Cross-Linking and Functional Analyses for Dimerization of a Cysteine Mutant of Glycine Transporter 1
Abstract
1. Introduction
2. Results
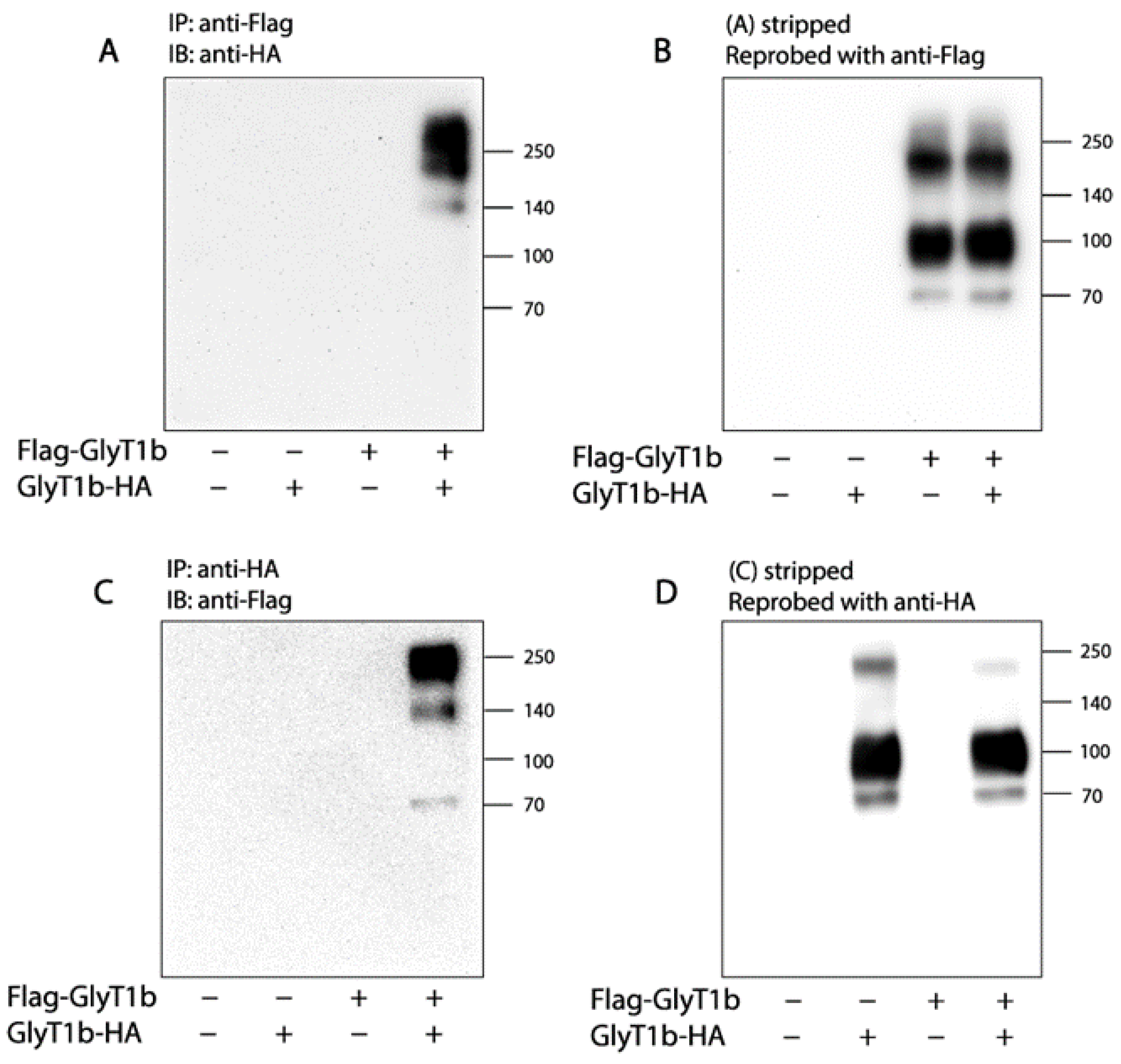
2.1. Association of FLAG-GlyT1 with GlyT1-HA
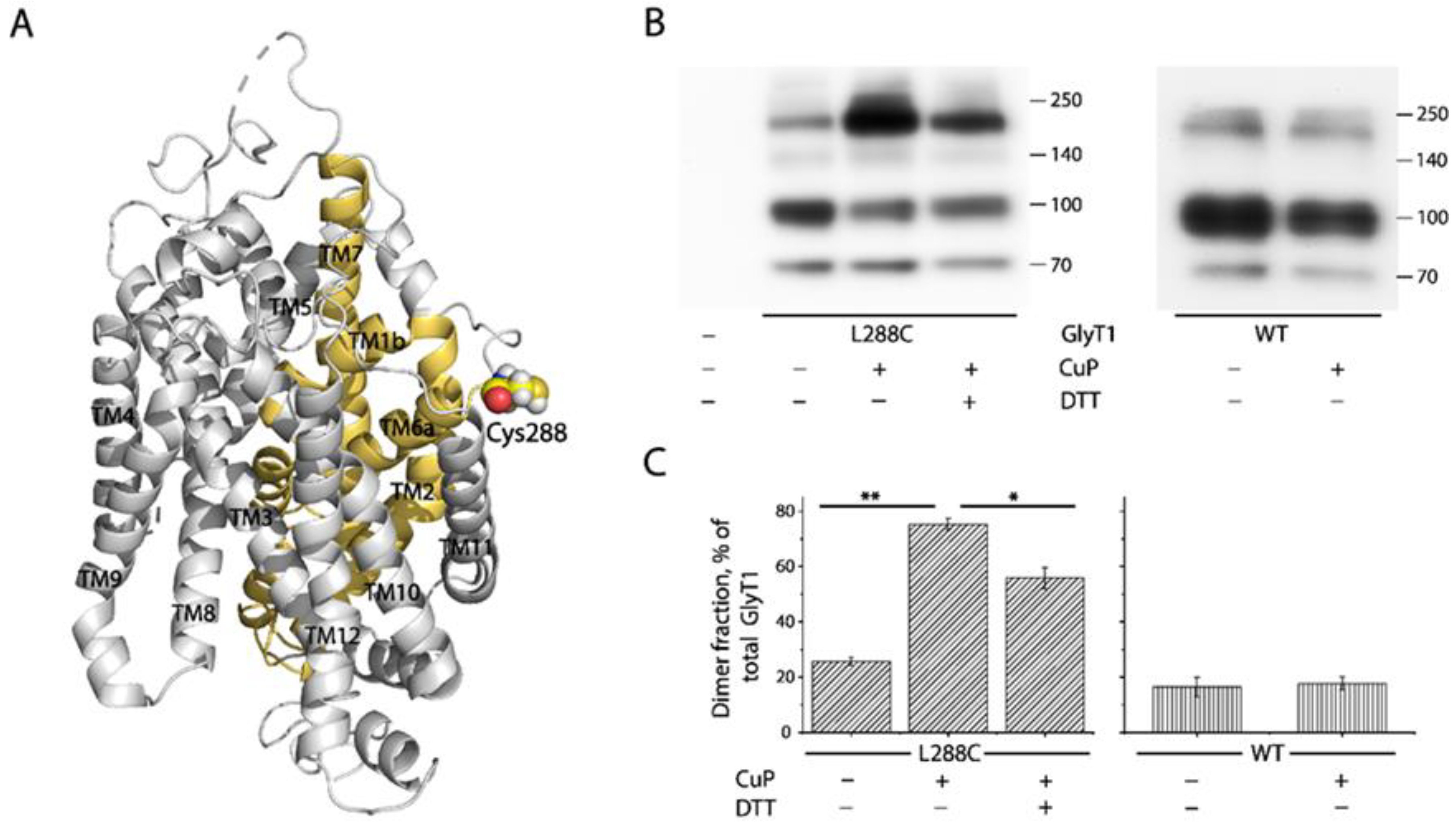
2.2. Cross-Linking of GlyT1 L288C
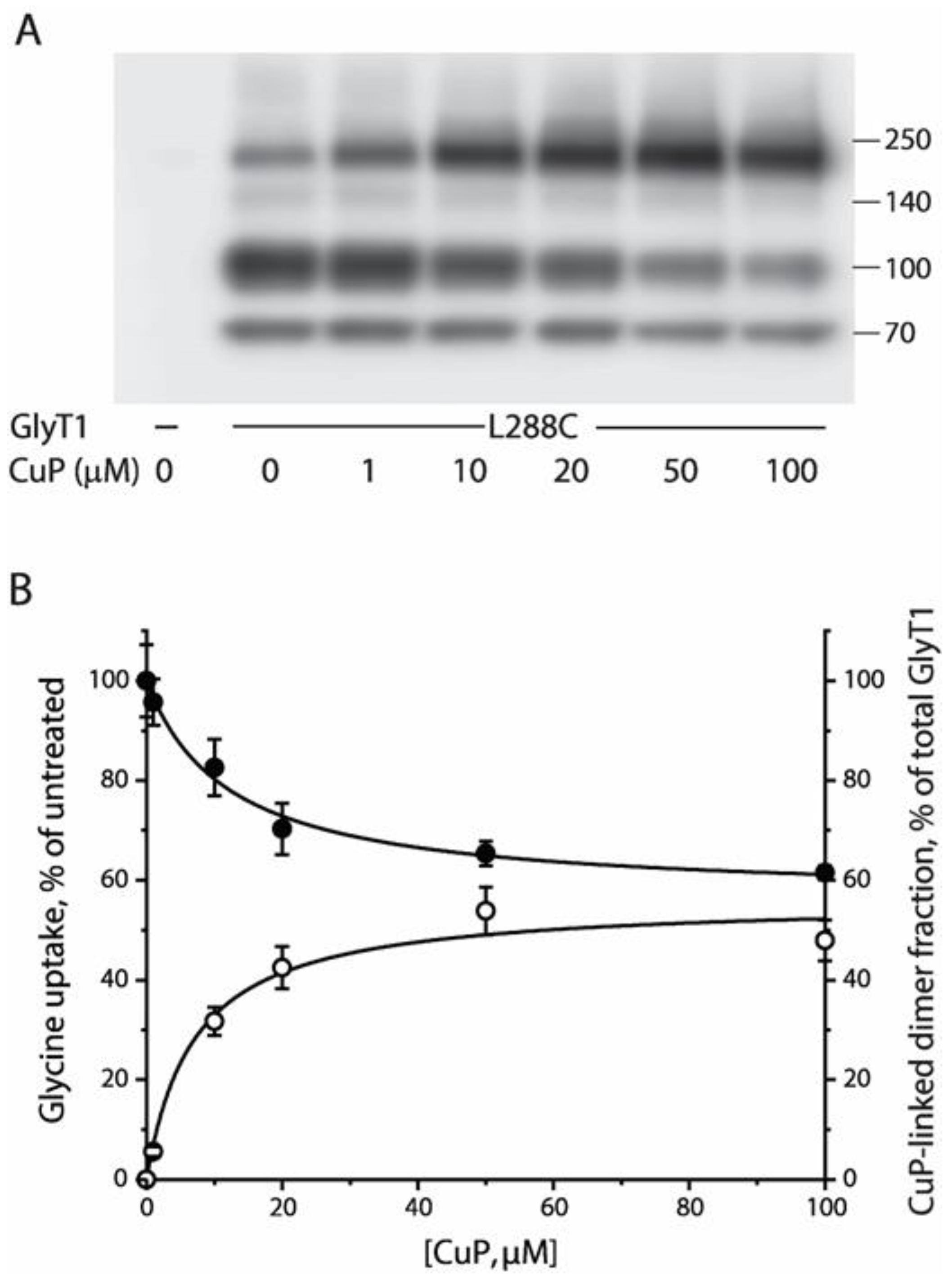
2.3. Functional Consequences of CuP Cross-Linking of L288C
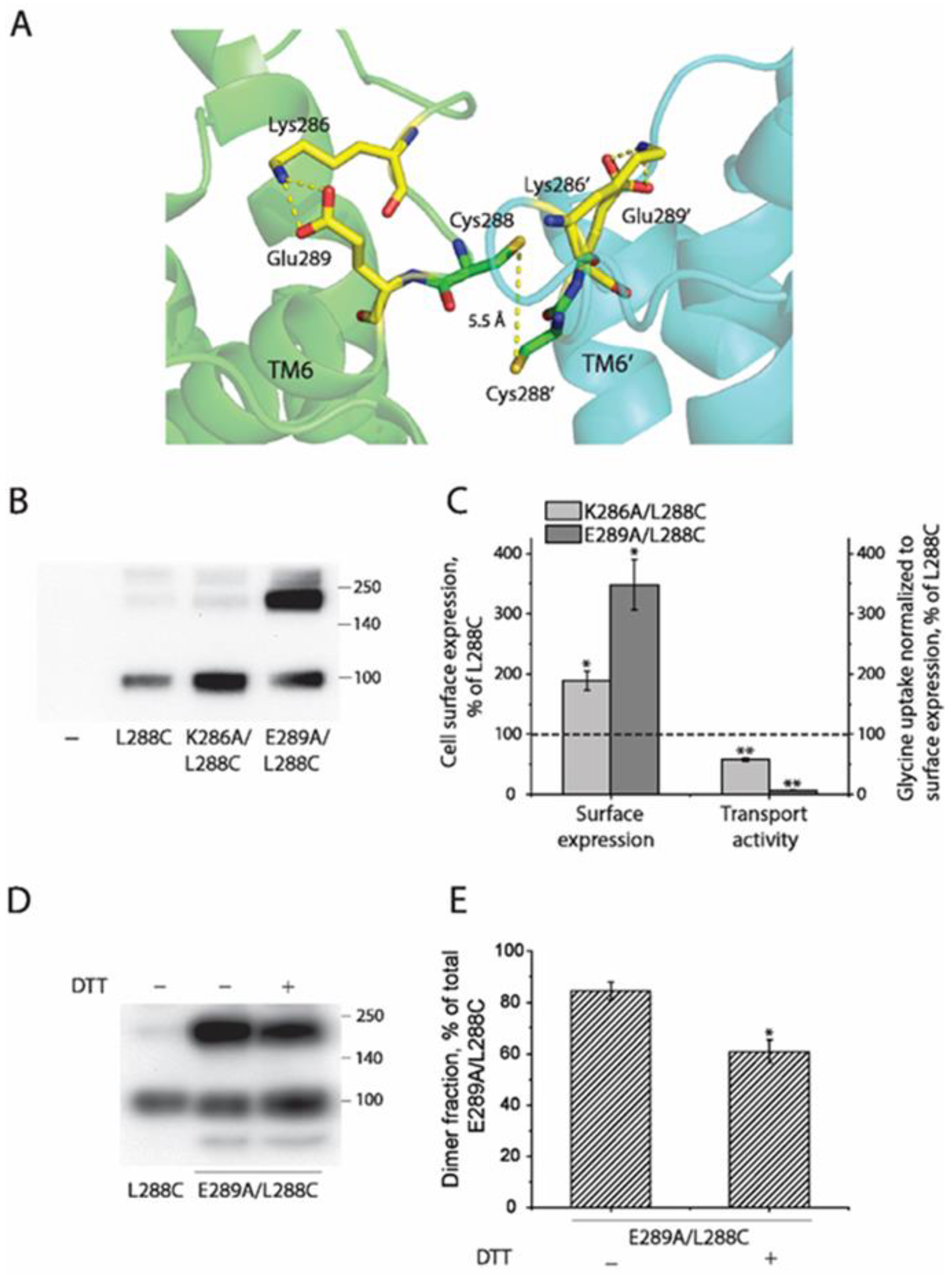
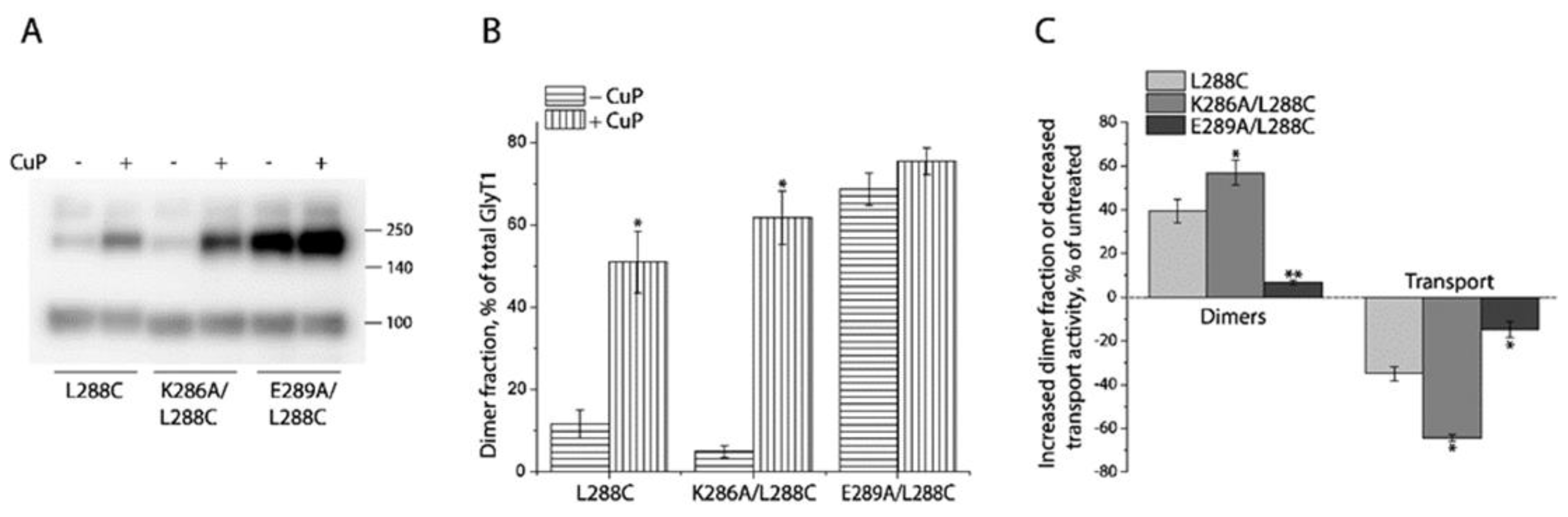
2.4. Influence of Intramolecular K286-E289 Ion Pair on CuP Cross-Linking of L288C
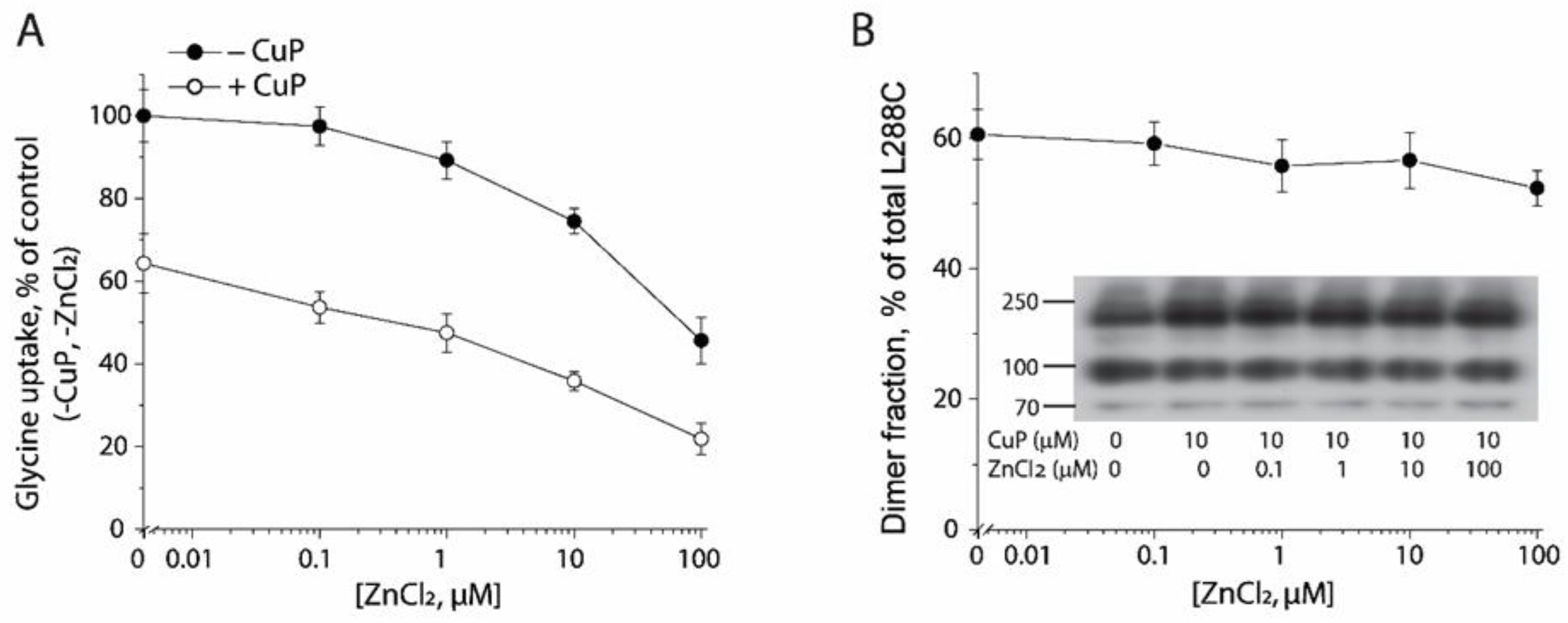
2.5. Influence of Zn2+ on CuP Cross-Linking of L288C
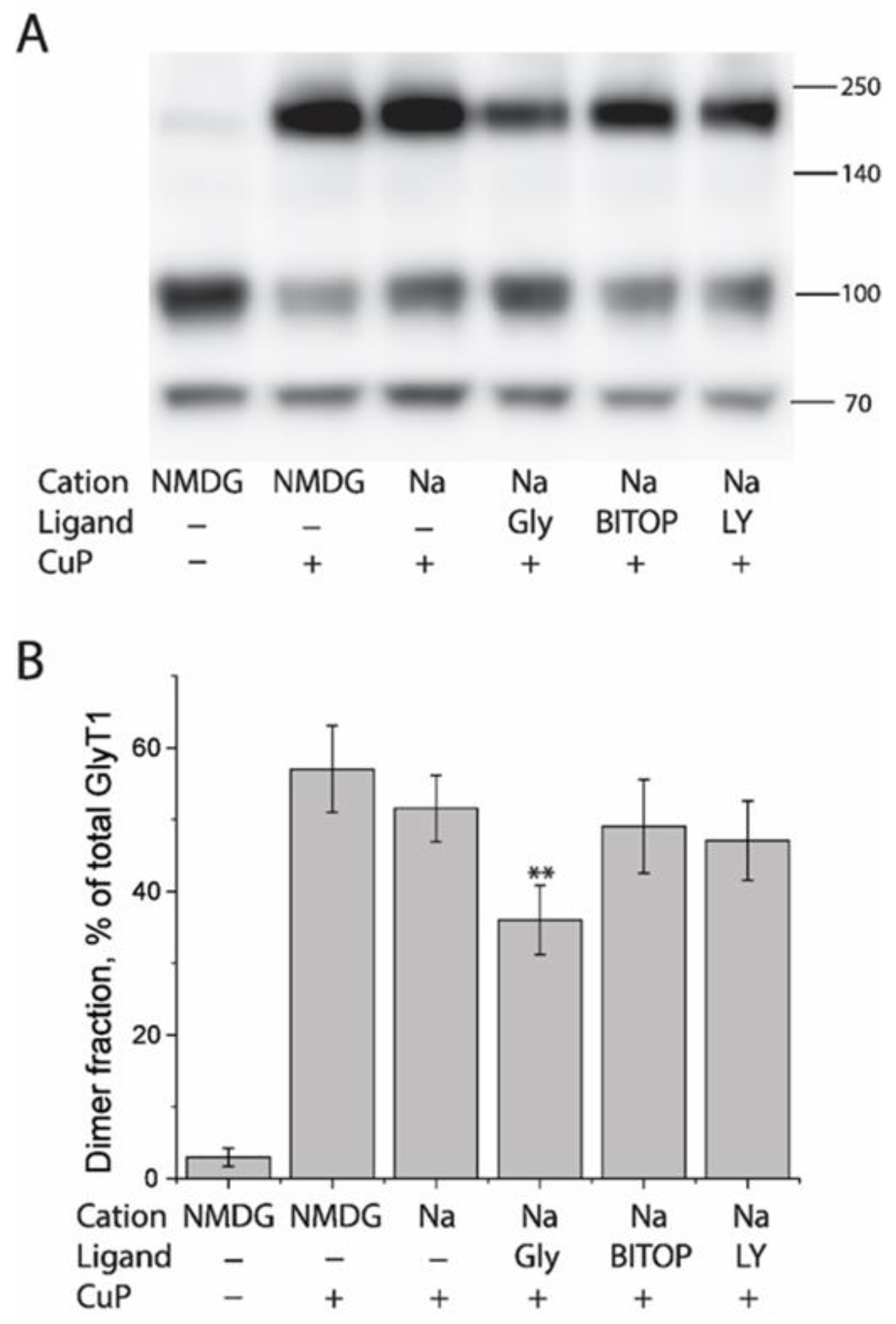
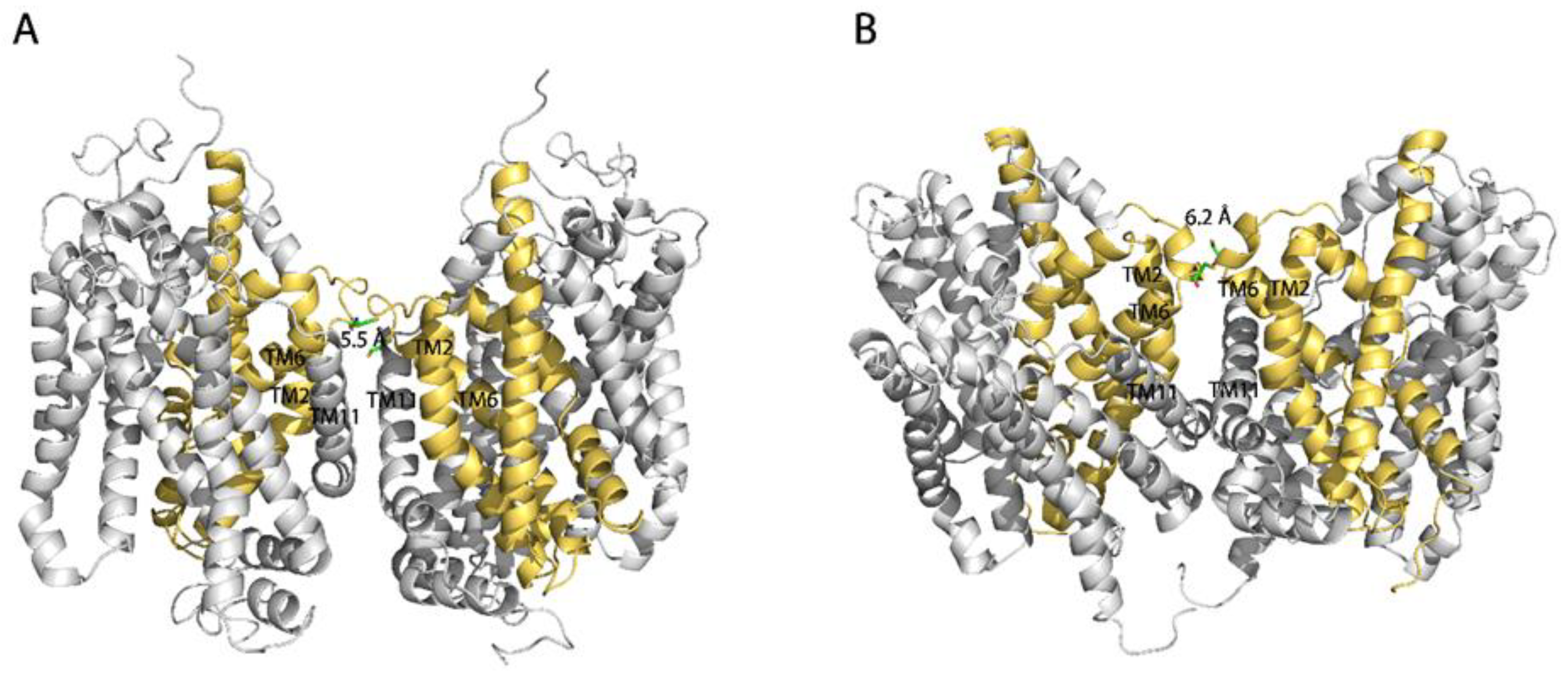
2.6. Influence of GlyT1 Conformation on CuP Cross-Linking of L288C
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Mutagenesis
4.3. Lentivirus and Stable Cell Line Preparation
4.4. Transport and CHIBA-3007 Binding Assays
4.5. Co-Immunoprecipitation and Immunoblotting
4.6. Biotinylation
4.7. Oxidative Cross-Linking of GlyT1b
4.8. Homology Modeling
4.9. Dimer Docking of GlyT1b
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Legendre, P. The glycinergic inhibitory synapse. Cell. Mol. Life Sci. 2001, 58, 760–793. [Google Scholar] [CrossRef] [PubMed]
- Zeilhofer, H.U.; Wildner, H.; Yevenes, G.E. Fast synaptic inhibition in spinal sensory processing and pain control. Physiol. Rev. 2012, 92, 193–235. [Google Scholar] [CrossRef] [PubMed]
- Zafra, F.; Ibanez, I.; Bartolome-Martin, D.; Piniella, D.; Arribas-Blazquez, M.; Gimenez, C. Glycine transporters and its coupling with NMDA receptors. Adv. Neurobiol. 2017, 16, 55–83. [Google Scholar] [PubMed]
- Eulenburg, V.; Armsen, W.; Betz, H.; Gomeza, J. Glycine transporters: Essential regulators of neurotransmission. Trends Biochem. Sci. 2005, 30, 325–333. [Google Scholar] [CrossRef]
- Berger, A.J.; Dieudonne, S.; Ascher, P. Glycine uptake governs glycine site occupancy at NMDA receptors of excitatory synapses. J. Neurophysiol. 1998, 80, 3336–3340. [Google Scholar] [CrossRef] [PubMed]
