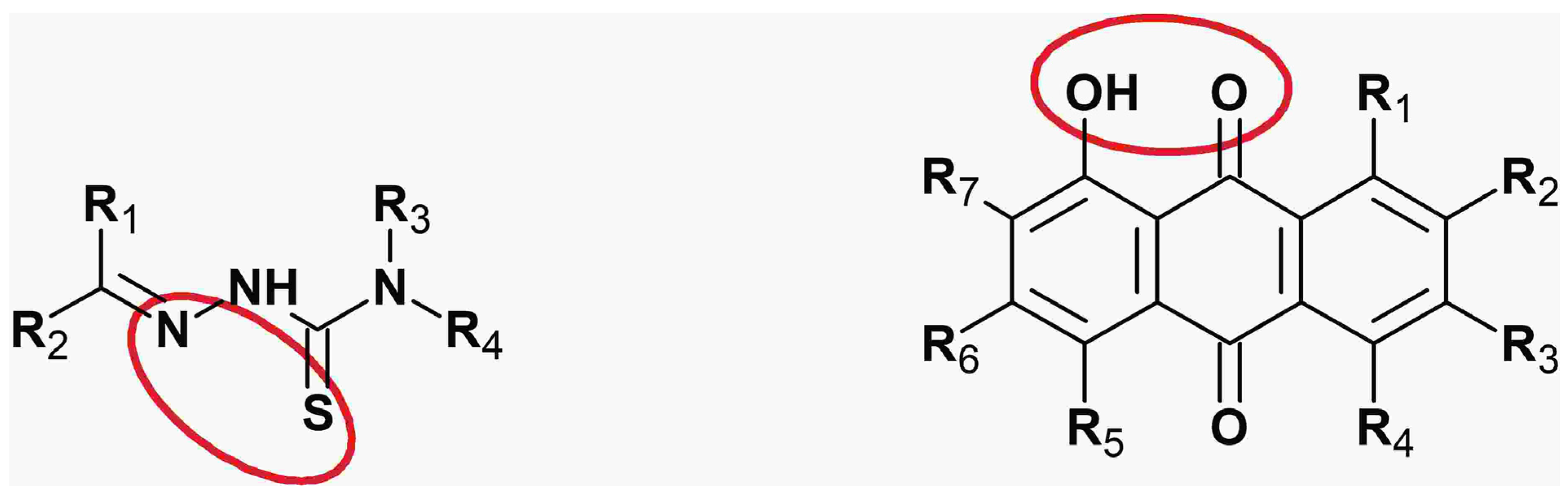
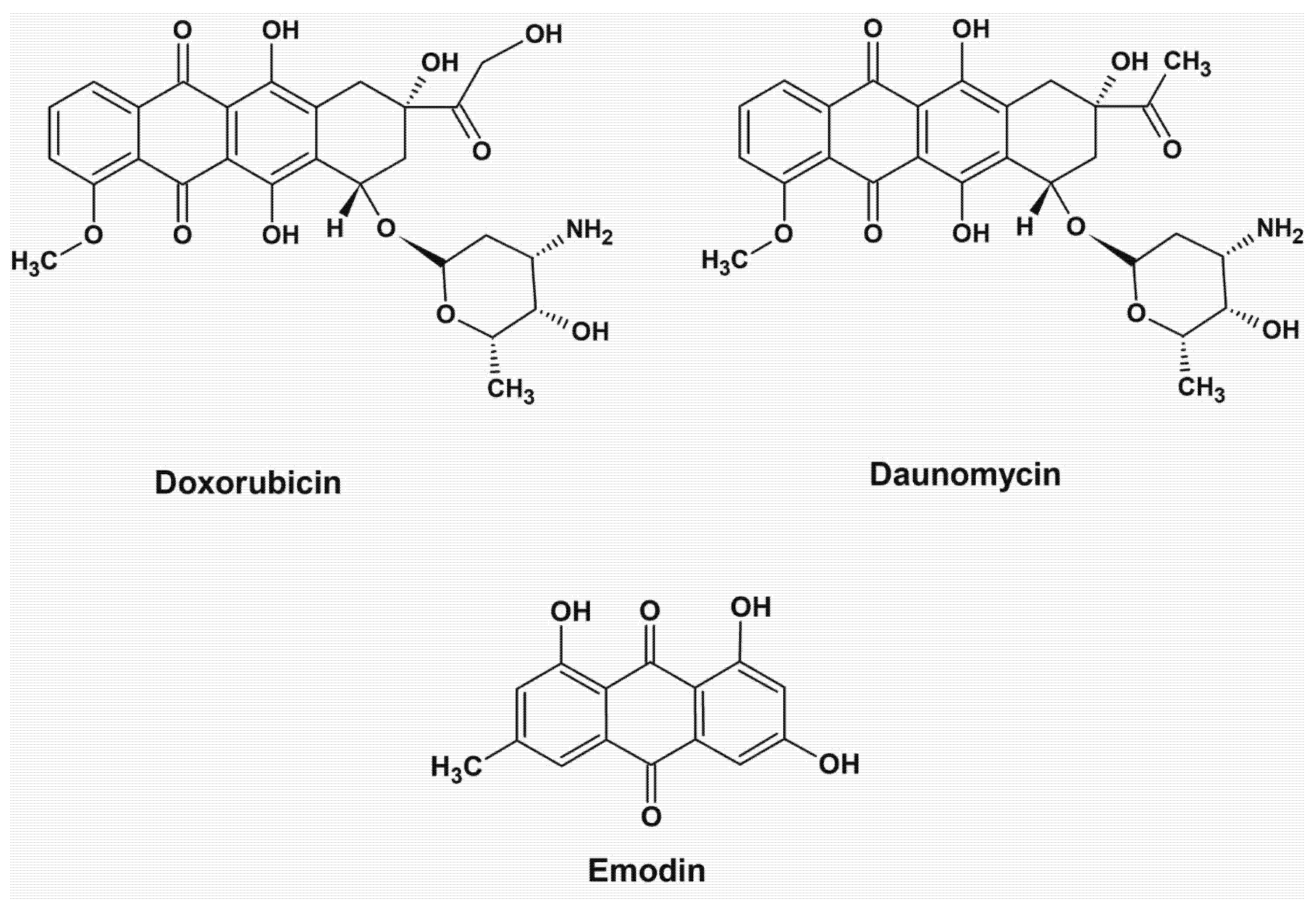
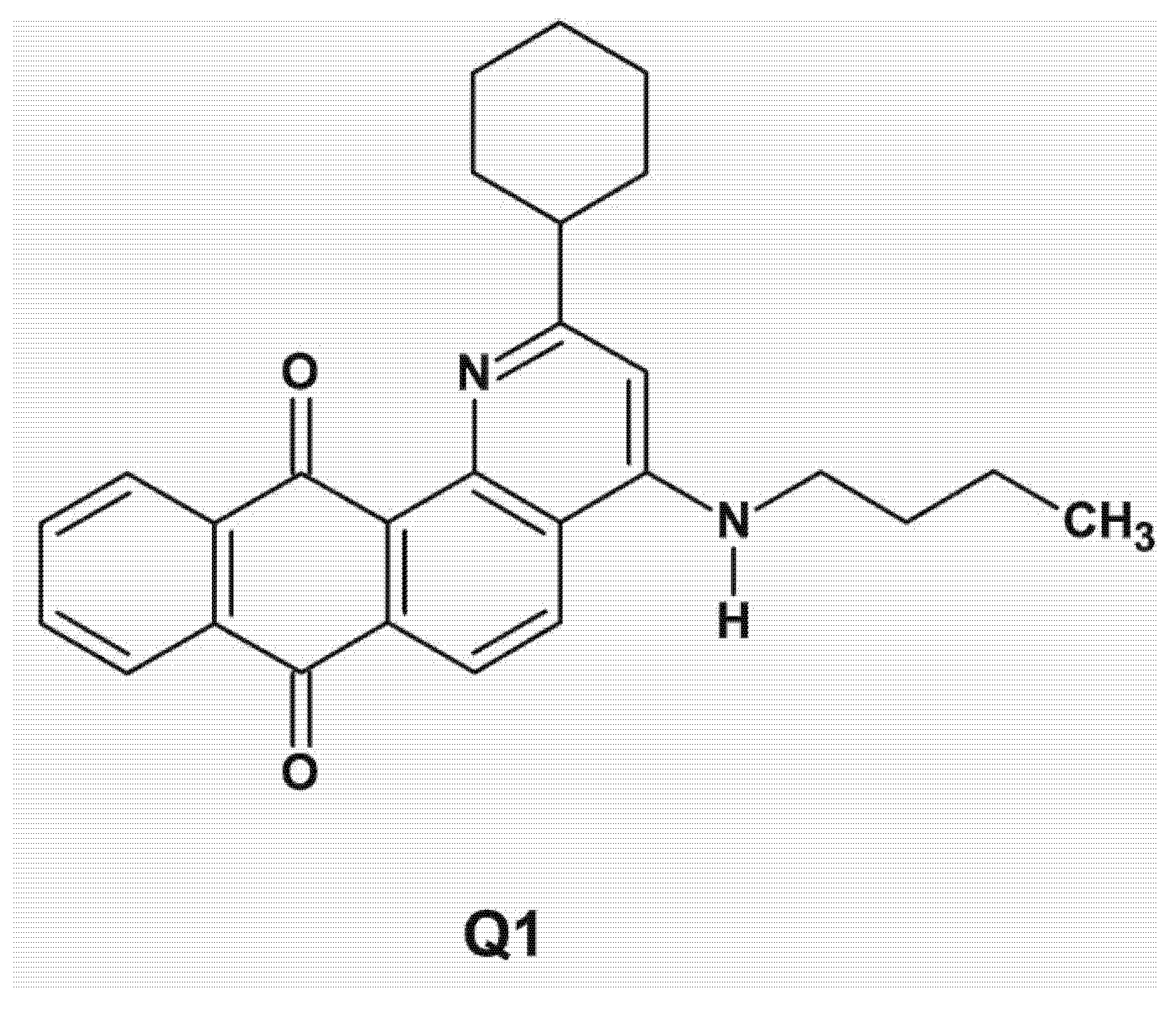
6. Anthraquinone Chelators
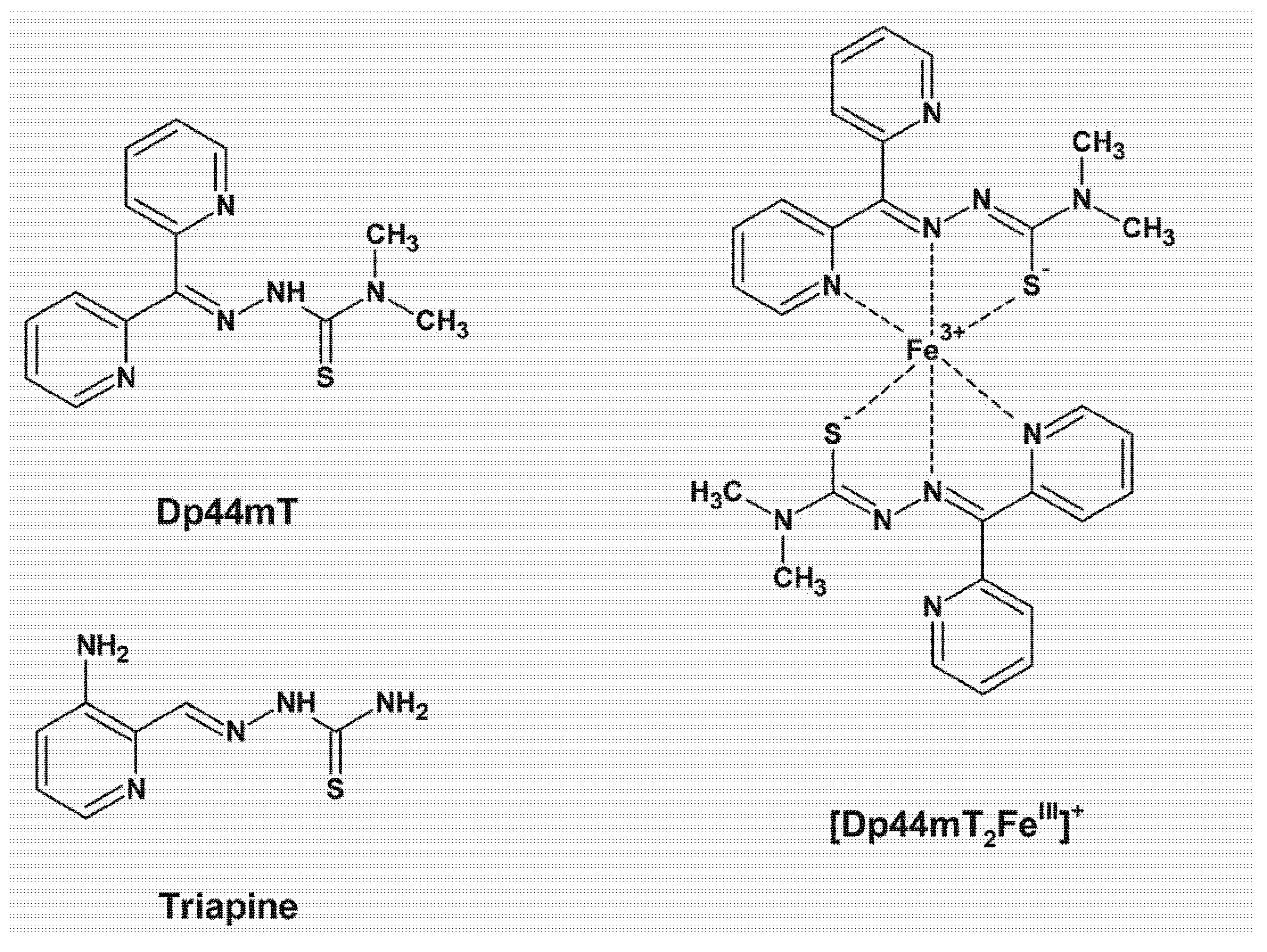
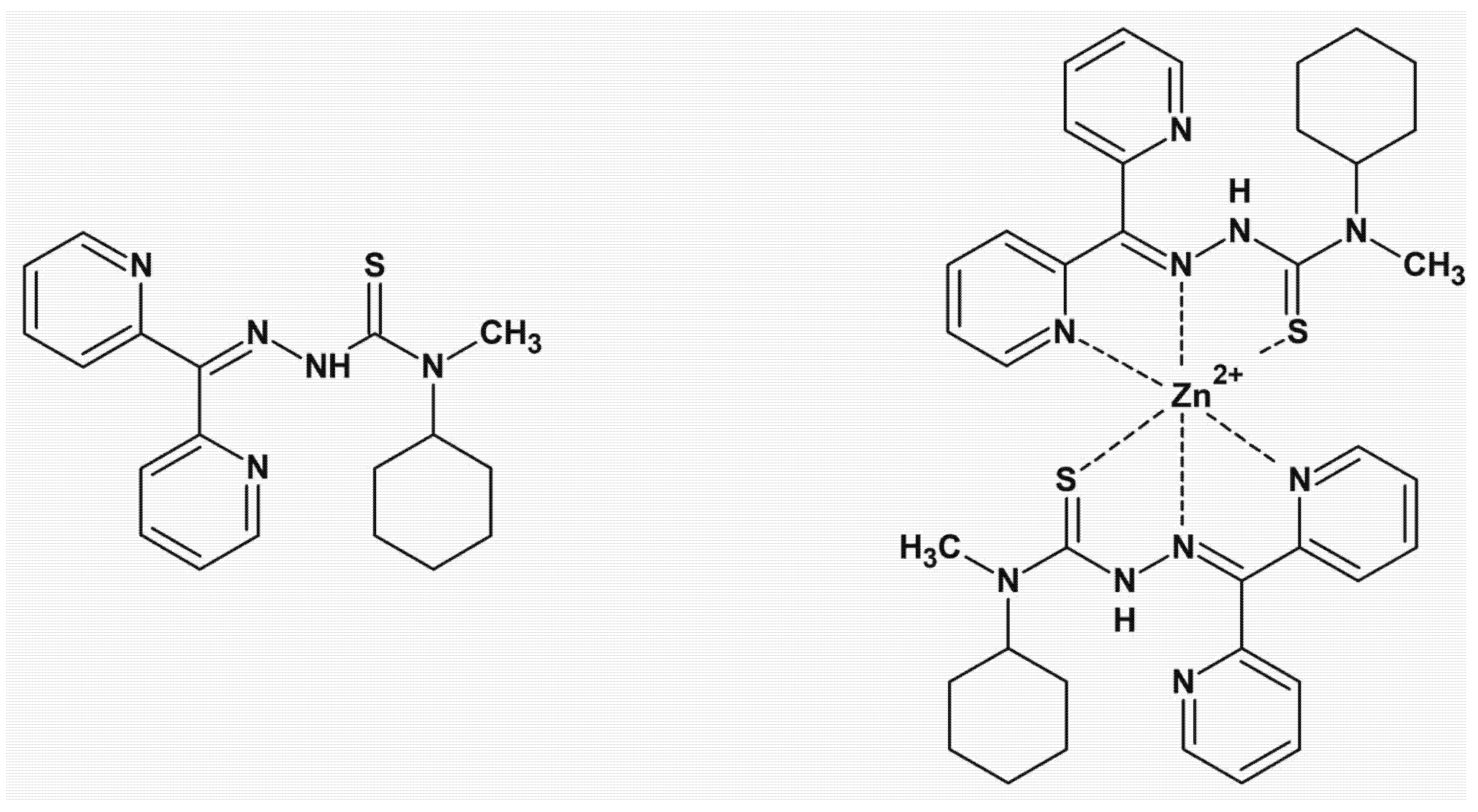
7. Thiosemicarbazone Chelators
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abeta | anti-amyloid beta aggregation; |
| AChE | anti-acetylcholinesterase; |
| anti-Aβ | inhibition of amyloid plaque deposits; |
| AoA | antioxidant activity; |
| Asc•− | ascorbyl anion radical; |
| AscH2 | ascorbic acid; |
| AscH• | ascorbyl radical; |
| AscH− | ascorbate anion; |
| CIDNP | chemically induced dynamic nuclear polarization; |
| Dox | Doxorubicin; |
| DpC | di-2-pyridylketone-4-cyclohexyl-4-methyl-3-thiosemicarbazone; |
| DHA | dehydroascorbate; |
| DHP | dihydropyridine; |
| DNA | deoxyribonucleic acid; |
| Dp44mT | di-2-pyridylketone-4,4,-dimethyl-3-thiosemicarbazone; |
| EPR | electron paramagnetic resonance; |
| GSH | glutathione; |
| HL1 | 2-((E)-3-(4-chlorophenyl)-1-phenylallylidene)-N-phenyl-hydrazinecarbothioamide; |
| HL4 | 2-((E)-3-(4-cyanophenyl)-1-phenylallylidene)-N-phenyl-hydrazinecarbothioamide; |
| H2O2 | hydrogen peroxide; |
| H3Ra | H3 receptor antagonism; |
| IND | investigational new drugs; |
| KHP | alpha-ketohydroxypyridines; |
| LA | linoleic acid; |
| logP | octanol/water partition coefficient; |
| L1 | deferiprone; |
| NADH | nicotinamide adenine dinucleotide; |
| NMR | nuclear magnetic resonance; |
| 1O2 | singlet oxygen; |
| OH | hydroxyl radical; |
| OOH | peroxyl radical; |
| Q1 | 2-phenyl-4-(butylamino)naphtho[2,3-h] quinoline-7,12-dione; |
| Q−• | semiquinone radical; |
| RNA | ribonucleic acid; |
| ROS | reactive oxygen species; |
| TSC | thiosemicarbazone; |
| UV-Vis | ultraviolet-visible. |
References
- Halliwell, B.; Gutteridge, J.M.C. Editorial: Free Radicals and Antioxidant Protection: Mechanisms and Significance in Toxicology and Disease. Hum. Exp. Toxicol. 1988, 7, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; Taylor & Francis: Abingdon, UK, 2005; ISBN 9780824753566. [Google Scholar]
- Kontoghiorghes, G.J. Iron Chelation in Biochemistry and Medicine. In Free radicals, Oxidant Stress and Drug Action; Rice-Evans, C., Ed.; Rechelieu Press: London, UK, 1987; pp. 277–303. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.; Cross, C.E. Free Radicals, Antioxidants, and Human Disease: Where Are We Now? J. Lab. Clin. Med. 1992, 119, 598–620. [Google Scholar] [PubMed]
- Ďuračková, Z. Some Current Insights into Oxidative Stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Stangherlin, A.; Reddy, A.B. Regulation of Circadian Clocks by Redox Homeostasis. J. Biol. Chem. 2013, 288, 26505–26511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santucci, R.; Sinibaldi, F.; Cozza, P.; Polticelli, F.; Fiorucci, L. Cytochrome c: An Extreme Multifunctional Protein with a Key Role in Cell Fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246. [Google Scholar] [CrossRef]
- Ramzan, R.; Vogt, S.; Kadenbach, B. Stress-Mediated Generation of Deleterious ROS in Healthy Individuals—Role of Cytochrome c Oxidase. J. Mol. Med. 2020, 98, 651–657. [Google Scholar] [CrossRef] [Green Version]
- Cabon, L.; Martinez-Torres, A.C.; Susin, S.A. Programmed Cell Death Comes in Many Flavors. Med. Sci. 2013, 29, 1117–1124. [Google Scholar] [CrossRef] [Green Version]
- Speer, R.E.; Karuppagounder, S.S.; Basso, M.; Sleiman, S.F.; Kumar, A.; Brand, D.; Smirnova, N.; Gazaryan, I.; Khim, S.J.; Ratan, R.R. Hypoxia-Inducible Factor Prolyl Hydroxylases as Targets for Neuroprotection by “Antioxidant” Metal Chelators: From Ferroptosis to Stroke. Free Radic. Biol. Med. 2013, 62, 26–36. [Google Scholar] [CrossRef]
- Feng, H.; Stockwell, B.R. Unsolved Mysteries: How Does Lipid Peroxidation Cause Ferroptosis? PLoS Biol. 2018, 16, e2006203. [Google Scholar] [CrossRef]
- Shah, R.; Shchepinov, M.S.; Pratt, D.A. Resolving the Role of Lipoxygenases in the Initiation and Execution of Ferroptosis. ACS Cent. Sci. 2018, 4, 387–396. [Google Scholar] [CrossRef]
- Hao, S.; Liang, B.; Huang, Q.; Dong, S.; Wu, Z.; He, W.; Shi, M. Metabolic Networks in Ferroptosis. Oncol. Lett. 2018, 15, 5405–5411. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular Mechanisms and Health Implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Yan, N.; Zhang, J.J. Iron Metabolism, Ferroptosis, and the Links with Alzheimer’s Disease. Front. Neurosci. 2020, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Martinet, W.; Coornaert, I.; Puylaert, P.; De Meyer, G.R.Y. Macrophage Death as a Pharmacological Target in Atherosclerosis. Front. Pharmacol. 2019, 10, 306. [Google Scholar] [CrossRef]
- Ravingerová, T.; Kindernay, L.; Barteková, M.; Ferko, M.; Adameová, A.; Zohdi, V.; Bernátová, I.; Ferenczyová, K.; Lazou, A. The Molecular Mechanisms of Iron Metabolism and Its Role in Cardiac Dysfunction and Cardioprotection. Int. J. Mol. Sci. 2020, 21, 7889. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Regulatory Molecules and Chelators Used for the Control of Essential and Toxic Metals in Health and Disease: From Molecular Interactions to Clinical Effects and Applications. Curr. Med. Chem. 2005, 12, 2661–2662. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Aldouri, M.A.; Hoffbrand, A.V.; Barr, J.; Wonke, B.; Kourouclaris, T.; Sheppard, L. Effective Chelation of Iron in β Thalassaemia with the Oral Chelator 1,2-Dimethyl-3-Hydroxypyrid-4-One. Br. Med. J. 1987, 295, 1509–1512. [Google Scholar] [CrossRef] [Green Version]
- Kolnagou, A.; Kleanthous, M.; Kontoghiorghes, G.J. Reduction of Body Iron Stores to Normal Range Levels in Thalassaemia by Using a Deferiprone/Deferoxamine Combination and Their Maintenance Thereafter by Deferiprone Monotherapy. Eur. J. Haematol. 2010, 85, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Baldari, S.; Di Rocco, G.; Toietta, G. Current Biomedical Use of Copper Chelation Therapy. Int. J. Mol. Sci. 2020, 21, 1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontoghiorghes, G.J.; Piga, A.; Hoffbrand, A.V. Cytotoxic Effects of the Lipophilic Iron Chelator Omadine. FEBS Lett. 1986, 204, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Kontoghiorghes, G.J.; Piga, A.; Hoffbrand, A.V. Cytotoxic and DNA-inhibitory Effects of Iron Chelators on Human Leukaemic Cell Lines. Hematol. Oncol. 1986, 4, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ganeshaguru, K.; Lally, J.M.; Piga, A.; Hoffbrand, A.V.; Kontoghiorghes, G.J. Cytotoxic Mechanisms of Iron Chelators. Drugs Today 1992, 28, 29–34. [Google Scholar]
