Declines in Reproductive Condition of Male Largemouth Bass (Micropterus salmoides) Following Seasonal Exposure to Estrogenic Endocrine-Disrupting Compounds
Abstract
1. Introduction
2. Results
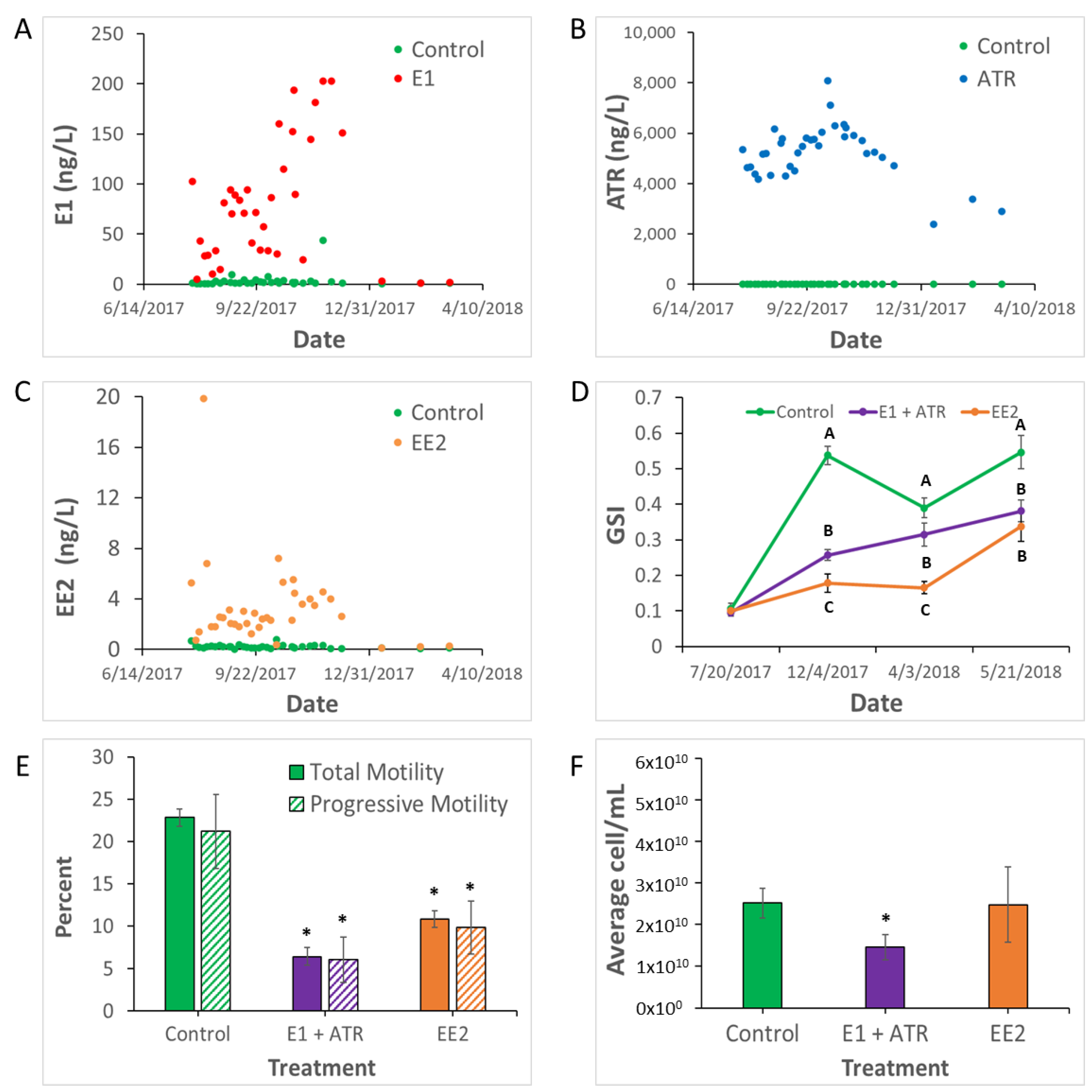
2.1. Exposure Analysis
2.2. Reproductive Condition
3. Discussion
4. Materials and Methods
4.1. Exposure and Animal Care
4.2. Dosing and Water Chemical Analysis
4.3. Fish Collection and Processing
4.4. Sperm Count Analysis
4.5. Sperm Motility Analysis
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hinck, J.E.; Blazer, V.S.; Schmitt, C.J.; Papoulias, D.M.; Tillitt, D.E. Widespread occurrence of intersex in black basses (Micropterus spp.) from U.S. rivers, 1995–2004. Aquat. Toxicol. 2009, 95, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Iwanowicz, L.R.; Blazer, V.S.; Pinkney, A.E.; Guy, C.P.; Major, A.M.; Munney, K.; Mierzykowski, S.; Lingenfelser, S.; Secord, A.; Patnode, K.; et al. Evidence of estrogenic endocrine disruption in smallmouth and largemouth bass inhabiting Northeast U.S. national wildlife refuge waters: A reconnaissance study. Ecotoxicol. Environ. Saf. 2016, 124, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Kellock, K.A.; Trushel, B.E.; Ely, P.C.; Jennings, C.A.; Bringolf, R.B. Survey of Intersex Largemouth Bass from Impoundments in Georgia USA. Trans. Am. Fish Soc. 2014, 143, 565–572. [Google Scholar] [CrossRef]
- Yonkos, L.T.; Friedel, E.A.; Fisher, D.J. Intersex (testicular oocytes) in largemouth bass (Micropterus salmoides) on the Delmarva Peninsula, USA. Environ. Toxicol. Chem. 2014, 33, 1163–1169. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Blazer, V.S.; Gray, J.L.; Focazio, M.J.; Young, J.A.; Alvarez, D.A.; Iwanowicz, L.R.; Foreman, W.T.; Furlong, E.T.; Speiran, G.K.; et al. Chemical contaminants in water and sediment near fish nesting sites in the Potomac River basin: Determining potential exposures to smallmouth bass (Micropterus dolomieu). Sci. Total Environ. 2013, 443, 700–716. [Google Scholar] [CrossRef]
- Hansen, S.P.; Messer, T.L.; Mittelstet, A.R. Mitigating the risk of atrazine exposure: Identifying hot spots and hot times in surface waters across Nebraska, USA. J. Environ. Manag. 2019, 250, 109424. [Google Scholar] [CrossRef]
- Smalling, K.L.; Devereux, O.H.; Gordon, S.E.; Phillips, P.J.; Blazer, V.S.; Hladik, M.L.; Kolpin, D.W.; Meyer, M.T.; Sperry, A.J.; Wagner, T. Environmental and anthropogenic drivers of contaminants in agricultural watersheds with implications for land management. Sci. Total Environ. 2021, 774, 145687. [Google Scholar] [CrossRef]
- McClure, C.M.; Smalling, K.L.; Blazer, V.S.; Sperry, A.J.; Schall, M.K.; Kolpin, D.W.; Phillips, P.J.; Hladik, M.L.; Wagner, T. Spatiotemporal variation in occurrence and co-occurrence of pesticides, hormones, and other organic contaminants in rivers in the Chesapeake Bay Watershed, United States. Sci. Total Environ. 2020, 728, 138765. [Google Scholar] [CrossRef]