- Marques, B.L.; Oliveira-Lima, O.C.; Carvalho, G.A.; de Almeida Chiarelli, R.; Ribeiro, R.I.; Parreira, R.C.; da Madeira Freitas, E.M.; Resende, R.R.; Klempin, F.; Ulrich, H.; et al. Neurobiology of glycine transporters: From molecules to behavior. Neurosci. Biobehav. Rev. 2020, 118, 97–110. [Google Scholar] [CrossRef]
- Sur, C.; Kinney, G.G. The therapeutic potential of glycine transporter-1 inhibitors. Expert Opin. Investig. Drugs 2004, 13, 515–521. [Google Scholar] [CrossRef]
- Yang, C.R.; Svensson, K.A. Allosteric modulation of NMDA receptor via elevation of brain glycine and D-serine: The therapeutic potentials for schizophrenia. Pharmacol. Ther. 2008, 120, 317–332. [Google Scholar] [CrossRef]
- Javitt, D.C. Glycine transport inhibitors and the treatment of schizophrenia. Biol. Psychiatry 2008, 63, 6–8. [Google Scholar] [CrossRef]
- Harvey, R.J.; Yee, B.K. Glycine transporters as novel therapeutic targets in schizophrenia, alcohol dependence and pain. Nat. Rev. Drug Discov. 2013, 12, 866–885. [Google Scholar] [CrossRef]
- Morita, K.; Motoyama, N.; Kitayama, T.; Morioka, N.; Kifune, K.; Dohi, T. Spinal antiallodynia action of glycine transporter inhibitors in neuropathic pain models in mice. J. Pharmacol. Exp. Ther. 2008, 326, 633–645. [Google Scholar] [CrossRef] [PubMed]
- Dohi, T.; Morita, K.; Kitayama, T.; Motoyama, N.; Morioka, N. Glycine transporter inhibitors as a novel drug discovery strategy for neuropathic pain. Pharmacol. Ther. 2009, 123, 54–79. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, L.; Barthel, F.; Brandenburger, T.; Neumann, E.; Bauer, I.; Eulenburg, V.; Werdehausen, R.; Hermanns, H. Glycine transporter GlyT1, but not GlyT2, is expressed in rat dorsal root ganglion--Possible implications for neuropathic pain. Neurosci. Lett. 2015, 600, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Rudnick, G. Bioenergetics of neurotransmitter transport. J. Bioenerg. Biomembr. 1998, 30, 173–185. [Google Scholar] [CrossRef]
- Rudnick, G. How do transporters couple solute movements? Mol. Membr. Biol. 2013, 30, 355–359. [Google Scholar] [CrossRef]