- Nutting, C.M.; Van Herpen, C.M.L.; Miah, A.B.; Bhide, S.A.; Machiels, J.P.; Buter, J.; Kelly, C.; De Raucourt, D.; Harrington, K.J. Phase II Study of 3-AP Triapine in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2009, 20, 1275–1279. [Google Scholar] [CrossRef]
- Wagner, R.R. Chemical and Biologic Approaches to the Therapy of Viral Diseases. Am. Rev. Respir. Dis. 1963, 88, 404–419. [Google Scholar] [CrossRef]
- Bove, F.; Fasano, A. Iron Chelation Therapy to Prevent the Manifestations of Aceruloplasminemia. Neurology 2015, 85, 1085–1086. [Google Scholar] [CrossRef]
- Romanova, A.S. Natural Anthraquinones. Farmatsiia 1968, 17, 71–76. [Google Scholar]
- Steere, A.N.; Byrne, S.L.; Chasteen, N.D.; Mason, A.B. Kinetics of Iron Release from Transferrin Bound to the Transferrin Receptor at Endosomal PH. Biochim. Biophys. Acta-Gen. Subj. 2012, 1820, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Pantopoulos, K.; Porwal, S.K.; Tartakoff, A.; Devireddy, L. Mechanisms of Mammalian Iron Homeostasis. Biochemistry 2012, 51, 5705–5724. [Google Scholar] [CrossRef] [PubMed]
- Gozzelino, R.; Arosio, P. Iron Homeostasis in Health and Disease. Int. J. Mol. Sci. 2016, 17, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, G.J.; Frazer, D.M. Current Understanding of Iron Homeostasis. Am. J. Clin. Nutr. 2017, 106 (Suppl.6), 1559S–1566S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLoughery, T.G. Iron Deficiency Anemia. Med. Clin. N. Am. 2017, 101, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Weatherall, D.J.; Clegg, J.B. Inherited Haemoglobin Disorders: An Increasing Global Health Problem. Bull. World Health Organ. 2001, 79, 704–712. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. How to Manage Iron Toxicity in Post-Allogeneic Hematopoietic Stem Cell Transplantation? Expert Rev. Hematol. 2020, 13, 299–302. [Google Scholar] [CrossRef] [Green Version]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-Cell Disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef]
- Fitzsimons, E.J.; Cullis, J.O.; Thomas, D.W.; Tsochatzis, E.; Griffiths, W.J.H. Diagnosis and Therapy of Genetic Haemochromatosis (Review and 2017 Update). Br. J. Haematol. 2018, 181, 293–303. [Google Scholar] [CrossRef]
- Milman, N.T.; Schioedt, F.V.; Junker, A.E.; Magnussen, K. Diagnosis and Treatment of Genetic HFE-Hemochromatosis: The Danish Aspect. Gastroenterol. Res. 2019, 12, 221–232. [Google Scholar] [CrossRef] [Green Version]
- Holm, R.H.; Kennepohl, P.; Solomon, E.I. Structural and Functional Aspects of Metal Sites in Biology. Chem. Rev. 1996, 2123, 2239–2314. [Google Scholar] [CrossRef]
- Erdman, J.W.; MacDonald, I.; Zeisel, S.H.; International Life Sciences Institute. Present Knowledge in Nutrition; International Life Sciences Institute: Washington, DC, USA; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2012; ISBN 9780470959176. [Google Scholar]
- Prohaska, J.R. Impact of Copper Limitation on Expression and Function of Multicopper Oxidases (Ferroxidases). Adv. Nutr. Int. Rev. J. 2011, 2, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Vashchenko, G.; MacGillivray, R. Multi-Copper Oxidases and Human Iron Metabolism. Nutrients 2013, 5, 2289–2313. [Google Scholar] [CrossRef] [Green Version]
- Best, K.; McCoy, K.; Gemma, S.; DiSilvestro, R.A. Copper Enzyme Activities in Cystic Fibrosis before and after Copper Supplementation plus or Minus Zinc. Metabolism 2004, 53, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.A.; DiSilvestro, R.A.; Coleman, M.; Wagner, T.L. Copper Supplementation of Adult Men: Effects on Blood Copper Enzyme Activities and Indicators of Cardiovascular Disease Risk. Metabolism 1997, 46, 1380–1383. [Google Scholar] [CrossRef]
- Daniel, K.G.; Harbach, R.H.; Guida, W.C.; Dou, Q.P. Copper Storage Diseases: Menkes, Wilsons, and Cancer. Front. Biosci. 2004, 9, 2652–2662. [Google Scholar] [CrossRef]
- Afanas’ev, I.B. Superoxide and Nitric Oxide in Pathological Conditions Associated with Iron Overload. The Effects of Antioxidants and Chelators. Curr. Med. Chem. 2005, 12, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial Effects from a Mechanistic Perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turan, B. Role of Antioxidants in Redox Regulation of Diabetic Cardiovascular Complications. Curr. Pharm. Biotechnol. 2010, 11, 819–836. [Google Scholar] [CrossRef]
- Ferrari, G.S.L.; Ferrari, C.K.B. Exercise Modulation of Total Antioxidant Capacity (TAC): Towards a Molecular Signature of Healthy Aging. Front. Life Sci. 2011, 5, 81–90. [Google Scholar] [CrossRef]
- Clopton, D.A.; Saltman, P. Copper-Specific Damage in Human Erythrocytes Exposed to Oxidative Stress. Biol. Trace Elem. Res. 1997, 56, 231–240. [Google Scholar] [CrossRef]
- Delangle, P.; Mintz, E. Chelation Therapy in Wilson’s Disease: From d-Penicillamine to the Design of Selective Bioinspired Intracellular Cu(i) Chelators. Dalt. Trans. 2012, 41, 6359. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, Toxicity and Oxidative Stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like Copper Redox Chemistry Revisited: Hydrogen Peroxide and Superoxide Mediation of Copper-Catalyzed Oxidant Production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- De Laat, J.; Gallard, H. Catalytic Decomposition of Hydrogen Peroxide by Fe (III) in Homogeneous Aqueous Solution: Mechanism and Kinetic Modeling. Environ. Sci. Technol. 1999, 33, 2726–2732. [Google Scholar] [CrossRef]
- Millero, F.J.; Johnson, R.L.; Vega, C.A.; Sharma, V.K.; Sotolongo, S. Effect of Ionic Interactions of the Rates of Reduction of Cu (II) with H2O2 in Aqueous Solutions. J. Solut. Chem. 1992, 21, 1271–1287. [Google Scholar] [CrossRef]
- Moffett, J.W.; Zika, R.G. Reaction Kinetics of Hydrogen Peroxide with Copper and Iron in Seawater. Environ. Sci. Technol. 1987, 21, 804–810. [Google Scholar] [CrossRef]
- Jomova, K.; Lawson, M.; Drostinova, L.; Lauro, P.; Poprac, P.; Brezova, V.; Michalik, M.; Lukes, V.; Valko, M. Protective Role of Quercetin against Copper(II)-Induced Oxidative Stress: A Spectroscopic, Theoretical and DNA Damage Study. Food Chem. Toxicol. 2017, 110, 340–350. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, S.; Jiang, G.; Jiang, G.; Tang, R. A CuII-Based Metal-Organic Framework as an Efficient Photocatalyst for Direct Hydroxylation of Benzene to Phenol in Aqueous Solution. Asian J. Org. Chem. 2018, 7, 165–170. [Google Scholar] [CrossRef]
