Rare Variants in Genes of the Cholesterol Pathway Are Present in 60% of Patients with Acute Myocardial Infarction
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Participants
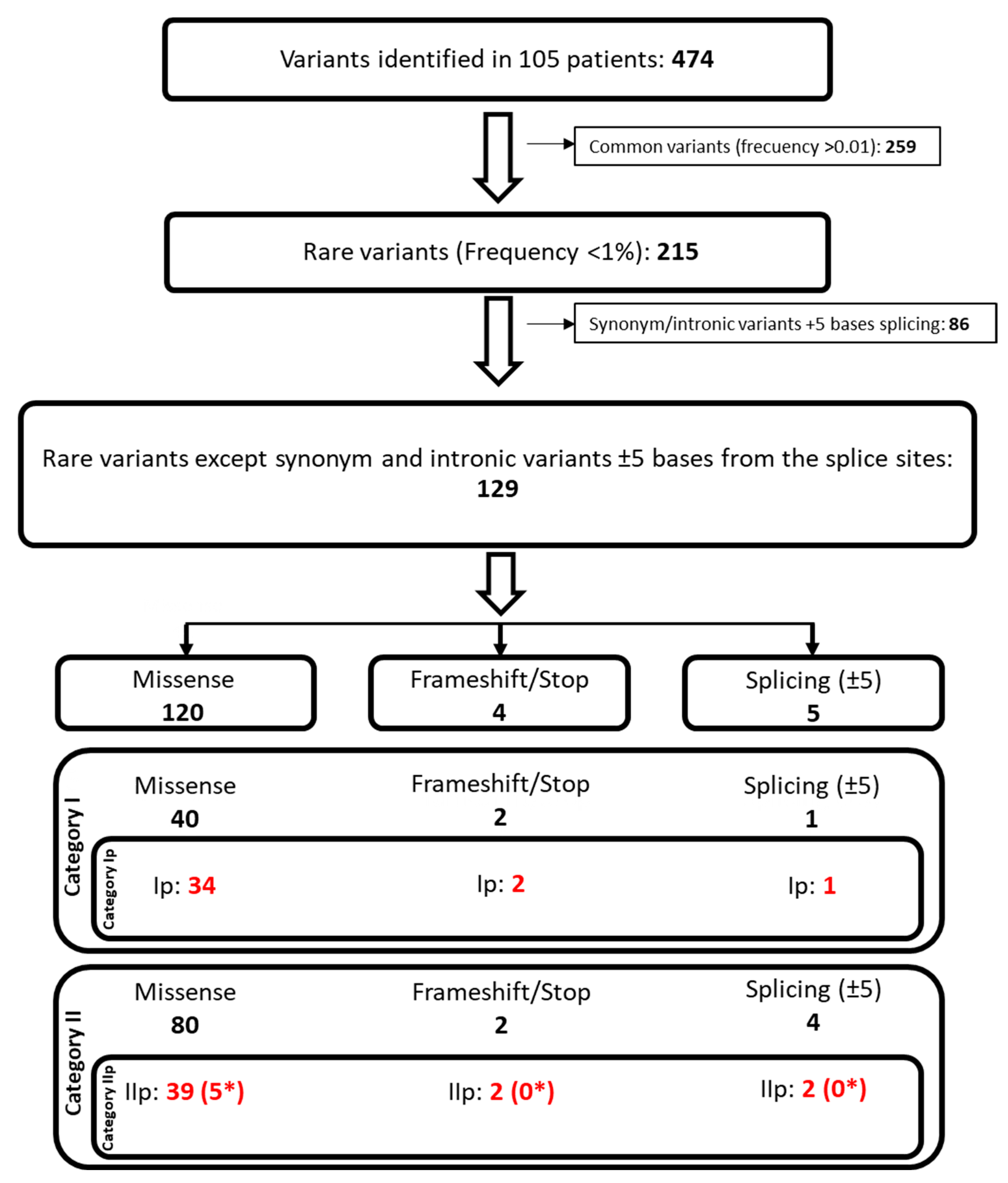
2.2. Classification of Variants Identified in the Cholesterol Pathway
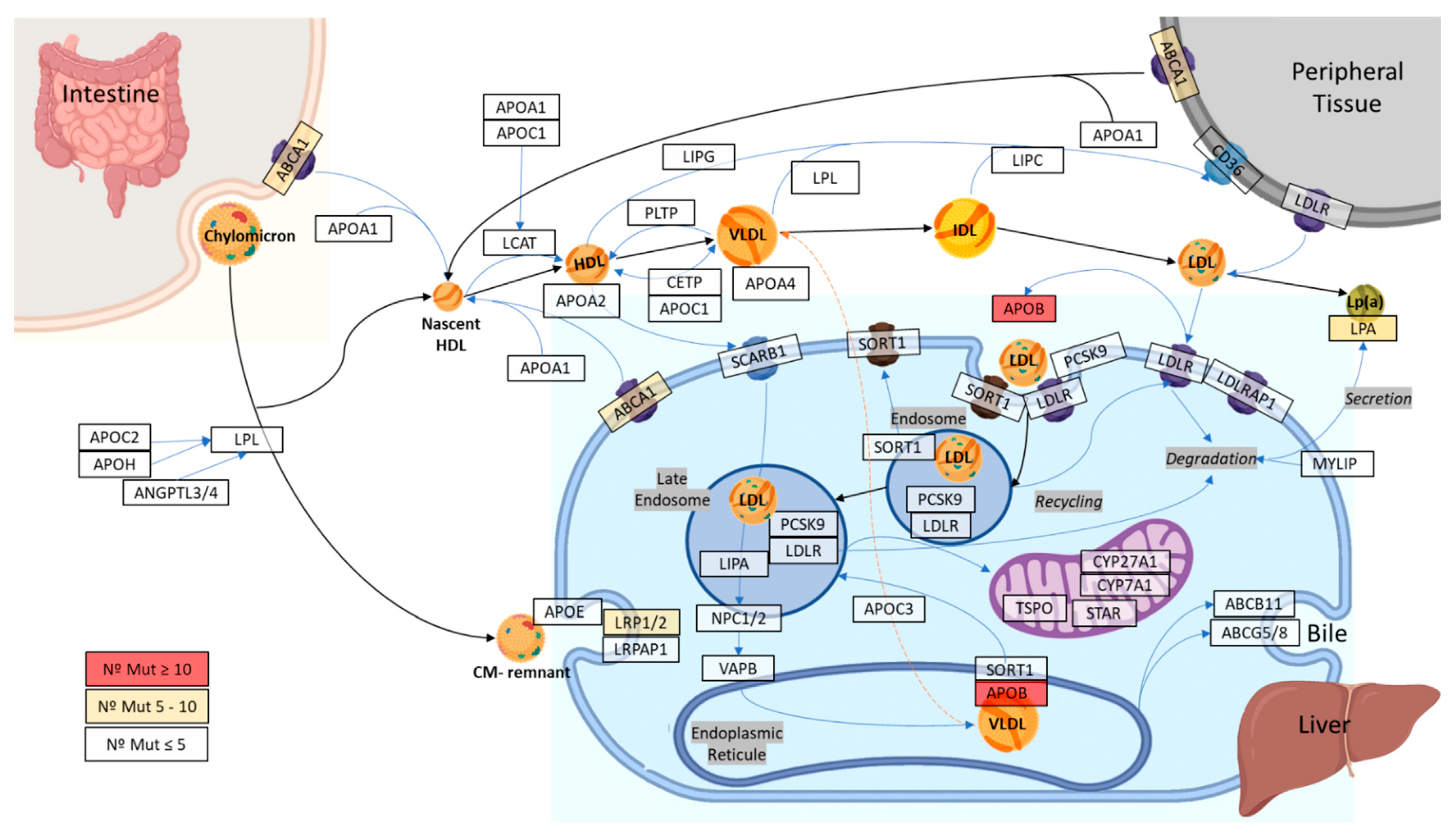
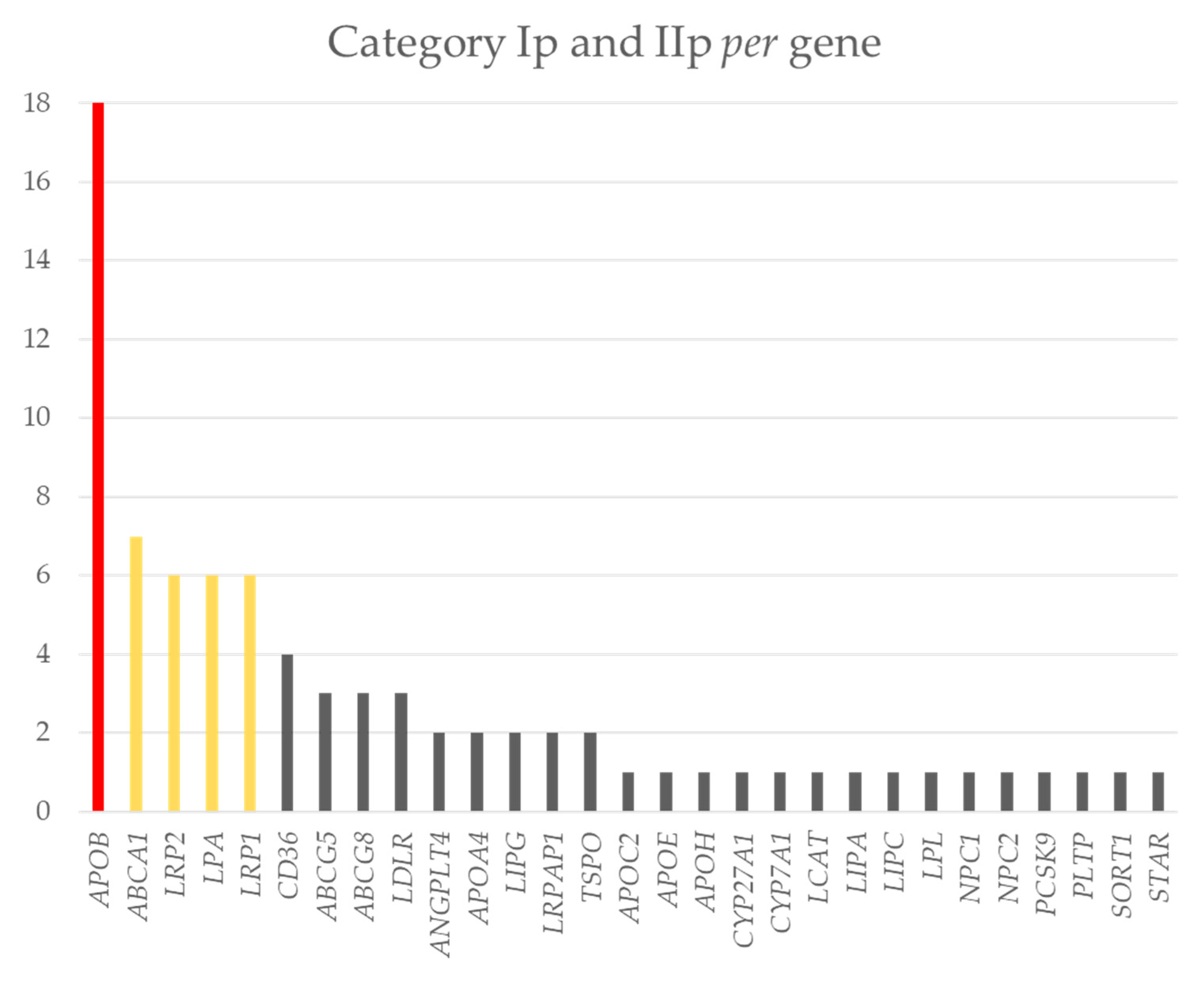
2.3. Distribution of the Variants of Interest in the Genes
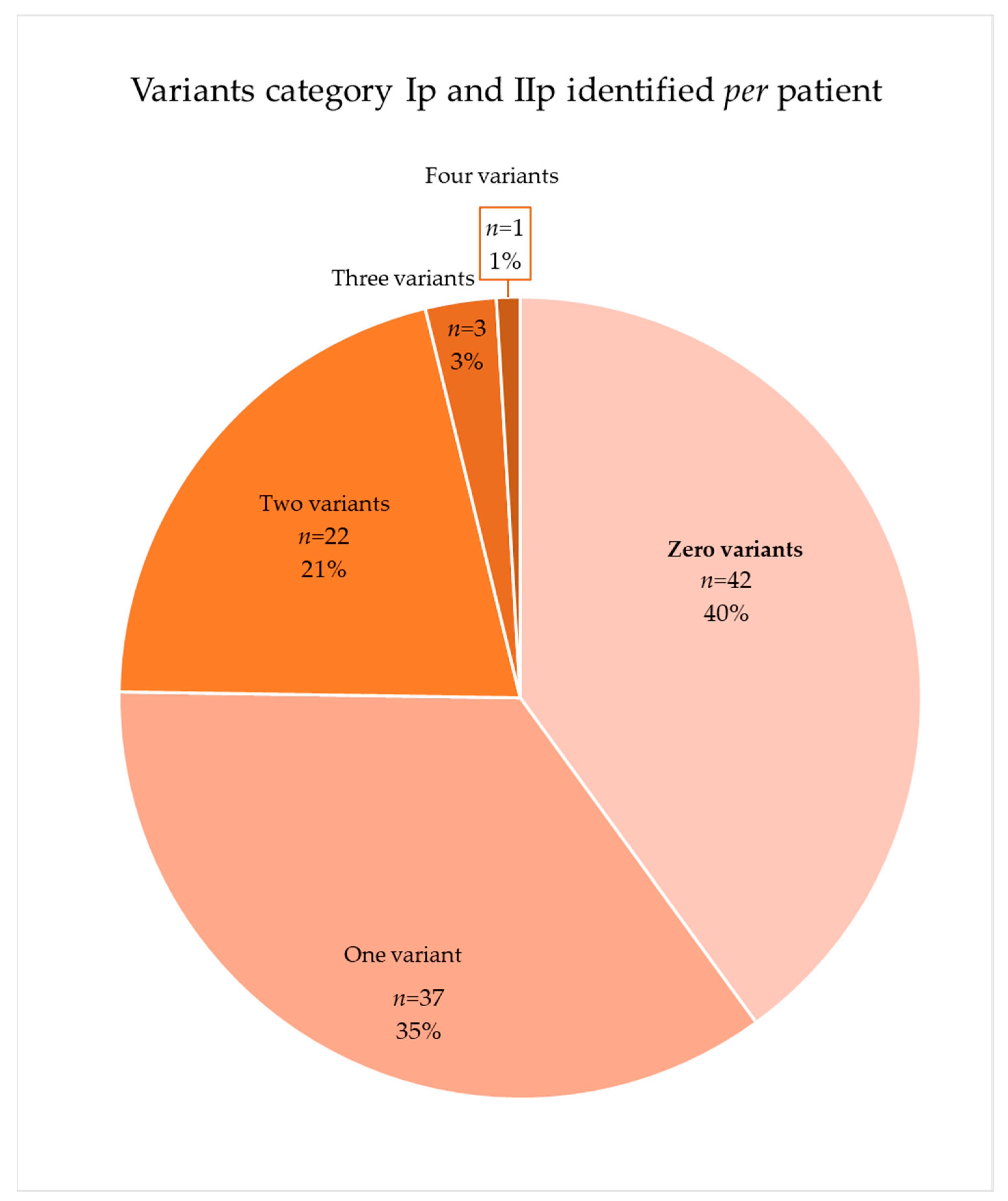
2.4. Distribution of the Variants of Interest in the Patients
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Selection of Genes Related to Cholesterol Metabolism
4.3. Next-Generation Sequencing
4.4. Variant Annotation and Classification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Writing Group Members; Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Després, J.P.; et al. Heart Disease and Stroke Statistics-2016 Update: A Report from the American Heart Association. Circulation 2016, 133, e38–e360, Erratum in Circulation 2016, 133, e599. [Google Scholar] [CrossRef]
- Feng, B.; Li, H. Genetic Polymorphism of Matrix Metalloproteinase-9 and Susceptibility to Myocardial Infarction: A Meta-Analysis. Dis. Markers 2022, 2022, 5507153. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Chang, C.C.; Hadley, T. Genetic Risk Stratification: A Paradigm Shift in Prevention of Coronary Artery Disease. JACC Basic Transl. Sci. 2021, 6, 287–304. [Google Scholar] [CrossRef] [PubMed]
- McPherson, R.; Tybjaerg-Hansen, A. Genetics of Coronary Artery Disease. Circ Res. 2016, 118, 564–578. [Google Scholar] [CrossRef]
- Musunuru, K.; Kathiresan, S. Surprises From Genetic Analyses of Lipid Risk Factors for Atherosclerosis. Circ Res. 2016, 118, 579–585. [Google Scholar] [CrossRef]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target. Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- Ference, B.A.; Kastelein, J.J.P.; Ray, K.K.; Ginsberg, H.N.; Chapman, M.J.; Packard, C.J.; Laufs, U.; Oliver-Williams, C.; Wood, A.M.; Butterworth, A.S.; et al. Association of Triglyceride-Lowering LPL Variants and LDL-C-Lowering LDLR Variants with Risk of Coronary Heart Disease. JAMA 2019, 321, 364–373. [Google Scholar] [CrossRef]
- Rać, M.E.; Suchy, J.; Kurzawski, G.; Kurlapska, A.; Safranow, K.; Rać, M.; Sagasz-Tysiewicz, D.; Krzystolik, A.; Poncyljusz, W.; Jakubowska, K.; et al. Polymorphism of the CD36 Gene and Cardiovascular Risk Factors in Patients with Coronary Artery Disease Manifested at a Young Age. Biochem. Genet. 2012, 50, 103–111. [Google Scholar] [CrossRef]
- Qi, L.P.; Chen, L.F.; Dang, A.M.; Li, L.Y.; Fang, Q.; Yan, X.W. Association between the ABCA1-565C/T gene promoter polymorphism and coronary heart disease severity and cholesterol efflux in the Chinese Han population. Genet. Test. Mol. Biomark. 2015, 19, 347–352. [Google Scholar] [CrossRef]
- Dalan, A.B.; Toptaş, B.; Buğra, Z.; Polat, N.; Yılmaz-Aydoğan, H.; Çimen, A.; Isbir, T. The effects of endothelial lipase gene (LIPG) variants on inflammation marker levels and atherosclerosis development. Mol. Biol. Rep. 2013, 40, 5143–5149. [Google Scholar] [CrossRef] [PubMed]
- Ranjith, N.; Pegoraro, R.J.; Rom, L. Lipid profiles and associated gene polymorphisms in young Asian Indian patients with acute myocardial infarction and the metabolic syndrome. Metab. Syndr. Relat. Disord. 2009, 7, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Cyrus, C.; Vatte, C.; Al-Nafie, A.; Chathoth, S.; Al-Ali, R.; Al-Shehri, A.; Akhtar, M.S.; Almansori, M.; Al-Muhanna, F.; Keating, B.; et al. The impact of common polymorphisms in CETP and ABCA1 genes with the risk of coronary artery disease in Saudi Arabians. Hum. Genom. 2016, 10, 8. [Google Scholar] [CrossRef]
- Kotaska, K.; Kolarova, J.; Kotrcova, K.; Cepova, J.; Prusa, R. Correlation between common genetic variants and risk factors associated with prediction of cardiovascular diseases in dyslipidemic patients. Genet. Test. Mol. Biomark. 2012, 16, 210–214. [Google Scholar] [CrossRef]
- Kotlęga, D.; Gołąb-Janowska, M.; Masztalewicz, M.; Ciećwież, S.; Nowacki, P. Association between selected gene polymorphisms and statin metabolism, risk of ischemic stroke and cardiovascular disorders. Postepy Hig. Med. Dosw. (Online) 2016, 70, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Trinder, M.; Paquette, M.; Cermakova, L.; Ban, M.R.; Hegele, R.A.; Baass, A.; Brunham, L.R. Polygenic Contribution to Low-Density Lipoprotein Cholesterol Levels and Cardiovascular Risk in Monogenic Familial Hypercholesterolemia. Circ. Genom. Precis. Med. 2020, 13, 515–523. [Google Scholar] [CrossRef]
- Karjalainen, J.P.; Mononen, N.; Hutri-Kähönen, N.; Lehtimäki, M.; Hilvo, M.; Kauhanen, D.; Juonala, M.; Viikari, J.; Kähönen, M.; Raitakari, O.; et al. New evidence from plasma ceramides links apoE polymorphism to greater risk of coronary artery disease in Finnish adults. J. Lipid Res. 2019, 60, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Tajima, T.; Morita, H.; Ito, K.; Yamazaki, T.; Kubo, M.; Komuro, I.; Momozawa, Y. Blood lipid-related low-frequency variants in LDLR and PCSK9 are associated with onset age and risk of myocardial infarction in Japanese. Sci. Rep. 2018, 8, 8107. [Google Scholar] [CrossRef]
- Do, R.; Stitziel, N.O.; Won, H.H.; Jørgensen, A.B.; Duga, S.; Angelica Merlini, P.; Kiezun, A.; Farrall, M.; Goel, A.; Zuk, O.; et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature 2015, 518, 102–106. [Google Scholar] [CrossRef]
- Awan, Z.; Choi, H.Y.; Stitziel, N.; Ruel, I.; Bamimore, M.A.; Husa, R.; Gagnon, M.H.; Wang, R.H.; Peloso, G.M.; Hegele, R.A.; et al. APOE p.Leu167del mutation in familial hypercholesterolemia. Atherosclerosis 2013, 231, 218–222. [Google Scholar] [CrossRef]