- Terry, D.S.; Kolster, R.A.; Quick, M.; LeVine, M.V.; Khelashvili, G.; Zhou, Z.; Weinstein, H.; Javitch, J.A.; Blanchard, S.C. A partially-open inward-facing intermediate conformation of LeuT is associated with Na+ release and substrate transport. Nat. Commun. 2018, 9, 230. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Tavoulari, S.; Sinning, S.; Aleksandrova, A.A.; Forrest, L.R.; Rudnick, G. Structural elements required for coupling ion and substrate transport in the neurotransmitter transporter homolog LeuT. Proc. Natl. Acad. Sci. USA 2018, 115, E8854–E8862. [Google Scholar] [CrossRef]
- Yamashita, A.; Singh, S.K.; Kawate, T.; Jin, Y.; Gouaux, E. Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature 2005, 437, 215–223. [Google Scholar] [CrossRef]
- Krishnamurthy, H.; Gouaux, E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 2012, 481, 469–474. [Google Scholar] [CrossRef]
- Fan, J.; Xiao, Y.; Quick, M.; Yang, Y.; Sun, Z.; Javitch, J.A.; Zhou, X. Crystal structures of LeuT reveal conformational dynamics in the outward-facing states. J. Biol. Chem. 2021, 296, 100609. [Google Scholar] [CrossRef]
- Penmatsa, A.; Wang, K.H.; Gouaux, E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 2013, 503, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.H.; Penmatsa, A.; Gouaux, E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 2015, 521, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.A.; Gouaux, E. Structural basis for recognition of diverse antidepressants by the human serotonin transporter. Nat. Struct. Mol. Biol. 2018, 25, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 2016, 532, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.A.; Yang, D.; Zhao, Z.; Wen, P.C.; Yoshioka, C.; Tajkhorshid, E.; Gouaux, E. Serotonin transporter-ibogaine complexes illuminate mechanisms of inhibition and transport. Nature 2019, 569, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Shahsavar, A.; Stohler, P.; Bourenkov, G.; Zimmermann, I.; Siegrist, M.; Guba, W.; Pinard, E.; Sinning, S.; Seeger, M.A.; Schneider, T.R.; et al. Structural insights into the inhibition of glycine reuptake. Nature 2021, 591, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Kilic, F.; Rudnick, G. Oligomerization of serotonin transporter and its functional consequences. Proc. Natl. Acad. Sci. USA 2000, 97, 3106–3111. [Google Scholar] [CrossRef]