- Stoyanovsky, D.A.; Tyurina, Y.Y.; Shrivastava, I.; Bahar, I.; Tyurin, V.A.; Protchenko, O.; Jadhav, S.; Bolevich, S.B.; Kozlov, A.V.; Vladimirov, Y.A.; et al. Iron Catalysis of Lipid Peroxidation in Ferroptosis: Regulated Enzymatic or Random Free Radical Reaction? Free Radic. Biol. Med. 2019, 133, 153–161. [Google Scholar] [CrossRef]
- Cao, J.Y.; Dixon, S.J. Mechanisms of Ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef] [Green Version]
- Repetto, M.; Semprine, J.; Boveris, A. Lipid Peroxidation: Chemical Mechanism, Biological Implications and Analytical Determination. In Lipid Peroxidation; Serra, J., Ed.; Intech: London, UK, 2012. [Google Scholar]
- Kim, W.Y.; Jo, E.J.; Eom, J.S.; Mok, J.; Kim, M.H.; Kim, K.U.; Park, H.K.; Lee, M.K.; Lee, K. Combined Vitamin C, Hydrocortisone, and Thiamine Therapy for Patients with Severe Pneumonia Who Were Admitted to the Intensive Care Unit: Propensity Score-Based Analysis of a before-after Cohort Study. J. Crit. Care 2018, 47, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.A.; Truwit, J.D.; Hite, R.D.; Morris, P.E.; Dewilde, C.; Priday, A.; Fisher, B.; Thacker, L.R.; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients with Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA—J. Am. Med. Assoc. 2019, 322, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Rosengrave, P.C.; Bayer, S.; Chambers, S.; Mehrtens, J.; Shaw, G.M. Hypovitaminosis C and Vitamin C Deficiency in Critically Ill Patients despite Recommended Enteral and Parenteral Intakes. Crit. Care 2017, 21, 300. [Google Scholar] [CrossRef] [Green Version]
- Berenson, J.R.; Matous, J.; Swift, R.A.; Mapes, R.; Morrison, B.; Yeh, H.S. A Phase I/II Study of Arsenic Trioxide/Bortezomib/Ascorbic Acid Combination Therapy for the Treatment of Relapsed or Refractory Multiple Myeloma. Clin. Cancer Res. 2007, 13, 1762–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subbarayan, P.R.; Lima, M.; Ardalan, B. Arsenic Trioxide/Ascorbic Acid Therapy in Patients with Refractory Metastatic Colorectal Carcinoma: A Clinical Experience. Acta Oncol. 2007, 46, 557–561. [Google Scholar] [CrossRef]
- Bael, T.E.; Peterson, B.L.; Gollob, J.A. Phase II Trial of Arsenic Trioxide and Ascorbic Acid with Temozolomide in Patients with Metastatic Melanoma with or without Central Nervous System Metastases. Melanoma Res. 2008, 18, 147–151. [Google Scholar] [CrossRef]
- Fillenbaum, G.G.; Kuchibhatla, M.N.; Hanlon, J.T.; Artz, M.B.; Pieper, C.F.; Schmader, K.E.; Dysken, M.W.; Gray, S.L. Dementia and Alzheimer’s Disease in Community-Dwelling Elders Taking Vitamin C and/or Vitamin E. Ann. Pharmacother. 2005, 39, 2009–2014. [Google Scholar] [CrossRef]
- Gokce, N.; Keaney, J.F.; Frei, B.; Holbrook, M.; Olesiak, M.; Zachariah, B.J.; Leeuwenburgh, C.; Heinecke, J.W.; Vita, J.A. Long-Term Ascorbic Acid Administration Reverses Endothelial Vasomotor Dysfunction in Patients with Coronary Artery Disease. Circulation 1999, 99, 3234–3240. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Karne, R.J.; Hall, G.; Campia, U.; Panza, J.A.; Cannon, R.O.; Wang, Y.; Katz, A.; Levine, M.; Quon, M.J. High-Dose Oral Vitamin C Partially Replenishes Vitamin C Levels in Patients with Type 2 Diabetes and Low Vitamin C Levels but Does Not Improve Endothelial Dysfunction or Insulin Resistance. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H137–H145. [Google Scholar] [CrossRef] [Green Version]
- Carr, A.C. A New Clinical Trial to Test High-Dose Vitamin C in Patients with COVID-19. Crit. Care 2020, 24, 133. [Google Scholar] [CrossRef] [Green Version]
- Macan, A.M.; Kraljević, T.G.; Raić-malić, S. Therapeutic Perspective of Vitamin C and Its Derivatives. Antioxidants 2019, 8, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidu, K.A. Vitamin C in Human Health and Disease Is Still a Mystery? An Overview. Nutr. J. 2003, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Galaris, D.; Pantopoulos, K. Oxidative Stress and Iron Homeostasis: Mechanistic and Health Aspects. Crit. Rev. Clin. Lab. Sci. 2008, 45, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Young, I.S.; Woodside, J.V. Antioxidants in Health and Disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Rahman, K. Studies on Free Radicals, Antioxidants, and Co-Factors. Clin. Interv. Aging 2007, 2, 219–236. [Google Scholar]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic Metals, Ascorbate and Free Radicals: Combinations to Avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef] [Green Version]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Phytochelators Intended for Clinical Use in Iron Overload, Other Diseases of Iron Imbalance and Free Radical Pathology. Molecules 2015, 20, 20841–20872. [Google Scholar] [CrossRef] [Green Version]
- Bielski, B.H.J.; Allen, A.O.; Schwarz, H.A. Mechanism of Disproportionation of Ascorbate Radicals. J. Am. Chem. Soc. 1981, 103, 3516–3518. [Google Scholar] [CrossRef]
- Bielski, B.H.J. Chemistry of Ascorbic Acid Radicals. In Ascorbic Acid: Chemistry, Metabolism, and Uses; Seib, P.A., Tolbert, B.M., Eds.; Advances in Chemistry: Washington, DC, USA, 1982; pp. 81–100. ISBN 9780841206328. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. The Antioxidants of Human Extracellular Fluids. Arch. Biochem. Biophys. 1990, 280, 1–8. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Hider, R.H. Iron and Redox Cycling. Do’s and Don’ts. Free Radic. Biol. Med. 2019, 133, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Kolnagou, A.; Kontoghiorghe, C.N.; Mourouzidis, L.; Timoshnikov, V.A.; Polyakov, N.E. Trying to Solve the Puzzle of the Interaction of Ascorbic Acid and Iron: Redox, Chelation and Therapeutic Implications. Medicines 2020, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Timoshnikov, V.A.; Kobzeva, T.; Selyutina, O.Y.; Polyakov, N.E.; Kontoghiorghes, G.J. Effective Inhibition of Copper-Catalyzed Production of Hydroxyl Radicals by Deferiprone. J. Biol. Inorg. Chem. 2019, 24, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Timoshnikov, V.A.; Kichigina, L.A.; Selyutina, O.Y.; Polyakov, N.E.; Kontoghiorghes, G.J. Antioxidant Activity of Deferasirox and Its Metal Complexes in Model Systems of Oxidative Damage: Comparison with Deferiprone. Molecules 2021, 26, 5064. [Google Scholar] [CrossRef]