- Haas, J.; Frese, K.S.; Peil, B.; Kloos, W.; Keller, A.; Nietsch, R.; Feng, Z.; Müller, S.; Kayvanpour, E.; Vogel, B.; et al. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur. Heart J. 2015, 36, 1123–1135a. [Google Scholar] [CrossRef] [PubMed]
- The Human Gene Mutation Database. Available online: https://www.hgmd.cf.ac.uk/ac/index.php (accessed on 21 July 2022).
- Service, S.K.; Teslovich, T.M.; Fuchsberger, C.; Ramensky, V.; Yajnik, P.; Koboldt, D.C.; Larson, D.E.; Zhang, Q.; Lin, L.; Welch, R.; et al. Re-sequencing expands our understanding of the phenotypic impact of variants at GWAS loci. PLoS Genet. 2014, 10, e1004147. [Google Scholar] [CrossRef]
- Frikke-Schmidt, R.; Nordestgaard, B.G.; Schnohr, P.; Steffensen, R.; Tybjaerg-Hansen, A. Mutation in ABCA1 predicted risk of ischemic heart disease in the Copenhagen City Heart Study Population. J. Am. Coll. Cardiol. 2005, 46, 1516–1520. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dron, J.S.; Wang, J.; Low-Kam, C.; Khetarpal, S.A.; Robinson, J.F.; McIntyre, A.D.; Ban, M.R.; Cao, H.; Rhainds, D.; Dubé, M.P.; et al. Polygenic determinants in extremes of high-density lipoprotein cholesterol. J. Lipid Res. 2017, 58, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.S.; Polisecki, E.Y.; Diffenderfer, M.R.; Asztalos, B.F.; Karathanasis, S.K.; Hegele, R.A.; Schaefer, E.J. Genetic and secondary causes of severe HDL deficiency and cardiovascular disease. J. Lipid Res. 2018, 59, 2421–2435. [Google Scholar] [CrossRef]
- Bodzioch, M.; Orsó, E.; Klucken, J.; Langmann, T.; Böttcher, A.; Diederich, W.; Drobnik, W.; Barlage, S.; Büchler, C.; Porsch-Ozcürümez, M.; et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 1999, 22, 347–351. [Google Scholar] [CrossRef]
- Sadananda, S.N.; Foo, J.N.; Toh, M.T.; Cermakova, L.; Trigueros-Motos, L.; Chan, T.; Liany, H.; Collins, J.A.; Gerami, S.; Singaraja, R.R.; et al. Targeted next-generation sequencing to diagnose disorders of HDL cholesterol. J. Lipid Res. 2015, 56, 1993–2001. [Google Scholar] [CrossRef]
- Kars, M.E.; Başak, A.N.; Onat, O.E.; Bilguvar, K.; Choi, J.; Itan, Y.; Çağlar, C.; Palvadeau, R.; Casanova, J.L.; Cooper, D.N.; et al. The genetic structure of the Turkish population reveals high levels of variation and admixture. Proc. Natl. Acad. Sci. USA 2021, 118, e2026076118, Erratum in Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Hong, S.H.; Rhyne, J.; Zeller, K.; Miller, M. ABCA1(Alabama): A novel variant associated with HDL deficiency and premature coronary artery disease. Atherosclerosis 2002, 164, 245–250. [Google Scholar] [CrossRef]
- Backman, J.D.; Li, A.H.; Marcketta, A.; Sun, D.; Mbatchou, J.; Kessler, M.D.; Benner, C.; Liu, D.; Locke, A.E.; Balasubramanian, S.; et al. Exome sequencing and analysis of 454,787 UK Biobank participants. Nature 2021, 599, 628–634. [Google Scholar] [CrossRef]
- Johansen, C.T.; Dubé, J.B.; Loyzer, M.N.; MacDonald, A.; Carter, D.E.; McIntyre, A.D.; Cao, H.; Wang, J.; Robinson, J.F.; Hegele, R.A. LipidSeq: A next-generation clinical resequencing panel for monogenic dyslipidemias. J. Lipid Res. 2014, 55, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Downes, K.; Megy, K.; Duarte, D.; Vries, M.; Gebhart, J.; Hofer, S.; Shamardina, O.; Deevi, S.V.V.; Stephens, J.; Mapeta, R.; et al. Diagnostic high-throughput sequencing of 2396 patients with bleeding, thrombotic, and platelet disorders. Blood 2019, 134, 2082–2091. [Google Scholar] [CrossRef]
- Hansel, B.; Carrié, A.; Brun-Druc, N.; Leclert, G.; Chantepie, S.; Coiffard, A.S.; Kahn, J.F.; Chapman, M.J.; Bruckert, E. Premature atherosclerosis is not systematic in phytosterolemic patients: Severe hypercholesterolemia as a confounding factor in five subjects. Atherosclerosis 2014, 234, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Dron, J.S.; Wang, J.; McIntyre, A.D.; Iacocca, M.A.; Robinson, J.F.; Ban, M.R.; Cao, H.; Hegele, R.A. Six years’ experience with LipidSeq: Clinical and research learnings from a hybrid, targeted sequencing panel for dyslipidemias. BMC Med. Genom. 2020, 13, 23. [Google Scholar] [CrossRef]
- Romeo, S.; Yin, W.; Kozlitina, J.; Pennacchio, L.A.; Boerwinkle, E.; Hobbs, H.H.; Cohen, J.C. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J. Clin. Investig. 2009, 119, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Marmontel, O.; Rollat-Farnier, P.A.; Wozny, A.S.; Charrière, S.; Vanhoye, X.; Simonet, T.; Chatron, N.; Collin-Chavagnac, D.; Nony, S.; Dumont, S.; et al. Development of a new expanded next-generation sequencing panel for genetic diseases involved in dyslipidemia. Clin. Genet. 2020, 98, 589–594. [Google Scholar] [CrossRef]
- Deeb, S.S.; Nevin, D.N.; Iwasaki, L.; Brunzell, J.D. Two novel apolipoprotein A-IV variants in individuals with familial combined hyperlipidemia and diminished levels of lipoprotein lipase activity. Hum. Mutat. 1996, 8, 319–325. [Google Scholar] [CrossRef]
- Radovica-Spalvina, I.; Latkovskis, G.; Silamikelis, I.; Fridmanis, D.; Elbere, I.; Ventins, K.; Ozola, G.; Erglis, A.; Klovins, J. Next-generation-sequencing-based identification of familial hypercholesterolemia-related mutations in subjects with increased LDL-C levels in a latvian population. BMC Med. Genet. 2015, 16, 86. [Google Scholar] [CrossRef][Green Version]
- Alves, A.C.; Benito-Vicente, A.; Medeiros, A.M.; Reeves, K.; Martin, C.; Bourbon, M. Further evidence of novel APOB mutations as a cause of familial hypercholesterolaemia. Atherosclerosis 2018, 277, 448–456. [Google Scholar] [CrossRef]
- Grzymski, J.J.; Elhanan, G.; Morales Rosado, J.A.; Smith, E.; Schlauch, K.A.; Read, R.; Rowan, C.; Slotnick, N.; Dabe, S.; Metcalf, W.J.; et al. Population genetic screening efficiently identifies carriers of autosomal dominant diseases. Nat. Med. 2020, 26, 1235–1239. [Google Scholar] [CrossRef]
