Glucose-6-Phosphate Dehydrogenases: The Hidden Players of Plant Physiology
Abstract
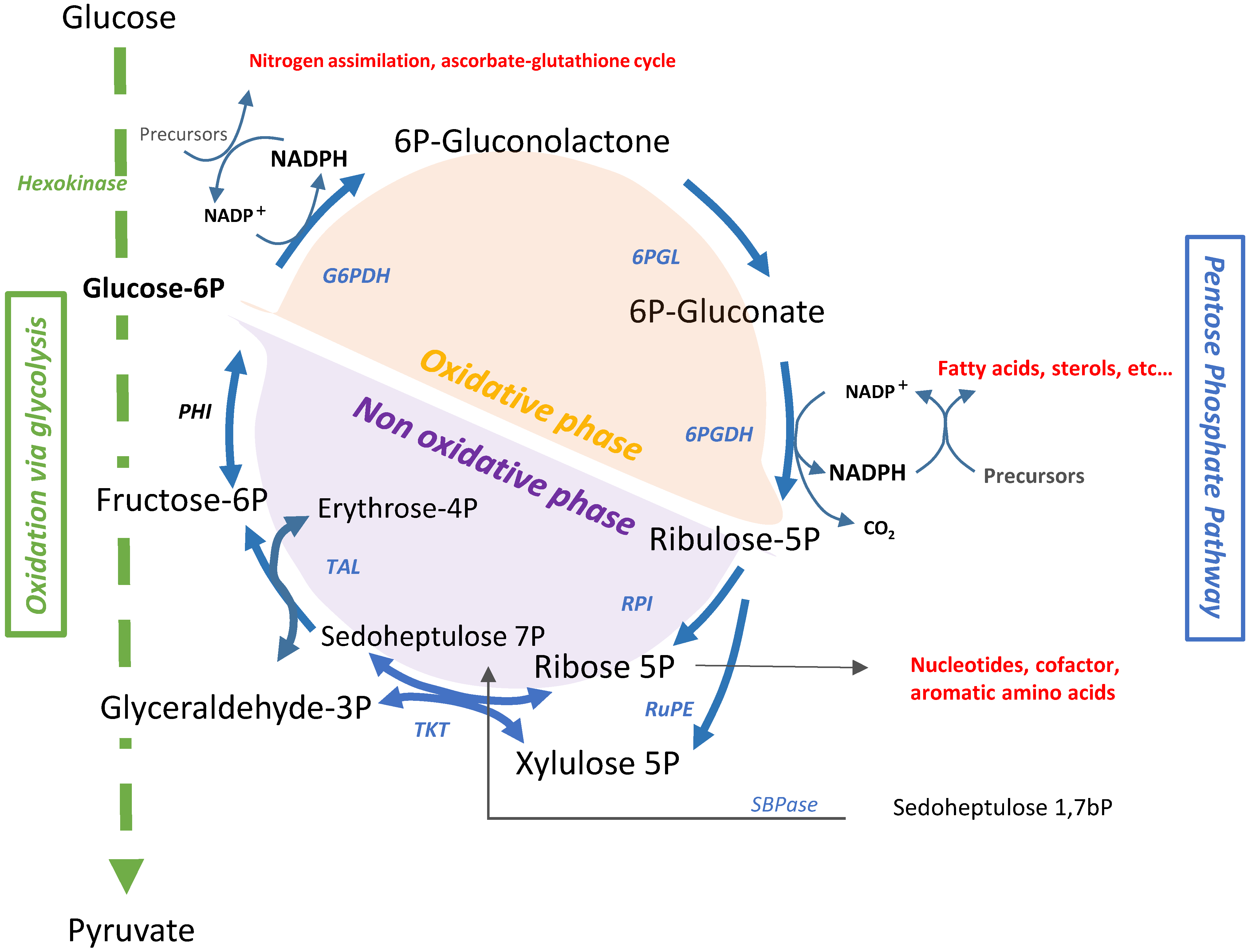
1. Introduction
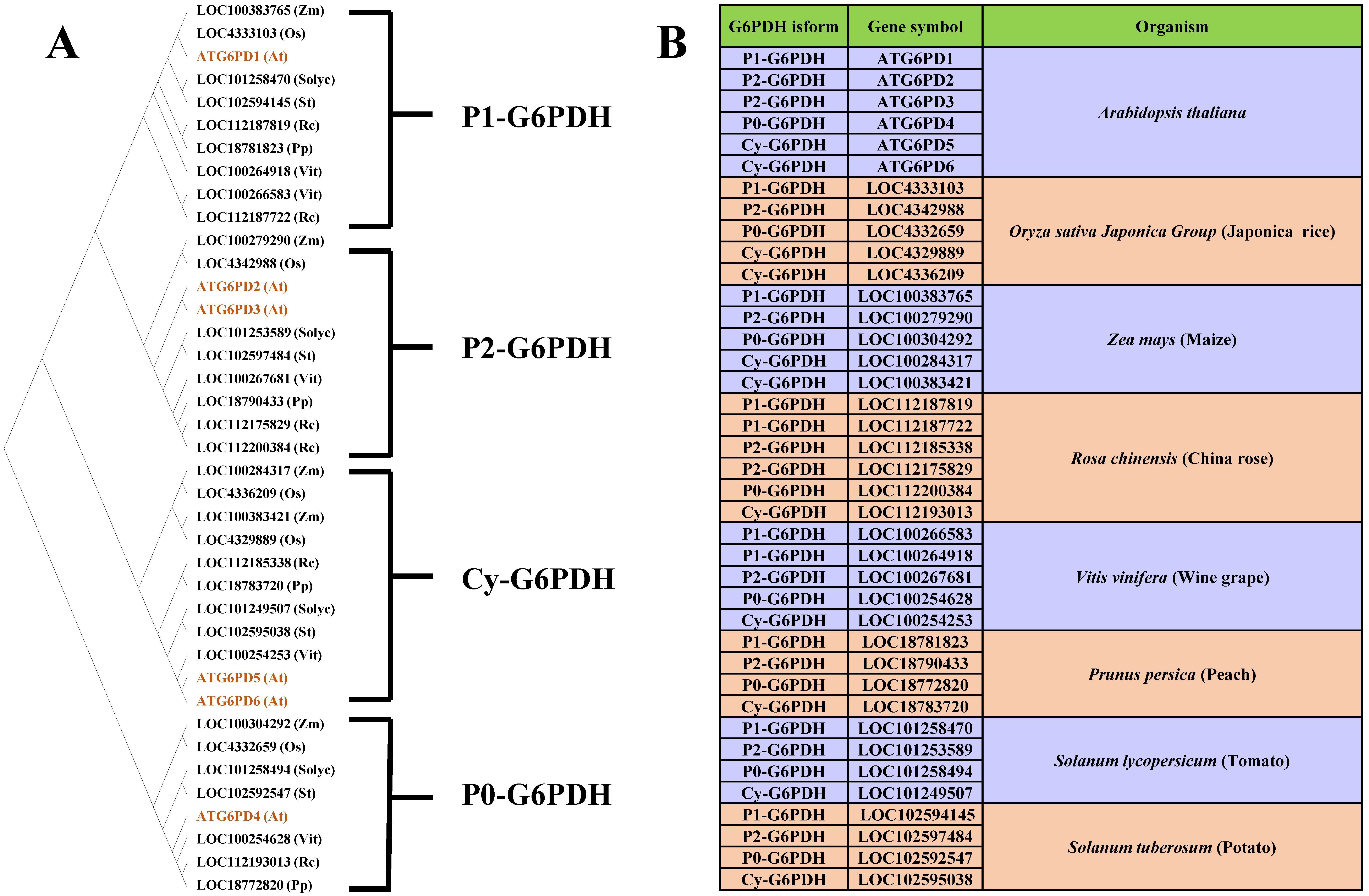
2. Classification and Regulation of G6PDH
2.1. P1-G6PDH
2.2. P2-G6PDH
2.3. The Enigmatic P0-G6PDH
2.4. Cytosolic G6PDH (Cy-G6PDH)
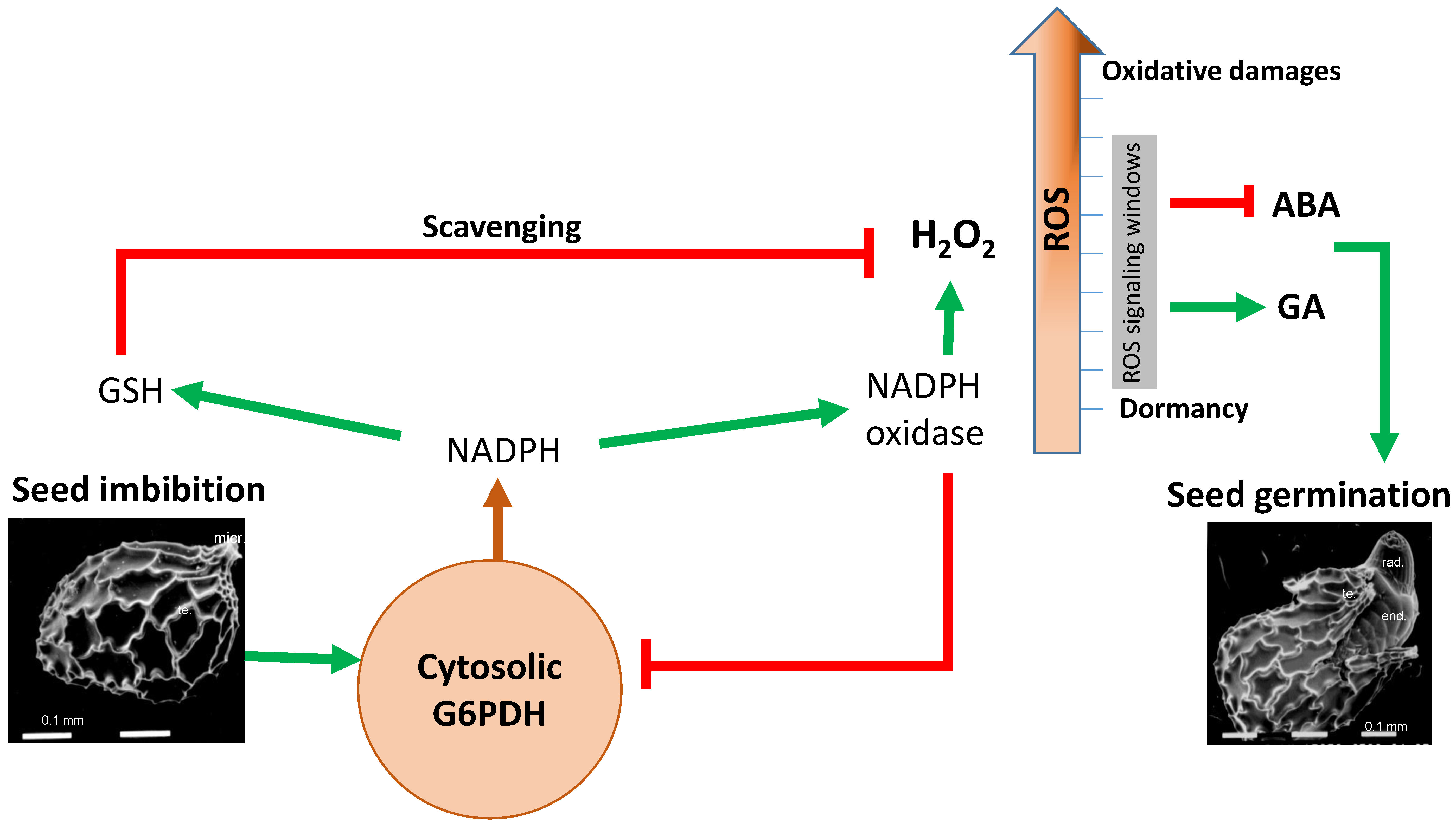
3. G6PDH and Seed Germination
4. G6PDHs and Nitrogen Assimilation
5. G6PDHs and Plant Branching
6. G6PDHs and Sugar Signaling
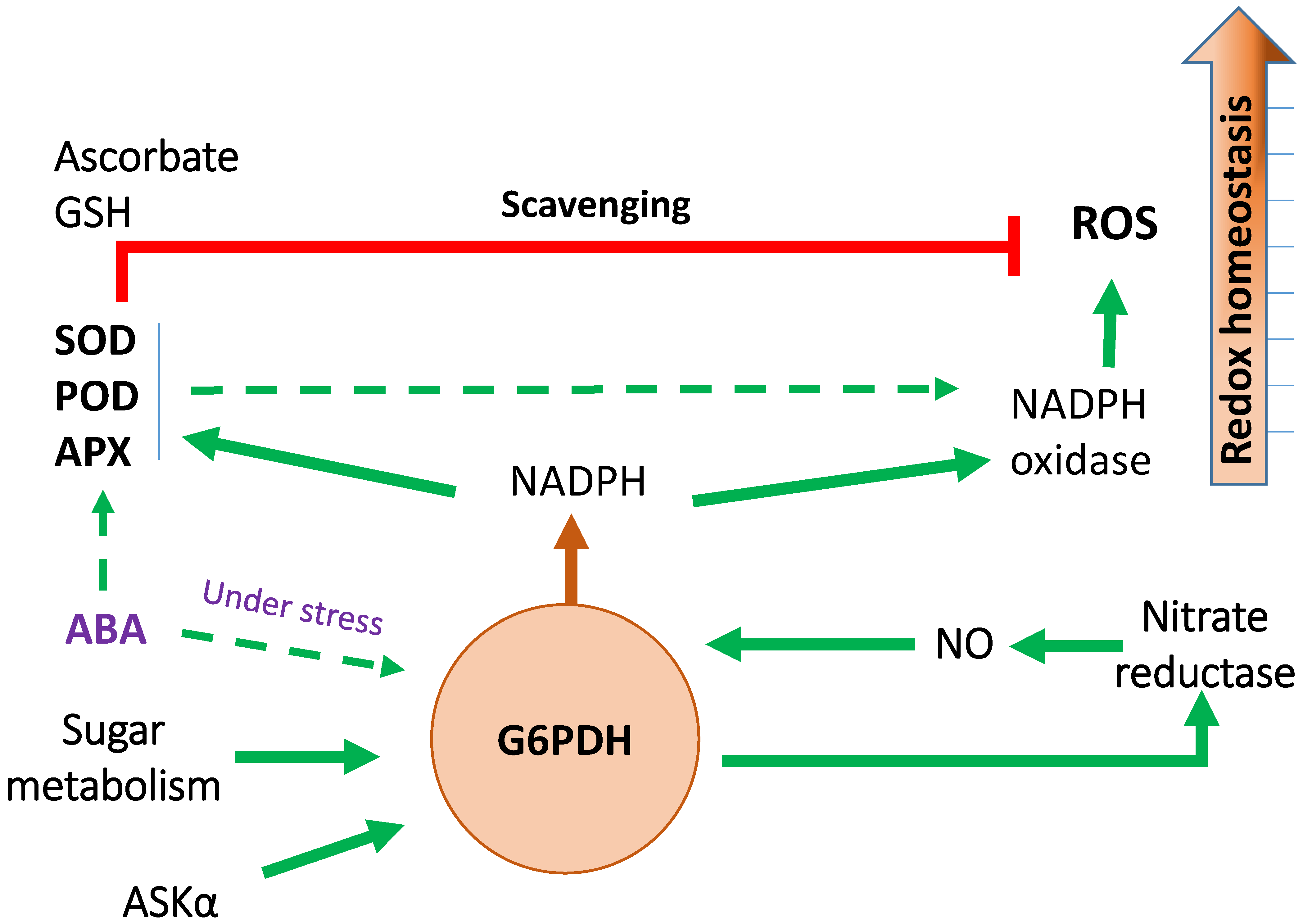
7. G6PDH and Abiotic Stress
7.1. Identification of Link between G6PDH and Abiotic Stress
7.2. Saline-Alkaline Stress and Aluminum Toxicity
7.3. Drought and Heat
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, J.F.; Blackmore, P.F.; Duke, C.C.; MacLeod, J.K. Fact, uncertainty and speculation concening the biochemistry of d-erythrose-4-phosphate and its metabolic roles. Int. J. Biochem. 1980, 12, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Copeland, L.E.S.; Turner, J.F. The Regulation of Glycolysis and the Pentose Phosphate Pathway. In Biochemistry of Metabolism; Academic Press: Cambridge, MA, USA, 1987; pp. 107–128. [Google Scholar]
- Esposito, S. Nitrogen assimilation, abiotic stress and glucose 6-phosphate dehydrogenase: The full circle of reductants. Plants 2016, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, R.D.; von Schaewen, A. Differential Regulation of Glucose-6-Phosphate Dehydrogenase Isoenzyme Activities in Potato. Plant Physiol. 2003, 133, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; Mach, R.L.; Mach-Aigner, A.R. The pentose phosphate pathway in industrially relevant fungi: Crucial insights for bioprocessing. Appl. Microbiol. Biotechnol. 2021, 105, 4017–4031. [Google Scholar] [CrossRef] [PubMed]
- Rashida, Z.; Laxman, S. The pentose phosphate pathway and organization of Metabolic Networks Enabling Growth Programs. Curr. Opin. Syst. Biol. 2021, 28, 100390. [Google Scholar] [CrossRef]
- Graeve, K.; von Schaewen, A.; Scheibe, R. Purification, characterization, and cDNA sequence of glucose-6-phosphate dehydrogenase from potato (Solatium tuberosum L.). Plant J. 1994, 5, 353–361. [Google Scholar] [CrossRef]
- von Schaewen, A.; Langenkamper, G.; Graeve, K.; Wenderoth, I.; Scheibe, R. Molecular characterization of the plastidic glucose-6-phosphate dehydrogenase from potato in comparison to its cytosolic counterpart. Plant Physiol. 1995, 109, 1327–1335. [Google Scholar] [CrossRef]
- Wendt, U.K.; Hauschild, R.; Lange, C.; Pietersma, M.; Wenderoth, I.; von Schaewen, A. Evidence for functional convergence of redox regulation in G6PDH isoforms of cyanobacteria and higher plants. Plant Mol. Biol. 1999, 40, 487–494. [Google Scholar] [CrossRef]
- Landi, S.; Nurcato, R.; De Lillo, A.; Lentini, M.; Grillo, S.; Esposito, S. Glucose-6-phosphate dehydrogenase plays a central role in the response of tomato (Solanum lycopersicum) plants to short and long-term drought. Plant Physiol. Biochem. 2016, 105, 79–89. [Google Scholar] [CrossRef]
- Heber, U.; Hallier, U.; Hudson, M.; Groeben, B.; Ernst, R.; Stang, H., II. Lokalisation von Enzymen des reduktiven und dem oxydativen Pentosephosphat-Zyklus in den Chloroplasten und Permeabilität der Chloroplasten-Membran gegenüber Metaboliten. Z. Für. Nat. B 1967, 22. [Google Scholar] [CrossRef]
- Schnarrenberger, C.; Oeser, A.; Tolbert, N.E. Two isoenzymes each of glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in spinach leaves. Arch. Biochem. Biophys. 1973, 154, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Wakao, S.; Benning, C. Genome-wide analysis of glucose-6-phosphate dehydrogenases in Arabidopsis. Plant J. 2005, 41, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.; He, W.; Sun, H.; Cui, C.; Wang, X.; Li, R.; Wang, X.; Bi, Y. Cytosolic glucose-6-phosphate dehydrogenases play a pivotal role in Arabidopsis seed development. Plant Physiol. Biochem. 2022, 186, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, L.; Li, Y.; Hou, J.; Huang, J.; Liang, W. Involvement of ABA-and H2O2-dependent cytosolic glucose-6-phosphate dehydrogenase in maintaining redox homeostasis in soybean roots under drought stress. Plant Physiol. Biochem. 2016, 107, 126–136. [Google Scholar] [CrossRef]