- Erdem, G.; Öner, C.; Önal, A.M.; Kisakürek, D.; Ögüs, A.Y. Free Radical Mediated Interaction of Ascorbic Acid and Ascorbate/Cu(II) with Viral and Plasmid DNAs. J. Biosci. 1994, 19, 9–17. [Google Scholar] [CrossRef]
- Gerster, H. High-Dose Vitamin C: A Risk for Persons with High Iron Stores? Int. J. Vitam. Nutr. Res. 1999, 69, 67–82. [Google Scholar] [CrossRef]
- Carr, A.; Frei, B. Does Vitamin C Act as a Pro-Oxidant under Physiological Conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef] [Green Version]
- Van Gorkom, G.N.Y.; Lookermans, E.L.; Van Elssen, C.H.M.J.; Bos, G.M.J. The Effect of Vitamin C (Ascorbic Acid) in the Treatment of Patients with Cancer: A Systematic Review. Nutrients 2019, 11, 977. [Google Scholar] [CrossRef] [Green Version]
- Borst, P. Mega-Dose Vitamin C as Therapy for Human Cancer? Proc. Natl. Acad. Sci. USA 2008, 105, 95. [Google Scholar] [CrossRef] [Green Version]
- Hoffer, L.J.; Levine, M.; Assouline, S.; Melnychuk, D.; Padayatty, S.J.; Rosadiuk, K.; Rousseau, C.; Robitaille, L.; Miller, W.H. Phase I Clinical Trial of i.v. Ascorbic Acid in Advanced Malignancy. Ann. Oncol. 2008, 19, 1969–1974. [Google Scholar] [CrossRef]
- Frei, B.; Lawson, S. Vitamin C and Cancer Revisited. Proc. Natl. Acad. Sci. USA 2008, 105, 11037–11038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.K.; Kumbhar, A.A.; Pokharel, Y.R.; Yadav, P.N. Anticancer Potency of Copper(II) Complexes of Thiosemicarbazones. J. Inorg. Biochem. 2020, 210, 111134. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Buc Calderon, P. Vitamin C (Ascorbate) and Redox Topics in Cancer. Antioxid. Redox Signal. 2021, 35, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Zümreoglu-Karan, B. The Coordination Chemistry of Vitamin C: An Overview. Coord. Chem. Rev. 2006, 250, 2295–2307. [Google Scholar] [CrossRef]
- Grillet, L.; Ouerdane, L.; Flis, P.; Hoang, M.T.T.; Isaure, M.P.; Lobinski, R.; Curie, C.; Mari, S. Ascorbate Efflux as a New Strategy for Iron Reduction and Transport in Plants. J. Biol. Chem. 2014, 289, 2515–2525. [Google Scholar] [CrossRef] [Green Version]
- Nayak, S.; Dash, A.C. Phenol-Amide Chelates of Iron(III). Kinetics and Mechanism of Reversible Formation of (Diaqua) (1,3) Bis (2-Hydroxybenzamido)Propaneiron(III) and Its Reactions with Thiocyanate, Azide, Imidazole, Sulphur(IV) and Ascorbic Acid in Aqueous Medium. Indian J. Chem.-Sect. A 2003, 42, 2427–2438. [Google Scholar]
- Ortega-Castro, J.; Frau, J.; Casasnovas, R.; Fernández, D.; Donoso, J.; Muñoz, F. High- and Low-Spin Fe(III) Complexes of Various AGE Inhibitors. J. Phys. Chem. A 2012, 116, 2961–2971. [Google Scholar] [CrossRef]
- Hininger, I.; Waters, R.; Osman, M.; Garrel, C.; Fernholz, K.; Roussel, A.M.; Anderson, R.A. Acute Prooxidant Effects of Vitamin C in EDTA Chelation Therapy and Long-Term Antioxidant Benefits of Therapy. Free Radic. Biol. Med. 2005, 38, 1565–1570. [Google Scholar] [CrossRef]
- Martell, A.E. Ascorbic Acid: Chemistry, Metabolism, and Uses. In Ascorbic Acid: Chemistry, Metabolism, and Uses; Seib, P.A., Tolbert, B.M., Eds.; ACS Publications: Washington, DC, USA, 1982; pp. 153–178. ISBN 780841206328. [Google Scholar]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases. Cells 2020, 9, 1456. [Google Scholar] [CrossRef]
- Keypour, H.; Silver, J.; Wilson, M.T.; Hamed, M.Y. Studies on the Reactions of Ferric Iron with Ascorbic Acid. A Study of Solution Chemistry Using Mössbauer Spectroscopy and Stopped-Flow Techniques. Inorg. Chim. Acta 1986, 125, 97–106. [Google Scholar] [CrossRef]
- Yuan, X.; Pham, A.N.; Xing, G.; Rose, A.L.; Waite, T.D. Effects of PH, Chloride, and Bicarbonate on Cu(I) Oxidation Kinetics at Circumneutral PH. Environ. Sci. Technol. 2012, 46, 1527–1535. [Google Scholar] [CrossRef]
- Mahata, S.; Mitra, I.; Mukherjee, S.; Venkata Pera, R.B.; Ghosh, G.K.; Linert, W.; Moi, S.C. Speciation Study of L-Ascorbic Acid and Its Chelated Cu(II) & Ni(II) Complexes: An Experimental and Theoretical Model of Complex Formation. S. Afr. J. Chem. 2019, 72, 229–236. [Google Scholar] [CrossRef]
- Moffett, J.W.; Zika, R.G. Oxidation Kinetics of Cu(I) in Seawater: Implications for Its Existence in the Marine Environment. Mar. Chem. 1983, 13, 239–251. [Google Scholar] [CrossRef]
- Hou, X.; Huang, X.; Ai, Z.; Zhao, J.; Zhang, L. Ascorbic Acid/Fe@Fe2O3: A Highly Efficient Combined Fenton Reagent to Remove Organic Contaminants. J. Hazard. Mater. 2016, 310, 170–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, D.Q.; Zhang, Y.J.; Pei, D.N.; Huang, G.X.; Liu, C.; Li, J.; Yu, H.Q. Degradation of Benzoic Acid in an Advanced Oxidation Process: The Effects of Reducing Agents. J. Hazard. Mater. 2020, 382, 121090. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, N.E.; Leshina, T.V.; Konovalova, T.A.; Kispert, L.D. Carotenoids as scavengers of free radicals in a fenton reaction: Antioxidants or pro-oxidants? Free Radic. Biol. Med. 2001, 31, 398–404. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Leshina, T.V.; Salakhutdinov, N.F.; Konovalova, T.A.; Kispert, L.D. Antioxidant and Redox Properties of Supramolecular Complexes of Carotenoids with β-Glycyrrhizic Acid. Free Radic. Biol. Med. 2006, 40, 1804–1809. [Google Scholar] [CrossRef]
- Salgado, P.; Melin, V.; Contreras, D.; Moreno, Y.; Mansilla, H.D. Fenton Reaction Driven by Iron Ligands. J. Chil. Chem. Soc. 2013, 58, 2096–2101. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Zhou, T.; Bai, R.; Xie, Y. Hydroxypyridinone-Based Iron Chelators with Broad-Ranging Biological Activities. J. Med. Chem. 2020, 63, 14470–14501. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Marques, S.M.; Chaves, S. Hydroxypyridinones as “Privileged” Chelating Structures for the Design of Medicinal Drugs. Coord. Chem. Rev. 2012, 256, 240–259. [Google Scholar] [CrossRef]
- Telpoukhovskaia, M.A.; Patrick, B.O.; Rodríguez-Rodríguez, C.; Orvig, C. In Silico to in Vitro Screening of Hydroxypyridinones as Acetylcholinesterase Inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G. The Design of Orally Active Iron Chelators for the Treatment of Thalassaemia. Ph.D. Thesis, University of Essex, Colchester, UK, 1982; pp. 1–243. Available online: https://www.pri.ac.cy/files/KGJ_thesis_1982.pdf (accessed on 22 December 2021).