- Kocabas, A.M.; Rudnick, G.; Kilic, F. Functional consequences of homo- but not hetero-oligomerization between transporters for the biogenic amine neurotransmitters. J. Neurochem. 2003, 85, 1513–1520. [Google Scholar] [CrossRef]
- Farhan, H.; Freissmuth, M.; Sitte, H.H. Oligomerization of neurotransmitter transporters: A ticket from the endoplasmic reticulum to the plasma membrane. Handb. Exp. Pharmacol. 2006, 175, 233–249. [Google Scholar]
- Anderluh, A.; Hofmaier, T.; Klotzsch, E.; Kudlacek, O.; Stockner, T.; Sitte, H.H.; Schutz, G.J. Direct PIP2 binding mediates stable oligomer formation of the serotonin transporter. Nat. Commun. 2017, 8, 14089. [Google Scholar] [CrossRef]
- Gupta, K.; Donlan, J.A.C.; Hopper, J.T.S.; Uzdavinys, P.; Landreh, M.; Struwe, W.B.; Drew, D.; Baldwin, A.J.; Stansfeld, P.J.; Robinson, C.V. The role of interfacial lipids in stabilizing membrane protein oligomers. Nature 2017, 541, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Zhen, J.; Reith, M.E.A. Functional properties of dopamine transporter oligomers after copper linking. J. Neurochem. 2018, 144, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.; Ponzoni, L.; Sorkina, T.; Lee, J.Y.; Zhang, S.; Sorkin, A.; Bahar, I. Trimerization of dopamine transporter triggered by AIM-100 binding: Molecular mechanism and effect of mutations. Neuropharmacology 2019, 161, 107676. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Kudlacek, O.; Baumgart, F.; Jaentsch, K.; Stockner, T.; Sitte, H.H.; Schutz, G.J. Dopamine transporter forms stable dimers in the live cell plasma membrane in a phosphatidylinositol 4,5-bisphosphate-independent manner. J. Biol. Chem. 2019, 294, 5632–5642. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, K.; Das, A.K.; Luethi, D.; Szollosi, D.; Schutz, G.J.; Reith, M.E.A.; Sitte, H.H.; Stockner, T. SLC6 transporter oligomerization. J. Neurochem. 