- Landi, S.; Capasso, G.; Esposito, S. Different G6PDH isoforms show specific roles in acclimation to cold stress at various growth stages of barley (Hordeum vulgare) and Arabidopsis thaliana. Plant Physiol. Biochem. 2021, 169, 190–202. [Google Scholar] [CrossRef]
- Lendzian, K.; Bassham, J.A. Regulation of glucose-6-phosphate dehydrogenase in spinach chloroplasts by ribulose 1,5-diphosphate and NADPH/NADP+ ratios. Biochim. Biophys. Acta 1975, 396, 260–275. [Google Scholar] [CrossRef]
- Anderson, L.E.; Duggan, J.X. Light modulation of glucose-6-phosphate dehydrogenase: Partial characterization of the light inactivation system and its effects on the properties of the chloroplastic and cytoplasmic forms of the enzyme. Plant Physiol. 1976, 58, 135–139. [Google Scholar] [CrossRef]
- Kruger, N.J.; von Schaewen, A. The oxidative pentose phosphate pathway: Structure and organisation. Curr. Opin. Plant Biol. 2003, 6, 236–246. [Google Scholar] [CrossRef]
- Lendzian, K. Modulation of glucose-6-phosphate dehydrogenase by NADPH, NADP+ and dithiothreitol at variable NADPH/NADP+ ratios in an illuminated reconstituted spinach (Spinacia oleracea L.) chloroplast system. Planta 1980, 148, 1–6. [Google Scholar] [CrossRef]
- Esposito, S.; Carfagna, S.; Massaro, G.; Vona, V.; Di Martino Rigano, V. Glucose-6-phosphate dehydrogenase in barley roots: Kinetic properties and localisation of the isoforms. Planta 2001, 212, 627–634. [Google Scholar] [CrossRef]
- Ferrara, M.; Guerriero, G.; Cardi, M.; Esposito, S. Purification and biochemical characterisation of a glucose-6-phosphate dehydrogenase from the psychrophilic green alga Koliella antarctica. Extremophiles 2013, 17, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Née, G.; Aumont-Nicaise, M.; Zaffagnini, M.; Nessler, S.; Valerio-Lepiniec, M.; Issakidis-Bourguet, E. Redox regulation of chloroplastic G6PDH activity by thioredoxin occurs through structural changes modifying substrate accessibility and cofactor binding. Biochem. J. 2014, 457, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Guerriero, G.; Vona, V.; Di Martino Rigano, V.; Carfagna, S.; Rigano, C. Glutamate synthase activities and protein changes in relation to nitrogen nutrition in barley: The dependence on different plastidic glucose-6P dehydrogenase isoforms. J. Exp. Bot. 2005, 56, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Hölscher, C.; Schwöppe, C.; von Schaewen, A. Alternative targeting of Arabidopsis plastidic glucose-6-phosphate dehydrogenase G6PD1 involves cysteine-dependent interaction with G6PD4 in the cytosol. Plant J. 2011, 66, 745–758. [Google Scholar] [CrossRef]
- Hölscher, C.; Meyer, T.; von Schaewen, A. Dual-targeting of Arabidopsis 6-phosphogluconolactonase 3 (PGL3) to chloroplasts and peroxisomes involves interaction with Trx m2 in the cytosol. Mol. Plant 2014, 7, 252–255. [Google Scholar] [CrossRef]
- Yang, Y.T.; Fu, Z.W.; Su, Y.C.; Zhang, X.; Li, G.Y.; Guo, J.L.; Que, Y.X.; Xu, L.P. A cytosolic glucose-6-phosphate dehydrogenase gene, ScG6PDH, plays a positive role in response to various abiotic stresses in sugarcane. Sci. Rep. 2014, 4, 7090. [Google Scholar] [CrossRef]
- Lansing, H.; Doering, L.; Fischer, K.; Baune, M.-C.; Schaewen, A.V. Analysis of potential redundancy among Arabidopsis 6-phosphogluconolactonase isoforms in peroxisomes. J. Exp. Bot. 2020, 71, 823–836. [Google Scholar] [CrossRef]
- Hölscher, C.; Lutterbey, M.-C.; Lansing, H.; Meyer, T.; Fischer, K.; von Schaewen, A. Defects in peroxisomal 6-phosphogluconate dehydrogenase isoform PGD2 prevent gametophytic interaction in Arabidopsis thaliana. Plant Physiol. 2016, 171, 192–205. [Google Scholar] [CrossRef]
- Kaur, N.; Hu, J. Defining the plant peroxisomal proteome: From Arabidopsis to rice. Front. Plant Sci. 2011, 2, 103. [Google Scholar] [CrossRef]
- Castiglia, D.; Cardi, M.; Landi, S.; Cafasso, D.; Esposito, S. Expression and characterization of a cytosolic glucose 6 phosphate dehydrogenase isoform from barley (Hordeum vulgare) roots. Protein Expr. Purif. 2015, 112, 8–14. [Google Scholar] [CrossRef]