- Leet, J.K.; Lee, L.S.; Gall, H.E.; Goforth, R.R.; Sassman, S.; Gordon, D.A.; Lazorchak, J.M.; Smith, M.E.; Jafvert, C.T.; Sepulveda, M.S. Assessing impacts of land-applied manure from concentrated animal feeding operations on fish populations and communities. Environ Sci Technol 2012, 46, 13440–13447. [Google Scholar] [CrossRef]
- Blazer, V.; Iwanowicz, D.; Walsh, H.; Sperry, A.; Iwanowicz, L.; Alvarez, D.; Brightbill, R.; Smith, G.; Foreman, W.; Manning, R. Reproductive health indicators of fishes from Pennsylvania watersheds: Association with chemicals of emerging concern. Environ Monit Assess 2014, 186, 6471–6491. [Google Scholar] [CrossRef]
- Gall, H.E.; Sassman, S.A.; Lee, L.S.; Jafvert, C.T. Hormone discharges from a midwest tile-drained agroecosystem receiving animal wastes. Environ Sci Technol 2011, 45, 8755–8764. [Google Scholar] [CrossRef] [PubMed]
- Leet, J.K.; Gall, H.E.; Sepulveda, M.S. A review of studies on androgen and estrogen exposure in fish early life stages: Effects on gene and hormonal control of sexual differentiation. J. Appl. Toxicol. 2011, 31, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Ankley, G.T.; Feifarek, D.; Blackwell, B.; Cavallin, J.E.; Jensen, K.M.; Kahl, M.D.; Poole, S.; Randolph, E.; Saari, T.; Villeneuve, D.L. Re-evaluating the Significance of Estrone as an Environmental Estrogen. Environ. Sci. Technol. 2017, 51, 4705–4713. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Silva, M.G.; Soares, A.; Freitas, R. Concentrations levels and effects of 17alpha-Ethinylestradiol in freshwater and marine waters and bivalves: A review. Environ. Res. 2020, 185, 109316. [Google Scholar] [CrossRef]
- Kidd, K.A.; Blanchfield, P.J.; Mills, K.H.; Palace, V.P.; Evans, R.E.; Lazorchak, J.M.; Flick, R.W. Collapse of a fish population after exposure to a synthetic estrogen. Proc. Natl. Acad Sci. USA 2007, 104, 8897–8901. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.A.; Papoulias, D.M.; Mehinto, A.; Kroll, K.J.; Denslow, N.D.; Tillitt, D.E. Gene expression profiles in male largemouth bass induced by chronic exposure to an environmentally relevant level of 17α-ethinylestradiol. In Proceedings of the 32nd Annual Meeting of the Society of Environmental Toxicology and Chemistry (SETAC) North America, Boston, MA, USA, 13–17 November 2011. [Google Scholar]
- Clugston, J.P. Centrarchid spawning in the Florida Everglades. Q. J. Fla. Acad Sci. 1966, 29, 137–143. [Google Scholar]
- Rosenblum, P.; Brandt, T.; Mayes, K.; Hutson, P. Annual cycles of growth and reproduction in hatchery–reared Florida largemouth bass, Micropterus salmoides floridanus, raised on forage or pelleted diets. J. Fish Biol. 1994, 44, 1045–1059. [Google Scholar] [CrossRef]