- Kontoghiorghes, G.J.; Evans, R.W. Site Specificity of Iron Removal from Transferrin by Alpha-Ketohydroxypyridine Chelators. FEBS Lett. 1985, 189, 141–144. [Google Scholar] [CrossRef] [Green Version]
- Kontoghiorghes, G.J. Design, Properties, and Effective Use of the Oral Chelator L1 and Other Alpha-Ketohydroxypyridines in the Treatment of Transfusional Iron Overload in Thalassemia. Ann. N. Y. Acad. Sci. 1990, 612, 339–350. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Orally Active Alpha-Ketohydroxypyridine Iron Chelators: Effects on Iron and Other Metal Mobilisations. Acta Haematol. 1987, 78, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Orally Active Alpha-Ketohydroxypyridine Iron Chelators: Studies in Mice-PubMed. Mol. Pharmacol. 1986, 30, 670–673. [Google Scholar]
- Kontoghiorghes, G.J.; Hoffbrand, A.V. Orally Active Alpha-Ketohydroxy Pyridine Iron Chelators Intended for Clinical Use: In Vivo Studies in Rabbits. Br. J. Haematol. 1986, 62, 607–613. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. The Study of Iron Mobilisation from Transferrin Using Alpha-Ketohydroxy Heteroaromatic Chelators. Biochim. Biophys. Acta 1986, 869, 141–146. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Iron Mobilisation from Lactoferrin by Chelators at Physiological PH. BBA-Gen. Subj. 1986, 882, 267–270. [Google Scholar] [CrossRef]
- Kontoghiorghes, J.; Chambers, S.; Hoffbrand, A.V. Comparative Study of Iron Mobilization from Haemosiderin, Ferritin and Iron(III) Precipitates by Chelators. Biochem. J. 1987, 241, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Kontoghiorghes, G.J. New Orally Active Alpha-Ketohydroxy Pyridine Chelators for the Treatment of Iron Overload. Birth Defects Orig. Artic. Ser. 1988, 23, 97–103. [Google Scholar]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, S.; Palmerini, E. Adjuvant and Neoadjuvant Combination Chemotherapy for Osteogenic Sarcoma. Curr. Opin. Oncol. 2007, 19, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M. Oxygen Radicals Generation and DNA Scission by Anticancer and Synthetic Quinones. Methods Enzymol. 1994, 233, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, B.; Mukherjee, S. Cancer Therapy Using Antibiotics. J. Cancer Ther. 2015, 6, 849–858. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, I.; Ahmed, S.; Anwar, Z.; Sheraz, M.A.; Sikorski, M. Photostability and Photostabilization of Drugs and Drug Products. Int. J. Photoenergy 2016, 2016, 8135608. [Google Scholar] [CrossRef] [Green Version]
- Girotti, A.W. Photodynamic Lipid Peroxidation in Biological Systems. Photochem. Photobiol. 1990, 51, 497–509. [Google Scholar] [CrossRef]
- Abdel-Kader, M.H. History of Photodynamic Therapy. In Photodynamic Therapy: From Theory to Application; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–22. ISBN 9783642396298. [Google Scholar]
- Štěrba, M.S.; Popelová, O.; Vávrová, A.; Jirkovský, E.; Kovaříková, P.; Geršl, V.; Šimůnek, T.S. Oxidative Stress, Redox Signaling, and Metal Chelation in Anthracycline Cardiotoxicity and Pharmacological Cardioprotection. Antioxid. Redox Signal. 2013, 18, 899–929. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; He, J.; Yu, W.; Li, Y.; Liu, Z.; Zhou, B.; Liu, Y. A Promising Anticancer Drug: A Photosensitizer Based on the Porphyrin Skeleton. RSC Med. Chem. 2020, 11, 427–437. [Google Scholar] [CrossRef]
- Zhu, H.; Sarkar, S.; Scott, L.; Danelisen, I.; Trush, M.A.; Jia, Z.; Li, Y.R. Doxorubicin Redox Biology: Redox Cycling, Topoisomerase Inhibition, and Oxidative Stress HHS Public Access. React. Oxigen Species 2016, 1, 189–198. [Google Scholar] [CrossRef]
- Frankel, E.N. Phenolic Antioxidant. In Lipid Oxidation; Elsevier: Amsterdam, The Netherlands, 2012; pp. 209–258. [Google Scholar]
- Bebbington, D.; Monck, N.J.T.; Gaur, S.; Palmer, A.M.; Benwell, K.; Harvey, V.; Malcolm, C.S.; Porter, R.H.P. 3,5-Disubstituted-4-Hydroxyphenyls Linked to 3-Hydroxy-2-Methyl-4(1H)-Pyridinone: Potent Inhibitors of Lipid Peroxidation and Cell Toxicity. J. Med. Chem. 2000, 43, 2779–2782. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Stanbury, D.M.; Bounds, P.L. Electrode Potentials of Partially Reduced Oxygen Species, from Dioxygen to Water. Free Radic. Biol. Med. 2010, 49, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Kleanthous, M.; Kontoghiorghe, C.N. The History of Deferiprone (L1) and the Paradigm of the Complete Treatment of Iron Overload in Thalassaemia. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020011. [Google Scholar] [CrossRef] [PubMed]
- Chaves, S.; Piemontese, L.; Hiremathad, A.; Santos, M.A. Hydroxypyridinone Derivatives: A Fascinating Class of Chelators with Therapeutic Applications—An Update. Curr. Med. Chem. 2017, 25, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Neocleous, K.; Kolnagou, A. Benefits and Risks of Deferiprone in Iron Overload in Thalassaemia and Other Conditions: Comparison of Epidemiological and Therapeutic Aspects with Deferoxamine. Drug Saf. 2003, 26, 553–584. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.; Pattichis, K.; Neocleous, K.; Kolnagou, A. The Design and Development of Deferiprone (L1) and Other Iron Chelators for Clinical Use: Targeting Methods and Application Prospects. Curr. Med. Chem. 2004, 11, 2161–2183. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.; Eracleous, E.; Economides, C.; Kolnagou, A. Advances in Iron Overload Therapies. Prospects for Effective Use of Deferiprone (L1), Deferoxamine, the New Experimental Chelators ICL670, GT56-252, L1NAll and Their Combinations. Curr. Med. Chem. 2005, 12, 2663–2681. [Google Scholar] [CrossRef]
- Sheppard, L.N.; Kontoghiorghes, G.J. Competition between Deferiprone, Desferrioxamine and Other Chelators for Iron and the Effect of Other Metals. Arzneimittelforschung 1993, 43, 659–663. [Google Scholar]
- Kontoghiorghes, G.J.; Jackson, M.J.; Lunec, J. In Vitro Screening of Iron Chelators Using Models of Free Radical Damage. Free Radic. Res. Commun. 1986, 2, 115–124. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Inhibition of Fe2+- and Fe3+-Induced Hydroxyl Radical Production by the Iron-Chelating Drug Deferiprone. Free Radic. Biol. Med. 2015, 78, 118–122. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kobzeva, T.V.; Polyakov, N.E.; Kontoghiorghes, G.J. Redox interactions of vitamin c and iron: Inhibition of the pro-oxidant activity by deferiprone. Int. J. Mol. Sci. 2020, 21, 3967. [Google Scholar] [CrossRef]
- Merkofer, M.; Kissner, R.; Hider, R.C.; Koppenol, W.H. Redox Properties of the Iron Complexes of Orally Active Iron Chelators CP20, CP502, CP509, and ICL670. Helv. Chim. Acta 2004, 87, 3021–3034. [Google Scholar] [CrossRef]
- Akrawinthawong, K.; Chaowalit, N.; Chatuparisuth, T.; Siritanaratkul, N. Effectiveness of Deferiprone in Transfusionindependent Beta-Thalassemia/HbE Patients. Hematology 2011, 16, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Pashalidis, I.; Kontoghiorghes, G.J. Competition Studies of L1-Deferiprone with Copper and Iron. Possible Implications on Efficacy, Toxicity and New Therapeutic Applications. Transfus. Sci. 2000, 23, 259–261. [Google Scholar] [CrossRef]
- Pashalidis, I.; Kontoghiorghes, G.J. Molecular Factors Affecting the Complex Formation between Deferiprone (L1) and Cu(II). Possible Implications on Efficacy and Toxicity. Arzneimittelforschung 2001, 51, 998–1003. [Google Scholar] [CrossRef]