- Wakao, S.; Andre, C.; Benning, C. Functional analyses of cytosolic glucose-6-phosphate dehydrogenases and their contribution to seed oil accumulation in Arabidopsis. Plant Physiol. 2008, 146, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Dal Santo, S.; Stampfl, H.; Krasensky, J.; Kempa, S.; Gibon, Y.; Petutschnig, E.; Rozhon, W.; Heuck, A.; Clausen, T.; Jonak, C. Stress-induced GSK3 regulates the redox stress response by phosphorylating glucose-6-phosphate dehydrogenase in Arabidopsis. Plant Cell 2012, 24, 3380–3392. [Google Scholar] [CrossRef] [PubMed]
- Fickenscher, K.; Scheibe, R. Purification and properties of the cytoplasmic glucose-6-phosphate dehydrogenase from pea leaves. Arch. Biochem. Biophys. 1986, 247, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Wenderoth, I.; Scheibe, R.; von Schaewen, A. Identification of the cysteine residues involved in redox modification of plant plastidic glucose-6-phosphate dehydrogenase. J. Biol. Chem. 1997, 272, 26985–26990. [Google Scholar] [CrossRef] [PubMed]
- Haschek, W.M.; Rousseaux, C.G.; Wallig, M.A.; Bolon, B.; Ochoa, R. Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Swamy, P.; Sandhyarani, C. Contribution of the pentose phosphate pathway and glycolytic pathway to dormancy breakage and germination of peanut (Arachis hypogaea L.) seeds. J. Exp. Bot. 1986, 37, 80–88. [Google Scholar] [CrossRef]
- Lacroix, L.; Jaswal, A. Metabolic changes in after-ripening seed of Prunus cerasus. Plant Physiol. 1967, 42, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; Wray, J. Correlated changes of some enzyme activities and cofactor and substrate contents of pea cotyledon tissue during germination. Biochem. J. 1968, 108, 437–444. [Google Scholar] [CrossRef]
- Kovacs, M.I.; Simpson, G.M. Dormancy and enzyme levels in seeds of wild oats. Phytochemistry 1976, 15, 455–458. [Google Scholar] [CrossRef]
- Gosling, P.G.; Ross, J.D. Pentose phosphate metabolism during dormancy breakage in Corylus avellana L. Planta 1980, 148, 362–366. [Google Scholar] [CrossRef]
- Adkins, S.W.; Ross, J.D. Studies in Wild Oat Seed Dormancy: II. Activities of Pentose Phosphate Pathway Dehydrogenases. Plant Physiol. 1981, 68, 15–17. [Google Scholar] [CrossRef]
- Côme, D.; Corbineau, F. Some aspects of metabolic regulation of seed germination and dormancy. In Recent Advances in the Development and Germination of Seeds; Springer: Berlin/Heidelberg, Germany, 1989; pp. 165–179. [Google Scholar]
- Ponnaiah, M.; Gilard, F.; Gakière, B.; El-Maarouf-Bouteau, H.; Bailly, C. Regulatory actors and alternative routes for Arabidopsis seed germination are revealed using a pathway-based analysis of transcriptomic datasets. Plant J. 2019, 99, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Barba-Espín, G.; Hernández, J.A.; Diaz-Vivancos, P. Role of H2O2 in pea seed germination. Plant Signal Behav. 2012, 7, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Yadav, S.; Sibi, G. Seed germination and maturation under the influence of hydrogen peroxide—A review. J. Crit. Rev. 2020, 7, 6–10. [Google Scholar]
- Leymarie, J.; Vitkauskaité, G.; Hoang, H.H.; Gendreau, E.; Chazoule, V.; Meimoun, P.; Corbineau, F.; El-Maarouf-Bouteau, H.; Bailly, C. Role of reactive oxygen species in the regulation of Arabidopsis seed dormancy. Plant Cell Physiol. 2012, 53, 96–106. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Chang, N.; Nan, W.; Wang, S.; Ruan, M.; Sun, L.; Li, S.; Bi, Y. Cytosolic Glucose-6-Phosphate Dehydrogenase Is Involved in Seed Germination and Root Growth Under Salinity in Arabidopsis. Front. Plant Sci. 2019, 10, 182. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Sun, L.; Ruan, M.; Li, S.; He, R.; Zhang, W.; Liang, C.; Wang, X.; Bi, Y. Involvement of G6PD5 in ABA response during seed germination and root growth in Arabidopsis. BMC Plant Biol. 2019, 19, 44. [Google Scholar] [CrossRef]
- Oracz, K.; Bouteau, H.E.M.; Farrant, J.M.; Cooper, K.; Belghazi, M.; Job, C.; Job, D.; Corbineau, F.; Bailly, C. ROS production and protein oxidation as a novel mechanism for seed dormancy alleviation. Plant J. 2007, 50, 452–465. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Meimoun, P.; Job, C.; Job, D.; Bailly, C. Role of protein and mRNA oxidation in seed dormancy and germination. Front. Plant Sci. 2013, 4, 77. [Google Scholar] [CrossRef]
- Muller, K.; Linkies, A.; Vreeburg, R.A.; Fry, S.C.; Krieger-Liszkay, A.; Leubner-Metzger, G. In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol. 2009, 150, 1855–1865. [Google Scholar] [CrossRef]