- Martyniuk, C.J.; Kroll, K.J.; Porak, W.F.; Steward, C.; Grier, H.J.; Denslow, N.D. Seasonal relationship between gonadotropin, growth hormone, and estrogen receptor mRNA expression in the pituitary gland of largemouth bass. Gen. Comp. Endocrinol. 2009, 163, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.W.; de Franca, L.R.; Lareyre, J.J.; Le Gac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef]
- Jenkins, J.A. Male germplasm in relation to environmental conditions: Synoptic focus on DNA. In Cryopreservation in Aquatic Species; The World Aquaculture Society: Baton Rouge, LA, USA, 2011; pp. 227–239. [Google Scholar]
- Jenkins, J.A.; Rosen, M.R.; Draugelis-Dale, R.O.; Echols, K.R.; Torres, L.; Wieser, C.M.; Kersten, C.A.; Goodbred, S.L. Sperm quality biomarkers complement reproductive and endocrine parameters in investigating environmental contaminants in common carp (Cyprinus carpio) from the Lake Mead National Recreation Area. Environ. Res. 2018, 163, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.M.H.; Barzegar-Fallah, S.; Rahdar, P.; Ahmadi, M.M.; Yavari, M.; Hatef, A.; Golshan, M.; Linhart, O. A Review on Environmental Contaminants-Related Fertility Threat in Male Fishes: Effects and Possible Mechanisms of Action Learned from Wildlife and Laboratory Studies. Animals 2021, 11, 2817. [Google Scholar] [CrossRef] [PubMed]
- Blazer, V.S.; Iwanowicz, L.R.; Henderson, H.; Mazik, P.M.; Jenkins, J.A.; Alvarez, D.A.; Young, J.A. Reproductive endocrine disruption in smallmouth bass (Micropterus dolomieu) in the Potomac River basin: Spatial and temporal comparisons of biological effects. Environ. Monit. Assess. 2012, 184, 4309–4334. [Google Scholar] [CrossRef] [PubMed]
- Martyniuk, C.J.; Feswick, A.; Munkittrick, K.R.; Dreier, D.A.; Denslow, N.D. Twenty years of transcriptomics, 17alpha-ethinylestradiol, and fish. Gen. Comp. Endocrinol. 2020, 286, 113325. [Google Scholar] [CrossRef]
- Leet, J.K.; Richter, C.A.; Gale, R.W.; Tillitt, D.E. Water Chemistry and Fish Metrics Data for Adult Largemouth Bass Exposed in Outdoor Ponds to 17alpha-ethinylestradiol or an Estrone-Atrazine Mixture; U.S. Geological Survey: Reston, VA, USA, 2022. [CrossRef]
- Jenkins, J.A.; Leet, J.K. Motility of Sperm from Adult Largemouth Bass Pond Exposure to 17 alpha-ethinylestradiol or Estrone-Atrazine Mixture (2018); U.S. Geological Survey: Reston, VA, USA, 2022. [CrossRef]
- Timmons, T.J.; Shelton, W.L.; Davies, W.D. Gonad Development, Fecundity, and Spawning Season of Largemouth Bass in Newly Impounded West Point Reservoir, Alabama-Georgia; US Department of the Interior, Fish and Wildlife Service: Washington, DC, USA, 1980; Volume 100.