- Kontoghiorghe, C.N.; Kontoghiorghes, G.J. Efficacy and Safety of Iron-Chelation Therapy with Deferoxamine, Deferiprone, and Deferasirox for the Treatment of Iron-Loaded Patients with Non-Transfusion-Dependent Thalassemia Syndromes. Drug Des. Devel. Ther. 2016, 10, 465–481. [Google Scholar] [CrossRef] [Green Version]
- Kontoghiorghes, G.J.; Graham Goddard, J.; Bardett, A.N.; Sheppard, L. Pharmacokinetic Studies in Humans with the Oral Iron Chelator 1,2-Dimethyl-3-Hydroxypyrid-4-One. Clin. Pharmacol. Ther. 1990, 48, 255–261. [Google Scholar] [CrossRef]
- Levy, M.; Llinas, R. Pilot Safety Trial of Deferiprone in 10 Subjects with Superficial Siderosis. Stroke 2012, 43, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Elincx-Benizri, S.; Glik, A.; Merkel, D.; Arad, M.; Freimark, D.; Kozlova, E.; Cabantchik, I.; Hassin-Baer, S. Clinical Experience with Deferiprone Treatment for Friedreich Ataxia. J. Child Neurol. 2016, 31, 1036–1040. [Google Scholar] [CrossRef]
- Zou, C.; Liu, X.; Xie, R.; Bao, Y.; Jin, Q.; Jia, X.; Li, L.; Liu, R. Deferiprone Attenuates Inflammation and Myocardial Fibrosis in Diabetic Cardiomyopathy Rats. Biochem. Biophys. Res. Commun. 2017, 486, 930–936. [Google Scholar] [CrossRef]
- Liu, J.L.; Fan, Y.G.; Yang, Z.S.; Wang, Z.Y.; Guo, C. Iron and Alzheimer’s Disease: From Pathogenesis to Therapeutic Implications. Front. Neurosci. 2018, 12, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Kontoghiorghes, G.J.; Efstathiou, A.; Ioannou-Loucaides, S.; Kolnagou, A. Chelators Controlling Metal Metabolism and Toxicity Pathways: Applications in Cancer Prevention, Diagnosis and Treatment. Hemoglobin 2008, 32, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Yoshino, M. Generation of Reactive Oxygen Species by Hydroxypyridone Compound/Iron Complexes. Redox Rep. 2020, 25, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, M.; Brindisi, M.; Sotgia, F.; Lisanti, M.P. Deferiprone (DFP) Targets Cancer Stem Cell (CSC) Propagation by Inhibiting Mitochondrial Metabolism and Inducing ROS Production. Cells 2020, 9, 1529. [Google Scholar] [CrossRef] [PubMed]
- Timoshnikov, V.A.; Klimentiev, V.I.; Polyakov, N.E.; Kontoghiorghes, G.J. Photoinduced Transformation of Iron Chelator Deferiprone: Possible Implications in Drug Metabolism and Toxicity. J. Photochem. Photobiol. A Chem. 2014, 289, 14–21. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2014 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2014, 10, 47–92. [Google Scholar] [CrossRef] [Green Version]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles: Intracellular Abeta and Synaptic Dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef] [Green Version]
- Ayton, S.; Lei, P.; Bush, A.I. Metallostasis in Alzheimer’s Disease. Free Radic. Biol. Med. 2013, 62, 76–89. [Google Scholar] [CrossRef]
- Gazova, Z.; Soukup, O.; Sepsova, V.; Siposova, K.; Drtinova, L.; Jost, P.; Spilovska, K.; Korabecny, J.; Nepovimova, E.; Fedunova, D.; et al. Multi-Target-Directed Therapeutic Potential of 7-Methoxytacrine-Adamantylamine Heterodimers in the Alzheimer’s Disease Treatment. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 607–619. [Google Scholar] [CrossRef]
- Hiremathad, A.; Chand, K.; Tolayan, L.; Rajeshwari; Keri, R.S.; Esteves, A.R.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Hydroxypyridinone-Benzofuran Hybrids with Potential Protective Roles for Alzheimer´s Disease Therapy. J. Inorg. Biochem. 2018, 179, 82–96. [Google Scholar] [CrossRef]
- Sheng, R.; Tang, L.; Jiang, L.; Hong, L.; Shi, Y.; Zhou, N.; Hu, Y. Novel 1-Phenyl-3-Hydroxy-4-Pyridinone Derivatives as Multifunctional Agents for the Therapy of Alzheimer’s Disease. ACS Chem. Neurosci. 2016, 7, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, M.; Khanam, R.; Vohora, D. Histamine H3 Receptor Antagonists in Relation to Epilepsy and Neurodegeneration: A Systemic Consideration of Recent Progress and Perspectives. Br. J. Pharmacol. 2012, 167, 1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.A.; Chand, K.; Chaves, S. Recent Progress in Repositioning Alzheimer’s Disease Drugs Based on a Multitarget Strategy. Future Med. Chem. 2016, 8, 2113–2142. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Marques, S.M.; Quintanova, C.; Silva, D.F.; Cardoso, S.M.; Chaves, S.; Santos, M.A. Multifunctional Iron-Chelators with Protective Roles against Neurodegenerative Diseases. Dalt. Trans. 2013, 42, 6058–6073. [Google Scholar] [CrossRef]
- Telpoukhovskaia, M.A.; Rodríguez-Rodríguez, C.; Cawthray, J.F.; Scott, L.E.; Page, B.D.G.; Alí-Torres, J.; Sodupe, M.; Bailey, G.A.; Patrick, B.O.; Orvig, C. 3-Hydroxy-4-Pyridinone Derivatives as Metal Ion and Amyloid Binding Agents. Metallomics 2014, 6, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Dikalov, S.; Alov, P.; Rangelova, D. Role of Iron Ion Chelation by Quinones in Their Reduction, Oh-Radical Generation, and Lipid Peroxidation. Biochem. Biophys. Res. Commun. 1993, 195, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Antebi, H.; Alcindor, L.G.; Teiger, E.; Perez, G.; Giudicelli, Y.; Nordmann, R.; Piwnica, A. Iron Chelation by Deferoxamine Inhibits Lipid Peroxidation during Cardiopulmonary Bypass in Humans. Circulation 1990, 82, 390–396. [Google Scholar]
- Yoshida, Y.; Furuta, S.; Niki, E. Effects of Metal Chelating Agents on the Oxidation of Lipids Induced by Copper and Iron. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1993, 1210, 81–88. [Google Scholar] [CrossRef]
- Alegría, A.E.; Santiago, G. Structural and Hydrophilicity Requirements in Quinone-Induced Lipid Peroxidation of Phosphatidylcholine Vesicles. Toxicol. Environ. Chem. 1998, 65, 1–4. [Google Scholar] [CrossRef]
- Markova, I.D.; Polyakov, N.E.; Selyutina, O.Y.; Fedenok, L.G.; Fedotov, K.Y.; Slepneva, I.A.; Leshina, T.V.; Pokrovsky, A.G.; Vasilieva, N.V.; Weiner, L.M. Light-Stimulated Generation of Free Radicals by Quinones-Chelators. Z. Phys. Chem. 2017, 231, 369–389. [Google Scholar] [CrossRef]
- Miura, T.; Muraoka, S.; Ogiso, T. Lipid Peroxidation of Rat Erythrocyte Membrane Induced by Adriamycin-Fe3+. Pharmacol. Toxicol. 1991, 69, 296–300. [Google Scholar] [CrossRef]
- Polyakov, N.; Leshina, T.; Fedenok, L.; Slepneva, I.; Kirilyuk, I.; Furso, J.; Olchawa, M.; Sarna, T.; Elas, M.; Bilkis, I.; et al. Redox-Active Quinone Chelators: Properties, Mechanisms of Action, Cell Delivery, and Cell Toxicity. Antioxid. Redox Signal. 2018, 28, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Powis, G. Free Radical Formation by Antitumor Quinones. Free Radic. Biol. Med. 1989, 6, 63–101. [Google Scholar] [CrossRef]
- Hopkins, R.Z. Superoxide in Biology and Medicine: An Overview. React. Oxig. Species 2016, 1, 99–109. [Google Scholar] [CrossRef]
- Thomas, C.E.; Aust, S.D. Release of Iron from Ferritin by Cardiotoxic Anthracycline Antibiotics. Arch. Biochem. Biophys. 1986, 248, 684–689. [Google Scholar] [CrossRef]
- Kwok, J.C.; Richardson, D.R. Anthracyclines Induce Accumulation of Iron in Ferritin in Myocardial and Neoplastic Cells: Inhibition of the Ferritin Iron Mobilization Pathway. Mol. Pharmacol. 2003, 63, 849–861. [Google Scholar] [CrossRef] [Green Version]