- Corbineau, F.; Xia, Q.; Bailly, C.; El-Maarouf-Bouteau, H. Ethylene, a key factor in the regulation of seed dormancy. Front. Plant Sci. 2014, 5, 539. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, N.; Liu, R.; Chen, M.; Zhang, J. H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J. Exp. Bot. 2010, 61, 2979–2990. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ruan, M.; Wan, Q.; He, W.; Yang, L.; Liu, X.; He, L.; Yan, L.; Bi, Y. Nitric oxide and hydrogen peroxide increase glucose-6-phosphate dehydrogenase activities and expression upon drought stress in soybean roots. Plant Cell Rep. 2020, 39, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kobrehel, K.; Wong, J.H.; Balogh, A.; Kiss, F.; Yee, B.C.; Buchanan, B.B. Specific reduction of wheat storage proteins by thioredoxin h. Plant Physiol. 1992, 99, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Wong, J.H.; Lee, Y.M.; Cho, M.-J.; Buchanan, B.B. A strategy for the identification of proteins targeted by thioredoxin. Proc. Natl. Acad. Sci. USA 2001, 98, 4794–4799. [Google Scholar] [CrossRef] [PubMed]
- Marx, C.; Wong, J.H.; Buchanan, B.B. Thioredoxin and germinating barley: Targets and protein redox changes. Planta 2003, 216, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Alkhalfioui, F.; Renard, M.; Vensel, W.H.; Wong, J.; Tanaka, C.K.; Hurkman, W.J.; Buchanan, B.B.; Montrichard, F. Thioredoxin-linked proteins are reduced during germination of Medicago truncatula seeds. Plant Physiol. 2007, 144, 1559–1579. [Google Scholar] [CrossRef]
- Montrichard, F.; Alkhalfioui, F.; Yano, H.; Vensel, W.H.; Hurkman, W.J.; Buchanan, B.B. Thioredoxin targets in plants: The first 30 years. J. Proteom. 2009, 72, 452–474. [Google Scholar] [CrossRef]
- Lozano, R.M.; Wong, J.H.; Yee, B.C.; Peters, A.; Kobrehel, K.; Buchanan, B.B. New evidence for a role for thioredoxin h in germination and seedling development. Planta 1996, 200, 100–106. [Google Scholar] [CrossRef]
- Née, G.; Zaffagnini, M.; Trost, P.; Issakidis-Bourguet, E. Redox regulation of chloroplastic glucose-6-phosphate dehydrogenase: A new role for f-type thioredoxin. FEBS Lett. 2009, 583, 2827–2832. [Google Scholar] [CrossRef]
- Nee, G.; Chatel-Innocenti, G.; Meimoun, P.; Leymarie, J.; Montrichard, F.; Satour, P.; Bailly, C.; Issakidis-Bourguet, E. A New Role for Plastid Thioredoxins in Seed Physiology in Relation to Hormone Regulation. Int. J. Mol. Sci. 2021, 22, 10395. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Rossiello, R.O.P. Mineral nitrogen in plant physiology and plant nutrition. Crit. Rev. Plant Sci. 1995, 14, 111–148. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, Y.; Xu, G. How does nitrogen shape plant architecture? J. Exp. Bot. 2020, 71, 4415–4427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, B.; Chu, C. Towards understanding the hierarchical nitrogen signalling network in plants. Curr. Opin. Plant Biol. 2020, 55, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Coruzzi, G.M. Primary N-assimilation into amino acids in Arabidopsis. In The Arabidopsis Book; American Society of Plant Biologists: Portland, OR, USA, 2003; Volume 2. [Google Scholar]
- Bussell, J.D.; Keech, O.; Fenske, R.; Smith, S.M. Requirement for the plastidial oxidative pentose phosphate pathway for nitrate assimilation in Arabidopsis. Plant J. 2013, 75, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Frungillo, L.; Skelly, M.J.; Loake, G.J.; Spoel, S.H.; Salgado, I. S-nitrosothiols regulate nitric oxide production and storage in plants through the nitrogen assimilation pathway. Nat. Commun. 2014, 5, 5401. [Google Scholar] [CrossRef]
- Bowsher, C.; Boulton, E.; Rose, J.; Nayagam, S.; Emes, M. Reductant for glutamate synthase in generated by the oxidative pentose phosphate pathway in non-photosynthetic root plastids. Annu. Rev. Plant Biol. 1992, 2, 893–898. [Google Scholar] [CrossRef]
- Esposito, S.; Massaro, G.; Vona, V.; Di Martino Rigano, V.; Carfagna, S. Glutamate synthesis in barley roots: The role of the plastidic glucose-6-phosphate dehydrogenase. Planta 2003, 216, 639–647. [Google Scholar] [CrossRef]