- Hou, Y.C.; Yu, H.C.; Martin, R.; Cirulli, E.T.; Schenker-Ahmed, N.M.; Hicks, M.; Cohen, I.V.; Jönsson, T.J.; Heister, R.; Napier, L.; et al. Precision medicine integrating whole-genome sequencing, comprehensive metabolomics, and advanced imaging. Proc. Natl. Acad. Sci. USA 2020, 117, 3053–3062. [Google Scholar] [CrossRef] [PubMed]
- Rimbert, A.; Daggag, H.; Lansberg, P.; Buckley, A.; Viel, M.; Kanninga, R.; Johansson, L.; Dullaart, R.P.F.; Sinke, R.; Al Tikriti, A.; et al. Low Detection Rates of Genetic FH in Cohort of Patients with Severe Hypercholesterolemia in the United Arabic Emirates. Front. Genet. 2022, 12, 809256. [Google Scholar] [CrossRef] [PubMed]
- Leren, T.P.; Berge, K.E. Identification of mutations in the apolipoprotein B-100 gene and in the PCSK9 gene as the cause of hypocholesterolemia. Clin. Chim. Acta 2008, 397, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.X.; Wu, N.Q.; Sun, D.; Liu, H.H.; Jin, J.L.; Li, S.; Guo, Y.L.; Zhu, C.G.; Gao, Y.; Dong, Q.T.; et al. Application of expanded genetic analysis in the diagnosis of familial hypercholesterolemia in patients with very early-onset coronary artery disease. J. Transl. Med. 2018, 16, 345. [Google Scholar] [CrossRef]
- Johansen, C.T.; Wang, J.; Lanktree, M.B.; Cao, H.; McIntyre, A.D.; Ban, M.R.; Martins, R.A.; Kennedy, B.A.; Hassell, R.G.; Visser, M.E.; et al. Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat. Genet. 2010, 42, 684–687. [Google Scholar] [CrossRef]
- Roa Garrido, J.; Carrasco Salas, P.; Toscano Pérez, C.; Arrobas Velilla, T.; Vázquez Rico, I.; Díaz Fernández, J.F. Genetics and biochemistry of familial hypercholesterolemia in Southwest of the Iberian Peninsula. Particularidades genéticas y bioquímicas de la hipercolesterolemia familiar en el suroeste de la Península Ibérica. Clin. Investig. Arterioscler. 2021, 33, 62–69. [Google Scholar] [CrossRef]
- Noto, D.; Spina, R.; Giammanco, A.; Barbagallo, C.M.; Ganci, A.; Scrimali, C.; Brucato, F.; Misiano, G.; Ciaccio, M.; Caldarella, R.; et al. Diagnosis of familial hypercholesterolemia in a large cohort of Italian genotyped hypercholesterolemic patients. Atherosclerosis 2022, 347, 63–67. [Google Scholar] [CrossRef]
- Batais, M.A.; Almigbal, T.H.; Shaik, N.A.; Alharbi, F.K.; Alharbi, K.K.; Ali Khan, I. Screening of common genetic variants in the APOB gene related to familial hypercholesterolemia in a Saudi population: A case-control study. Medicine 2019, 98, e14247. [Google Scholar] [CrossRef]
- Lancellotti, S.; Di Leo, E.; Penacchioni, J.Y.; Balli, F.; Viola, L.; Bertolini, S.; Calandra, S.; Tarugi, P. Hypobetalipoproteinemia with an apparently recessive inheritance due to a “de novo” mutation of apolipoprotein B. Biochim. Biophys. Acta 2004, 1688, 61–67. [Google Scholar] [CrossRef]
- Khlebus, E.; Kutsenko, V.; Meshkov, A.; Ershova, A.; Kiseleva, A.; Shevtsov, A.; Shcherbakova, N.; Zharikova, A.; Lankin, V.; Tikhaze, A.; et al. Multiple rare and common variants in APOB gene locus associated with oxidatively modified low-density lipoprotein levels. PLoS ONE 2019, 14, e0217620. [Google Scholar] [CrossRef]
- Zeissig, S.; Dougan, S.K.; Barral, D.C.; Junker, Y.; Chen, Z.; Kaser, A.; Ho, M.; Mandel, H.; McIntyre, A.; Kennedy, S.M.; et al. Primary deficiency of microsomal triglyceride transfer protein in human abetalipoproteinemia is associated with loss of CD1 function. J. Clin. Investig. 2010, 120, 2889–2899. [Google Scholar] [CrossRef] [PubMed]
- Hegele, R.A.; Connelly, P.W.; Maguire, G.F.; Huff, M.W.; Leiter, L.; Wolfe, B.M.; Evans, A.J.; Little, J.A. An apolipoprotein CII mutation, CIILys19—Thr’ identified in patients with hyperlipidemia. Dis. Markers 1991, 9, 73–80. [Google Scholar] [PubMed]
- Sanghera, D.K.; Wagenknecht, D.R.; McIntyre, J.A.; Kamboh, M.I. Identification of structural mutations in the fifth domain of apolipoprotein H (beta 2-glycoprotein I) which affect phospholipid binding. Hum. Mol. Genet. 1997, 6, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Eicher, J.D.; Chami, N.; Kacprowski, T.; Nomura, A.; Chen, M.H.; Yanek, L.R.; Tajuddin, S.M.; Schick, U.M.; Slater, A.J.; Pankratz, N.; et al. Platelet-Related Variants Identified by Exomechip Meta-analysis in 157,293 Individuals. Am. J. Hum. Genet. 2016, 99, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Iotchkova, V.; Huang, J.; Morris, J.A.; Jain, D.; Barbieri, C.; Walter, K.; Min, J.L.; Chen, L.; Astle, W.; Cocca, M.; et al. Discovery and refinement of genetic loci associated with cardiometabolic risk using dense imputation maps. Nat. Genet. 2016, 48, 1303–1312, Erratum in Nat. Genet. 2018, 50, 1752. [Google Scholar] [CrossRef]
- Flesch, B.K.; Scherer, V.; Opitz, A.; Ochmann, O.; Janson, A.; Steitz, M.; Zeiler, T. Platelet CD36 deficiency is present in 2.6% of Arabian individuals and can cause NAIT and platelet refractoriness. Transfusion 2021, 61, 1932–1942. [Google Scholar] [CrossRef]
- Pilo, B.; de Blas, G.; Sobrido, M.J.; Navarro, C.; Grandas, F.; Barrero, F.J.; Moya, M.A.; Jimenez-Escrig, A. Neurophysiological study in cerebrotendinous xanthomatosis. Muscle Nerve 2011, 43, 531–536. [Google Scholar] [CrossRef]
- Mozas, P.; Castillo, S.; Tejedor, D.; Reyes, G.; Alonso, R.; Franco, M.; Saenz, P.; Fuentes, F.; Almagro, F.; Mata, P.; et al. Molecular characterization of familial hypercholesterolemia in Spain: Identification of 39 novel and 77 recurrent mutations in LDLR. Hum. Mutat. 2004, 24, 187. [Google Scholar] [CrossRef]
- Leren, T.P.; Bogsrud, M.P. Molecular genetic testing for autosomal dominant hypercholesterolemia in 29,449 Norwegian index patients and 14,230 relatives during the years 1993–2020. Atherosclerosis 2021, 322, 61–66. [Google Scholar] [CrossRef]
- Hobbs, H.H.; Brown, M.S.; Goldstein, J.L. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum. Mutat. 1992, 1, 445–466. [Google Scholar] [CrossRef]