2021, 157, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Sorkina, T.; Cheng, M.H.; Bagalkot, T.R.; Wallace, C.; Watkins, S.C.; Bahar, I.; Sorkin, A. Direct coupling of oligomerization and oligomerization-driven endocytosis of the dopamine transporter to its conformational mechanics and activity. J. Biol. Chem. 2021, 296, 100430. [Google Scholar] [CrossRef] [PubMed]
- Zeppelin, T.; Pedersen, K.B.; Berglund, N.A.; Periole, X.; Schiott, B. Effect of palmitoylation on the dimer formation of the human dopamine transporter. Sci. Rep. 2021, 11, 4164. [Google Scholar] [CrossRef]
- Hastrup, H.; Karlin, A.; Javitch, J.A. Symmetrical dimer of the human dopamine transporter revealed by cross-linking Cys-306 at the extracellular end of the sixth transmembrane segment. Proc. Natl. Acad. Sci. USA 2001, 98, 10055–10060. [Google Scholar] [CrossRef]
- Bartholomaus, I.; Milan-Lobo, L.; Nicke, A.; Dutertre, S.; Hastrup, H.; Jha, A.; Gether, U.; Sitte, H.H.; Betz, H.; Eulenburg, V. Glycine transporter dimers: Evidence for occurrence in the plasma membrane. J. Biol. Chem. 2008, 283, 10978–10991. [Google Scholar] [CrossRef]
- Cheng, M.H.; Garcia-Olivares, J.; Wasserman, S.; DiPietro, J.; Bahar, I. Allosteric modulation of human dopamine transporter activity under conditions promoting its dimerization. J. Biol. Chem. 2017, 292, 12471–12482. [Google Scholar] [CrossRef]
- Gur, M.; Cheng, M.H.; Zomot, E.; Bahar, I. Effect of dimerization on the dynamics of neurotransmitter:sodium symporters. J. Phys. Chem. B 2017, 121, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, K.; Morley, A.N.; Szollosi, D.; Wassenaar, T.A.; Sitte, H.H.; Stockner, T. Dopamine transporter oligomerization involves the scaffold domain, but spares the bundle domain. PLoS Comput. Biol. 2018, 14, e1006229. [Google Scholar] [CrossRef] [PubMed]
- Ju, P.; Aubrey, K.R.; Vandenberg, R.J. Zn2+ inhibits glycine transport by glycine transporter subtype 1b. J. Biol. Chem. 2004, 279, 22983–22991. [Google Scholar] [CrossRef] [PubMed]
- Norregaard, L.; Frederiksen, D.; Nielsen, E.O.; Gether, U. Delineation of an endogenous zinc-binding site in the human dopamine transporter. EMBO J. 1998, 17, 4266–4273. [Google Scholar] [CrossRef] [PubMed]