- Myers, C.E.; Mcguire, W.P.; Liss, R.H.; Ifrim, I.; Grotzinger, K.; Young, R.C. Adriamycin: The Role of Lipid Peroxidation in Cardiac Toxicity and Tumor Response. Science 1977, 197, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Hrelia, S.; Fiorentini, D.; Maraldi, T.; Angeloni, C.; Bordoni, A.; Biagi, P.L.; Hakim, G. Doxorubicin Induces Early Lipid Peroxidation Associated with Changes in Glucose Transport in Cultured Cardiomyocytes. Biochim. Biophys. Acta-Biomembr. 2002, 1567, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Oleinick, N.L.; Morris, R.L.; Belichenko, I. The Role of Apoptosis in Response to Photodynamic Therapy: What, Where, Why, and How. Photochem. Photobiol. Sci. 2002, 1, 1–21. [Google Scholar] [CrossRef]
- MacCormack, M.A. Photodynamic Therapy. Adv. Dermatol. 2006, 22, 219–258. [Google Scholar] [CrossRef]
- Keyal, U.; Bhatta, A.K.; Zhang, G.; Wang, X.L. Present and Future Perspectives of Photodynamic Therapy for Cutaneous Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2019, 80, 765–773. [Google Scholar] [CrossRef]
- Shafirstein, G.; Bellnier, D.; Oakley, E.; Hamilton, S.; Potasek, M.; Beeson, K.; Parilov, E. Interstitial Photodynamic Therapy—A Focused Review. Cancers 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakravarty, A.R.; Roy, M. Photoactivated DNA Cleavage and Anticancer Activity of 3D Metal Complexes. In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2011; Volume 57, pp. 119–202. [Google Scholar]
- Garai, A.; Pant, I.; Bhattacharyya, A.; Kondaiah, P.; Chakravarty, A.R. Mitochondria-Targeted Anticancer Activity of BODIPY-Appended Iron(III) Catecholates in Red Light. ChemistrySelect 2017, 2, 11686–11692. [Google Scholar] [CrossRef]
- Devi, L.R.; Raza, M.K.; Musib, D.; Ramu, V.; Devi, J.; Roy, M. Nucleus Targeting Anthraquinone-Based Copper (II) Complexes as the Potent PDT Agents: Synthesis, Photo-Physical and Theoretical Evaluation. Inorg. Chim. Acta 2020, 500, 119208. [Google Scholar] [CrossRef]
- Hudson, L.; Rashdan, E.; Bonn, C.A.; Chavan, B.; Rawlings, D.; Birch-Machin, M.A. Individual and Combined Effects of the Infrared, Visible, and Ultraviolet Light Components of Solar Radiation on Damage Biomarkers in Human Skin Cells. FASEB J. 2020, 34, 3874–3883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabłońska-Trypuć, A.; Świderski, G.; Krętowski, R.; Lewandowski, W. Newly Synthesized Doxorubicin Complexes with Selected Metals—Synthesis, Structure and Anti-Breast Cancer Activity. Molecules 2017, 22, 1106. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Yang, Y.; He, P.; Fang, Y. Spectroscopic Studies of Copper(II) and Iron(II) Complexes of Adriamycin. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2000, 56, 581–587. [Google Scholar] [CrossRef]
- Rahimipour, S.; Gescheidt, G.; Bilkis, I.; Fridkin, M.; Weiner, L. Towards the Efficiency of Pharmacologically Active Quinoid Compounds: Electron Transfer and Formation of Reactive Oxygen Species. Appl. Magn. Reson. 2010, 37, 629–648. [Google Scholar] [CrossRef]
- Roy, S.; Mondal, P.; Sengupta, P.S.; Dhak, D.; Santra, R.C.; Das, S.; Guin, P.S. Spectroscopic, Computational and Electrochemical Studies on the Formation of the Copper Complex of 1-Amino-4-Hydroxy-9,10-Anthraquinone and Effect of It on Superoxide Formation by NADH Dehydrogenase. Dalt. Trans. 2015, 44, 5428–5440. [Google Scholar] [CrossRef]
- Silveira-Dorta, G.; Monzón, D.M.; Crisóstomo, F.P.; Martín, T.; Martín, V.S.; Carrillo, R. Oxidation with Air by Ascorbate-Driven Quinone Redox Cycling. Chem. Commun. 2015, 51, 7027–7030. [Google Scholar] [CrossRef] [Green Version]
- Verrax, J.; Beck, R.; Dejeans, N.; Glorieux, C.; Sid, B.; Pedrosa, R.C.; Benites, J.; Vasquez, D.; Valderrama, J.A.; Buc Calderon, P. Redox-Active Quinones and Ascorbate: An Innovative Cancer Therapy That Exploits the Vulnerability of Cancer Cells to Oxidative Stress. Anticancer Agents Med. Chem. 2011, 11, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Taper, H.S. Altered Deoxyribonuclease Activity in Cancer Cells and Its Role in Non Toxic Adjuvant Cancer Therapy with Mixed Vitamins C and K3. Anticancer Res. 2008, 28, 2727–2732. [Google Scholar] [PubMed]
- Pfau, C.J. The Thiosemicarbazones. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 1982; pp. 147–204. [Google Scholar]
- Pavan, F.R.; Maia, P.I.D.S.; Leite, S.R.; Deflon, V.M.; Batista, A.A.; Sato, D.N.; Franzblau, S.G.; Leite, C.Q. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti-Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Kizilcikli, I.; Kurt, Y.D.; Akkurt, B.; Genel, A.Y.; Birteksöz, S.; Ötük, G.; Ülküseven, B. Antimicrobial Activity of a Series of Thiosemicarbazones and Their Zn II and PdII Complexes. Folia Microbiol. 2007, 52, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Matsa, R.; Makam, P.; Kaushik, M.; Hoti, S.L.; Kannan, T. Thiosemicarbazone Derivatives: Design, Synthesis and in Vitro Antimalarial Activity Studies. Eur. J. Pharm. Sci. 2019, 137, 104986. [Google Scholar] [CrossRef]
- Mannargudi, M.B.; Deb, S. Clinical Pharmacology and Clinical Trials of Ribonucleotide Reductase Inhibitors: Is It a Viable Cancer Therapy? J. Cancer Res. Clin. Oncol. 2017, 143, 1499–1529. [Google Scholar] [CrossRef]
- Kunos, C.A.; Chu, E.; Beumer, J.H.; Sznol, M.; Ivy, S.P. Phase I Trial of Daily Triapine in Combination with Cisplatin Chemotherapy for Advanced-Stage Malignancies. Cancer Chemother. Pharmacol. 2017, 79, 201–207. [Google Scholar] [CrossRef]
- Kunos, C.A.; Sherertz, T.M. Long-Term Disease Control with Triapine-Based Radiochemotherapy for Patients with Stage IB2-IIIB Cervical Cancer. Front. Oncol. 2014, 4, 184. [Google Scholar] [CrossRef] [Green Version]
- Zeidner, J.F.; Karp, J.E.; Blackford, A.L.; Smith, B.D.; Gojo, I.; Gore, S.D.; Levis, M.J.; Carraway, H.E.; Greer, J.M.; Percy Ivy, S.; et al. A Phase II Trial of Sequential Ribonucleotide Reductase Inhibition in Aggressive Myeloproliferative Neoplasms. Haematologica 2014, 99, 672–678. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, A.; Ling, Y.; Martin, L.K.; Wei, L.; Phelps, M.A.; Liu, Z.; Harper, E.J.; Ivy, S.P.; Wu, X.; Zhou, B.-S.; et al. A Phase I Study of Prolonged Infusion of Triapine in Combination with Fixed Dose Rate Gemcitabine in Patients with Advanced Solid Tumors. Investig. New Drugs 2013, 31, 685–695. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Kalinowski, D.S.; Kovacevic, Z.; Siafakas, A.R.; Jansson, P.J.; Stefani, C.; Lovejoy, D.B.; Sharpe, P.C.; Bernhardt, P.V.; Richardson, D.R. Thiosemicarbazones from the Old to New: Iron Chelators That Are More than Just Ribonucleotide Reductase Inhibitors. J. Med. Chem. 2009, 52, 5271–5294. [Google Scholar] [CrossRef]
- Thelander, L.; Gräslund, A. Mechanism of Inhibition of Mammalian Ribonucleotide Reductase by the Iron Chelate of 1-Formylisoquinoline Thiosemicarbazone. Destruction of the Tyrosine Free Radical of the Enzyme in an Oxygen-Requiring Reaction-PubMed. J. Biol. Chem. 1983, 258, 4063–4066. [Google Scholar] [CrossRef]