- Redinbaugh, M.G.; Campbell, W.H. Nitrate regulation of the oxidative pentose phosphate pathway in maize (Zea mays L.) root plastids: Induction of 6-phosphogluconate dehydrogenase activity, protein and transcript levels. Plant Sci. 1998, 134, 129–140. [Google Scholar] [CrossRef]
- Wang, R.; Okamoto, M.; Xing, X.; Crawford, N.M. Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1,000 rapidly responding genes and new linkages to glucose, trehalose-6-phosphate, iron, and sulfate metabolism. Plant Physiol. 2003, 132, 556–567. [Google Scholar] [CrossRef]
- Wang, R.; Tischner, R.; Gutiérrez, R.A.; Hoffman, M.; Xing, X.; Chen, M.; Coruzzi, G.; Crawford, N.M. Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiol. 2004, 136, 2512–2522. [Google Scholar] [CrossRef]
- Lejay, L.; Wirth, J.; Pervent, M.; Cross, J.M.-F.; Tillard, P.; Gojon, A. Oxidative Pentose Phosphate Pathway-Dependent Sugar Sensing as a Mechanism for Regulation of Root Ion Transporters by Photosynthesis. Plant Physiol. 2008, 146, 2036–2053. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.; George, G.; Ongaro, V.; Williamson, L.; Willetts, B.; Ljung, K.; McCulloch, H.; Leyser, O. Auxin and strigolactone signaling are required for modulation of Arabidopsis shoot branching by nitrogen supply. Plant Physiol. 2014, 166, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Kohler, E.; Barrach, H.; Neubert, D. Inhibition of NADP dependent oxidoreductases by the 6-aminonicotinamide analogue of NADP. FEBS Lett. 1970, 6, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Garlick, A.P.; Moore, C.; Kruger, N.J. Monitoring flux through the oxidative pentose phosphate pathway using [1-14C] gluconate. Planta 2002, 216, 265–272. [Google Scholar] [CrossRef]
- Chaput, V.; Martin, A.; Lejay, L. Redox metabolism: The hidden player in carbon and nitrogen signaling? J. Exp. Bot. 2020, 71, 3816–3826. [Google Scholar] [CrossRef]
- Neuhaus, H.; Emes, M. Nonphotosynthetic metabolism in plastids. Annu. Rev. Plant Biol. 2000, 51, 111. [Google Scholar] [CrossRef]
- Kumar, V.; Mills, D.J.; Anderson, J.D.; Mattoo, A.K. An alternative agriculture system is defined by a distinct expression profile of select gene transcripts and proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 10535–10540. [Google Scholar] [CrossRef]
- Schneider, A.; Godin, C.; Boudon, F.; Demotes-Mainard, S.; Sakr, S.; Bertheloot, J. Light regulation of axillary bud outgrowth along plant axes: An overview of the roles of sugars and hormones. Front. Plant Sci. 2019, 10, 1296. [Google Scholar] [CrossRef]
- Barbier, F.F.; Cao, D.; Fichtner, F.; Weiste, C.; Pérez-Garcia, M.-D.; Caradeuc, M.; Le Gourrierec, J.; Sakr, S.; Beveridge, C.A. HEXOKINASE1 signalling promotes shoot branching and interacts with cytokinin and strigolactone pathways. New Phytol. 2021, 231, 1088–1104. [Google Scholar] [CrossRef]
- Tao, Y.; An, L.; Xiao, F.; Li, G.; Ding, Y.; Paul, M.; Liu, Z.H. Integration of embryo–endosperm interaction into a holistic and dynamic picture of seed development using a rice mutant with notched-belly kernels. Crop J. 2021, 10, 729–742. [Google Scholar] [CrossRef]
- Rabot, A.; Henry, C.; Ben Baaziz, K.; Mortreau, E.; Azri, W.; Lothier, J.; Hamama, L.; Boummaza, R.; Leduc, N.; Pelleschi-Travier, S. Insight into the role of sugars in bud burst under light in the rose. Plant Cell Physiol. 2012, 53, 1068–1082. [Google Scholar] [CrossRef]
- Barbier, F.; Péron, T.; Lecerf, M.; Perez-Garcia, M.-D.; Barrière, Q.; Rolčík, J.; Boutet-Mercey, S.; Citerne, S.; Lemoine, R.; Porcheron, B.; et al. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J. Exp. Bot. 2015, 66, 2569–2582. [Google Scholar] [CrossRef] [PubMed]
- Rabot, A.; Portemer, V.; Péron, T.; Mortreau, E.; Leduc, N.; Hamama, L.; Coutos-Thévenot, P.; Atanassova, R.; Sakr, S.; Le Gourrierec, J. Interplay of sugar, light and gibberellins in expression of Rosa hybrida vacuolar invertase 1 regulation. Plant Cell Physiol. 2014, 55, 1734–1748. [Google Scholar] [CrossRef] [PubMed]
- Demotes-Mainard, S.; Huché-Thélier, L.; Morel, P.; Boumaza, R.; Guérin, V.; Sakr, S. Temporary water restriction or light intensity limitation promotes branching in rose bush. Sci. Hortic. 2013, 150, 432–440. [Google Scholar] [CrossRef]
- Crespel, L.; Le Bras, C.; Amoroso, T.; Unda Ulloa, M.G.; Morel, P.; Sakr, S. Genotype× Light quality interaction on rose architecture. Agronomy 2020, 10, 913. [Google Scholar] [CrossRef]