- Loland, C.J.; Norregaard, L.; Gether, U. Defining proximity relationships in the tertiary structure of the dopamine transporter. Identification of a conserved glutamic acid as a third coordinate in the endogenous Zn2+-binding site. J. Biol. Chem. 1999, 274, 36928–36934. [Google Scholar] [CrossRef] [PubMed]
- Norgaard-Nielsen, K.; Norregaard, L.; Hastrup, H.; Javitch, J.A.; Gether, U. Zn2+ site engineering at the oligomeric interface of the dopamine transporter. FEBS Lett. 2002, 524, 87–91. [Google Scholar] [CrossRef]
- Perry, K.W.; Falcone, J.F.; Fell, M.J.; Ryder, J.W.; Yu, H.; Love, P.L.; Katner, J.; Gordon, K.D.; Wade, M.R.; Man, T.; et al. Neurochemical and behavioral profiling of the selective GlyT1 inhibitors ALX5407 and LY2365109 indicate a preferential action in caudal vs. cortical brain areas. Neuropharmacology 2008, 55, 743–754. [Google Scholar] [CrossRef]
- Shen, H.Y.; van Vliet, E.A.; Bright, K.A.; Hanthorn, M.; Lytle, N.K.; Gorter, J.; Aronica, E.; Boison, D. Glycine transporter 1 is a target for the treatment of epilepsy. Neuropharmacology 2015, 99, 554–565. [Google Scholar] [CrossRef]
- Umbricht, D.; Alberati, D.; Martin-Facklam, M.; Borroni, E.; Youssef, E.A.; Ostland, M.; Wallace, T.L.; Knoflach, F.; Dorflinger, E.; Wettstein, J.G.; et al. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: A randomized, double-blind, proof-of-concept study. JAMA Psychiatry 2014, 71, 637–646. [Google Scholar] [CrossRef]
- Pinard, E.; Borroni, E.; Koerner, A.; Umbricht, D.; Alberati, D. Glycine Transporter type I (GlyT1) inhibitor, bitopertin: A journey from lab to patient. Chimia 2018, 72, 477–484. [Google Scholar] [CrossRef]
- Horiuchi, M.; Nicke, A.; Gomeza, J.; Aschrafi, A.; Schmalzing, G.; Betz, H. Surface-localized glycine transporters 1 and 2 function as monomeric proteins in Xenopus oocytes. Proc. Natl. Acad. Sci. USA 2001, 98, 1448–1453. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Uchendu, S.; Leone, V.; Bradshaw, R.T.; Sangwa, N.; Forrest, L.R.; Rudnick, G. Chloride-dependent conformational changes in the GlyT1 glycine transporter. Proc. Natl. Acad. Sci. USA 2021, 118, e2017431118. [Google Scholar] [CrossRef] [PubMed]