- Bringolf, R.B.; Belden, J.B.; Summerfelt, R.C. Effects of atrazine on fathead minnow in a short-term reproduction assay. Environ. Toxicol. Chem. 2004, 23, 1019–1025. [Google Scholar] [CrossRef]
- Montgomery, T.M.; Brown, A.C.; Gendelman, H.K.; Ota, M.; Clotfelter, E.D. Exposure to 17alpha-ethinylestradiol decreases motility and ATP in sperm of male fighting fish Betta splendens. Environ. Toxicol. 2014, 29, 243–252. [Google Scholar] [CrossRef]
- Parrott, J.L.; Blunt, B.R. Life-cycle exposure of fathead minnows (Pimephales promelas) to an ethinylestradiol concentration below 1 ng/L reduces egg fertilization success and demasculinizes males. Environ. Toxicol. 2005, 20, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Schultz, I.R.; Skillman, A.; Nicolas, J.M.; Cyr, D.G.; Nagler, J.J. Short-term exposure to 17α-ethynylestradiol decreases the fertility of sexually maturing male rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 2003, 22, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Denny, J.S.; Tapper, M.A.; Schmieder, P.K.; Hornung, M.W.; Jensen, K.M.; Ankley, G.T.; Henry, T.R. Comparison of relative binding affinities of endocrine active compounds to fathead minnow and rainbow trout estrogen receptors. Environ. Toxicol. Chem. 2005, 24, 2948–2953. [Google Scholar] [CrossRef]
- Hatef, A.; Alavi, S.M.; Golshan, M.; Linhart, O. Toxicity of environmental contaminants to fish spermatozoa function in vitro—A review. Aquat. Toxicol. 2013, 140–141, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Cleary, J.A.; Tillitt, D.E.; Vom Saal, F.S.; Nicks, D.K.; Claunch, R.A.; Bhandari, R.K. Atrazine induced transgenerational reproductive effects in medaka (Oryzias latipes). Environ. Pollut. 2019, 251, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Goodbred, S.L.; Patino, R.; Torres, L.; Echols, K.R.; Jenkins, J.A.; Rosen, M.R.; Orsak, E. Are endocrine and reproductive biomarkers altered in contaminant-exposed wild male Largemouth Bass (Micropterus salmoides) of Lake Mead, Nevada/Arizona, USA? Gen. Comp. Endocrinol. 2015, 219, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Leet, J.K.; Greer, J.; Richter, C.A.; Iwanowicz, I.; Spinard, E.; McDondald, J.; Conway, C.; Gale, R.; Tillitt, D.E.; Hansen, J. Exposure to 17α-ethinylestradiol results in differential susceptibility of largemouth bass (Micropterus salmoides) to bacterial infection. Environ. Sci. Technol. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leet, J.K.; Richter, C.A.; Gale, R.W.; Tillitt, D.E.; Jenkins, J.A. Declines in Reproductive Condition of Male Largemouth Bass (Micropterus salmoides) Following Seasonal Exposure to Estrogenic Endocrine-Disrupting Compounds. Int. J. Mol. Sci. 2022, 23, 16131. https://doi.org/10.3390/ijms232416131
Leet JK, Richter CA, Gale RW, Tillitt DE, Jenkins JA. Declines in Reproductive Condition of Male Largemouth Bass (Micropterus salmoides) Following Seasonal Exposure to Estrogenic Endocrine-Disrupting Compounds. International Journal of Molecular Sciences. 2022; 23(24):16131. https://doi.org/10.3390/ijms232416131
Chicago/Turabian StyleLeet, Jessica K., Catherine A. Richter, Robert W. Gale, Donald E. Tillitt, and Jill A. Jenkins. 2022. "Declines in Reproductive Condition of Male Largemouth Bass (Micropterus salmoides) Following Seasonal Exposure to Estrogenic Endocrine-Disrupting Compounds" International Journal of Molecular Sciences 23, no. 24: 16131. https://doi.org/10.3390/ijms232416131
APA StyleLeet, J. K., Richter, C. A., Gale, R. W., Tillitt, D. E., & Jenkins, J. A. (2022). Declines in Reproductive Condition of Male Largemouth Bass (Micropterus salmoides) Following Seasonal Exposure to Estrogenic Endocrine-Disrupting Compounds. International Journal of Molecular Sciences, 23(24), 16131. https://doi.org/10.3390/ijms232416131