- Wang, M.; Le Gourrierec, J.; Jiao, F.; Demotes-Mainard, S.; Perez-Garcia, M.-D.; Ogé, L.; Hamama, L.; Crespel, L.; Bertheloot, J.; Chen, J. Convergence and divergence of sugar and cytokinin signaling in plant development. Int. J. Mol. Sci. 2021, 22, 1282. [Google Scholar] [CrossRef]
- Wang, M.; Ogé, L.; Pérez-Garcia, M.D.; Launay-Avon, A.; Clément, G.; Le Gourrierec, J.; Hamama, L.; Sakr, S. Antagonistic Effect of Sucrose Availability and Auxin on Rosa Axillary Bud Metabolism and Signaling, Based on the Transcriptomics and Metabolomics Analysis. Front. Plant Sci. 2022, 13, 830840. [Google Scholar] [CrossRef]
- Djennane, S.; Hibrand-Saint Oyant, L.; Kawamura, K.; Lalanne, D.; Laffaire, M.; Thouroude, T.; Chalain, S.; Sakr, S.; Boumaza, R.; Foucher, F.; et al. Impacts of light and temperature on shoot branching gradient and expression of strigolactone synthesis and signalling genes in rose. Plant Cell Environ. 2014, 37, 742–757. [Google Scholar] [CrossRef]
- Fichtner, F.; Barbier, F.F.; Annunziata, M.G.; Feil, R.; Olas, J.J.; Mueller-Roeber, B.; Stitt, M.; Beveridge, C.A.; Lunn, J.E. Regulation of shoot branching in arabidopsis by trehalose 6-phosphate. New Phytol. 2021, 229, 2135–2151. [Google Scholar] [CrossRef]
- Wang, M.; Pérez-Garcia, M.-D.; Davière, J.-M.; Barbier, F.; Ogé, L.; Gentilhomme, J.; Voisine, L.; Péron, T.; Launay-Avon, A.; Clément, G.; et al. Outgrowth of the axillary bud in rose is controlled by sugar metabolism and signalling. J. Exp. Bot. 2021, 72, 3044–3060. [Google Scholar] [CrossRef]
- Porcher, A.; Guérin, V.; Montrichard, F.; Lebrec, A.; Lothier, J.; Vian, A. Ascorbate glutathione-dependent H2O2 scavenging is an important process in axillary bud outgrowth in rosebush. Ann. Bot. 2020, 126, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Porcher, A.; Guérin, V.; Leduc, N.; Lebrec, A.; Lothier, J.; Vian, A. Ascorbate–glutathione pathways mediated by cytokinin regulate H2O2 levels in light-controlled rose bud burst. Plant Physiol. 2021, 186, 910–928. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ogé, L.; Voisine, L.; Pérez-Garcia, M.-D.; Jeauffre, J.; Hibrand Saint-Oyant, L.; Grappin, P.; Hamama, L.; Sakr, S. Posttranscriptional Regulation of RhBRC1 (Rosa hybrida BRANCHED1) in Response to Sugars is Mediated via its Own 3′ Untranslated Region, with a Potential Role of RhPUF4 (Pumilio RNA-Binding Protein Family). Int. J. Mol. Sci. 2019, 20, 3808. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Martínez, J.A.; Poza-Carrión, C.; Cubas, P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell 2007, 19, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sheen, J. Dynamic and diverse sugar signaling. Curr. Opin. Plant Biol. 2016, 33, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Chen, Q.; Chen, L.; Liu, D.; Yang, H.; Xu, C.; Hong, J.; Li, J.; Ding, Y.; Sakr, S.; et al. Sink Strength Promoting Remobilization of Non-Structural Carbohydrates by Activating Sugar Signaling in Rice Stem during Grain Filling. Int. J. Mol. Sci. 2022, 23, 4864. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, Q.; Chen, L.; Yang, H.; Zhu, M.; Ding, Y.; Li, W.; Liu, Z.; Jiang, Y.; Li, G. Efficiency of Sucrose to Starch Metabolism Is Related to the Initiation of Inferior Grain Filling in Large Panicle Rice. Front. Plant Sci. 2021, 12, 732867. [Google Scholar] [CrossRef]
- Sakr, S.; Wang, M.; Dédaldéchamp, F.; Pérez-Garcia, M.-D.; Ogé, L.; Hamama, L.; Atanassova, R. The Sugar-Signaling Hub: Overview of Regulators and Interaction with the Hormonal and Metabolic Network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef]
- Moore, B.; Zhou, L.; Rolland, F.; Hall, Q.; Cheng, W.H.; Liu, Y.X.; Hwang, I.; Jones, T.; Sheen, J. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 2003, 300, 332–336. [Google Scholar] [CrossRef]
- Bellegarde, F.; Maghiaoui, A.; Boucherez, J.; Krouk, G.; Lejay, L.; Bach, L.; Gojon, A.; Martin, A. The Chromatin Factor HNI9 and ELONGATED HYPOCOTYL5 Maintain ROS Homeostasis under High Nitrogen Provision. Plant Physiol. 2019, 180, 582–592. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Wendt, U.K.; Wenderoth, I.; Tegeler, A.; Von Schaewen, A. Molecular characterization of a novel glucose-6-phosphate dehydrogenase from potato (Solanum tuberosum L.). Plant J. 2000, 23, 723–733. [Google Scholar] [CrossRef] [PubMed]