- Van der Schoot, V.; Haer-Wigman, L.; Feenstra, I.; Tammer, F.; Oerlemans, A.J.M.; van Koolwijk, M.P.A.; van Agt, F.; Arens, Y.H.J.M.; Brunner, H.G.; Vissers, L.E.L.M.; et al. Lessons learned from unsolicited findings in clinical exome sequencing of 16,482 individuals. Eur. J. Hum. Genet. 2022, 30, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Loux, N.; Saint-Jore, B.; Collod, G.; Dairou, F.; Benlian, P.; Truffert, J.; Dastugue, B.; Douste-Blazy, P.; de Gennes, J.L.; Junien, C. Screening for new mutations in the LDL receptor gene in seven French familial hypercholesterolemia families by the single strand conformation polymorphism method. Hum. Mutat. 1992, 1, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Chora, J.R.; Medeiros, A.M.; Alves, A.C.; Bourbon, M. Analysis of publicly available LDLR, APOB, and PCSK9 variants associated with familial hypercholesterolemia: Application of ACMG guidelines and implications for familial hypercholesterolemia diagnosis. Genet. Med. 2018, 20, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Martín-Campos, J.M.; Plana, N.; Figueras, R.; Ibarretxe, D.; Caixàs, A.; Esteve, E.; Pérez, A.; Bueno, M.; Mauri, M.; Roig, R.; et al. Autosomal dominant hypercholesterolemia in Catalonia: Correspondence between clinical-biochemical and genetic diagnostics in 967 patients studied in a multicenter clinical setting. J. Clin. Lipidol. 2018, 12, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Vinje, T.; Wierød, L.; Leren, T.P.; Strøm, T.B. Prevalence of cholesteryl ester storage disease among hypercholesterolemic subjects and functional characterization of mutations in the lysosomal acid lipase gene. Mol. Genet. Metab. 2018, 123, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Singaraja, R.R.; Sivapalaratnam, S.; Hovingh, K.; Dubé, M.P.; Castro-Perez, J.; Collins, H.L.; Adelman, S.J.; Riwanto, M.; Manz, J.; Hubbard, B.; et al. The impact of partial and complete loss-of-function mutations in endothelial lipase on high-density lipoprotein levels and functionality in humans. Circ. Cardiovasc. Genet. 2013, 6, 54–62. [Google Scholar] [CrossRef]
- Motazacker, M.M.; Peter, J.; Treskes, M.; Shoulders, C.C.; Kuivenhoven, J.A.; Hovingh, G.K. Evidence of a polygenic origin of extreme high-density lipoprotein cholesterol levels. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1521–1528, Erratum in Arterioscler. Thromb. Vasc. Biol. 2013, 33, e128. [Google Scholar] [CrossRef]
- Edmondson, A.C.; Brown, R.J.; Kathiresan, S.; Cupples, L.A.; Demissie, S.; Manning, A.K.; Jensen, M.K.; Rimm, E.B.; Wang, J.; Rodrigues, A.; et al. Loss-of-function variants in endothelial lipase are a cause of elevated HDL cholesterol in humans. J. Clin. Investig. 2009, 119, 1042–1050. [Google Scholar] [CrossRef]
- Cole, J.; Blackhurst, D.M.; Solomon, G.A.E.; Ratanjee, B.D.; Benjamin, R.; Marais, A.D. Atherosclerotic cardiovascular disease in hyperalphalipoproteinemia due to LIPG variants. J. Clin. Lipidol. 2021, 15, 142–150.e2. [Google Scholar] [CrossRef]
- Morgan, B.M.; Brown, A.N.; Deo, N.; Harrop, T.W.R.; Taiaroa, G.; Mace, P.D.; Wilbanks, S.M.; Merriman, T.R.; Williams, M.J.A.; McCormick, S.P.A. Nonsynonymous SNPs in LPA homologous to plasminogen deficiency mutants represent novel null apo(a) alleles. J. Lipid Res. 2020, 61, 432–444. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Natarajan, P.; Klarin, D.; Won, H.H.; Peloso, G.M.; Stitziel, N.O.; Nomura, A.; Zekavat, S.M.; Bick, A.G.; et al. Phenotypic Characterization of Genetically Lowered Human Lipoprotein(a) Levels. J Am Coll Cardiol. 2016, 68, 2761–2772. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.L.; Vogler, G.; Missinato, M.A.; Li, X.; Nielsen, T.; Zeng, X.I.; Martinez-Fernandez, A.; Walls, S.M.; Kervadec, A.; Kezos, J.N.; et al. Patient-specific genomics and cross-species functional analysis implicate LRP2 in hypoplastic left heart syndrome. Elife 2020, 9, e59554. [Google Scholar] [CrossRef] [PubMed]
- Park, W.D.; O’Brien, J.F.; Lundquist, P.A.; Kraft, D.L.; Vockley, C.W.; Karnes, P.S.; Patterson, M.C.; Snow, K. Identification of 58 novel mutations in Niemann-Pick disease type C: Correlation with biochemical phenotype and importance of PTC1-like domains in NPC1. Hum. Mutat. 2003, 22, 313–325. [Google Scholar] [CrossRef]
- Sriretnakumar, V.; Harripaul, R.; Vincent, J.B.; Kennedy, J.L.; So, J. Enrichment of pathogenic variants in genes associated with inborn errors of metabolism in psychiatric populations. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2019, 180, 46–54. [Google Scholar] [CrossRef]
- Picillo, M.; Amboni, M.; Bruni, A.; Maletta, R.; Barone, P. Prevalence of heterozygous mutations in Niemann-Pick type C genes in a cohort of progressive supranuclear palsy. Park. Relat. Disord. 2020, 79, 9–10. [Google Scholar] [CrossRef]
- Abouelhoda, M.; Faquih, T.; El-Kalioby, M.; Alkuraya, F.S. Revisiting the morbid genome of Mendelian disorders. Genome Biol. 2016, 17, 235. [Google Scholar] [CrossRef] [PubMed]
- Samani, N.J.; Hengstenberg, C.; Mangino, M.; Mayer, B.; Dixon, R.J.; Meitinger, T.; Braund, P.; Wichmann, H.E.; Barrett, J.H.; König, I.R.; et al. Genomewide association analysis of coronary artery disease. N. Engl. J. Med. 2007, 357, 443–453. [Google Scholar] [CrossRef]
- Kathiresan, S.; Voight, B.F.; Purcell, S.; Musunuru, K.; Ardissino, D.; Mannucci, P.M.; Anand, S.; Engert, J.C.; Samani, N.J.; Schunkert, H.; et al. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat. Genet. 2009, 41, 334–341. [Google Scholar] [CrossRef]
- Schunkert, H.; König, I.R.; Kathiresan, S.; Reilly, M.P.; Assimes, T.L.; Holm, H.; Preuss, M.; Stewart, A.F.; Barbalic, M.; Gieger, C.; et al. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 2011, 43, 333–338. [Google Scholar] [CrossRef]