- Richardson, D.R.; Sharpe, P.C.; Lovejoy, D.B.; Senaratne, D.; Kalinowski, D.S.; Islam, M.; Bernhardt, P.V. Dipyridyl Thiosemicarbazone Chelators with Potent and Selective Antitumor Activity Form Iron Complexes with Redox Activity. J. Med. Chem. 2006, 49, 6510–6521. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, D.S.; Richardson, D.R. The Evolution of Iron Chelators for the Treatment of Iron Overload Disease and Cancer. Pharmacol. Rev. 2005, 57, 547–583. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, D.S.; Quach, P.; Richardson, D.R. Thiosemicarbazones: The New Wave in Cancer Treatment. Future Med. Chem. 2009, 1, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Malarz, K.; Mrozek-Wilczkiewicz, A.; Serda, M.; Rejmund, M.; Polanski, J.; Musiol, R. The Role of Oxidative Stress in Activity of Anticancer Thiosemicarbazones. Oncotarget 2018, 9, 17689–17710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, D.R.; Kalinowski, D.S.; Richardson, V.; Sharpe, P.C.; Lovejoy, D.B.; Islam, M.; Bernhardt, P.V. 2-Acetylpyridine Thiosemicarbazones Are Potent Iron Chelators and Antiproliferative Agents: Redox Activity, Iron Complexation and Characterization of Their Antitumor Activity. J. Med. Chem. 2009, 52, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, D.S.; Yu, Y.; Sharpe, P.C.; Islam, M.; Liao, Y.T.; Lovejoy, D.B.; Kumar, N.; Bernhardt, P.V.; Richardson, D.R. Design, Synthesis, and Characterization of Novel Iron Chelators: Structure-Activity Relationships of the 2-Benzoylpyridine Thiosemicarbazone Series and Their 3-Nitrobenzoyl Analogues as Potent Antitumor Agents. J. Med. Chem. 2007, 50, 3716–3729. [Google Scholar] [CrossRef]
- Heffeter, P.; Pape, V.F.S.; Enyedy, É.A.; Keppler, B.K.; Szakacs, G.; Kowol, C.R. Anticancer Thiosemicarbazones: Chemical Properties, Interaction with Iron Metabolism, and Resistance Development. Antioxid. Redox Signal. 2019, 30, 1062–1082. [Google Scholar] [CrossRef]
- Hasinoff, B.B.; Patel, D. The Iron Chelator Dp44mT Does Not Protect Myocytes against Doxorubicin. J. Inorg. Biochem. 2009, 103, 1093–1101. [Google Scholar] [CrossRef]
- Gonzálvez, M.A.; Algarra, A.G.; Basallote, M.G.; Bernhardt, P.V.; Fernández-Trujillo, M.J.; Martínez, M. Proton-Assisted Air Oxidation Mechanisms of Iron(Ii) Bis-Thiosemicarbazone Complexes at Physiological PH: A Kinetico-Mechanistic Study. Dalt. Trans. 2019, 48, 16578–16587. [Google Scholar] [CrossRef]
- Jansson, P.J.; Hawkins, C.L.; Lovejoy, D.B.; Richardson, D.R. The Iron Complex of Dp44mT Is Redox-Active and Induces Hydroxyl Radical Formation: An EPR Study. J. Inorg. Biochem. 2010, 104, 1224–1228. [Google Scholar] [CrossRef] [PubMed]
- Stacy, A.E.; Palanimuthu, D.; Bernhardt, P.V.; Kalinowski, D.S.; Jansson, P.J.; Richardson, D.R. Zinc(II)-Thiosemicarbazone Complexes Are Localized to the Lysosomal Compartment Where They Transmetallate with Copper Ions to Induce Cytotoxicity. J. Med. Chem. 2016, 59, 4965–4984. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, E.M.; Seebacher, N.A.; Arzuman, L.; Kovacevic, Z.; Lane, D.J.R.; Richardson, V.; Merlot, A.M.; Lok, H.; Kalinowski, D.S.; Sahni, S.; et al. Lysosomal Membrane Stability Plays a Major Role in the Cytotoxic Activity of the Anti-Proliferative Agent, Di-2-Pyridylketone 4,4-Dimethyl-3-Thiosemicarbazone (Dp44mT). Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 1665–1681. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, I.R.; Pinheiro, I.D.S.; Dos Santos, A.D.L.; Echevarria, A.; Goulart, C.M.; Guedes, G.P.; Da Costa, N.A.; Silva, B.M.D.O.E.; Riger, C.J.; Neves, A.P. Synthesis of copper(II) and zinc(II) complexes with chalcone–thiosemicarbazone hybrid ligands: X-ray crystallography, spectroscopy and yeast activity. Transit. Met. Chem. 2018, 43, 739–751. [Google Scholar] [CrossRef]
- Ohui, K.; Stepanenko, I.; Besleaga, I.; Babak, M.V.; Stafi, R.; Darvasiova, D.; Giester, G.; Pósa, V.; Enyedy, E.A.; Vegh, D.; et al. Triapine Derivatives Act as Copper Delivery Vehicles to Induce Deadly Metal Overload in Cancer Cells. Biomolecules 2020, 10, 1336. [Google Scholar] [CrossRef]
- Santoro, A.; Vileno, B.; Palacios, Ò.; Peris-Díaz, M.D.; Riegel, G.; Gaiddon, C.; Krȩzel, A.; Faller, P. Reactivity of Cu(II)–, Zn(II)– and Fe(II)–Thiosemicarbazone Complexes with Glutathione and Metallothionein: From Stability to Dissociation to Transmetallation. Metallomics 2019, 11, 994–1004. [Google Scholar] [CrossRef]
- Chen, G.; Niu, C.; Yi, J.; Sun, L.; Cao, H.; Fang, Y.; Jin, T.; Li, Y.; Lou, C.; Kang, J.; et al. Novel Triapine Derivative Induces Copper-Dependent Cell Death in Hematopoietic Cancers. J. Med. Chem. 2019, 62, 3107–3121. [Google Scholar] [CrossRef]
- Kowol, C.R.; Heffeter, P.; Miklos, W.; Gille, L.; Trondl, R.; Cappellacci, L.; Berger, W.; Keppler, B.K. Mechanisms Underlying Reductant-Induced Reactive Oxygen Species Formation by Anticancer Copper(II) Compounds. J. Biol. Inorg. Chem. 2012, 17, 409–423. [Google Scholar] [CrossRef] [Green Version]
- Kolesar, J.; Brundage, R.C.; Pomplun, M.; Alberti, D.; Holen, K.; Traynor, A.; Ivy, P.; Wilding, G. Population Pharmacokinetics of 3-Aminopyridine-2-Carboxaldehyde Thiosemicarbazone (Triapine®) in Cancer Patients. Cancer Chemother. Pharmacol. 2011, 67, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Pitucha, M.; Korga-Plewko, A.; Czylkowska, A.; Rogalewicz, B.; Drozd, M.; Iwan, M.; Kubik, J.; Humeniuk, E.; Adamczuk, G.; Karczmarzyk, Z.; et al. Influence of Complexation of Thiosemicarbazone Derivatives with Cu (II) Ions on Their Antitumor Activity against Melanoma Cells. Int. J. Mol. Sci. 2021, 22, 3104. [Google Scholar] [CrossRef]
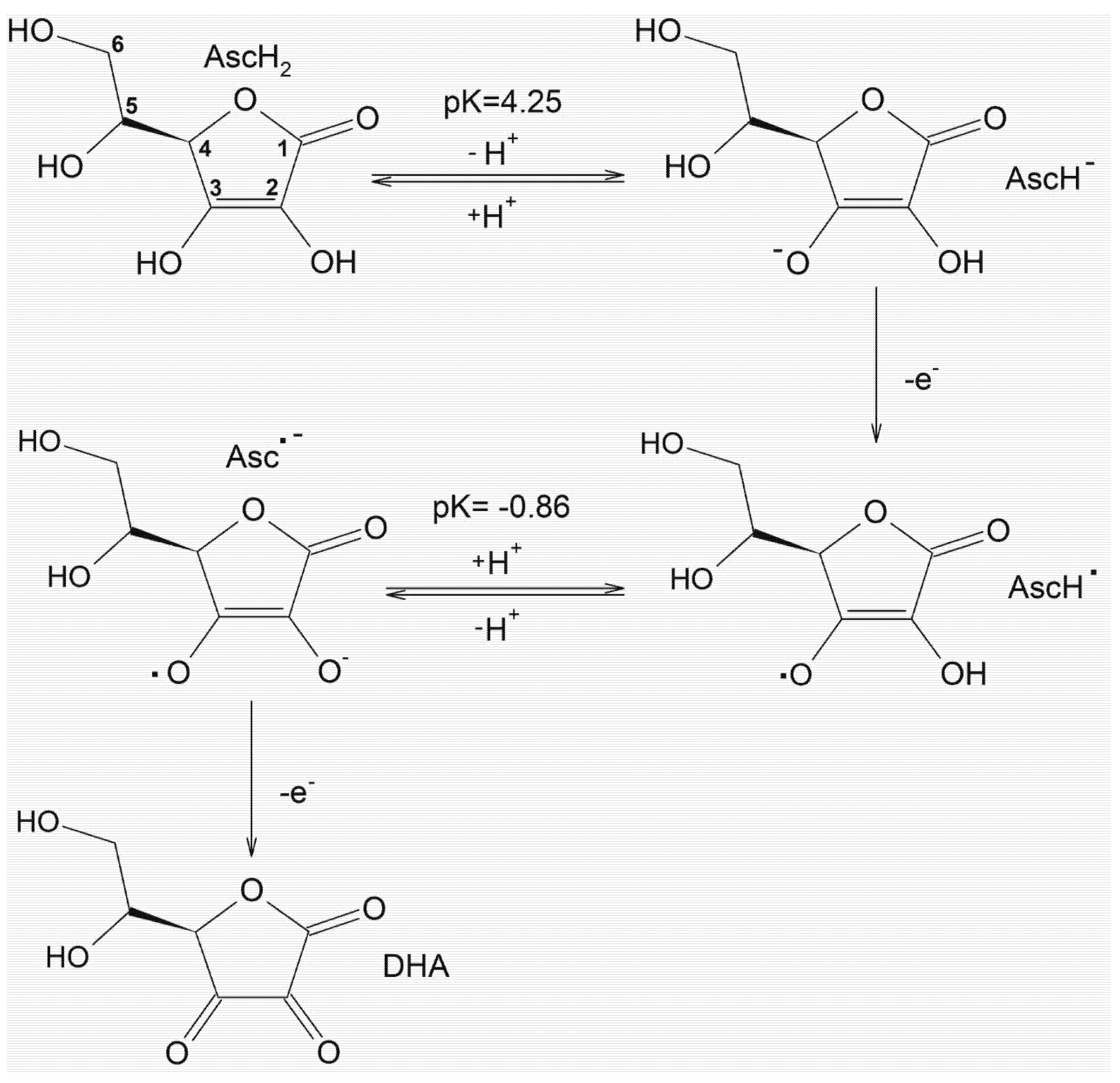

| Radical | k (M−1s−1) |
|---|---|
| •OH (hydroxyl) | 1.1 × 1010 |
| RO• (tert-butyl alkoxyl radical) | 1.6 × 109 |
| ROO• (alkyl peroxyl radical, e.g., CH3OO•) | 1–2 × 106 |
| GS• (glutathiol radical) | 6 × 108 |
| TO• (tocopheroxyl radical) | 2 × 105 |
| Asc•− (dismutation) | 2 × 105 |
| O2•−/•OOH | 1 × 105 |
| Chelator | Types of Anti-Alzheimer Activity | References * | |
|---|---|---|---|
 | R1,R2 = H,  | AoA AChE Abeta | [117] |
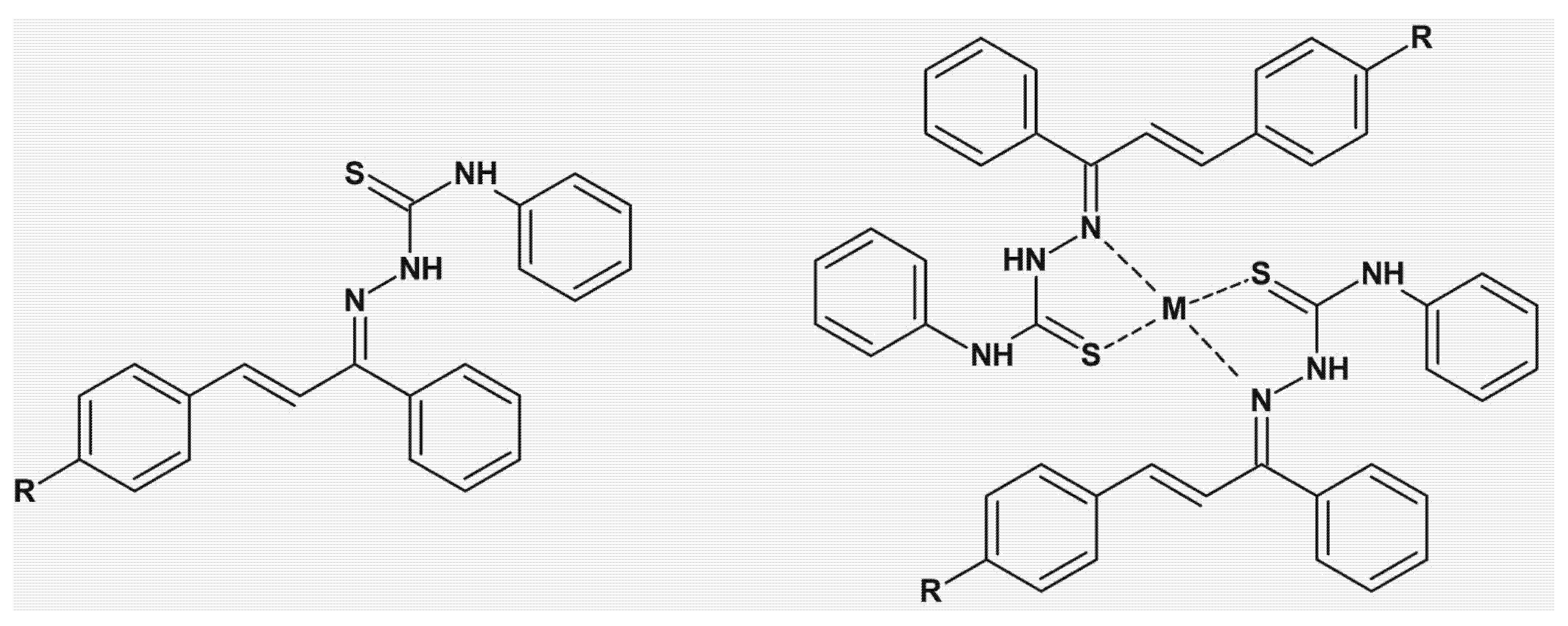
 | n = 2–4 R1 = H, Bn R2 = H, OCH3 | AoA AChE Abeta | [167,168] |
 | n = 3–5 NR1R2 = 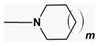 , m = 0,1 , m = 0,1NR1R2 = 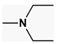 | AoA Abeta H3Ra | [169,170] |
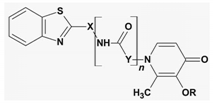 | n = 1 X,Y = -CH2Ph, -Ph, -(CH2)n n = 0 X = Ph R = H, Bn | AoA AChE Abeta | [117,171] |
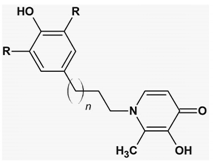 | n = 1–3 R = t-Bu, OCH3 | AoA | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timoshnikov, V.A.; Selyutina, O.Y.; Polyakov, N.E.; Didichenko, V.; Kontoghiorghes, G.J. Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine. Int. J. Mol. Sci. 2022, 23, 1247. https://doi.org/10.3390/ijms23031247
Timoshnikov VA, Selyutina OY, Polyakov NE, Didichenko V, Kontoghiorghes GJ. Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine. International Journal of Molecular Sciences. 2022; 23(3):1247. https://doi.org/10.3390/ijms23031247
Chicago/Turabian StyleTimoshnikov, Viktor A., Olga Yu. Selyutina, Nikolay E. Polyakov, Victoria Didichenko, and George J. Kontoghiorghes. 2022. "Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine" International Journal of Molecular Sciences 23, no. 3: 1247. https://doi.org/10.3390/ijms23031247
APA StyleTimoshnikov, V. A., Selyutina, O. Y., Polyakov, N. E., Didichenko, V., & Kontoghiorghes, G. J. (2022). Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine. International Journal of Molecular Sciences, 23(3), 1247. https://doi.org/10.3390/ijms23031247