- Latka, K.; Bajda, M. Analysis of binding determinants for different classes of competitive and noncompetitive inhibitors of glycine transporters. Int. J. Mol. Sci. 2022, 23, 8050. [Google Scholar] [CrossRef] [PubMed]
- Hastrup, H.; Sen, N.; Javitch, J.A. The human dopamine transporter forms a tetramer in the plasma membrane: Cross-linking of a cysteine in the fourth transmembrane segment is sensitive to cocaine analogs. J. Biol. Chem. 2003, 278, 45045–45048. [Google Scholar] [CrossRef] [PubMed]
- Elegheert, J.; Behiels, E.; Bishop, B.; Scott, S.; Woolley, R.E.; Griffiths, S.C.; Byrne, E.F.X.; Chang, V.T.; Stuart, D.I.; Jones, E.Y.; et al. Lentiviral transduction of mammalian cells for fast, scalable and high-level production of soluble and membrane proteins. Nat. Protoc. 2018, 13, 2991–3017. [Google Scholar] [CrossRef]
- Olivares, L.; Aragón, C.; Giménez, C.; Zafra, F. Carboxyl terminus of the glycine transporter GLYT1 is necessary for correct processing of the protein. J. Biol. Chem. 1994, 269, 28400–28404. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Rudnick, G. Cysteine-scanning mutagenesis of serotonin transporter intracellular loop 2 suggests an alpha-helical conformation. J. Biol. Chem. 2005, 280, 30807–30813. [Google Scholar] [CrossRef]
- Sato, Y.; Zhang, Y.W.; Androutsellis-Theotokis, A.; Rudnick, G. Analysis of transmembrane domain 2 of rat serotonin transporter by cysteine scanning mutagenesis. J. Biol. Chem. 2004, 279, 22926–22933. [Google Scholar] [CrossRef]
- Li, L.B.; Chen, N.; Ramamoorthy, S.; Chi, L.; Cui, X.N.; Wang, L.C.; Reith, M.E. The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J. Biol. Chem. 2004, 279, 21012–21020. [Google Scholar] [CrossRef]