- Lu, X.; Wang, L.; Chen, S.; He, L.; Yang, X.; Shi, Y.; Cheng, J.; Zhang, L.; Gu, C.C.; Huang, J.; et al. Genome-wide association study in Han Chinese identifies four new susceptibility loci for coronary artery disease. Nat. Genet. 2012, 44, 890–894. [Google Scholar] [CrossRef]
- Deloukas, P.; Kanoni, S.; Willenborg, C.; Farrall, M.; Assimes, T.L.; Thompson, J.R.; Ingelsson, E.; Saleheen, D.; Erdmann, J.; Goldstein, B.A.; et al. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat. Genet. 2013, 45, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.P.; Peloso, G.M.; Pirruccello, J.P.; Johansen, C.T.; Dubé, J.B.; Larach, D.B.; Ban, M.R.; Dallinge-Thie, G.M.; Gupta, N.; Boehnke, M.; et al. Targeted exonic sequencing of GWAS loci in the high extremes of the plasma lipids distribution. Atherosclerosis 2016, 250, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Van der Harst, P.; Verweij, N. Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ. Res. 2018, 122, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.S.; Xie, C.; Paré, G.; Montpetit, A.; Rangarajan, S.; McQueen, M.J.; Cordell, H.J.; Keavney, B.; Yusuf, S.; Hudson, T.J.; et al. Genetic variants associated with myocardial infarction risk factors in over 8000 individuals from five ethnic groups: The Interheart Genetics Study. Circ. Cardiovasc. Genet. 2009, 2, 16–25. [Google Scholar] [CrossRef]
- Brænne, I.; Kleinecke, M.; Reiz, B.; Graf, E.; Strom, T.; Wieland, T.; Fischer, M.; Kessler, T.; Hengstenberg, C.; Meitinger, T.; et al. Systematic analysis of variants related to familial hypercholesterolemia in families with premature myocardial infarction. Eur. J. Hum. Genet. 2016, 24, 191–197. [Google Scholar] [CrossRef]
- Dai, X.; Wiernek, S.; Evans, J.P.; Runge, M.S. Genetics of coronary artery disease and myocardial infarction. World J. Cardiol. 2016, 8, 1–23. [Google Scholar] [CrossRef]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H., Jr.; Hobbs, H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N. Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef]
- Song, C.; Pedersen, N.L.; Reynolds, C.A.; Sabater-Lleal, M.; Kanoni, S.; Willenborg, C.; CARDIoGRAMplusC4D Consortium; Syvänen, A.C.; Watkins, H.; Hamsten, A.; et al. Genetic variants from lipid-related pathways and risk for incident myocardial infarction. PLoS ONE 2013, 8, e60454. [Google Scholar] [CrossRef]
- Helgadottir, A.; Gretarsdottir, S.; Thorleifsson, G.; Hjartarson, E.; Sigurdsson, A.; Magnusdottir, A.; Jonasdottir, A.; Kristjansson, H.; Sulem, P.; Oddsson, A.; et al. Variants with large effects on blood lipids and the role of cholesterol and triglycerides in coronary disease. Nat. Genet. 2016, 48, 634–639. [Google Scholar] [CrossRef]
- Thormaehlen, A.S.; Schuberth, C.; Won, H.H.; Blattmann, P.; Joggerst-Thomalla, B.; Theiss, S.; Asselta, R.; Duga, S.; Merlini, P.A.; Ardissino, D.; et al. Systematic cell-based phenotyping of missense alleles empowers rare variant association studies: A case for LDLR and myocardial infarction. PLoS Genet. 2015, 11, e1004855. [Google Scholar] [CrossRef]
- Alves, A.C.; Azevedo, S.; Benito-Vicente, A.; Graça, R.; Galicia-Garcia, U.; Barros, P.; Jordan, P.; Martin, C.; Bourbon, M. LDLR variants functional characterization: Contribution to variant classification. Atherosclerosis 2021, 329, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Behbodikhah, J.; Ahmed, S.; Elyasi, A.; Kasselman, L.J.; De Leon, J.; Glass, A.D.; Reiss, A.B. Apolipoprotein B and Cardiovascular Disease: Biomarker and Potential Therapeutic Target. Metabolites 2021, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Dagli-Hernandez, C.; Borges, J.B.; Marçal, E.D.S.R.; de Freitas, R.C.C.; Mori, A.A.; Gonçalves, R.M.; Faludi, A.A.; de Oliveira, V.F.; Ferreira, G.M.; Bastos, G.M.; et al. Genetic Variant ABCC1 rs45511401 Is Associated with Increased Response to Statins in Patients with Familial Hypercholesterolemia. Pharmaceutics 2022, 14, 944. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.M.; Alves, A.C.; Bourbon, M. Mutational analysis of a cohort with clinical diagnosis of familial hypercholesterolemia: Considerations for genetic diagnosis improvement. Genet. Med. 2016, 18, 316–324. [Google Scholar] [CrossRef]
- Dewey, F.E.; Murray, M.F.; Overton, J.D.; Habegger, L.; Leader, J.B.; Fetterolf, S.N.; O’Dushlaine, C.; Van Hout, C.V.; Staples, J.; Gonzaga-Jauregui, C.; et al. Distribution and clinical impact of functional variants in 50,726 whole-exome sequences from the DiscovEHR study. Science 2016, 354, aaf6814. [Google Scholar] [CrossRef]
- Alves, A.C.; Etxebarria, A.; Soutar, A.K.; Martin, C.; Bourbon, M. Novel functional APOB mutations outside LDL-binding region causing familial hypercholesterolaemia. Hum. Mol. Genet. 2014, 23, 1817–1828. [Google Scholar] [CrossRef]
- Hayat, M.; Kerr, R.; Bentley, A.R.; Rotimi, C.N.; Raal, F.J.; Ramsay, M. Genetic associations between serum low LDL-cholesterol levels and variants in LDLR, APOB, PCSK9 and LDLRAP1 in African populations. PLoS ONE 2020, 15, e0229098, Erratum in PLoS ONE 2021, 16, e0249478. [Google Scholar] [CrossRef]
- Benn, M. Apolipoprotein B levels, APOB alleles, and risk of ischemic cardiovascular disease in the general population, a review. Atherosclerosis 2009, 206, 17–30. [Google Scholar] [CrossRef]
- Cohen, J.; Pertsemlidis, A.; Kotowski, I.K.; Graham, R.; Garcia, C.K.; Hobbs, H.H. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 2005, 37, 161–165, Erratum in Nat. Genet. 2005, 37, 328. [Google Scholar] [CrossRef]
- Henkel, A.S.; Kavesh, M.H.; Kriss, M.S.; Dewey, A.M.; Rinella, M.E.; Green, R.M. Hepatic overexpression of abcb11 promotes hypercholesterolemia and obesity in mice. Gastroenterology 2011, 141, 1404–1411.e2. [Google Scholar] [CrossRef]