- De Freitas-Silva, L.; Rodríguez-Ruiz, M.; Houmani, H.; da Silva, L.C.; Palma, J.M.; Corpas, F.J. Glyphosate-induced oxidative stress in Arabidopsis thaliana affecting peroxisomal metabolism and triggers activity in the oxidative phase of the pentose phosphate pathway (OxPPP) involved in NADPH generation. J. Plant Physiol. 2017, 218, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liao, J.; Zheng, X.; Chen, Y.; Ren, H. Low-level free nitrous acid efficiently inhibits the conjugative transfer of antibiotic resistance by altering intracellular ions and disabling transfer apparatus. Water Res. 2019, 158, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Yoshioka, M.; Nomura, H.; Tone, C.; Nakajima, K.; Nakane, E.; Doke, N.; Yoshioka, H. A plastidic glucose-6-phosphate dehydrogenase is responsible for hypersensitive response cell death and reactive oxygen species production. J. Gen. Plant Pathol. 2011, 77, 152–162. [Google Scholar] [CrossRef]
- Kano, A.; Fukumoto, T.; Ohtani, K.; Yoshihara, A.; Ohara, T.; Tajima, S.; Izumori, K.; Tanaka, K.; Ohkouchi, T.; Ishida, Y.; et al. The rare sugar d-allose acts as a triggering molecule of rice defence via ROS generation. J. Exp. Bot. 2013, 64, 4939–4951. [Google Scholar] [CrossRef] [PubMed]
- Zaka, R.; Vandecasteele, C.M.; Misset, M.T. Effects of low chronic doses of ionizing radiation on antioxidant enzymes and G6PDH activities in Stipa capillata (Poaceae). J. Exp. Bot. 2002, 53, 1979–1987. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, S.; Guo, H.; Zhang, Z.; Chen, X. Functional analysis of PsG6PDH, a cytosolic glucose-6-phosphate dehydrogenase gene from Populus suaveolens, and its contribution to cold tolerance improvement in tobacco plants. Biotechnol. Lett. 2013, 35, 1509–1518. [Google Scholar] [CrossRef]
- Li, C.; Wei, M.; Ge, Y.; Zhao, J.; Chen, Y.; Hou, J.; Cheng, Y.; Chen, J.; Li, J. The role of glucose-6-phosphate dehydrogenase in reactive oxygen species metabolism in apple exocarp induced by acibenzolar-S-methyl. Food Chem. 2020, 308, 125663. [Google Scholar] [CrossRef]
- Wei, M.; Ge, Y.; Li, C.; Han, X.; Qin, S.; Chen, Y.; Tang, Q.; Li, J. G6PDH regulated NADPH production and reactive oxygen species metabolism to enhance disease resistance against blue mold in apple fruit by acibenzolar-S-methyl. Postharvest Biol. Technol. 2019, 148, 228–235. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Hu, Y.; Hu, W.; Bi, Y. Glucose-6-phosphate dehydrogenase plays a pivotal role in tolerance to drought stress in soybean roots. Plant Cell Rep. 2013, 32, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.; Liu, W.; Li, P.; Kong, L.; Ren, W.; Wu, H.; Tu, Y. Degradation of pyrene by immobilized microorganisms in saline-alkaline soil. J. Environ. Sci. 2012, 24, 1662–1669. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Chuamnakthong, S.; Nampei, M.; Ueda, A. Characterization of Na+ exclusion mechanism in rice under saline-alkaline stress conditions. Plant Sci. 2019, 287, 110171. [Google Scholar] [CrossRef] [PubMed]
- Cardi, M.; Castiglia, D.; Ferrara, M.; Guerriero, G.; Chiurazzi, M.; Esposito, S. The effects of salt stress cause a diversion of basal metabolism in barley roots: Possible different roles for glucose-6-phosphate dehydrogenase isoforms. Plant Physiol. Biochem. 2015, 86, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, Y.; Sasakuma, T. Specific expression of glucose-6-phosphate dehydrogenase (G6PDH) gene by salt stress in wheat (Triticum aestivum L.). Plant Sci. 2000, 158, 53–60. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, P.; Zhang, W.; Bi, Y. Cytoplasmic glucose-6-phosphate dehydrogenase plays an important role in the silicon-enhanced alkaline tolerance in highland barley. Funct. Plant Biol. 2021, 48, 119–130. [Google Scholar] [CrossRef]
- Feng, R.; Wang, X.; He, L.; Wang, S.; Li, J.; Jin, J.; Bi, Y. Identification, Characterization, and Stress Responsiveness of Glucose-6-phosphate Dehydrogenase Genes in Highland Barley. Plants 2020, 9, 1800. [Google Scholar] [CrossRef]