- Chen, N.; Reith, M.E. Substrates dissociate dopamine transporter oligomers. J. Neurochem. 2008, 105, 910–920. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Beglov, D.; Bohnuud, T.; Mottarella, S.E.; Xia, B.; Hall, D.R.; Vajda, S. How good is automated protein docking? Proteins 2013, 81, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Vajda, S.; Kozakov, D. Accounting for pairwise distance restraints in FFT-based protein-protein docking. Bioinformatics 2016, 32, 3342–3344. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein-protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Vajda, S.; Yueh, C.; Beglov, D.; Bohnuud, T.; Mottarella, S.E.; Xia, B.; Hall, D.R.; Kozakov, D. New additions to the ClusPro server motivated by CAPRI. Proteins 2017, 85, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Desta, I.T.; Porter, K.A.; Xia, B.; Kozakov, D.; Vajda, S. Performance and its limits in rigid body protein-protein docking. Structure 2020, 28, 1071–1081.e3. [Google Scholar] [CrossRef] [PubMed]
| Km (μM) | Vmax (pmol/mg/min) | Cell Surface Expression (% of WT) | Kd (nM) | Bmax (pmol/mg) | |
|---|---|---|---|---|---|
| WT | 38.6 ± 2.6 | 1413 ± 40 | 100 | 5.70 ± 0.47 | 2.76 ± 0.09 |
| L288C | 43.6 ± 2.5 | 918.5 ± 20 * | 68.3 ± 5.3 * | 7.38 ± 0.43 | 1.73 ± 0.07 * |
| L288C + CuP | 54.1 ± 1.8 | 598.8 ± 17 # | nd | 20.6 ± 1.4 # | 1.88 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, H.; Zhang, Y.-W. Cross-Linking and Functional Analyses for Dimerization of a Cysteine Mutant of Glycine Transporter 1. Int. J. Mol. Sci. 2022, 23, 16157. https://doi.org/10.3390/ijms232416157
Wang J, Liu H, Zhang Y-W. Cross-Linking and Functional Analyses for Dimerization of a Cysteine Mutant of Glycine Transporter 1. International Journal of Molecular Sciences. 2022; 23(24):16157. https://doi.org/10.3390/ijms232416157
Chicago/Turabian StyleWang, Jingru, Hanhe Liu, and Yuan-Wei Zhang. 2022. "Cross-Linking and Functional Analyses for Dimerization of a Cysteine Mutant of Glycine Transporter 1" International Journal of Molecular Sciences 23, no. 24: 16157. https://doi.org/10.3390/ijms232416157
APA StyleWang, J., Liu, H., & Zhang, Y.-W. (2022). Cross-Linking and Functional Analyses for Dimerization of a Cysteine Mutant of Glycine Transporter 1. International Journal of Molecular Sciences, 23(24), 16157. https://doi.org/10.3390/ijms232416157