- Su, X.; Peng, D.Q. New insights into ANGPLT3 in controlling lipoprotein metabolism and risk of cardiovascular diseases. Lipids Health Dis. 2018, 17, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.D.; Chen, X.C.; Yang, Y.N.; Gao, X.M.; Ma, X.; Huang, Y.; Li, X.M.; Gai, M.T.; Liu, F.; Pan, S.; et al. Apolipoprotein A1 is associated with SYNTAX score in patients with a non-ST segment elevation myocardial infarction. Lipids Health Dis. 2019, 18, 159. [Google Scholar] [CrossRef] [PubMed]
- Birjmohun, R.S.; Dallinga-Thie, G.M.; Kuivenhoven, J.A.; Stroes, E.S.; Otvos, J.D.; Wareham, N.J.; Luben, R.; Kastelein, J.J.; Khaw, K.T.; Boekholdt, S.M. Apolipoprotein A-II is inversely associated with risk of future coronary artery disease. Circulation 2007, 116, 2029–2035. [Google Scholar] [CrossRef]
- Gautier, T.; Deckert, V.; Aires, V.; Le Guern, N.; Proukhnitzky, L.; Patoli, D.; Lemaire, S.; Maquart, G.; Bataille, A.; Xolin, M.; et al. Human apolipoprotein C1 transgenesis reduces atherogenesis in hypercholesterolemic rabbits. Atherosclerosis 2021, 320, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, T.A.; Mohamed, R.H.; Hashem, R.M. Association of lipoprotein lipase and apolipoprotein C-III genes polymorphism with acute myocardial infarction in diabetic patients. Mol. Cell. Biochem. 2011, 354, 141–150. [Google Scholar] [CrossRef]
- Meiner, V.; Friedlander, Y.; Milo, H.; Sharon, N.; Ben-Avi, L.; Shpitzen, S.; Leitersdorf, E.; Siscovick, D.S.; Schwartz, S.M. Cholesteryl ester transfer protein (CETP) genetic variation and early onset of non-fatal myocardial infarction. Ann. Hum. Genet. 2008, 72 Pt 6, 732–741. [Google Scholar] [CrossRef]
- Lee, C.; Cui, Y.; Song, J.; Li, S.; Zhang, F.; Wu, M.; Li, L.; Hu, D.; Chen, H. Effects of familial hypercholesterolemia-associated genes on the phenotype of premature myocardial infarction. Lipids Health Dis. 2019, 18, 95. [Google Scholar] [CrossRef]
- Weissglas-Volkov, D.; Calkin, A.C.; Tusie-Luna, T.; Sinsheimer, J.S.; Zelcer, N.; Riba, L.; Tino, A.M.; Ordoñez-Sánchez, M.L.; Cruz-Bautista, I.; Aguilar-Salinas, C.A.; et al. The N342S MYLIP polymorphism is associated with high total cholesterol and increased LDL receptor degradation in humans. J. Clin. Investig. 2011, 121, 3062–3071. [Google Scholar] [CrossRef]
- Stanislovaitiene, D.; Lesauskaite, V.; Zaliuniene, D.; Smalinskiene, A.; Gustiene, O.; Zaliaduonyte-Peksiene, D.; Tamosiunas, A.; Luksiene, D.; Petkeviciene, J.; Zaliunas, R. SCARB1 single nucleotide polymorphism (rs5888) is associated with serum lipid profile and myocardial infarction in an age- and gender-dependent manner. Lipids Health Dis. 2013, 12, 24. [Google Scholar] [CrossRef]
- Silbernagel, N.; Walecki, M.; Schäfer, M.K.; Kessler, M.; Zobeiri, M.; Rinné, S.; Kiper, A.K.; Komadowski, M.A.; Vowinkel, K.S.; Wemhöner, K.; et al. The VAMP-associated protein VAPB is required for cardiac and neuronal pacemaker channel function. FASEB J. 2018, 32, 6159–6173. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443, Erratum in Nature 2021, 597, E3–E4. [Google Scholar] [CrossRef] [PubMed]
- Sherry, S.T.; Ward, M.H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Akinrinade, O.; Ollila, L.; Vattulainen, S.; Tallila, J.; Gentile, M.; Salmenperä, P.; Koillinen, H.; Kaartinen, M.; Nieminen, M.S.; Myllykangas, S.; et al. Genetics and genotype-phenotype correlations in Finnish patients with dilated cardiomyopathy. Eur. Heart J. 2015, 36, 2327–2337. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods 2014, 11, 361–362. [Google Scholar] [CrossRef] [PubMed]
- Bromberg, Y.; Rost, B. SNAP: Predict effect of non-synonymous polymorphisms on function. Nucleic Acids Res. 2007, 35, 3823–3835. [Google Scholar] [CrossRef]
- Sim, N.L.; Kumar, P.; Hu, J.; Henikoff, S.; Schneider, G.; Ng, P.C. SIFT web server: Predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012, 40, W452–W457. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Capriotti, E.; Fariselli, P. PhD-SNPg: A webserver and lightweight tool for scoring single nucleotide variants. Nucleic Acids Res. 2017, 45, W247–W252. [Google Scholar] [CrossRef]
- Reese, M.G.; Eeckman, F.H.; Kulp, D.; Haussler, D. Improved splice site detection in Genie. J. Comput. Biol. 1997, 4, 311–323. [Google Scholar] [CrossRef]
- Hebsgaard, S.M.; Korning, P.G.; Tolstrup, N.; Engelbrecht, J.; Rouzé, P.; Brunak, S. Splice site prediction in Arabidopsis thaliana pre-mRNA by combining local and global sequence information. Nucleic Acids Res. 1996, 24, 3439–3452. [Google Scholar] [CrossRef]
- Wang, M.; Marín, A. Characterization and prediction of alternative splice sites. Gene 2006, 366, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Rentzsch, P.; Schubach, M.; Shendure, J.; Kircher, M. CADD-Splice-improving genome-wide variant effect prediction using deep learning-derived splice scores. Genome Med. 2021, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Houdayer, C.; Dehainault, C.; Mattler, C.; Michaux, D.; Caux-Moncoutier, V.; Pagès-Berhouet, S.; d’Enghien, C.D.; Laugé, A.; Castera, L.; Gauthier-Villars, M.; et al. Evaluation of in silico splice tools for decision-making in molecular diagnosis. Hum. Mutat. 2008, 29, 975–982. [Google Scholar] [CrossRef] [PubMed]
| All Patients (n = 105) | With Mutations (n = 63) | Without Mutations (n = 42) | ||
|---|---|---|---|---|
| Age (years) | 57.89 ± 12.12 | 55.35 ± 11.61 | 59.29 ± 12.31 | |
| Sex (M, %) | 80 | 74.6 | 88.1 | |
| Dyslipidemia (%) | 50.50 | 46.30 | 57.14 | |
| Treatment of dyslipidemia (%) | ||||
| Hypertension (%) | 46.66 | 42.85 | 52.38 | |
| Diabetes (%) | 17.15 | 17.46 | 16.66 | |
| Tobacco (%) | 69.52 | 74.60 | 61.90 | |
| AMI Localization (%) | Anterior | 56 | 60,30 | 50 |
| Septal | 2 | 1.50 | 2.40 | |
| Inferior | 35 | 30.20 | 42.80 | |