- Cardi, M.; Chibani, K.; Cafasso, D.; Rouhier, N.; Jacquot, J.-P.; Esposito, S. Abscisic acid effects on activity and expression of barley (Hordeum vulgare) plastidial glucose-6-phosphate dehydrogenase. J. Exp. Bot. 2011, 62, 4013–4023. [Google Scholar] [CrossRef]
- Hu, Y.; You, J.; Li, J.; Wang, C. Loss of cytosolic glucose-6-phosphate dehydrogenase increases the susceptibility of Arabidopsis thaliana to root-knot nematode infection. Ann. Bot. 2018, 123, 37–46. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, Y.; Huang, S.; Yu, J.; Wang, X.; Xin, D.; Li, X.; Liu, Y.; Dai, Y.; Qi, Z.; et al. Genome-Wide Analysis of the Glucose-6-Phosphate Dehydrogenase Family in Soybean and Functional Identification of GmG6PDH2 Involvement in Salt Stress. Front. Plant Sci. 2020, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, R.; Wan, Q.; Xie, G.; Bi, Y. Glucose-6-Phosphate Dehydrogenase Plays a Pivotal Role in Nitric Oxide-Involved Defense Against Oxidative Stress Under Salt Stress in Red Kidney Bean Roots. Plant Cell Physiol. 2007, 48, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hou, J.; Li, Y.; Zhang, Y.; Huang, J.; Liang, W. Nitric oxide-mediated cytosolic glucose-6-phosphate dehydrogenase is involved in aluminum toxicity of soybean under high aluminum concentration. Plant Soil 2017, 416, 39–52. [Google Scholar] [CrossRef]
- Huang, J.; Han, R.; Ji, F.; Yu, Y.; Wang, R.; Hai, Z.; Liang, W.; Wang, H. Glucose-6-phosphate dehydrogenase and abscisic acid mediate programmed cell death induced by aluminum toxicity in soybean root tips. J. Hazard. Mater. 2022, 425, 127964. [Google Scholar] [CrossRef]
- Tian, Y.; Peng, K.; Bao, Y.; Zhang, D.; Meng, J.; Wang, D.; Wang, X.; Cang, J. Glucose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase genes of winter wheat enhance the cold tolerance of transgenic Arabidopsis. Plant Physiol. Biochem. 2021, 161, 86–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, M.; Cheng, L.; Lin, Y.; Chen, Q.; Sun, B.; Gu, X.; Wang, Y.; Li, M.; Luo, Y.; et al. Identification of the Cytosolic Glucose-6-Phosphate Dehydrogenase Gene from Strawberry Involved in Cold Stress Response. Int. J. Mol. Sci. 2020, 21, 7322. [Google Scholar] [CrossRef]
- Lei, D.; Lin, Y.; Luo, M.; Zhao, B.; Tang, H.; Zhou, X.; Yao, W.; Zhang, Y.; Wang, Y.; Li, M.; et al. Genome-Wide Investigation of G6PDH Gene in Strawberry: Evolution and Expression Analysis during Development and Stress. Int. J. Mol. Sci. 2022, 23, 4728. [Google Scholar] [CrossRef]
- Gong, H.; Chen, G.; Li, F.; Wang, X.; Hu, Y.; Bi, Y. Involvement of G6PDH in heat stress tolerance in the calli from Przewalskia tangutica and Nicotiana tabacum. Biol. Plant. 2012, 56, 422–430. [Google Scholar] [CrossRef]
- Santiago, J.P.; Soltani, A.; Bresson, M.M.; Preiser, A.L.; Lowry, D.B.; Sharkey, T.D. Contrasting anther glucose-6-phosphate dehydrogenase activities between two bean varieties suggest an important role in reproductive heat tolerance. Plant Cell Environ. 2021, 44, 2185–2199. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Wang, M.; Nicolas, M.; Ogé, L.; Pérez-Garcia, M.-D.; Crespel, L.; Li, G.; Ding, Y.; Le Gourrierec, J.; Grappin, P.; et al. Glucose-6-Phosphate Dehydrogenases: The Hidden Players of Plant Physiology. Int. J. Mol. Sci. 2022, 23, 16128. https://doi.org/10.3390/ijms232416128
Jiang Z, Wang M, Nicolas M, Ogé L, Pérez-Garcia M-D, Crespel L, Li G, Ding Y, Le Gourrierec J, Grappin P, et al. Glucose-6-Phosphate Dehydrogenases: The Hidden Players of Plant Physiology. International Journal of Molecular Sciences. 2022; 23(24):16128. https://doi.org/10.3390/ijms232416128
Chicago/Turabian StyleJiang, Zhengrong, Ming Wang, Michael Nicolas, Laurent Ogé, Maria-Dolores Pérez-Garcia, Laurent Crespel, Ganghua Li, Yanfeng Ding, José Le Gourrierec, Philippe Grappin, and et al. 2022. "Glucose-6-Phosphate Dehydrogenases: The Hidden Players of Plant Physiology" International Journal of Molecular Sciences 23, no. 24: 16128. https://doi.org/10.3390/ijms232416128
APA StyleJiang, Z., Wang, M., Nicolas, M., Ogé, L., Pérez-Garcia, M.-D., Crespel, L., Li, G., Ding, Y., Le Gourrierec, J., Grappin, P., & Sakr, S. (2022). Glucose-6-Phosphate Dehydrogenases: The Hidden Players of Plant Physiology. International Journal of Molecular Sciences, 23(24), 16128. https://doi.org/10.3390/ijms232416128