| Posterior | 1 | 1.50 | 0 | |
| Lateral | 4 | 5 | 2.40 | |
| Indeterminate | 2 | 1.50 | 2.40 | |
| Vessels affected (%) | 1 | 51 | 47.60 | 57.20 |
| 2 | 22 | 22.20 | 21.40 | |
| 3 | 27 | 30.20 | 21.40 | |
| Time of Ischemia (%) | <120 min | 17 | 13 | 21.40 |
| 120–360 min | 76 | 81 | 71.50 | |
| >360 min | 7 | 6 | 7.10 |
| Gene | Position | dbSNP Code | c.HGVS | Type | p.HGVS | References | Described Pathology in HGMD |
|---|---|---|---|---|---|---|---|
| ABCA1 | 9:107558416 | rs528270977 | c.5300A>G | Missense | p.Y1767C | [23] | Reduced total cholesterol |
| 9:107589238 | rs138880920 | c.2328G>C | Missense | p.K776N | [24,25,26] | Increased risk of ischemic heart disease | |
| 9:107599376 | rs9282543 | c.1196T>C | Missense | p.V399A | [27,28,29] | Tangier disease | |
| 9:107646756 | rs145183203 | c.254C>T | Missense | p.P85L | [29,30,31] | HDL deficiency | |
| ABCG5 | 2:44065739 | rs56204478 | c.80G>C | Missense | p.G27A | [32,33] | Hypercholesterolaemia |
| ABCG8 | 2:44101610 | rs370422066 | c.1476T>A | Stop gained | p.Y492* | [34] | Phytosterolaemia |
| 2:44102301 | rs761153163 | c.1505C>T | Missense | p.P502L | [35] | Sitosterolaemia | |
| ANGPTL4 | 19:8436373 | rs140744493 | c.1006C>T | Missense | p.R336C | [28,36,37] | Lower plasma triglyceride level |
| APOA4 | 11:116691720 | rs147577451 | c.1054A>T | Missense | p.N352Y | [34] | High triglyceride |
| 11:116692293 | rs12721043 | c.481G>T | Missense | p.A161S | [32,37,38] | Hyperlipidaemia | |
| APOB | 2:21225354 | rs72654423 | c.12940A>G | Missense | p.I4314V | [39] | Hypercholesterolaemia |
| 2:21228263 | rs61744153 | c.11477C>T | Missense | p.T3826M | [32,40,41] | Hypertriglyceridaemia | |
| 2:21228339 | rs12713540 | c.11401T>A | Missense | p.S3801T | [40] | Hypercholesterolaemia | |
| 2:21230828 | rs72653098 | c.8912A>C | Missense | p.N2971T | [42,43] | Familial hypercholesterolemia | |
| 2:21231278 | rs72653095 | c.8462C>T | Missense | p.P2821L | [44,45] | Hypocholesterolaemia | |
| 2:21232455 | rs72653092 | c.7285T>A | Missense | p.S2429T | [46,47,48] | Hypertriglyceridaemia | |
| 2:21234674 | rs151009667 | c.5066G>A | Missense | p.R1689H | [46,49] | Hypertriglyceridaemia | |
| 2:21238367 | rs12713843 | c.3383G>A | Missense | p.R1128H | [29,50,51] | Hypobetalipoproteinaemia | |
| 2:21238413 | rs12713844 | c.3337G>C | Missense | p.D1113H | [37,51,52] | Hypobetalipoproteinaemia | |
| 2:21249682 | rs12714192 | c.2222C>A | Missense | p.T741N | [37] | Dyslipidaemia | |
| 2:21260934 | rs6752026 | c.433C>T | Missense | p.P145S | [37] | Dyslipidaemia | |
| APOC2 | 19:45452024 | rs120074114 | c.122A>C | Missense | p.K41T | [29,37,53] | Apolipoprotein C2 deficiency |
| APOE | 19:45411110 | rs769452 | c.137T>C | Missense | p.L46P | [48] | Hypercholesterolaemia |
| APOH | 17:64210599 | rs150652035 | c.973T>G | Missense | p.C325G | [54,55,56] | Apolipoprotein H deficiency |
| CD36 | 7:80292426 | rs138897347 | c.550G>A | Missense | p.D184N | [57] | CD36 deficiency |
| CYP27A1 | 2:219679730 | rs374507635 | c.1573C>T | Stop gained | p.Q525* | [58] | Cerebrotendinous xanthomatosis |
| LDLR | 19:11217352 | rs143992984 | c.806G>A | Missense | p.G269D | [48,59,60] | Hypercholesterolaemia |
| 19:11227604 | rs137929307 | c.1775G>A | Missense | p.G592E | [48,61,62] | Hypercholesterolaemia | |
| 19:11233886 | rs45508991 | c.2177C>T | Missense | p.T726I | [63,64,65] | Hypercholesterolaemia | |
| LIPA | 10:90988005 | rs544080483 | c.380G>A | Missense | p.R127Q | [66] | Hypercholesterolaemia |
| LIPG | 18:47109939 | rs138438163 | c.1171G>A | Missense | p.E391K | [34,67,68] | Higher plasma HDL cholesterol |
| 18:47109955 | rs77960347 | c.1187A>G | Missense | p.N396S | [34,69,70] | Higher plasma HDL cholesterol | |
| LPA | 6:160966559 | rs139145675 | c.5311C>T | Missense | p.R1771C | [71] | Plasminogen deficiency |
| 6:160969693 | rs143431368 | c.4974-2A>G | Splice acceptor | - | [31,72] | Lowered human lipoprotein(a) levels | |
| LRP2 | 2:170042245 | rs35734447 | c.9613A>G | Missense | p.N3205D | [73] | Hypoplastic left heart syndrome |
| NPC2 | 14:74953134 | rs151220873 | c.88G>A | Missense | p.V30M | [74,75,76] | Niemann-Pick disease, type C2 |
| SORT1 | 1:109910100 | rs61797119 | c.370A>G | Missense | p.I124V | [32,77] | Hypercholesterolaemia |
| Gene | Position | dbSNP Code | cHGVS | Type | pHGVS | In Silico Prediction Programs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M.T. | SNAP2 | SIFT | PP2 | PhD-SNP | BDGP | NetGene2 | ASSP | C. Splice | ||||||
| ABCA1 | 9:107549242 | rs1230573600 | c.6220G>A | Missense | p.G2074S | PP (0.999) | PP (69) | PP (0.00) | PP (0.999) | PP (7) | ||||
| 9:107550232 | 9:107550232 | c.6173C>G | Missense | p.A2058G | PP (0.999) | PP (31) | PP (0.00) | PP (0.998) | PP (6) | |||||
| 9:107599296 | rs201586430 | c.1276T>C | Missense | p.F426L | PP (0.999) | PP (50) | PP (0.00) | PP (0.999) | PP (4) | |||||
| ABCG5 | 2:44040359 | rs137996263 | c.1852T>C | Missense | p.S618P | PP (0.998) | PP (51) | PP (0.00) | PP (0.945) | PP (8) | ||||
| 2:44051085 | 2:44051085 | c.1291C>G | Missense | p.P431A | PP (0.999) | PP (9) | NPP (0.94) | PP (1) | NPP (4) | |||||
| ANGPTL4 | 19:8435981 | rs866158597 | c.703G>A | Missense | p.V235M | PP (0.999) | PP (28) | NPP (0.14) | PP (0.999) | PP (7) | ||||
| APOB | 2:21225938 | 2:21225938 | c.12356C>A | Missense | p.A4119D | NPP (0.999) | PP (56) | PP (0.00) | PP (0.989) | PP (1) | ||||
| 2:21227295 | rs1458765902 | c.11933T>C | Missense | p.I3978T | PP (0.999) | PP (63) | PP (0.00) | PP (0.977) | PP (0) | |||||
| 2:21229970 | rs146178619 | c.9770A>G | Missense | p.N3257S | PP (0.984) | PP (4) | PP (0.00) | NPP (0.073) | PP (5) | |||||
| 2:21230600 | rs61742323 | c.9140C>T | Missense | p.T3047M | NPP (0.999) | PP (17) | PP (0.00) | PP (0.625) | NPP (6) | |||||
| 2:21233163 | 2:21233163 | c.6577G>T | Missense | p.D2193Y | NPP (0.999) | PP (42) | PP (0.00) | NPP (0.396) | PP (0) | |||||
| 2:21238007 | rs61736761 | c.3634C>A | Missense | p.L1212M | NPP (0.989) | PP (10) | PP (0.00) | PP (1) | NPP (7) | |||||
| 2:21246505 | rs773987185 | c.2496G>A | Missense | p.M832I | PP (0.738) | NPP (-55) | PP (0.03) | PP (0.592) | NPP (4) | |||||
| CD36 | 7:80276161 | rs754478799 | c.107del | Frameshift variant | p.K36Rfs*41 | PP (1) | - | - | - | - | ||||
| 7:80293767 | rs201715989 | c.655G>T | Missense | p.D219Y | PP (0.998) | PP (47) | PP (0.01) | NPP (0.139) | PP (9) | |||||
| 7:80299343 | rs748146857 | c.818+5G>A | Splicing variant | - | PP (1) | Diff. 24.24% | Diff. 34.04% | Diff. 11.06% | PP (21.3) | |||||
| CYP7A1 | CYP7A1/8:59405037 | rs149291486 | c.1090C>T | Missense | p.R364W | PP (0.999) | PP (93) | PP (0.00) | PP (1) | PP (9) | ||||
| LCAT | 16:67976376 | rs1186446170 | c.638A>G | Missense | p.Y213C | PP (0.989) | PP (29) | PP (0.01) | PP (1) | PP (6) | ||||
| LIPC | 15:58838165 | rs540524619 | c.799G>T | Missense | p.G267C | PP (0.999) | PP (60) | PP (0.01) | PP (1) | PP (8) | ||||
| LPA | 6:160966559 | rs139145675 | c.5311C>T | Missense | p.R1771C | PP (0.999) | PP (20) | PP (0.00) | PP (1) | PP (7) | ||||
| 6:160969591 | rs757921434 | c.5074C>T | Stop gained | p.R1692* | PP (1) | - | - | - | - | |||||
| 6:160998167 | rs200099994 | c.4631+1G>A | Splicing variant | - | PP (1) | Diff. >20% | - | Diff. >20% | PP (31) | |||||
| 6:161006084 | rs76144756 | c.4283C>T | Missense | p.P1428L | NPP (0.996) | PP (33) | PP (0.02) | PP (1) | PP (5) | |||||
| LPL | 8:19819628 | rs116403115 | c.1325T>G | Missense, | p.V442G | PP (0.999) | PP (22) | PP (0.02) | PP (1) | NPP (3) | ||||
| LRP1 | 12:57549979 | rs750499142 | c.1330C>T | Missense | p.R444C | PP (0.999) | PP (44) | PP (0.00) | PP (1) | PP (8) | ||||
| 12:57577915 | rs141826184 | c.5977C>T | Missense | p.R1993W | PP (0.971) | PP (58) | PP (0.00) | PP (1) | PP (7) | |||||
| 12:57587039 | rs113379328 | c.7636G>A | Missense | p.G2546S | PP (0.996) | NPP (-19) | NPP (0.58) | PP (0.742) | PP (3) | |||||
| 12:57599365 | rs149488896 | c.11495G>C | Missense | p.G3832A | PP (0.997) | PP (26) | PP (0.04) | PP (0.999) | NPP (2) | |||||
| 12:57601936 | rs755903131 | c.11975G>A | Missense | p.R3992H | PP (0.999) | PP (6) | NPP (0.22) | PP (0.998) | PP (7) | |||||
| 12:57606021 | rs142605462 | c.13471G>C | Missense | p.D4491H | PP (0.999) | PP (25) | NPP (0.15) | PP (1) | NPP (4) | |||||
| LRP2 | 2:169997031 | rs746070288 | c.13133C>T | Missense | p.P4378L | PP (0.999) | PP (27) | NPP (0.17) | PP (1) | PP (1) | ||||
| 2:170034493 | rs145432614 | c.10213G>A | Missense | p.G3405R | PP (0.999) | PP (28) | NPP (0.48) | PP (0.907) | PP (4) | |||||
| 2:170037997 | rs1248351989 | c.10130A>C | Missense | p.D3377A | PP (0.999) | PP (38) | NPP (0.25) | PP (1) | PP (1) | |||||
| 2:170058335 | rs750566206 | c.8255G>A | Missense | p.R2752Q | PP (0.999) | PP (14) | PP (0.00) | PP (0.999) | PP (5) | |||||
| 2:170163815 | rs142594441 | c.403G>A | Missense | p.D135N | PP (0.999) | PP (2) | PP (0.00) | PP (1) | PP (7) | |||||
| LRPAP1 | 4:3519802 | rs760183295 | c.710G>A | Missense | p.R237H | PP (0.999) | PP (13) | - | PP (0.546) | NPP (3) | ||||
| 4:3521804 | rs141393177 | c.466C>T | Missense | p.H156Y | PP (0.996) | PP (10) | - | PP (0.934) | NPP (9) | |||||
| NPC1 | 18:21152041 | rs762610198 | c.284C>T | Missense | p.S95F | PP (0.999) | PP (23) | PP (0.01) | NPP (0.022) | PP (3) | ||||
| PCSK9 | 1:55521765 | 1:55521765 | c.899C>T | Missense | p.A300V | PP (0.999) | PP (66) | PP (0.01) | PP (1) | PP (7) | ||||
| PLTP | 20:44530943 | rs6065903 | c.1138C>T | Missense | p.R380W | NPP (0.551) | PP (68) | PP (0.00) | PP (1) | NPP (6) | ||||
| STAR | 8:38005810 | rs748942681 | c.214G>A | Missense | p.E72K | PP (0.999) | PP (65) | PP (0.02) | NPP (0.358) | PP (1) | ||||
| TSPO | 22:43557122 | rs746919529 | c.247G>C | Missense | p.G83R | PP (0.999) | PP (1) | NPP (0.28) | PP (1) | PP (4) | ||||
| 22:43557156 | rs142445069 | c.281C>T | Missense | p.A94V | PP (0.999) | PP (37) | NPP (0.36) | PP (0.805) | PP (5) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan-Lizcano, R.; Mariñas-Pardo, L.; Núñez, L.; Rebollal-Leal, F.; López-Vázquez, D.; Pereira, A.; Molina-Nieto, A.; Calviño, R.; Vázquez-Rodríguez, J.M.; Hermida-Prieto, M. Rare Variants in Genes of the Cholesterol Pathway Are Present in 60% of Patients with Acute Myocardial Infarction. Int. J. Mol. Sci. 2022, 23, 16127. https://doi.org/10.3390/ijms232416127
Pan-Lizcano R, Mariñas-Pardo L, Núñez L, Rebollal-Leal F, López-Vázquez D, Pereira A, Molina-Nieto A, Calviño R, Vázquez-Rodríguez JM, Hermida-Prieto M. Rare Variants in Genes of the Cholesterol Pathway Are Present in 60% of Patients with Acute Myocardial Infarction. International Journal of Molecular Sciences. 2022; 23(24):16127. https://doi.org/10.3390/ijms232416127
Chicago/Turabian StylePan-Lizcano, Ricardo, Luis Mariñas-Pardo, Lucía Núñez, Fernando Rebollal-Leal, Domingo López-Vázquez, Ana Pereira, Aranzazu Molina-Nieto, Ramón Calviño, Jose Manuel Vázquez-Rodríguez, and Manuel Hermida-Prieto. 2022. "Rare Variants in Genes of the Cholesterol Pathway Are Present in 60% of Patients with Acute Myocardial Infarction" International Journal of Molecular Sciences 23, no. 24: 16127. https://doi.org/10.3390/ijms232416127
APA StylePan-Lizcano, R., Mariñas-Pardo, L., Núñez, L., Rebollal-Leal, F., López-Vázquez, D., Pereira, A., Molina-Nieto, A., Calviño, R., Vázquez-Rodríguez, J. M., & Hermida-Prieto, M. (2022). Rare Variants in Genes of the Cholesterol Pathway Are Present in 60% of Patients with Acute Myocardial Infarction. International Journal of Molecular Sciences, 23(24), 16127. https://doi.org/10.3390/ijms232416127








